Abstract
The autonomic nervous system (ANS) plays an important role in the initiation and maintenance of atrial fibrillation (AF). However, the meaning of higher heart rate variability (HRV) in predicting AF remains unclear. Among 2100 patients in the Holter registry, a total of 782 hypertensive patients were included in this study. Baseline HRV was measured by time domain and frequency domain methods using 24-h Holter monitoring. The primary outcome was the development of AF. During an average follow-up of 1.1 years, 44 patients developed AF. Higher HRV parameters including high-frequency (P < 0.001), the square root of the mean squared differences of successive NN intervals (P < 0.001), and the percentage of NN intervals that are more than 50 ms different from the previous interval (P < 0.001) were associated with the occurrence of AF in univariate analysis. Premature atrial contractions burden, lower baseline heart rate, age, hemodialysis, coronary artery disease, and chronic heart failure were also associated with AF. In Cox regression analysis, higher HRV (representing excessive autonomic fluctuation) was an independent risk factor for AF. Excessive autonomic fluctuation represented by higher HRV in patients with hypertension was associated with an increased risk of AF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia requiring medical therapy1. Various pathophysiological mechanisms for the development of AF have been studied2. Recently, there has been an increasing evidence that the dysfunction of the autonomic nervous system (ANS) including the sympathetic and parasympathetic nervous systems, and the interaction between sympathetic and parasympathetic nervous systems are involved in the pathogenesis of AF3.
Despite the increasing evidence of an association between dysfunction of ANS and AF, whether abnormalities in the ANS can predict the development of AF remains unclear. Several population-based studies showed that lower heart rate variability (HRV) was associated with an increased risk of new-onset AF4,5,6. However, another study reported that higher HRV value was associated with the incident AF7. The electrocardiography (ECG) recording time used to analyze HRV and analysis methods differed among the studies, leading to inconsistent results.
Hypertension is the most common cardiovascular risk factor in patients with AF8. Previous studies reported that HRV was not only associated with cerebrovascular diseases such as stroke and myocardial infarction but also relatively mild disease such as hypertension and anxiety disorders9,10,11. The objective of this study was to investigate whether HRV using 24-h Holter monitoring could predict the development of AF in patients with hypertension.
Methods
Study population
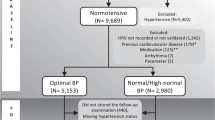
The study population was selected from the Kangwon National University Holter registry. A total of 2100 patients underwent 24-h Holter monitoring between May 2018 and April 2019. Patients were eligible for this study if they were in sinus rhythm at baseline 24-h Holter monitoring and had HRV data. The exclusion criteria were: (1) < 18 years of age, (2) had persistent AF or paroxysmal AF lasting more than 30 s at baseline 24-h Holter monitoring, (3) had temporary or permanent pacemaker, (4) had complete atrioventricular block, or (5) < 22 h of recording. After excluding 480 patients who met the exclusion criteria, 1620 patients were identified. Among the 1620 patients, 782 patients with hypertension were finally analyzed.
This retrospective observational cohort study was conducted in accordance with the principles of the Declaration of Helsinki. The Institutional Review Board of Kangwon National University Hospital approved the study protocol (KNUH-2020-04-021-001). The need for informed consent was waived for this retrospective study by the Institutional Review Board of Kangwon National University Hospital. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study design and data collection
Baseline demographic data, cardiovascular risk factors, medications, and clinical outcomes were retrospectively analyzed by reviewing the medical records. The study population was divided into two groups according to the presence of hypertension. Holter data including the HRV parameters and cardiovascular risk factors were compared. Whether HRV parameters could predict the occurrence of AF during the follow-up period in patients with hypertension was also determined.
Study outcome
The study outcome was the development of AF during follow-up. The development of AF was defined as a standard 12-lead ECG recording or Holter recording with ≥ 30 s of AF. The development of AF was evaluated by reviewing the medical records from our hospital. All ECG and 24-h Holter monitoring data were interpreted by one cardiologist.
