Abstract
Left atrial enlargement is an independent risk factor for ischemic stroke in patients with atrial fibrillation. Little is known regarding the association between nighttime blood pressure variability and left atrial enlargement in patients with atrial fibrillation and preserved ejection fraction. The study population consisted of 140 consecutive patients with atrial fibrillation (mean age 64 ± 10 years) with preserved ejection fraction (≥50%). Nighttime blood pressure was measured at hourly intervals, using a home blood pressure monitoring device. Nighttime blood pressure variability was expressed as the standard deviation of all readings. Left atrial volume index was measured using the modified Simpson’s biplane method with transthoracic echocardiography. Multiple regression analysis indicated that nighttime mean systolic/diastolic blood pressure and its variability remained independently associated with left atrial enlargement after adjustment for age, sex, anti-hypertensive medication class, and left ventricular mass index (P < 0.01). When patients were divided into four groups according to nighttime blood pressure and its variability, the group with higher nighttime blood pressure and its variability had significantly larger left atrial volume than the group with lower nighttime blood pressure and its variability (46.6 ml/m2 vs. 35.0 ml/m2, P < 0.0001). Higher nighttime blood pressure and its variability are associated with left atrial enlargement. The combination of nighttime blood pressure and its variability has additional predictive value for left atrial enlargement. Intensive intervention for these high-risk patients may avoid or delay progression of left atrial enlargement and reduce the risk of stroke.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common arrhythmia, and its prevalence and incidence rates are gradually increasing worldwide [1, 2]. AF is a strong risk factor for stroke, which is linked to poor prognosis and higher healthcare costs [3, 4]. Although direct oral anticoagulant therapy markedly reduces the incidence of stroke and mortality, a substantial number of patients are still exposed to the risk of cerebral infarction [5, 6]. Therefore, the identification of other therapeutic targets is expected to improve long-term morbidity or mortality in patients with AF.
Left atrial (LA) enlargement, which is a subclinical abnormality that is common in patients with AF, has been identified as an independent risk factor for ischemic stroke [7, 8]. Moreover, LA enlargement is associated with the severity of neurologic deficit after ischemic stroke [9]. In general, LA enlargement is a progressive process; therefore, the identification of therapeutic targets to inhibit or slow the progression of LA enlargement may help improve long-term morbidity or mortality in patients with AF.
Blood pressure (BP) has proven to be an important determinant of LA enlargement [10]. Conventional single-BP measurement cannot reflect the patient’s intrinsic BP characteristics because of inadequate sampling and the possibility of falsely elevated reading due to emotional components, such as the white coat effect [11]. An alternative method is nighttime BP monitoring, which allows multiple measurements under more stable and standardized conditions. Several studies demonstrated that target organ damage or prognosis is more closely associated with nighttime BP than with daytime BP or single BP measurements [12,13,14,15,16]. Moreover, multiple BP measurements provide information about variability, which is not obtained from a single BP measurement. Increased nighttime BP variability was proven to be associated with atherosclerosis and subsequent stroke [11, 13, 17, 18]. However, the association between nighttime BP variables and LA enlargement has not been addressed in patients with AF.
The aim of the present study was to assess the correlation between nighttime BP variables (average values and variability) and LA enlargement in patients with AF and preserved ejection fraction (EF).
Methods
Study population
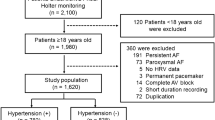
This study was designed as a cross-sectional study of prospectively collected data and conducted at Osaka City University Hospital. The study population consisted of 140 consecutive patients with AF (mean age, 64 ± 10 years) with preserved EF (EF ≥ 50%) who were scheduled to undergo catheter ablation or cardioversion between April 2013 and December 2016. We excluded patients with moderate or severe valvular heart disease. Among the variables used in the analyses, hypertension was defined as systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, and/or antihypertensive medication use. Dyslipidemia was defined as low-density lipoprotein cholesterol level ≥ 140 mg/dl and/or use of lipid-lowering medication. Diabetes mellitus was defined as fasting glucose level ≥ 126 mg/dl, glycated hemoglobin A1c level ≥ 6.5%, and/or current use of insulin or oral hypoglycemic agents. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the hospital ethics committee of Osaka City University Graduate School of Medicine. Written informed consent was obtained from all patients.
