Abstract
Purpose
To assess the proportion of maculopathy detectable only on optical coherence tomography (OCT) versus slit lamp indirect ophthalmoscopy (SLIO) during cataract assessment.
Methods
Population: Consecutive patients attending cataract assessments. Data collection: All patients underwent OCT and SLIO. SLIO findings were recorded before reviewing OCT. Scans were examined to compare with recorded SLIO findings. Primary outcome: analyse the proportion of eyes with maculopathy missed by SLIO. Secondary outcome: to assess the proportion of patients with maculopathy on OCT, the incidence of maculopathy in the fellow eye on OCT and proportion with cataracts too dense to allow SLIO or OCT.
Results
Six hundred twenty-six patients were enroled. Eighty (12.8%) had maculopathy detectable only on OCT which included: 26 (4.2%) epiretinal membrane (ERM), 25 (4%) dry age-related macular degeneration (AMD), 19 (3%) vitreomacular traction (VMT), 5 (0.8%) lamellar macular hole (LMH), 2 (0.3%) cystoid macular oedema (CMO) and 1 (0.2%) wet AMD. 166 (26.5%) had maculopathy on OCT, of which only 48 (7.7%) had known history of maculopathy. In fellow eyes, 29 (4.6%) had significant findings and 29 (4.6%) were unable to have SLIO or OCT due to dense cataract.
Conclusions
A quarter of the patients had occult maculopathy. One-tenth of the occult maculopathy were missed without OCT, with ERM, dry AMD, VMT, LMH, CMO and wet AMD being the primary missed diagnosis. Less than 5% had occult maculopathy in fellow eye, and <5% had dense cataracts where neither SLIO nor OCT was not possible.
Similar content being viewed by others
Introduction
Subtle macular pathologies are often the cause of an unhappy patient following routine cataract surgery [1, 2]. Binocular indirect slit-lamp [3] evaluation of the posterior pole (with a +90/+78 dioptre [D] lens) is an essential skill for an ophthalmologist. Still, the power of resolution of the naked eye limits its usefulness in certain situations. Also, certain subclinical conditions that may become significant postoperatively [such as cystoid macular oedema (CMO) or an epiretinal membrane (ERM)] may be missed on routine preoperative slit lamp indirect ophthalmoscopic evaluation (SLIO) [4,5,6].
Optical coherence tomography (OCT) has become an essential tool for surgeons to diagnose clinical and subclinical macular lesions and monitor treatment outcomes in patients with macular pathologies [2, 7]. While cataract surgeons note the role of corneal topography [8], the ocular surface [9], and endothelium [10] in their preoperative planning of cataract surgery, objective documentation of macular integrity is vital to a satisfactory post-operative outcome. It is also known that spectral-domain OCT can pick up vitreoretinal interface abnormalities [ERM, lamellar macular holes (LMH), etc.], which may often be missed during an SLIO [1, 11,12,13]. Klein and associates [2] have demonstrated the utility of the posterior segment spectral-domain OCT scan in determining eligibility for multifocal and toric lenses.
We sought to investigate the proportion of occult macular pathology detectable preoperatively only on OCT of the macula compared to SLIO. In addition, this study was also designed to assess the overall incidence of maculopathy on OCT in the eye referred for cataract surgery, the incidence of maculopathy in the fellow eye on the OCT and the proportion of patients where SLIO or OCT was not possible due to density of the cataract.
Methods
This was a prospective cross-sectional study of consecutive patients attending cataract assessment clinics at the Sussex Eye Hospital in the first week of November 2020. That week of activity was dedicated to cataract assessment as part of work to decrease waiting times for patients in the wake of the first wave of the COVID-19 pandemic and the shutdown of non-emergency services. The study followed the guidelines of the tenets of the Declaration of Helsinki. It was approved by the local audit department at the Sussex Eye Hospital, University Hospitals Sussex NHS Foundation Trust, Brighton, United Kingdom, and informed verbal consent was obtained from the patients. Included patients were the ones who were referred for cataract surgery and attended the cataract assessment clinic. Patients who were too morbid to have an SLIO and an OCT scan were excluded from the study.
