Abstract
Objectives
To determine whether pars plana vitrectomy (PPV) or cataract surgery should be performed first in concurrent epiretinal membrane (ERM) and cataract treatment with respect to visual outcome and pseudophakic cystoid macular oedema (PCMO) incidence.
Methods
Patients who underwent PPV and cataract surgery sequentially at a tertiary medical centre were retrospectively recruited. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) measured from optical coherence tomography (OCT) images collected before and after each surgery were documented. OCT-defined PCMO incidence and its influence on visual outcomes were analysed.
Results
In total, 259 and 159 eyes received PPV (ERM-CATA) before and after cataract surgery (CATA-ERM), respectively. The ERM-CATA group had better final BCVA (logMAR: 0.274 vs. 0.558, p < 0.001) and greater BCVA gain (logMAR VA change: −0.379 vs. −0.220, p = 0.001) than did the CATA-ERM group. Baseline BCVA was positively correlated with final BCVA (p < 0.001), whereas baseline CMT, final CMT, and postoperative CMT changes were not. PCMO incidence did not differ significantly between the two groups (15.4% vs. 19.5%, p = 0.287), and final BCVA changes did not significantly differ between eyes with and without PCMO. PCMO incidence was much higher (29.40% vs. 16.30%, p = 0.008) in eyes with baseline CMT ≥ 500 μm.
Conclusions
When managing ERM and cataract, PPV should be performed before cataract surgery to yield better visual outcomes. Both surgical sequences yield similar PCMO rates. Greater baseline CMT is a risk factor for PCMO after cataract surgery.
Similar content being viewed by others
Introduction
Cataract is a major cause of visual impairment in current aging society, accounting for one-third of blindness cases worldwide [1, 2]. Since the advent of small-incision surgical techniques, pseudophakic cystoid macular oedema (PCMO) has become less prevalent. However, PCMO remains a common complication with an incidence at 0.1–2.35% that leads to unexpected visual decline after cataract surgery [3, 4]. The mechanism underlying PCMO involves postoperative vitreous body changes and inflammatory mediators released because of blood–aqueous barrier damage after cataract extraction [3, 5, 6]. PCMO has many well-established risk factors, such as retinal vein occlusion (RVO), diabetic macular oedema (DMO), active uveitis, and intraoperative posterior capsular tear, and the epiretinal membrane (ERM) is an especially crucial risk factor [4, 7,8,9,10,11]. For patients with ERM and cataract, the mainstay treatments are pars plana vitrectomy (PPV) with ERM peeling and phacoemulsification cataract surgery either performed sequentially or simultaneously. Although combined surgery has several advantages, including lower costs, shorter vision recovery time, and better visualization of the peripheral retina during PPV, Dugas et al. reported no significant difference in visual improvement and macular thickness reduction between combined and consecutive surgery in a multicentre retrospective study of 174 cases [12]. Nevertheless, consecutive surgery is still most commonly applied in Taiwan, which is covered by Taiwan’s National Health Insurance programme and is highly accessible. However, the sequence in which consecutive surgery should be conducted—that is, whether beginning with ERM or with cataract surgery yields better visual outcome, macular thickness reduction, and associated risk of PCMO—remains unclear.
Therefore, this study aimed to compare these two sequences with respect to visual outcome and macular thickness in patients with concurrent ERM and cataract. The incidence, associated factors, and influence of PCMO on visual outcome were also assessed.
Materials and methods
Participants
The medical records of patients with concurrent idiopathic ERM and cataract in Taipei Veterans General Hospital (TVGH), Taiwan, were retrospectively collected and reviewed. We included patients who had concurrent ERM and cataract at initial visit reporting symptomatic visual impairment, either with or without metamorphopsia, and consequently undergone PPV with ERM removal and cataract surgery as two separate surgeries at any time between January 1, 2010, and December 31, 2018, and had been followed-up for more than 3 months after their last surgery. Only the first-operated eye was included if both eyes of the same patient received sequential surgeries. Eyes with previous or recent retinal detachment; postoperative infection; secondary epiretinal membrane; the interval between sequential surgeries longer than 3 years; and macular copathology, specifically full-thickness macular hole, vitreomacular traction syndrome, and a history of receiving combined phacovitrectomy, were excluded. This study adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of TVGH. The need to collect written informed consent from the participants was waived for this retrospective study. The patients were divided into two groups depending on the sequence of surgery: ERM-CATA and CATA-ERM; these surgeries included sequential PPV with ERM peeling and phacoemulsification. All patients received both surgeries at a single institution (TVGH) from several retinal specialists during the aforementioned period.