Heart rate variability
HRV data were acquired by 24-h Holter monitoring (MARS, GE Healthcare, Chicago, Illinois, United States) and measured by frequency domain and time domain methods. Fast Fourier Transform (FFT) (a non-parametric method of spectral estimation) was used to convert single-lead ECG signals to power spectral densities. The cubic spline-interpolated NN interval function was sampled at 1024 samples/300 s or 3.413 samples/s. NN interval ratios of < 0.80 or > 1.20 and NN intervals of < 150 ms or > 5000 ms were excluded prior to HRV analysis. The power spectral density included very low-frequency (VLF) component (0.0033–0.0400 Hz), low-frequency (LF) component (0.0400–0.1500 Hz), high-frequency (HF) component (0.1500–0.4000 Hz), and the ratio of two components (LF/HF ratio). The time domain methods included the standard deviation of the NN interval (SDNN), the standard deviation of all 5-min average NN interval (SDANN), the average standard deviation of all 5-min NN interval (ASDNN), the square root of the mean squared differences of successive NN intervals (rMSSD), the percentage of NN intervals that are more than 50 ms different from the previous interval (pNN50), and the count of intervals that were more than 50 ms different from the previous interval (BB50).
Statistics
Continuous variables are expressed as mean ± standard deviation or median and interquartile range. Categorical variables are expressed as frequency and percentage. To evaluate the difference in HRV values and clinical characteristics according to the occurrence of AF, Student’s unpaired t test was used for normally distributed data and Mann–Whitney test was used for skewed data. Categorical variables were analyzed with chi-square test or Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was used to select the cut-off value between the HRV parameter and the occurrence of AF. Cox regression analysis was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk factors associated with the occurrence of AF. Calculations were performed using the Statistical Package for the Social Sciences version 20.0 for Windows (IBM Corp., Armonk, NY, USA). A P-value less than 0.05 was considered statistically significant.
Results
Among the 2100 patients who underwent 24-h Holter monitoring between May 2018 and April 2019, 480 patients were excluded due to the following: 120 patients were younger than 18 years old, 191 patients had persistent AF, 73 patients had paroxysmal AF, 5 patients had no HRV data, 3 patients had permanent pacemaker, 14 patients had complete atrioventricular block, 2 patients had insufficient recording, and 72 patients performed Holter monitoring repeatedly (Fig. 1). After excluding 480 patients who met the exclusion criteria and 838 patients without hypertension, 782 patients with hypertension were finally analyzed.
Baseline clinical characteristics
The baseline clinical characteristics of the study population are shown in Table 1. The baseline characteristics and cardiovascular risk factors according to the occurrence of AF in patients with hypertension are shown in Table 2. The patients with AF occurrence were older, with more end stage kidney disease on dialysis, coronary artery disease, chronic heart failure, and history of AF. However, there was no significant difference in gender, diabetes, or history of stroke between the two groups.
Heart rate variability and Holter data
LF and HF component in the frequency domain method and ASDNN, rMSSD, pNN50, and BB50 in the time domain method were higher in the AF occurrence group (Table 3). Mean heart rate was faster in the no AF occurrence group (P = 0.002). However, the burden of premature atrial contractions (PAC) was significantly higher in AF occurrence group (P < 0.001).
Risk factors for AF occurrence
During an average follow-up of 1.1 years, 44 patients developed AF. Among the 44 patients who developed AF during the follow-up period, 27 (61.4%) patients were found to have paroxysmal AF and 17 (38.6%) patients were found to have persistent AF at the initial AF identification. Of the 27 patients found to have paroxysmal AF, 10 patients had a history of AF, and 10 of the 17 patients found to have persistent AF had a history of AF.