Blood pressure assessment
We defined casual BP as the value measured at the time of admission for catheter ablation or cardioversion by trained nurses, using an oscillometric device (ES-H55, Terumo Corporation, Tokyo, Japan), with the patient in a sitting position and after 5 min rest. Nighttime BP was measured using a home BP monitoring device (HEM-5041, Omron Healthcare, Kyoto, Japan) during the first night of hospitalization. Therefore, both measurements were performed before catheter ablation or cardioversion. The device was set to automatically record BP at hourly intervals during sleep hours (from 11 pm to 6 am). The mean systolic/diastolic BP in a sleep period was calculated. Nighttime BP variability was calculated as the standard deviation (SD) of all nighttime BP readings.
Echocardiography
Transthoracic echocardiography examinations were performed by the investigators who were blinded to BP status before catheter ablation or cardioversion using commercially available systems equipped with a high-frequency transducer. All patients underwent a comprehensive examination, and anatomic measurements were made according to the recommendations of the American Society of Echocardiography [19]. We evaluated LA volume with the biplane modified Simpson method and LA volume was indexed by the body surface area [19]. Left ventricular mass was calculated using the Devereux formula and indexed by body surface area [20].
Statistical analysis
Continuous variables are expressed as mean values ± standard deviation and categorical variables are reported as percentages. Linear regression analysis was performed to examine the correlation between LA volume index and clinical, echocardiographic, and BP variables. Multiple regression analysis adjusted for age, gender, anti-hypertensive medication class, and left ventricular index was carried out to identify BP variables associated with LA volume index. BP variables were entered into multiple regression analysis separately. A P value of <0.05 was considered statistically significant for all tests.
Results
Study cohort
Among the 161 patients with AF and preserved EF, 21 did not have nighttime BP data because of refusal (n = 19) or inability to complete the test (n = 2). This left a final sample size of 140 (mean age 64 ± 10 years; 74% male), of whom 82 (59%) had paroxysmal and 58 (41%) had persistent AF. Clinical characteristics are listed in Table 1. BP and echocardiographic indices are shown in Table 2. The percentage of antihypertensive medications use was 66% (only in the morning; 46%, only in the evening; 4%, and both in the morning and in the evening; 16%). The study population consisted of patients with hypertension (74%) that was well controlled (nighttime mean systolic BP, 124 ± 17 mmHg), and a moderately dilated LA (LA volume index, 40.4 ± 12.9 ml/m2), without coexistence of left ventricular hypertrophy (left ventricular mass index, 80.6 ± 14.2 g/m2) [19]. Heart rate was also well controlled (nighttime average heart rate, 65.9 ± 14.1 bpm) and 99.3% of the patients had controlled heart rate (nighttime mean heart rate < 110 bpm) which is based on the European Society of Cardiology guideline 2016 [21]. Figure 1 is a representative raw data, regarding the patients with same mean BP values but their variability was different, to show the importance of repeated measurements in AF patients.
A representative raw data regarding the patients with same mean BP values but their variability was different. Nighttime systolic/diastolic BP values in patient with higher variability (shown by solid line with rhombus). Nighttime systolic/diastolic BP values in patient with lower variability (shown by dashed line with triangle)
Nighttime BP variables associated with LA volume index
Correlation between LA volume index and coronary risk factors, echocardiographic parameters, and BP parameters are presented in Table 3. Univariate analyses indicated that age (r = 0.27, P < 0.01), hypertension (r = 0.30, P < 0.0001), BNP (r = 0.48, P < 0.0001), E/e′ (r = 0.35, P < 0.0001), and left ventricular mass index (r = 0.38, P < 0.0001) were positively associated with LA volume index. Moreover, casual systolic BP (r = 0.22, P < 0.01), nighttime mean systolic BP (r = 0.41, P < 0.0001), nighttime systolic BP SD (r = 0.28, P < 0.001), nighttime mean diastolic BP (r = 0.36, P < 0.0001), and nighttime diastolic BP SD (r = 0.30, P < 0.001) were positively correlated with LA volume index. However, the duration of AF (19.0 ± 30.0 months) was not associated with LA volume index (r = 0.10, P = 0.45) in the subgroup of patients with persistent AF. Table 4 shows the BP variables associated with LA volume index by multiple regression analyses. Nighttime mean systolic/diastolic BP and their variability remained independently associated with LA volume index after adjustment for age, sex, anti-hypertensive medication class, and left ventricular mass index, while casual systolic/diastolic BP was not (Model 1). Similar results were observed in Model 2, which is an in Model 1 plus the timing of antihypertensive medication, and Model 3, which is an in Model 1 plus ejection fraction and E/e’.