Surgical procedure
A standard 23-gauge three-port PPV was performed using the Alcon Constellation Vision System (Alcon Laboratories, Fort Worth, TX, USA). The vitreous space and retina were visualized with a contact lens viewing system during surgery. The ERM was lifted and peeled using microforceps with or without intravitreal injection of Brilliant Blue G or Indocyanine Green for membrane staining. The peripheral retina was scrupulously checked for any retinal breaks; if a retinal break was spotted, cryotherapy or an endolaser was applied. Cataract surgery was conducted using the modern phacoemulsification technique with an Alcon Infiniti phacoemulsification unit (Alcon Laboratories, Fort Worth, TX, USA) or the Alcon Constellation Vision System followed by posterior chamber intraocular lens (IOL) insertion.
Outcome assessment
Visual acuity was indicated by best-corrected visual acuity (BCVA), which was measured using a projected-light arithmetic Snellen chart and subsequently converted to the logarithm (base 10) of the minimum angle of resolution (logMAR). The BCVA of every participant was collected at the nearest time point before each surgery and at the last visit before December 31, 2018. ERM and macular thickness were evaluated using spectral-domain optical coherence tomography (SD-OCT). SD-OCT measurements were taken using an Avanti Widefield Optical Coherence Tomography (OCT) unit (Optovue, Fremont, CA, USA), and OCT images were retrieved at the nearest visit before and after each surgery and at the last visit before December 31, 2018. Macular thickness was defined as the distance between the internal limiting membrane and inner border of the retinal pigment epithelium; it was measured using a 6 mm × 6 mm circular macular mapping (ETDRS macular map) centred on the fovea, which was divided into three concentric rings and nine regions. The central macular thickness (CMT) was measured at the central 1-mm area. The changes in BCVA and CMT after each surgery were calculated. The final changes in BCVA and CMT after sequential surgeries were defined as the difference in BCVA and CMT between the initial and final visits. PCMO was defined as new-onset macular extracellular cystoid changes after cataract surgery, as indicated in SD-OCT [13]. Eyes with postoperative macular thickening but without any emerging cystoid changes were not considered as having PCMO. The OCT images were also interpreted by a second examiner (YM Huang). Baseline systemic and ocular diseases were recorded for analysis to identify possible factors associated with PCMO.
Statistical analysis
Student’s t test was used to compare vision and macular parameters between the two groups. These parameters were BCVA and CMT, specifically their baseline values, final values, and change after individual and both surgeries. Baseline binary categorical variables and PCMO incidence were analysed in a chi-square test for inter-group comparison. Pearson correlation analysis was used to identify possible associations between final BCVA and ocular factors. Correlation strength based on the correlation coefficient (r) was reported. Univariate and multivariate logistic regressions were conducted to determine factors associated with PCMO. Variables in the univariate model with p ≤ 0.20 were included in the multivariate model. Odds ratios (OR) and their 95% confidence interval (CI) were reported. Cases with the missing data were omitted and the remaining complete data were analysed. Statistical significance was indicated by a two-tailed p value of ≤0.05. Data analysis was conducted using SPSS Software (IBM, Chicago, IL, USA), version 19.
Results
A total of 418 eyes from 418 East Asian patients were included in this retrospective cohort, with 259 eyes in the ERM-CATA group and 159 eyes in the CATA-ERM group (Table 1). The baseline demographic data indicated that compared with the CATA-ERM group, the ERM-CATA group was younger (age: 67.13 ± 6.74 vs. 74.62 ± 9.22 years, p < 0.001) and had a larger proportion of women (65.6% vs. 50.3%, p = 0.002). The interval between the two surgeries was similar between the two groups (321.19 vs. 284.07 days, p = 0.186). With regard to systemic and ocular factors, compared with the ERM-CATA group, the CATA-ERM group had significantly more patients with diabetic mellitus (DM: 14.3% vs. 30.8%, p < 0.001), hypertension (31.3% vs. 53.5%, p < 0.001), cardiovascular disease (4.2% vs. 11.3%, p = 0.009), DMO (1.5% vs. 7.5%, p = 0.003), diabetic retinopathy (1.9% vs. 8.2%, p = 0.005), and age-related macular degeneration (AMD: 1.5% vs. 5.7%, p = 0.038).