The univariate analysis showed that the traditional risk factors such as age, coronary artery disease, hemodialysis, and chronic heart failure were associated with an increased risk of AF (Table 4). Several HRV parameters including VLF (P = 0.002), LF (P < 0.001), HF (P < 0.001), rMSSD (P < 0.001), and pNN50 (P < 0.001) were associated with AF occurrence. History of AF, the mean heart rate, and PAC count was also associated with AF occurrence. ROC analysis for HF, rMSSD, pNN50, and PAC count as predictors of AF occurrence revealed areas under the curve of 0.7033, 0.7045, 0.6911, and 0.7060, respectively (all P < 0.001, Fig. 2). The best HF cut off value of 11.1 for AF occurrence resulted in a sensitivity of 65.9% and a specificity of 62.9%. The sensitivity and specificity of the rMSSD at a cut off value of 29.5 for AF occurrence were 61.4% and 62.9%, respectively. In the case of pNN50 at a cut off value of 7.0, the sensitivity and specificity were 61.4% and 58.5%, respectively. Satisfying any one of these three HRV cut off values was associated with the occurrence of AF (P < 0.001, Table 5). The Kaplan–Meier estimates of AF occurrence according to any risk of HRV parameters are presented in Fig. 3 (log rank P < 0.001). Cox regression analysis showed that older age, higher PAC burden, hemodialysis, coronary artery disease, history of AF, and any risk of HRV parameters were independent predictors for the occurrence of AF (Table 6).
ROC curve of heart rate variability and premature atrial contractions to predict the occurrence of atrial fibrillation. ROC, Receiver operating characteristic; HF, high frequency; rMSSD, square root of the mean squared differences of successive NN interval; pNN50, the percentage of RR intervals more than 50 ms different from the previous interval; PAC, premature atrial contraction; AUC, area under the curve.
Kaplan–Meier estimate of AF-free survival according to the presence of any risk of HRV. AF, atrial fibrillation; HRV, heart rate variability. Any risk of HRV meant that at least one of the abnormal HRV parameters (HF, rMSSD, and pNN50) were present. AF, atrial fibrillation; HRV, heart rate variability.
Discussion
The main finding of this study was that higher HRV in patients with hypertension could predict the development of AF independent of demographics or known cardiovascular risk factors. HF, rMSSD, and pNN50 were notably associated with the occurrence of AF, along with age, hemodialysis, and high PAC burden, which are traditional risk factors for AF. Our findings suggest that higher HRV representing abnormal autonomic fluctuation is associated with a higher risk of AF development. Separately, patients with hypertension had more comorbidities and lower HRV than those without hypertension.
The ANS plays an important role in the initiation and maintenance of AF through atrial electrical remodeling12,13. A previous study showed that increased vagal activity could cause shortening of the atrial effective refractory periods12. Another study revealed that AF could be initiated by premature beats during vagal stimulation14. Moreover, autonomic fluctuations preceding AF are common15,16. Most studies evaluating these phenomena used HRV parameters to estimate dysfunction of ANS15,16. HRV is a quantitative method used to measure the balance between the sympathetic and parasympathetic nervous system17,18. HRV indicates fluctuations in autonomic inputs to the heart rather than the mean level of autonomic tones. Therefore, not only lower HRV values, but also higher HRV values might indicate abnormal conditions.
Two previous long-term follow-up studies showed that lower HRV was associated with an increased risk of AF4,5. However, another study reported that the association between lower SDNN and incident AF was not significant after adjusting for traditional risks factor for AF6. One recently published study showed that not only lower HRV but also higher rMSSD was associated with the development of AF7. These inconsistent results might be due to differences in ECG recording time and the study population used in HRV analysis. In our study, higher HRV parameters were associated with the development of AF, contrary to the results of some previous studies. There are several possible explanations for these differences. First, our study focused on hypertensive patients, unlike previous studies using general population4,5,6. Hypertensive patients generally have lower HRV than healthy people10. In our study, patients with hypertension had more comorbidities and lower HRV, consistent with other studies (Supplementary Tables S1 and S2). Since the mean HRV value might be higher in the general population than in hypertensive patients, higher HRV values might not be significantly different enough to predict AF. Second, we used 24-h Holter monitoring to measure HRV. In general, the time domain methods are ideal for long-term ECG recording, whereas the frequency domain methods are preferred to the time domain methods for short-term recording17. In the two previous long-term follow-up studies, short-term (2-min4 and 45-min5) ECG recording was used. Therefore, the HRV parameters measured by time domain methods were more appropriate in our study. Third, a closer look at the previous Atherosclerosis Risk in Communities (ARIC) cohort study reported that lower HRV was associated with an increased risk of AF, a figure in that report showed that higher HRV values were also associated with the occurrence of AF. However, this association between higher HRV values and the occurrence of AF was not addressed in previous ARIC cohort study4.