The combination of nighttime BP and its variability to predict LA volume index
When all patients were divided into four groups according to mean nighttime BP values and their variability, the group with higher mean nighttime BP (mean nighttime BP ≥ 120/70 mmHg, which is based on the European Society of Hypertension guideline 2013 [22], and higher nighttime BP variability (SD ≥ 12.2/7.9 mmHg, which is based on the previous study [11]) had significantly larger LA volume index than the group with lower mean nighttime BP (mean nighttime BP < 120/70 mmHg) and lower nighttime BP variability (SD < 12.2/7.9 mmHg) (46.6 ± 13.5 ml/m2 vs. 35.0 ± 8.8 ml/m2, P < 0.0001, Fig. 2).
The combination of nighttime blood pressure (BP) and its variability to predict left atrial volume index (LAVI). Group A: the group with lower mean nighttime BP and lower nighttime BP variability Group B: the group with lower mean nighttime BP and higher nighttime BP variability Group C: the group with higher mean nighttime BP and lower nighttime BP variability Group D: the group with higher nighttime BP variability and higher nighttime BP variability A) vs. D): P < 0.0001 B) vs. D): P < 0.01 C) vs. D): P < 0.01
Other BP parameters and LA volume index
We do not have ABPM data and office BP was available only in 78% of patients. On the other hand, since we routinely measure cardio-ankle vascular index (CAVI) to assess the arterial stiffness, all patients have daytime BP variables which were measured to calculate CAVI. Daytime mean systolic and diastolic BP were 130 ± 15 and 84 ± 10 mmHg, respectively. Daytime mean systolic BP (r = 0.31, P < 0.001) and daytime mean diastolic BP (r = 0.24, P < 0.01) were significantly associated with LA volume index. Multiple regression analysis indicated that daytime mean systolic BP (β-coefficient = 0.27, P < 0.001) and daytime mean diastolic BP (β-coefficient = 0.28, P < 0.001) remained independently associated with LA volume index after adjustment for age, sex, anti-hypertensive medication class, and left ventricular mass index.
Arterial stiffness and LA volume index
There was no significant association between CAVI data (8.8 ± 1.2) and LA volume index (r = 0.15, P = 0.09). Multiple regression analysis in which CAVI was included in the same model indicated nighttime mean systolic/diastolic BP and its variability remained independently associated with LA volume index (Table 4, Model 4).
Effects of nighttime BP on left ventricular mass and cardiac function
Nighttime mean systolic BP (β-coefficient = 0.34, P < 0.0001), and nighttime mean diastolic BP (β-coefficient = 0.17, P < 0.05) were significantly associated with left ventricular mass index after adjustment for age, sex, anti-hypertensive medication, whereas nighttime systolic BP SD (β-coefficient = 0.11, P = 0.18) and nighttime diastolic BP SD (β-coefficient = 0.15, P = 0.07) were not associated with left ventricular mass index. As for diastolic function (i.e., E/e′), only nighttime mean systolic BP was associated with E/e′ (β-coefficient = 0.27, P < 0.01), whereas nighttime mean diastolic BP and BP variability were not associated with E/e′. All of the BP variables were not associated with ejection fraction.
Discussion
In the present study, we demonstrated that all of the nighttime BP variables were significantly associated with LA enlargement, which carries an increased stroke risk, after adjustment for well-known risk factors. Moreover, the combination of nighttime mean BP and its variability improved such prediction. To the best of our knowledge, this is the first study to demonstrate that nighttime mean BP and marked fluctuations of BP during sleep are associated with LA enlargement in patients with AF and preserved EF.
