Abstract
Objective
A non-interventional, longitudinal, retrospective follow-up study to assess CsA-induced nephrotoxicity (IN) and its reversibility after withdrawal in patients exhibiting a bilateral chronic posterior uveitis (CPU) associated with cystoid macular oedema (CMO) in at least one eye. Data from medical records between 1986 and 2013.
Methods
Primary outcome was the renal tolerance during and after CsA treatment assessed by plasma creatinine concentration and glomerular filtration rate (GFR) estimated by Chronic Kidney Disease Epidemiology (CKD-Epi) formula. Secondary outcomes were CsA through concentration, occurrence of cancers and ophthalmologic efficacy assessed by three parameters including CMO, vitreous inflammation, and best-corrected visual acuity BVCA changes.
Results
One hundred forty-three patients were followed for renal tolerance. Underlying diseases were Birdshot retinochoroiditis (n = 67), Behçet disease (n = 9), probable sarcoidosis (n = 23), sympathetic ophthalmia (n = 3), idiopathic (n = 41). After CsA discontinuation in 115 patients (mean treatment duration of 5.9 ± 3.8 years) mean plasma creatinine concentration was 82.2 ± 14.2 µmol/L versus 82.1 ± 14.1 µmol/L at baseline, mean GFR was 79.4 ± 13.9 mL/min versus 82.5 ± 14.3 mL/min at baseline, with no significant difference (respectively p = 0.91 and p = 0.09). Blood pressure did not significantly change during follow-up. CMO was completely resorbed in at least one eye, in 70.8% patients (n = 72) at 6 months, in 71.4% patients (n = 49) at 10 years and in 54.2% patients (n = 24) at 20 years. BCVA did not statistically change over time.
Conclusion
Early and long-term monitoring of renal tolerance and dual adjustment of CsA doses in inflammatory stages of CPU were associated with reversible CsA IN. CsA could be effective in the treatment of CMO in CPU patients.
Similar content being viewed by others
Introduction
Auto-immune chronic posterior uveitis (CPU) is a rare, chronic inflammatory disease. Because of its low incidence and the low severity of initial symptoms, the diagnosis can be delayed, increasing the risk of irreversible tissue damage and poor functional prognosis. The prevalence of CPU in European and Northern American populations is estimated between 0.1 and 1.7 per 100,000. CPU is predominant in Caucasian populations [1, 2].
Cystoid macular oedema (CMO), retinal vasculitis, and choroidal and/or retinal neovascularization complicate CPU in respectively 63–84%, 40% and 13–14% of cases [3]. Although up to 20% of cases seem to be self-limited types of the disease, the overall evolution in long-term follow-up studies appears to be defined by successive inflammatory exacerbations leading to progressive visual loss. Due to the lack of consensus, initial and/or emergency treatment is often based on systemic corticosteroids, used in an inflammatory context, and as a bridging therapy immunosuppressive treatment. However, corticosteroids appear to be inconsistently effective for sustaining remission, when used at the usual doses and are responsible for many well-known adverse events (AEs) [4].
Therefore, steroid-sparing immunosuppressive treatments, including methotrexate, mycophenolate mofetil [5], ciclosporin A (CsA) and more recently biotherapies such as anti-Tumour Necrosis Factor (TNF) alpha [6], are the essential options, following therapeutics algorithms previously published [7, 8].
CsA is a calcineurin inhibitor, widely used in solid organ transplant and in auto-immune diseases. Its immunosuppressive effect results from decreased Interleukin-2 release and inhibition of T-cell proliferation [9].
CsA use is mainly limited by its AEs, especially nephrotoxicity and high blood pressure (HBP), which is potentially worsened by long-term corticosteroids often associated [10, 11]. CsA-induced nephrotoxicity (CsA-IN) results from two mechanisms: acute renal injury, induced by renal vasoconstriction, that is usually reversible, and chronic impairment, in which vasoconstriction leads to structural damage and interstitial fibrosis [12, 13] with typical pearl-like myocytes in the arteriolar walls. These long-term AEs might be unspecific and lead to dose reduction, exposing potentially to uncontrolled disease in auto-immune disorders [14].
CsA being efficient in T-cell-mediated pathologies has been used in CPU with reported efficacy in controlling the disease [15, 16]. In other pathological contexts, such as chronic inflammatory arthritis, systemic diseases or chronic dermatitis, a decrease in glomerular filtration rate (GFR) has been shown to be the most commonly reported AEs of CsA (48% of cases), with a decrease in plasma creatinine concentration after CsA discontinuation in most cases [17, 18]. Similarly, a new onset of high BP and dyslipidaemia are common under CsA treatment [19].