Parasympathetic dominance is associated with an increased propensity for AF, and vagal-mediated paroxysmal AF is preferentially seen in young individuals who have structurally normal heart19. Generally, HF components are predominantly modulated by the parasympathetic nervous system, whereas LF components are considered a marker of sympathetic modulation20,21. HRV represents the degree of autonomic fluctuations rather than the mean level of autonomic tones. Therefore, relatively higher HRV could be considered excessive fluctuations in the ANS rather than a physiologic condition. In other words, even though hypertensive patients generally had low HRV than those without hypertension, hypertensive patients with comparatively higher HRV might be considered to have disproportionate fluctuations in the ANS. A previous study7 showed that higher rMSSD was associated with incident AF could be interpreted from this point of view.
We enrolled patients with sinus rhythm at baseline 24-h Holter monitoring. Therefore, patients with paroxysmal AF were enrolled in this study. Sixty-six of 782 (8.4%) hypertensive patients had a history of AF. Perhaps more patients with a history of AF might be included due to its asymptomatic feature. Among 66 patients with a history of AF, AF recurred in 20 patients. A history of AF was an independent risk factor for AF occurrence as well as higher HRV in this study. When we examine a patient in an outpatient clinic, it is difficult to know whether the patient has a history of AF. Therefore, it is important to predict the occurrence of AF regardless of a history of AF, and the HRV value would be one of the factors that could predict the occurrence of AF.
This study had several limitations. First, this was a retrospective cohort study. We could not fully evaluate many other risk factors for AF, including obstructive sleep apnea, body weight, alcohol consumption, or physical activity. Second, the duration of follow-up was relatively short and the degree of effort to identify AF varied from patient to patient. Since most patients with AF are asymptomatic, it is difficult to detect AF with an intermittent ECG test. Regular 24-h Holter monitoring could not be performed due to the limitations of the retrospective study. Therefore, AF incidence in our study was low. Third, our study enrolled patients undergoing hemodialysis. Because blood pressure and autonomic tone change before and after dialysis, short-term ECG recordings are generally preferred to avoid environmental influences. However, since the number of dialysis patients in our study was very small, we analyzed HRV data using 24-h Holter recordings. Finally, since HRV parameters measured by frequency domain methods were made by 24-h Holter recording, there might be a problem of stationarity.
In conclusion, higher HF, rMSSD, and pNN50 in patients with hypertension as surrogate markers for excessive autonomic fluctuation could predict the occurrence of AF. Frequent AF screening should be considered for hypertensive patients with higher HRV values and other risk factors for AF. Further large scale, prospective studies are needed to verify our findings.
Data availability
The datasets used and/or analyzed in the present study can be shared on reasonable request.
References
Tsang, T. S. & Gersh, B. J. Atrial fibrillation: An old disease, a new epidemic. Am. J. Med. 113, 432–435. https://doi.org/10.1016/s0002-9343(02)01245-7 (2002).
Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498. https://doi.org/10.1093/eurheartj/ehaa612 (2021).
Xi, Y. & Cheng, J. Dysfunction of the autonomic nervous system in atrial fibrillation. J. Thorac. Dis. 7, 193–198. https://doi.org/10.3978/j.issn.2072-1439.2015.01.12 (2015).
Agarwal, S. K. et al. Cardiac autonomic dysfunction and incidence of atrial fibrillation: Results from 20 years follow-up. J. Am. Coll. Cardiol. 69, 291–299. https://doi.org/10.1016/j.jacc.2016.10.059 (2017).