There is growing evidence that show the superiority of nighttime BP over daytime BP in predicting the progression of atherosclerosis, target organ damage, and cardiovascular outcome [11,12,13,14, 16, 18] The Pressioni Alteriose Monitorate e Loro Associazioni (PAMELA) study reported that nighttime BP was superior to daytime BP in predicting all-cause and cardiovascular mortality in the general population [14]. Similar results were observed for hypertensive patients, when both nighttime and daytime BP were entered simultaneously into the model [12]. Hansen et al. reported nighttime BP was a stronger predictor of cardiovascular events and total mortality than daytime BP in hypertensive patients as well as in individuals randomly selected from multi-ethnic population studies [16]. Similarly, Iwata et al. revealed that only nighttime systolic BP variability was associated with the presence of large plaque in the aortic arch, which is the risk factor for cerebral infarction, in the general population [13]. Several studies reported that nighttime BP variability, rather than daytime BP variability, was associated with cardiac events [18], and both all-cause and cardiovascular mortality [11]. The mechanisms underlying these evidence remain poorly understood. However, there are considerable mechanisms that could explain the impact of nighttime BP over daytime BP. Nighttime BP is less affected by confounding factors such as mental and physical stress, which may trigger neuroendocrine activation and sympathetic stimulation; therefore, nighttime BP may reflect the patient’s intrinsic BP characteristics than daytime BP.
In our study, higher nighttime systolic/diastolic BP was significantly associated with LA enlargement. This finding is consistent with previous studies showing the importance of arterial hypertension in predicting LA enlargement [10, 23]. Tsioufis et al. reported that office systolic BP was associated with LA dimension in patients with hypertension and normal sinus rhythm [24]. Similarly, Cuspidi et al. showed that mean nighttime systolic BP correlated with LA dimension in individuals with hypertension who were in sinus rhythm [25]. In addition, Doménech et al. revealed that nocturnal systolic/diastolic BP was an independent predictor of LA dimension in patients with non-valvular AF [26]. However, these studies were based on conventional LA dimensional assessment and did not provide detailed information on LA volume, which is a better predictor of cardiovascular outcomes [27]. Moreover, few studies have explored the effect of BP variability on LA enlargement. Furthermore, previous studies primarily focused on patients with sinus rhythm, and few studies to date have evaluated patients with AF.
In our study, higher nighttime systolic/diastolic variability was significantly associated with LA enlargement. These findings are consistent with those of previous studies showing the importance of BP variability in predicting LA enlargement [28, 29]. Cipollini et al. reported that 24-h BP variability was associated with LA dimension after adjustment for considerable cofounders in newly diagnosed hypertension with normal sinus rhythm [28]. Similarly, Tadic et al. reported that nighttime BP variability was associated with LA volume in patients with hypertension who were in sinus rhythm [29]. However, no studies to date have explored the impact of BP variability on LA volume index in patients with AF.
Our study revealed that mean nighttime systolic/diastolic BP and marked fluctuations during sleep are associated with LA volume in patients with AF and preserved EF. Considering our results, we speculated that higher nighttime BP and its variability play an important role in the progression of LA enlargement and subsequent stroke in patients with AF and preserved EF. The mechanisms underlying our findings remain poorly understood. There are, however, two considerable mechanisms that could explain the impact of BP variability on LA enlargement. First, BP variability itself may have a direct effect on LA enlargement. Exaggerated BP variability was proven to be associated with activation of cardiac angiotensin II, which leads to LA remodeling through its ability to promote LA fibrosis [30, 31]. Similarly, Tadic et al. reported that BP variability itself is associated with LA enlargement independent of BP level and left ventricular diastolic function in patients with sinus rhythm [29]. Moreover, Cipollini et al. reported that LA enlargement without left ventricular hypertrophy is an early alteration in patients with sinus rhythm showing exaggerated BP fluctuation, because the thin LA wall, rather than left ventricular wall, is subject to BP variability [28]. Second, since increased BP variability contributes, independently of BP level, to the development of left ventricular hypertrophy through mechanosensitive pathway (p125 focal adhesion kinase and p38 mitogen-activated protein kinase) or autocrine pathway (local cardiac renin and transforming growth factor-β1) [32], increased left ventricular stiffness, even within the normal limits of wall thickness, may indirectly affect the adaptive process of the LA (i.e. LA enlargement) in patients with higher nighttime BP variability [32,33,34].