Nowadays, although effective, CsA is less used in CPU because of its associated nephrotoxicity [20]. Indeed, an irreversible loss of renal function has been reported in CPU patients with healthy native kidneys treated with long-term CsA therapy for CPU, mostly Birdshot retinochoroidopathy [3]. We have previously shown in a longer study that the effect of CsA on renal function was partially reversible after CsA discontinuation with BP normalization and a significant recovery of renal function [21]. Therefore, we assessed renal function in a larger cohort of patients with previously healthy kidneys, long-term exposed to CsA.
The aim of this study was to assess CsA-IN in a large cohort of CPU patients with healthy kidneys treated on the long term and its potential reversibility after treatment discontinuation and the ophthalmologic efficacy on CMO. To our knowledge, this is the first long-term study of CsA safety and tolerance in the treatment of CPU patients.
Methods
Study design
A retrospective follow-up study was conducted to assess the long-term renal tolerance and the ophthalmologic efficacy of CsA.
Study population
Data were retrospectively collected from the medical files of all patients with CPU, diagnosed in several Ophthalmology centres in France, referred to our ophthalmology department (National Reference Center for Rare Diseases), and then referred to the Nephrology department for CsA initiation during the last decades (from 1986 to 2013). Patients were followed for at least 2 years after CsA withdrawal (Fig. 1a). Nephrological work up made for each patient before inclusion showed absence of chronic renal disease and all patients had a GFR > 60 mL/min/1.73 m2, normal urine testing and absence of renal abnormality in imaging showing no cysts and no obstruction on the ducts.
a Flow chart. b Multidisciplinary protocol of treatment and follow-up of renal tolerance and ophthalmologic efficacy. c Long-term monitoring of plasma creatinine concentration in patients who discontinued CsA (n = 115). ***p value <0.001. 1: Baseline: (corresponding to a GFR level mean CKD-Epi of 80(±14.6) mL/min). 2: Zenith of plasma creatinine concentration: (CKD-Epi 55 ± 19.2) mL/min. 3: Nadir of plasma creatinine concentration: (CKD-Epi mean 80 ± 18.4). 4: End of the follow-up. 5: CsA discontinuation. d Long-term monitoring of plasma creatinine concentration in patients who continued CsA (n = 28). ***p value <0.001, NS not significant. 1: Baseline. 2: Zenith of plasma creatinine concentration. 3: Nadir of plasma creatinine concentration. 4: End of the follow-up.
Outcome measurements
We aimed to analyze in depth the renal function of all patients whether under CsA or have discontinued CsA from the period of 1986 to 2013. The primary outcome was the renal tolerance, defined by the change in plasma creatinine concentration (N 44–80 µmol/L) and the estimated GFR (N > 90 mL/min), using the Chronic Kidney Disease Epidemiology (CKD-Epi) formula [22]. Plasma creatinine concentration is expressed in mg/dL, according to CsA dose and trough concentration. Blood tests were centralized in Pitié-Salpêtrière university hospital laboratory. The level of plasma creatinine concentration was always measured in the same hospital laboratory. We considered the percent of augmentation of plasma creatinine concentration compared to baseline. Secondary outcomes were the BP, the measurement of CsA C0, as the CsA trough concentration that is the lowest concentration in the whole day, measured on blood sample usually drawn the morning, and ophthalmologic efficacy.
Patients were assessed by ophthalmologists and nephrologists during their follow-up visits every 3 months during the first year of CsA treatment, and then every 6 months or yearly until CsA discontinuation and beyond, with a monitoring of C0 CsA trough concentration, biological renal assessment (Fig. 1b) and imaging for cancer detection each year. High daily doses were required at initial time to control the ocular inflammation. Then doses were slowly tapered while scheduled ophthalmological examinations at 1, 3, 6 months after initiation. This way allowed to determine the minimal efficient dose while searching to avoid inflammatory recurrence and long-term nephrotoxicity. The CsA trough concentration target was 100 ng/mL by analogy with results shown in a cohort of renal-transplant patients, that showed a good tolerance to long-term cyclosporine therapy without evidence of progressive toxic nephropathy [23].
In the first 6 months, we tolerated a 50% increasing of plasma creatinine concentration (the time needed to obtain a control of the ocular inflammation), which corresponded to a mean decrease of −30% in GFR.