Visual outcome
The baseline BCVA (logMAR: 0.655 vs. 0.711, p = 0.154) and proportion of subjective metamorphopsia (18.53% vs. 20.13%, p = 0.301) were similar between the two groups (Table 2). Compared with the CATA-ERM group, the ERM-CATA group had significantly better final BCVA at the last visit (logMAR: 0.274 vs. 0.558, p < 0.001) and greater final BCVA gain (ΔlogMAR: −0.379 vs. −0.220, p = 0.001). In a subgroup analysis excluding eyes with imbalanced ocular factors between groups – DM, DM retinopathy and AMD – the ERM-CATA group had a better final BCVA (logMAR: 0.272 vs. 0.509, p < 0.001) and final BCVA gain (ΔlogMAR: −0.382 vs. −0.239, p = 0.004) compared with the CATA-ERM group. After cataract surgery alone, the ERM-CATA group had better visual improvement (ΔlogMAR: −0.358 vs. −0.032, p < 0.001). Conversely, after PPV alone, visual improvement was much greater in the CATA-ERM group (ΔlogMAR: −0.018 vs. −0.137, p = 0.002). In the Pearson correlation analysis, final BCVA exhibited a moderate to strong correlation with various parameters, specifically baseline BCVA (r = 0.456; p < 0.001), visual acuity (VA) gain after cataract surgery alone (r = 0.503; p < 0.001), and VA gain after both surgeries (r = 0.638; p < 0.001). Nonetheless, baseline CMT, final CMT, and postoperative CMT changes exhibited very weak or no correlation with final BCVA (Supplementary materials Table 1).
Macular outcome
Baseline CMT (497.00 vs. 470.81 µm, p = 0.031) and extent of CMT reduction after sequential surgeries (−139.45 vs. −113.34 µm, p = 0.046) were greater in the ERM-CATA group than in the CATA-ERM group, but the groups did not significantly differ with respect to final CMT. Compared with the ERM-CATA group, the CATA-ERM group had a larger increase in CMT after cataract surgery (6.39 vs. 53.93 µm, p < 0.001; Table 2).
PCMO
In total, 17.0% of patients developed PCMO after cataract surgery (40/259 in ERM-CATA group; 31/159 in CATA-ERM group); the groups did not significantly differ with respect to PCMO incidence (15.4% vs. 19.5%, p = 0.287; Table 2; representative cases are shown in Supplementary materials Fig. 1). As indicated in the multivariate regression, PCMO was significantly associated with DMO (OR: 13.295; 95% CI: 1.170–151.040; p = 0.037), RVO (OR: 3.608; 95% CI: 1.130–11.521; p = 0.030), and baseline CMT (OR: 1.005; 95% CI: 1.002–1.009; p = 0.003) (Table 3).
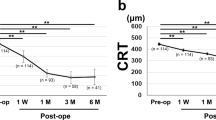
Eyes with and without PCMO did not significantly differ with respect to baseline BCVA, final BCVA, or changes in VA after individual or both surgeries. Compared with non-PCMO eyes, in PCMO eyes, CMT gain after cataract surgery was larger regardless of group. Furthermore, compared with non-PCMO eyes, PCMO eyes in the ERM-CATA group had greater baseline CMT and greater CMT reduction after PPV (Fig. 1 and Supplementary materials Table 2).
A Comparison of BCVA changes after individual and both surgeries between the two groups. B Comparison of CMT changes after individual and both surgeries between the two groups. C In the ERM-CATA group, serial changes in BCVA are shown with the presence and absence of PCMO. D In the ERM-CATA group, serial changes in CMT are shown with the presence and absence of PCMO. E In the CATA-ERM group, serial changes in BCVA are shown with the presence and absence of PCMO. F In the CATA-ERM group, serial changes in CMT are shown with the presence and absence of PCMO. Error bars show standard deviation.
In eyes with baseline CMT ≥ 500 µm, the incidence of PCMO was 29.40% and significantly higher than the 16.30% in eyes with baseline CMT < 500 µm (p = 0.008). PCMO incidence was also significantly higher in eyes with baseline BCVA < 0.3 than in eyes with baseline BCVA ≥ 0.3 (22.80% vs. 14.70%, p = 0.004, Fig. 2).
A PCMO rate segmented by baseline CMT. B PCMO rate segmented by baseline BCVA. a Median value of baseline CMT in the dataset was nearly 500 µm. Similarly, the median value of baseline BCVA was close to logMAR 0.523, equivalent to approximately 0.3 (6/20) in Snellen chart BCVA. Therefore, we set the cutoff value for baseline CMT to 500 µm and that for baseline BCVA to 0.3 to reveal their associations with PCMO. Error bars show 95% CI.