Previous studies reported that arterial stiffness, which increases left ventricular filling pressure and LA pressure, was also determinant of LA size [35, 36]. However, adding CAVI as the index of arterial stiffness to the model did not significantly affect the results of multiple regression analysis. These results suggest that nighttime BP variables have a stronger association with LA volume index than arterial stiffness.
Although some previous studies, including European Society of Hypertension positon paper [37], reported the acceptable accuracy of automated BP measurement including the proportion of reading error, the variability of BP, and the repeatability coefficients in AF patients [26, 38], we need to take into account some limitations. First, because the R–R interval in each cardiac cycle is associated with ventricular filling time, stroke volume, and, thereby, BP in patients with AF [39], repeated measurements are necessary to improve the accuracy. Second, because automated diastolic BP measurement in patients with AF is reported to be slightly higher than that obtained with the manual measurement or in patients with sinus rhythm [40], careful consideration is necessary to interpret diastolic BP variables.
Limitations
Our study has several limitations. First, because we used cross-sectional data, we could not evaluate the causal relationship between BP variables and LA enlargement. Prospective studies would be necessary to assess whether BP variables indeed predict LA enlargement in patients with AF and preserved EF. Second, although some previous studies, including European Society of Hypertension position paper [37], reported the possible value of automated BP measurement in AF patients [26, 38], the accuracy of automated BP measuring device is less optimal in the setting of AF and this may affect the result. Third, although we focused only on nighttime BP variables, 24-h ambulatory BP assessments, which we lack in this study, may be better to assess BP variability. Forth, although we adjusted for the most pertinent variables that may affect LA enlargement, some confounding factors may have been incompletely adjusted for. Fifth, although we selected the first night of hospitalization for nighttime BP assessment to avoid the impact of preoperative assessment such as enhanced computed tomography with contrast medium or transesophageal echocardiography which was performed under sedation, the level of anxiety, which is highest on the first night of hospitalization, may have induced an increase in the BP variability and thus a bias of the data.
In conclusion, higher nighttime BP and its variability are independently associated with LA enlargement, which carries a greater risk of ischemic stroke in patients with AF and preserved EF. Moreover, the combination of nighttime BP and its variability had additional predictive value for LA enlargement. These findings support the hypothesis that intensive intervention for these high-risk patients may avoid or delay progression of LA enlargement and consequently reduce the risk of subsequent stroke.
References
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47.
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5.
Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154: 1449–57.
Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–92.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62.
Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD, Jr., Kopecky SL, Tsang TS, Seward JB. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26:2556–61.
Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–24.
Kim TW, Jung SW, Song IU, Koo J, Choi HS, Lee KS, Park JW, Park HJ, Kim JS. Left atrial dilatation is associated with severe ischemic stroke in men with non-valvular atrial fibrillation. J Neurol Sci. 2015;354:97–102.
McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121:667–74.
Palatini P, Reboldi G, Beilin LJ, Casiglia E, Eguchi K, Imai Y, Kario K, Ohkubo T, Pierdomenico SD, Schwartz JE, Wing L, Verdecchia P. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure-International Study. Hypertension. 2014;64:487–93.
Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61.
Iwata S, Jin Z, Schwartz JE, Homma S, Elkind MS, Rundek T, Sacco RL, Di Tullio MR. Relationship between ambulatory blood pressure and aortic arch atherosclerosis. Atherosclerosis. 2012;221:427–31.
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–83.
Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35:695–701.
Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10.
Iwata S, Sugioka K, Fujita S, Ito A, Matsumura Y, Hanatani A, Takagi M, Di Tullio MR, Homma S, Yoshiyama M. Aortic arch atherosclerosis in patients with severe aortic stenosis can be argued by greater day-by-day blood pressure variability. Atherosclerosis. 2015;241:42–47.
Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20:154–61.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:e14.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force M. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357.