The primary outcome measure of our study was to analyse the proportion of eyes with subtle maculopathy missed by SLIO compared to OCT macula. The secondary outcome measure assessed the overall incidence of maculopathy in the eye referred for cataract surgery on OCT, the incidence of maculopathy in the fellow eye on OCT, and the proportion of patients with cataracts too dense to allow SLIO or OCT.
Only senior surgeons regularly performing and experienced in cataract surgery were appointed to assess these patients in the cataract assessment clinics. During their appointment, data on the eye referred for assessment, patient age, best-corrected visual acuity (BCVA), known prior history of maculopathy, and prior retinal surgery were collected on a special data collection sheet for each patient. Each patient was dilated with G. Tropicamide 1% minims (Bausch & Lomb, USA). A macular OCT was performed on either a Spectralis®, Heidelberg Engineering GmbH, Germany or Topcon DRI Triton®, Topcon Medical Systems inc., USA. The senior surgeon was asked to note the SLIO findings in the data collection sheet before assessing the macular OCTs on the central digital database. The clinicians were asked not to look at the OCT scans whilst writing their initial examination findings. Still, they were then made available to the clinicians when discussing potential listing for surgery. The OCT scans of each patient’s macula were examined by an independent grader (GM) who had a particular interest in the retina to compare with the recorded SLIO findings.
All patients underwent a best-corrected Snellen visual acuity, intraocular pressure measurement, optical biometry and dilated fundoscopic examination by the senior surgeon. Biometry was performed by an experienced technician with IOLMaster 500® Carl Zeiss, Germany or LenStar®, Haag Streit, Germany and OCT images were recorded as a fast macular radial line scan which captured on either Heidelberg Spectralis® (Heidelberg Engineering GmbH, Germany) or Topcon DRI Triton® (Topcon Medical Systems inc., USA). Where biometry was not possible on the optical device due to density of cataract, A-scan ultrasound biometry (Acutome®, Keeler, UK) was performed.
All datasheets from the clinical sessions were collected from all the notes, and any further findings from a review of the OCT imaging were added by a single observer (GM), this was then transcribed into a Microsoft 365 Excel spreadsheet (Microsoft Corp, Medmont, USA) for further analysis using Statsdirect v3 (Statsdirect Ltd, Wirral, UK). Snellen visual acuity was converted to LogMAR units for statistical analysis, using the following for non-numeric values: counting fingers = 2.0 LogMAR, hand movements = 2.3 LogMAR, light perception = 2.7 LogMAR, and no light perception = 3.0 LogMAR. Demographics and frequency distribution were plotted. Categorical variables are expressed as absolute and relative frequencies. Continuous variables are expressed as the mean ± standard deviation (SD) and the minimum and maximum values. Normality was tested using the Shapiro–Wilk test and accordingly for continuous data t-test or Mann–Whitney U test and Kruskal Wallis test analysed parametric and non-parametric data. Patients in whom an OCT scan was not present or analysable were excluded from sensitivity analysis. P value <0.05 was considered significant.
Results
Of 655 patients referred for cataract assessment, 626 attended and were eligible for inclusion and collected data during the study period. The baseline characteristics are detailed below (Table 1) and their BCVA (Fig. 1).
The average age of patients with maculopathy (78.92 ± 8.39 years) was significantly higher than both those without maculopathy (73.03 ± 10.0 years p < 0.01) and those for whom OCT acquisition was not possible (71.74 ± 12.0 years p < 0.01). There was no significant difference between the group without maculopathy and the group in whom an OCT was not possible (p = 0.47), the group without maculopathy also had slightly better average BCVA (0.58 ± 0.53 vs 0.66 ± 0.56 (p = 0.08)), but the differences were not statistically significant. The group in which it was not possible to obtain an OCT image had an average acuity of 1.66 ± 0.80, which was significantly worse than both the groups with and without maculopathy (p ≤ 0.01) Of the subpopulation with maculopathy, there was no significant difference in age between those who had visible maculopathy and those on whom it was missed on SLIO (77.7 5 ± 9.66 vs 79.14 ± 8.68 years (p = 0.32)); similarly, there was a difference in visual acuity, with the subgroup of visible maculopathy having better acuity than those missed (0.65 ± 0.56 vs 0.66 ± 0.57 (p = 0.87)), but the differences were not statistically significant.