Discussion
To the best of our knowledge, this is the first study to compare visual outcomes between the two surgical sequences of conducting ERM before cataract surgery or vice versa. In our cohort, both BCVA and CMT improved after either surgical sequence, but final BCVA and VA gains from baseline were much better in the ERM-CATA group. Compared with the ERM-CATA group, the CATA-ERM group had a significantly greater increase in CMT after cataract surgery, but differences in PCMO incidence were nonsignificant. This finding implies that for patients undergoing cataract surgery first, the presence of ERM can cause greater retinal thickening after cataract surgery. Yiu et al. reported a compatible result: combined phacovitrectomy yielded greater postoperative CMT and less CMT reduction compared with PPV alone; this result was due to greater postoperative inflammation and PCMO after phacoemulsification [14]. Therefore, cataract surgery can increase postoperative CMT, especially before ERM removal, and lead to subclinical macular oedema or even visually significant PCMO [15, 16]. Our finding of increased CMT partially explained the limited postcataract surgery VA gain and inferior final visual outcome in the CATA-ERM group compared with those in the ERM-CATA group.
There were several baseline demographic differences between the two groups of patients. Compared with the CATA-ERM group, the ERM-CATA group had more young patients and women, possibly because the incidence of ERM in women is higher and because young patients tend to be more willing and physically able to undergo PPV, which is more complicated and time-consuming than cataract surgery is [17]. Baseline CMT was also greater in the ERM-CATA group than in the CATA-ERM group, generally indicating more severe ERM and that clinicians may prefer performing PPV first to revive vision; thus, the postoperative changes in CMT were greater in the ERM-CATA group, but the final CMT after both surgeries did not differ between the two groups. Compared with the ERM-CATA group, the CATA-ERM group had a greater proportion of patients with systemic and ocular diseases attributable to advanced age. The mean interval between the two surgeries was similar between the two groups. The factors of advanced age and a higher proportion of ocular diseases may have adversely affected postoperative vision in the CATA-ERM group. In order to minimize ocular factors imbalance, a subgroup analysis excluding eyes with DMO, DM retinopathy, and AMD in both groups was done, showing a consistently better final VA and final VA changes in ERM-CATA group. Because the baseline vision was statistically similar between the two groups, the order of performing PPV and cataract surgery may substantially contribute to the difference in vision improvement. In addition, baseline BCVA and VA changes after surgery were positively correlated with final BCVA, whereas the factors of baseline CMT, final CMT, and postoperative CMT changes were less likely to influence the final visual outcome. These findings are similar to those of a previous study [12]. Our study suggests that although several baseline demographic differences existed between the two groups which may affect the decision of surgical sequence, performing PPV before cataract surgery gave rise to a better final visual outcome if other ocular confounding factors were excluded. Furthermore, Luu et al. demonstrated a natural history of idiopathic ERM and showed that lens status may be associated with the timing of ERM surgery in patients with baseline BCVA of 20/40 or greater [18]. Phakic eyes with ERM had worse baseline BCVA and more rapid visual decline, yet they were less likely to receive ERM surgery than pseudophakic eyes with ERM. The authors attributed lens opacity to the difference of visual decline between phakic and pseudophakic eyes because the baseline CMT and rate of CMT increase during follow-up were similar between different lens status. Hence, when considering ERM surgery for patients with combined cataract and ERM, the impact of ERM on visual function was a stronger contributing factor than lens opacity. This was also reflected in our study by the suboptimal VA gains after cataract surgery in the CATA-ERM group that required subsequent ERM surgery.
Regardless of whether PPV or cataract surgery was undertaken first, vision improved greatly after the second surgery, compared with the first performed one. Dugas et al. also reported more significant vision improvement after the second surgery in the consecutive management of ERM and cataract [12]. Both in our and Dugas’ study, the lesser visual acuity gain after the first surgery were derived from postoperative visual acuity recorded several months after the first surgery, reflecting the long-term and stable visual effect of the first surgery. A possible explanation is that, first, the vision gradually declined after the first and before the second surgery; second, the eyes included were more likely to have inferior visual improvement of the first surgery, requiring further intervention for the second disease. Considering these findings, we recommend that both surgeries should be performed and PPV would be better undertaken before cataract surgery in eyes with concurrent ERM and cataract to yield superior visual outcomes.