Cuspidi C, Rescaldani M, Sala C. Prevalence of echocardiographic left-atrial enlargement in hypertension: a systematic review of recent clinical studies. Am J Hypertens. 2013;26:456–64.
Tsioufis C, Stougiannos P, Taxiarchou E, Skiadas I, Chatzis D, Thomopoulos C, Lalos S, Stefanadis C, Kallikazaros I. The interplay between haemodynamic load, brain natriuretic peptide and left atrial size in the early stages of essential hypertension. J Hypertens. 2006;24:965–72.
Cuspidi C, Meani S, Valerio C, Fusi V, Catini E, Sala C, Zanchetti A. Ambulatory blood pressure, target organ damage and left atrial size in never-treated essential hypertensive individuals. J Hypertens. 2005;23:1589–95.
Domenech M, Berruezo A, Molina I, Mont L, Coca A. Nighttime ambulatory blood pressure is associated with atrial remodelling and neurohormonal activation in patients with idiopathic atrial fibrillation. Rev Esp Cardiol. 2013;66:458–63.
Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–23.
Cipollini F, Arcangeli E, Seghieri G. Left atrial dimension is related to blood pressure variability in newly diagnosed untreated hypertensive patients. Hypertens Res. 2016;39:583–7.
Tadic M, Cuspidi C, Ilic I, Suzic-Lazic J, Zivanovic V, Jozika L, Celic V. The relationship between blood pressure variability, obesity and left atrial phasic function in hypertensive population. Int J Cardiovasc Imaging. 2016;32:603–12.
Kudo H, Kai H, Kajimoto H, Koga M, Takayama N, Mori T, Ikeda A, Yasuoka S, Anegawa T, Mifune H, Kato S, Hirooka Y, Imaizumi T. Exaggerated blood pressure variability superimposed on hypertension aggravates cardiac remodeling in rats via angiotensin II system-mediated chronic inflammation. Hypertension. 2009;54:832–8.
Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr., Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–32.
Martinka P, Fielitz J, Patzak A, Regitz-Zagrosek V, Persson PB, Stauss HM. Mechanisms of blood pressure variability-induced cardiac hypertrophy and dysfunction in mice with impaired baroreflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R767–76.
Cioffi G, Mureddu GF, Stefenelli C, de Simone G. Relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J Hypertens. 2004;22:1589–96.
Leoncini G, Viazzi F, Storace G, Deferrari G, Pontremoli R. Blood pressure variability and multiple organ damage in primary hypertension. J Hum Hypertens. 2013;27:663–70.
Janwanishstaporn S, Boonyasirinant T. Correlation between aortic stiffness and left atrial volume index in hypertensive patients. Clin Exp Hypertens. 2016;38:160–5.
Lantelme P, Laurent S, Besnard C, Bricca G, Vincent M, Legedz L, Milon H. Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis. 2008;101:35–40.
O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E,Fagard R, Graves J,Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y, European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–68.
Vazquez-Rodriguez B, Pita-Fernandez S, Regueiro-Lopez M, Garcia-Pedreira D, Carro-Rodriguez MJ, Perez-Rivas G, de la Iglesia-Martinez F. Concordance between automatic and manual recording of blood pressure depending on the absence or presence of atrial fibrillation. Am J Hypertens. 2010;23:1089–94.
Wang XX, Shuai W, Hong K, Xu J, Li JX, Li P, Cheng XS, Su H. How to evaluate BP measurements using the oscillometric method in atrial fibrillation: the value of pulse rate variation. Hypertens Res. 2016;39:588–92.
Stergiou GS, Kollias A, Destounis A, Tzamouranis D. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Norioka, N., Iwata, S., Ito, A. et al. Greater nighttime blood pressure variability is associated with left atrial enlargement in atrial fibrillation patients with preserved ejection fraction. Hypertens Res 41, 614–621 (2018). https://doi.org/10.1038/s41440-018-0060-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0060-2
This article is cited by
-
The blood pressure variability in patients with cryptogenic stroke
The Egyptian Heart Journal (2022)
-
Validation of an ambulatory blood pressure monitoring device employing a novel method to detect atrial fibrillation
Hypertension Research (2022)