Of the 626 patients attending, 564 patients were listed for cataract surgery. Three out of 564 were referred for a further appointment before the cataract surgery in their referred eye, one of whom had neovascular age-related macular degeneration (AMD), one with vitreomacular traction (VMT), and another with ERM.
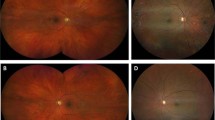
Of the 626 patients, 105 (16.8%) had discordance between their SLIO findings and the OCT scans. This was in the form of occult maculopathy detectable only on OCT in 80 (12.8%) patients but also included reassuringly normal scans when subtle maculopathy with dry AMD or ERM was suspected on SLIO in 13 (2.1%) patients. There were six patients in whom there was a normal OCT scan when SLIO was not possible, and six patients with normal SLIO when no OCT scan was possible. One patient with dry and neovascular AMD was diagnosed with OCT when SLIO was not possible. Compared with SLIO, OCT picked up 26 (4.2%) more patients with ERM (Fig. 2A), 19 (3%) more with VMT (Fig. 2B), 25 (4%) more with dry AMD (Fig. 2C), 5 (0.8%) more with LMH (Fig. 2D), 2 (0.3%) more with CMO and 1 (0.2%) more with wet AMD. Table 2 shows the frequency of pathological findings in both groups and the sensitivity and specificity of SLIO compared to OCT for detecting maculopathy, assuming these are 100% for OCT.
A Example of a case where epiretinal membrane was missed without OCT. B Example of a case where vitreomacular traction was missed without OCT. C Example of a case where mild dry age-related macular degeneration was missed without OCT. D Example of a case where lamellar macular hole was missed due without OCT.
Of the 626 patients, 166 patients (26.5%) had maculopathy on OCT, of which only 48 (7.7%) had known the previous history of maculopathy. The most frequent macular pathology was dry AMD in 101 patients (16%), followed by ERM in 44 patients (7.0%), VMT in 19 (3.0%), lamellar macular hole in 6 (1.0%), diabetic maculopathy in 3 (0.5%), CMO in 3 (0.5%), myopic maculopathy in 2 (0.3%), a single case of previously known and treated diabetic macular oedema (DMO) was present (0.2%), as was a single case of neovascular AMD. In the OCT scans of the fellow eye of each patient, there were 29 (4.6%) patients with findings deemed to be visually significant and requiring further appointments. 21 (3.4%) had ERM, 4 (0.6%) had VMT, 3 (0.5%) had neovascular AMD, and 1 (0.2%) had a suspected central serous retinopathy (CSR). Neither SLIO nor OCT scanning was possible in 29 (4.6%).
Discussion
Our study found that the overall incidence of maculopathy on OCT during cataract assessment clinics in the eye to be operated on was 26.5%, with dry AMD showing the highest incidence, followed by ERM, VMT, LMH, diabetic maculopathy, CMO, myopic maculopathy and least with neovascular AMD. Whereas 4.6% of fellow eyes also were detected to have maculopathy on OCT, requiring further appointments with the highest incidence of ERM followed by VMT, neovascular AMD and CSR. However, 12.8% of the patients had occult maculopathy detectable only on OCT. As shown in our results, routine SLIO is likely to miss 48.2% of patients with maculopathy, with VMT being tough to diagnose. It further potentially overestimates the prevalence of dry AMD in 2% and ERM in 0.3%.