Perkiomaki, J. et al. Heart rate variability findings as a predictor of atrial fibrillation in middle-aged population. J. Cardiovasc. Electrophysiol. 25, 719–724. https://doi.org/10.1111/jce.12402 (2014).
Singh, J. P. et al. Is baseline autonomic tone associated with new onset atrial fibrillation?: Insights from the Framingham heart study. Ann. Noninvasive Electrocardiol. 9, 215–220. https://doi.org/10.1111/j.1542-474X.2004.93550.x (2004).
Habibi, M. et al. Resting heart rate, short-term heart rate variability and incident atrial fibrillation (from the Multi-Ethnic Study of Atherosclerosis (MESA)). Am. J. Cardiol. 124, 1684–1689. https://doi.org/10.1016/j.amjcard.2019.08.025 (2019).
Giugliano, R. P. et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 369, 2093–2104. https://doi.org/10.1056/NEJMoa1310907 (2013).
Kemp, A. H., Quintana, D. S., Felmingham, K. L., Matthews, S. & Jelinek, H. F. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS ONE 7, e30777. https://doi.org/10.1371/journal.pone.0030777 (2012).
Park, S. B., Lee, B. C. & Jeong, K. S. Standardized tests of heart rate variability for autonomic function tests in healthy Koreans. Int. J. Neurosci. 117, 1707–1717. https://doi.org/10.1080/00207450601050097 (2007).
Hillebrand, S. et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace 15, 742–749. https://doi.org/10.1093/europace/eus341 (2013).
Yang, D. et al. Vagal stimulation promotes atrial electrical remodeling induced by rapid atrial pacing in dogs: Evidence of a noncholinergic effect. Pacing Clin. Electrophysiol. 34, 1092–1099. https://doi.org/10.1111/j.1540-8159.2011.03133.x (2011).
Arora, R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: Implications for therapies targeting the atrial autonomic nervous system. Circ. Arrhythm. Electrophysiol. 5, 850–859. https://doi.org/10.1161/CIRCEP.112.972273 (2012).
Moe, G. K. & Mendez, C. Basis of pharmacotherapy of cardiac arrhythmias. Mod. Concepts Cardiovasc. Dis. 31, 739–744 (1962).
Bettoni, M. & Zimmermann, M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 105, 2753–2759. https://doi.org/10.1161/01.cir.0000018443.44005.d8 (2002).
Lombardi, F. et al. Autonomic nervous system and paroxysmal atrial fibrillation: A study based on the analysis of RR interval changes before, during and after paroxysmal atrial fibrillation. Eur. Heart J. 25, 1242–1248. https://doi.org/10.1016/j.ehj.2004.05.016 (2004).
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065 (1996).
Goldberger, J. J. Sympathovagal balance: How should we measure it?. Am. J. Physiol. 276, H1273-1280. https://doi.org/10.1152/ajpheart.1999.276.4.H1273 (1999).
Khan, A. A., Lip, G. Y. H. & Shantsila, A. Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system. Eur. J. Clin. Investig. 49, e13174. https://doi.org/10.1111/eci.13174 (2019).
Pagani, M. et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59, 178–193. https://doi.org/10.1161/01.res.59.2.178 (1986).
Montano, N. et al. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90, 1826–1831. https://doi.org/10.1161/01.cir.90.4.1826 (1994).
Funding
This study was supported by a research grant (Grant No. 2020-001) funded by Gangwon Heart Association.
Author information
Authors and Affiliations
Contributions
K.J.C., S.H.K., and K.R.L. conceptualized, designed the research and drafted the manuscript. S.H.K. and K.J.C. collected data. K.R.L. and K.J.C. analyzed data. K.J.C., J.H.S., D.R.R., B.K.L., and B.R.C. performed research and interpreted the data. S.H.K and K.R.L contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.H., Lim, K.R., Seo, JH. et al. Higher heart rate variability as a predictor of atrial fibrillation in patients with hypertension. Sci Rep 12, 3702 (2022). https://doi.org/10.1038/s41598-022-07783-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07783-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.