In our study, PCMO developed in 71 eyes (incidence: 17.0%) with no statistical difference between the two groups. Patients with ERM had a 5-fold greater risk of PCMO compared with patients with no risk factors [10]. Schaub et al. reported a 15.7% incidence of OCT-defined PCMO in the presence of ERM; furthermore, subclinical cases might have been included, which resulted in an incidence rate that is similar to ours—because we also defined PCMO by using SD-OCT images [7, 13]. Conversely, the postoperative incidence of PCMO (whether visually significant or not) is reported to be 4–11% in the general population [3, 5, 6, 16]. Vitreous traction, blood–retinal barrier (BRB) disruption, and postoperative inflammatory reaction are key factors associated with PCMO [3, 5, 6, 19]. Despite the release of macular traction force after PPV with ERM peeling, we noted that PCMO incidence did not significantly decrease in the ERM-CATA group in which ERM was removed before cataract surgery, suggesting that the vitreoretinal microenvironment of ERM may not be completely altered and that the BRB is not fully restored by membrane peeling after PPV. In a small prospective study, Mylonas et al. also reported significantly increased CMT and a 26% incidence of PCMO after cataract surgery for eyes with previous PPV and internal limiting membrane peeling [20]. Jiramongkolchai et al. reported an even higher PCMO incidence of 30% at 6 months after cataract surgery in the eyes of patients with diabetes and previous vitrectomy; the authors attributed the high incidence to intraocular inflammation [21]. However, due to the transient and self-limited course of PCMO, it did not cause a significant difference in final BCVA or VA gain after two-step sequential surgery compared with non-PCMO eyes in literature as well as in our study [4, 19].
In the present study, DMO, RVO and baseline CMT were associated with PCMO in the logistic regression. Furthermore, baseline and final CMT were higher in eyes with PCMO, and PCMO incidence was significantly increased in eyes with baseline CMT ≥ 500 µm. Although the exact role of cytokines in promoting the macular oedema of ERM remains largely unknown, we speculated from our findings that eyes with more severe ERM are more susceptible to inflammatory mediators released by cataract surgery in spite of removed ERM after PPV [6, 8, 19, 22,23,24]. Furthermore, eyes with baseline BCVA < 0.3 had a much higher incidence of PCMO, possibly because they were more likely to have ERM with greater baseline CMT (results not shown), a positive predictor of PCMO. Therefore, patients with ERM and low baseline BCVA (<0.3) or high baseline CMT (≥500 µm) may be more susceptible to PCMO.
The limitations of this study pertain primarily to its retrospective nature. First, we could not account for various confounding factors. Second, our findings had limited statistical power due to our small sample size. Third, the visual acuity was assessed by Snellen chart with arithmetic ordering in the outpatient setting which may overlook vision changes in the poor visual acuity ranges, especially at time after the first and before the second surgery. In addition, ERM and cataract severity had no corresponding quantitative data. Furthermore, we calculated VA changes in terms of differences in perioperative BCVA instead of VA at the time of PCMO occurrence; thus, we did not calculate the transient VA decrease due to macular oedema. Because our two groups differed in age, future studies should investigate age as a factor by using a larger age-matched prospective sample and measure vision by a logarithmic visual acuity chart. Despite these limitations, however, our study elucidated outcomes on the order of PPV and cataract surgery in patients with concomitant ERM and cataract; our findings can aid clinicians in managing the two common ocular diseases.
In conclusion, when managing concurrent ERM and cataract, performing PPV with membrane peeling before, rather than after, cataract surgery yields better visual outcomes but similar PCMO incidence. PCMO incidence is higher in eyes with ERM and greater baseline CMT.
Summary
What was known before
-
Pseudophakic cystoid macular oedema is a common complication after cataract surgery that may adversely impact vision, and epiretinal membrane is known to be a crucial risk factor.
-
Surgical management of concurrent epiretinal membrane and cataract consists of pars plana vitrectomy and cataract surgery, either consecutively or simultaneously, while the two ways did not differ in the effect of vision improvement.
-
In consecutive treatment of epiretinal membrane and cataract, it is unclear which surgery to start with, concerning visual outcome and incidence of pseudophakic cystoid macular oedema.
What this study adds
-
When managing concurrent epiretinal membrane and cataract, performing pars plana vitrectomy before cataract surgery offers a better visual outcome but similar rate of pseudophakic cystoid macular oedema relative to performing cataract surgery prior to pars plana vitrectomy.
-
Higher preoperative central macular thickness of epiretinal membrane is associated with higher incidence of pseudophakic cystoid macular oedema.
References
Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56:6762–9.