Due to considerable overlap in risk factors for cataracts and macular disease, we would expect them to coexist in a significant number of eyes [14]. Advanced-technology intraocular lens (IOL) surgeons need a reliable strategy to screen for macular pathology in preoperative patients to inform them more realistically of the expected visual outcome. Macular disease is a relative contraindication to multifocal IOLs because both conditions compromise contrast sensitivity, a problem that is exacerbated by low illumination conditions [15]. In a smaller case series of 265 eyes evaluated for multifocal and toric IOL implantation, Klein and associates found occult macular pathology in 13% of eyes using preoperative spectral-domain OCT, the common pathologies being AMD (6%), ERM (4%) and oedema (1%) [2]. These findings are not unlike ours’, but they detected fewer patients with AMD than our study. A retrospective study was done in a Chinese population undergoing cataract surgery [16] also found a 25% rate of occult maculopathy detected only on OCT with ERM as a leading cause. A study by Zafar et al. [17], including 155 Pakistani patients with normal fundus examinations, reported macular pathologies identified only by OCT in 10.9%, with AMD having a slightly higher prevalence than ERM (3.2% versus 2.6%). Similarly, Pinto et al. [18] had macular alterations in 7% of patients (out of 614) that only OCT identified, and the most common pathologies diagnosed only by OCT were ERM (3.3%) followed by AMD (0.7%). Conversely, Moreira Neto et al. [19] reported a more significant difference in the prevalence of these aetiologies (AMD 6.9% and ERM 3.5%). Another study [19] enroled 98 Brazilian cataract patients, and the preoperative OCT increased the detection of macular abnormalities to 21.4% versus the 11.2% indicated only by the fundus examination. Abdelmassih et al. [20] found that OCT only detected 17% of occult maculopathy. The disparity between these papers is possibly due to the different populations and sample sizes [5, 21]. These previous papers corroborate our findings, indicating the importance of incorporating macular OCT in the preoperative evaluation for surgery. In addition to identifying and documenting the macular abnormality in this scenario, OCT is also necessary for follow-up after surgery.
There have been studies investigating the incidence of retinal pathology in patients referred for cataract surgery, and in those settings, the addition of OCT added value to the process [2, 20, 22,23,24,25]. The incidence of occult maculopathy in these studies ranges between 4.6–40.6% [22, 25], with AMD as the most common and ERM the second most common finding (in our cohort VMT was the third most common). In a population-based study, the Beaver Dam Eye Study group [4] screened 2980 eyes of individuals aged 63–102 years with spectral-domain OCT. The principal objective was to find the epidemiology of vitreoretinal interface abnormalities in the ageing population. The prevalence of ERM, VMT and LMH was 34.1%, 1.6% and 3.6% compared to the overall incidence in our study with 7%, 3% and 1%, respectively. The Beaver Dam Eye Study also reported that the prevalence of macular cysts, ERM, and VMT increased with age, the prevalence of paravascular cysts (PVC) decreased with age, and the prevalence of LMHs was not associated with age [4]. In another study, Abdelmassih et al. [20] found the incidence of AMD in 61.7%, ERM in 27.1% and LMHs in 2.9% of eyes on OCT scan in their study on 401 eyes (245 patients).
OCT has shown a higher sensitivity for detecting these conditions than SLIO alone [11], a factor that may be exacerbated when a clinically significant cataract is impeding the view. As well as discussing these findings with the patient, knowledge of their presence allows for a better-informed consenting process and can adjust the patient’s post-operative expectations. Prior knowledge of retinal pathology will also expedite appropriate referrals within ophthalmology services and decrease ‘on-the-day’ cancellations for cataract patients in pooled operating lists shared by multiple surgeons. Thus, improving the experience of their patient journey through the hospital service. Surgically, it may also change the post-operative management, with the surgeon prescribing a topical non-steroidal anti-inflammatory drop in addition to a steroid or planning a further post-operative check to monitor for the development of macular oedema. In some patients with positioning difficulties. OCT scanning will not always be possible, and some cataracts may be too dense to allow an image to be captured. Whilst this is a limitation of OCT scanning, it will also likely limit the fundal view through SLIO. However, if there are no positioning difficulties, there may be a minority of patients who can still be assessed on OCT successfully even when the SLIO is not possible due to the cataract density, as found in our study.
Whilst there are apparent advantages of scanning all patients for cataract assessment on an OCT, one can argue that it may be time-consuming in busy clinics and expensive to maintain an OCT with the appropriate technician and servicing. However, given the evidence from our study, the improvement in the quality of care by enhancing the predictability of the outcomes of the cataract surgery based on preoperative occult maculopathy and reducing post-operative unhappiness in patients by appropriate preoperative counselling based on these occult maculopathies outweighs the time and monitory investment in the OCT for routine cataract assessment clinics. It is further difficult to prescribe which patients are likely to have occult maculopathy and benefit from OCT without potentially missing pathology.
The prospective nature of our study and the large sample size therein are its main strengths, as is the real-world scenario of multiple clinicians assessing cataracts for pooled surgical lists. The study is limited by including the preoperative assessment and the clinicians being aware of the study being undertaken, which may have biased their examination. For analysis of all factors, recording visual acuity in Snellen is limiting, but there was no significant difference in the average acuities between groups where a full assessment was completed. Review of OCT imaging by a single assessor will not be 100% sensitive and specific but was considered as such for the purpose of statistical comparison. Utilisation of a consensus grading system and multimodal imaging would further improve the detection of maculopathy, but would not be practical to adopt in a real-world clinic setting. It would be worthwhile to evaluate the long-term impact of these occult macular lesions detectable only on the OCT on post-operative visual outcomes and the need for further intervention in future studies. Cost-benefit analysis can be integrated into future studies and will change as newer technologies are adopted that incorporate OCT into biometry equipment.
In summary, patients may have underlying maculopathy present when undergoing cataract surgery which is not visible on SLIO alone. Our study concludes that although clinical evaluation remains the mainstay of detection of significant macular lesions in cataract patients undergoing preoperative evaluation, a macular OCT scan is equally essential to ensure no ocular maculopathy is missed in the eye to be operated or the fellow eye. Knowledge of the underlying maculopathy will allow for an improved consenting process, informed decision-making with the patient, expectation of visual prognosis, and recovery following cataract surgery, all, in turn, improving the quality of care. We propose macular OCT to be an essential tool in the armamentarium of routine cataract assessment clinics.
Summary
What is known about this topic
-
Following cataract surgery, post-operative macular oedema may present both as an unwelcome surprise for the patient and surgeon, as well as the first recognition of undiagnosed macular pathology.
-
Such pathology can be present in populations varying from 4–40% and may be challenging to diagnose on slit-lamp biomicroscopy alone.
-
OCT is not widely used in all hospitals routinely for all cases of cataract preassessment.
What this study adds
-
OCT should be used in all cases for cataract preassessment.
-
A quarter of the patients presenting for cataract assessment had maculopathy on OCT. One-tenth of the maculopathy was missed without OCT, with ERM, dry AMD, VMT, LMH, CMO and wet AMD is the main missed diagnosis.
-
Less than 5% had maculopathy in fellow eye, and <5% had dense cataract where neither SLIO nor OCT was possible.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Hirnschall N, Leisser C, Radda S, Maedel S, Findl O. Macular disease detection with a swept-source optical coherence tomography-based biometry device in patients scheduled for cataract surgery. J Cataract Refract Surg. 2016;42:530–6.
Klein BR, Brown EN, Casden RS. Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J Cataract Refract Surg. 2016;42:537–41.
Kraushar MF. Simplified binocular indirect ophthalmoscopy as a routine office procedure. Ann Ophthalmol. 1974;6:475–8.
Meuer SM, Myers CE, Klein BE, Swift MK, Huang Y, Gangaputra S, et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology. 2015;122:787–95.
Ng CH, Cheung N, Wang JJ, Islam AF, Kawasaki R, Meuer SM, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118:694–9.
Takamura Y, Kubo E, Akagi Y. Analysis of the effect of intravitreal bevacizumab injection on diabetic macular edema after cataract surgery. Ophthalmology. 2009;116:1151–7.
van Velthoven ME, van der Linden MH, de Smet MD, Faber DJ, Verbraak FD. Influence of cataract on optical coherence tomography image quality and retinal thickness. Br J Ophthalmol. 2006;90:1259–62.
Virgili G, Menchini F, Casazza G, Hogg R, Das RR, Wang X, et al. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD008081.
Szakats I, Sebestyen M, Toth E, Purebl G. Dry eye symptoms, patient-reported visual functioning, and health anxiety influencing patient satisfaction after cataract surgery. Curr Eye Res. 2017;42:832–6.
Gogate P, Wood M. Recognising ‘high-risk’ eyes before cataract surgery. Community Eye Health. 2008;21:12–4.
Do DV, Cho M, Nguyen QD, Shah SM, Handa JT, Campochiaro PA, et al. The impact of optical coherence tomography on surgical decision making in epiretinal membrane and vitreomacular traction. Trans Am Ophthalmol Soc. 2006;104:161–6.
Wolf S, Wolf-Schnurrbusch U. Spectral-domain optical coherence tomography use in macular diseases: a review. Ophthalmologica. 2010;224:333–40.