Lou L, Ye X, Xu P, Wang J, Xu Y, Jin K, et al. Association of sex with the global burden of cataract. JAMA Ophthalmol. 2018;136:116–21.
Grzybowski A, Sikorski BL, Ascaso FJ, Huerva V. Pseudophakic cystoid macular edema: update 2016. Clin Inter Aging. 2016;11:1221–9.
Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33:1550–8.
Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012;23:26–32.
Han JV, Patel DV, Squirrell D, McGhee CN. Cystoid macular oedema following cataract surgery: a review. Clin Exp Ophthalmol. 2019;47:346–56.
Schaub F, Adler W, Enders P, Koenig MC, Koch KR, Cursiefen C, et al. Preexisting epiretinal membrane is associated with pseudophakic cystoid macular edema. Graefes Arch Clin Exp Ophthalmol. 2018;256:909–17.
Lobo C. Pseudophakic cystoid macular edema. Ophthalmologica. 2012;227:61–7.
Zur D, Fischer N, Tufail A, Mones J, Loewenstein A. Postsurgical cystoid macular edema. Eur J Ophthalmol. 2011;21:S62–8.
Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC, et al. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 Eyes. Ophthalmology 2016;123(2):316–23.
Hardin JS, Gauldin DW, Soliman MK, Chu CJ, Yang YC, Sallam AB. Cataract surgery outcomes in eyes with primary epiretinal membrane. JAMA Ophthalmol. 2018;136:148–54.
Dugas B, Ouled-Moussa R, Lafontaine PO, Guillaubey A, Berrod JP, Hubert I, et al. Idiopathic epiretinal macular membrane and cataract extraction: combined versus consecutive surgery. Am J Ophthalmol. 2010;149:302–6.
Kim SJ, Belair M-L, Bressler NM, Dunn JP, Thorne JE, Kedhar SR, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 2008;28:870–6.
Yiu G, Marra KV, Wagley S, Krishnan S, Sandhu H, Kovacs K, et al. Surgical outcomes after epiretinal membrane peeling combined with cataract surgery. Br J Ophthalmol. 2013;97:1197–201.
Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004;30:752–60.
Perente I, Utine CA, Ozturker C, Cakir M, Kaya V, Eren H, et al. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res. 2007;32:241–7.
Xiao W, Chen X, Yan W, Zhu Z, He M. Prevalence and risk factors of epiretinal membranes: a systematic review and meta-analysis of population-based studies. BMJ Open. 2017;7:e014644.
Luu K-Y, Koenigsaecker T, Yazdanyar A, Mukkamala L, Durbin-Johnson BP, Morse LS, et al. Long-term natural history of idiopathic epiretinal membranes with good visual acuity. Eye 2019;33:714–23.
Guo S, Patel S, Baumrind B, Johnson K, Levinsohn D, Marcus E, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015;60:123–37.
Mylonas G, Sacu S, Deak G, Dunavoelgyi R, Buehl W, Georgopoulos M, et al. Macular edema following cataract surgery in eyes with previous 23-gauge vitrectomy and peeling of the internal limiting membrane. Am J Ophthalmol. 2013;155:253–9.
Jiramongkolchai K, Lalezary M, Kim SJ. Influence of previous vitrectomy on incidence of macular oedema after cataract surgery in diabetic eyes. Br J Ophthalmol. 2011;95:524–9.
Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, et al. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018;63:20–68.
Zandi S, Tappeiner C, Pfister IB, Despont A, Rieben R, Garweg JG. Vitreal cytokine profile differences between eyes with epiretinal membranes or macular holes. Invest Ophthalmol Vis Sci. 2016;57:6320–6.
Applewhite BP, Babapoor-Farrokhran S, Poon D, Hassan SJ, Wellmann E, Ying HS, et al. Lack of evidence for vasoactive and inflammatory mediators in the promotion of macular edema associated with epiretinal membranes. Sci Rep. 2017;7:1–10.
Author information
Authors and Affiliations
Contributions
YMH and SJC designed the study. YCC and YMH analysed and interpreted the data. YCC wrote the initial draft. YCC, YMH, and SJC revised the manuscript. AFL and SJC supervised the study. All authors undertook a final review and approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, YC., Chen, SJ., Li, AF. et al. Visual outcomes and incidence of pseudophakic cystoid macular oedema in eyes with cataract and idiopathic epiretinal membrane after two-step sequential surgery. Eye 36, 1597–1603 (2022). https://doi.org/10.1038/s41433-021-01673-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01673-0