Yehoshua Z, Rosenfeld PJ, Gregori G, Penha F. Spectral domain optical coherence tomography imaging of dry age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2010;41:S6–S14.
Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54:ORSF5–ORSF13.
Leyland M, Zinicola E. Multifocal versus monofocal intraocular lenses in cataract surgery: a systematic review. Ophthalmology. 2003;110:1789–98.
Huang X, Zhang Z, Wang J, Meng X, Chen T, Wu Z. Macular assessment of preoperative optical coherence tomography in ageing Chinese undergoing routine cataract surgery. Sci Rep. 2018;8:5103.
Zafar S, Siddiqui MAR, Shahzad R, Shahzad MH. Swept-source optical coherence tomography to screen for macular pathology in eyes having routine cataract surgery. J Cataract Refract Surg. 2017;43:324–7.
Pinto WP, Rabello LP, Ventura MC, Rocha CS, Ventura BV. Prevalence of macular abnormalities identified only by optical coherence tomography in Brazilian patients with cataract. J Cataract Refract Surg. 2019;45:915–8.
Moreira Neto CA, Moreira Junior CA, Moreira AT. Optical coherence tomography in patients undergoing cataract surgery. Arq Bras Oftalmol. 2015;78:241–5.
Abdelmassih Y, El-Khoury S, Georges S, Guindolet D, Gabison E, Cochereau I. Preoperative spectral-domain optical coherence tomography in patients having cataract surgery. J Cataract Refract Surg. 2018;44:610–4.
Aung KZ, Makeyeva G, Adams MK, Chong EW, Busija L, Giles GG, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013;33:1026–34.
Creese K, Ong D, Zamir E. Should macular optical coherence tomography be part of routine preoperative cataract assessment? Clin Exp Ophthalmol. 2012;40:e118–9.
Enright NJ, Catran A, Perera N, Cheng Y, Adler P. Yield of routine pre-cataract surgery macular optical coherence tomography in finding clinically undetected macular pathology. Clin Exp Ophthalmol. 2017;45:829–31.
McKeague M, Sharma P, Ho AC. Evaluation of the macula prior to cataract surgery. Curr Opin Ophthalmol. 2018;29:4–8.
Weill Y, Hanhart J, Zadok D, Smadja D, Gelman E, Abulafia A. Patient management modifications in cataract surgery candidates following incorporation of routine preoperative macular optical coherence tomography. J Cataract Refract Surg. 2020;47:78–82.
Acknowledgements
We are grateful to Jamal Sawan, Mohamed Abdeldaim, Shankar Khanal, Patrick Idam for their help in data collection. We are thankful to the following surgeons for assisting in this project and helping us with the data collection: Victoria Barrett, Paul Brittain, Dominic Heath, Edward Hughes, Amanda Lewis, Haifa Madi, Gabriela Ortiz and Robert Purbrick. We thank preassessment and management teams at the Sussex Eye Hospital, University Hospitals Sussex NHS Foundation Trust for their support. We thank Topcon, UK for loaning the Topcon DRI Triton® free of charge for the purpose of this cataract assessment week to allow us to get through the large number of patients.
Author information
Authors and Affiliations
Contributions
GM: protocol preparation, data collection, analysis, manuscript drafting and final approval. RO, SK and ZA: data collection, analysis, manuscript drafting and final approval. MAN: concept design, audit approval, protocal preparation, data collection, analysis, manuscript drafting, supervision and final approval.
Corresponding author
Ethics declarations
Competing interests
None of the authors have any financial interest in any products or procedures mentioned in this study. Other unrelated disclosures are as below: GM: Lecture fee from Allergan, Ireland SK: Travel grant from Bayer. MAN: Research grants from Alcon Laboratories, USA; European Society of Cataract & Refractive Surgery; Johnson & Johnson, USA; Rayner Intraocular lenses, UK; Ziemer, Switzerland. Lecture fees from Alcon Laboratories, USA. Consultant to Hoya. Travel grants from Alcon Laboratories, USA & Bausch & Lomb, USA.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murphy, G., Owasil, R., Kanavati, S. et al. Preoperative fundoscopy versus optical coherence tomography to detect occult maculopathy during cataract surgery preassessment. Eye 37, 665–669 (2023). https://doi.org/10.1038/s41433-022-02027-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02027-0