Further to minimize the CsA chronic exposure, the doses of CsA were adapted thanks to a dual ophthalmological and nephrological follow-up (Fig. 1b). The scheduled follow-up included plasma creatinine concentration analysis at 1 week, 1 month, 3 months, and 6 months and then every year.
The adjustment was performed as following: when plasma creatinine concentration was increasing, the CsA trough concentration was tested. If CsA trough concentration was higher than 100 ng/mL, the CsA dose was decreased. If in addition there was no ophthalmological efficiency, the CsA was stopped.
In case of ophthalmological inefficiency related to a low CsA trough concentration rate <100 ng/mL, the dose of CsA was increased.
If the increase in plasma creatinine concentration was >50% and GFR < 30% the CsA trough concentration was checked. If it was below or around the range of 100 ng/mL, CsA was stopped. If CsA trough concentration >100 ng/ml, the dose of CsA was adjusted by decreasing CsA daily dose and checking the plasma creatinine concentration after. If still high with low CsA trough concentration, it was decided to stop CsA and switch to another immunosuppressant or to an immunomodulator such as interferon alpha
The analysis of ophthalmologic efficacy was assessed by two masked observers, it was focused on CMO change and was performed at 6 months, 10 years, 20 years, and at the end of the follow-up. The primary parameter of effectiveness is inflammatory CMO change defined as positive, partial, and negative response. Its diagnosis was based on intra-retinal cysts in OCT or on hyperfluorescent cysts filling in FA for the oldest cases. The secondary ophthalmological outcome was the change in vitreous inflammation noted according to the SUN [24]. The tertiary outcome was the best-corrected visual acuity (BCVA) assessed on decimal and Snellen charts converted into LogMAR.
The occurrence of cancer and the cause of mortality, when available, were recorded until the end of the follow-up.
Statistical analysis
The final analysis was performed once all data were recorded and verified by two persons. Continuous data are presented as the median (range) or mean ± standard deviation, as appropriate. Categorical data are presented as numbers and percentages of total. Univariate analysis was performed using a Fisher’s exact test or a χ2 test, as appropriate, to compare categorical variables, and Student t tests were used to compare continuous variables. A two-tailed P value <0.05 was considered statistically significant. Statistical analysis was performed using R software (version 3.2.0; R Foundation for Statistical Computing, Vienna, Austria).
Subgroup analysis was performed to assess renal changes in patients who were still treated with CsA at the end of the study, and in those whose treatment was prematurely discontinued.
Ethics and consent
As this study was not in the scope of the French regulation applicable to clinical trials, no submission to IRB/Ethics Committee was needed. The study was conducted according to the French data protection law in force at the time conducting the study. The research adhered to the tenets of the Declaration of Helsinki.
Results
Between 1986 and 2016, 143 CPU patients were treated with CsA and treatment was initiated between 1986 and 2013. Thus, 143 patients were included in the analysis. Underlying diseases were Birdshot retinochoroiditis (n = 67), Behcet’s disease (n = 9), probable sarcoidosis (n = 23), sympathetic ophthalmia (n = 3), idiopathic (n = 41), Patterns of uveitis were panuveitis (n = 53), posterior uveitis (n = 90) and bilateral involvement in all patients. Median time from uveitis diagnosis to CsA administration was 24 months.
Patient characteristics
Table 1 CsA was initiated at the dose of 5 mg/kg/day or 3 mg/kg/day, and gradually increased according to its trough concentration and renal tolerance assessed by estimated GFR using the CKD-Epi formula using centralized plasma creatinine concentration (Fig. 1a). CsA dose was decreased in 143 patients (100%) and discontinued in 115 (80.4%) patients after 5.9 ± 3.8 years.
No difference was found at baseline between patients who discontinued CsA and patients who continued CsA throughout the study.
Renal tolerance
Long-term follow-up of plasma creatinine concentration in patients who discontinued CsA (Table 1 and Fig. 1c)
CsA was discontinued in 115 patients after a mean time of 5.9 ± 3.8 years. CsA was discontinued in three circumstances (Fig. 1b). An increase in plasma creatinine concentration of more than 50% (on average plasma creatinine concentration increased from 80 µmol/L (baseline) to 120 µmol/L (maximum)) was considered significant corresponding on average to a degradation of −30% in GFR (with a loss of 25 mL/min/1.73 m2 on average). For this cohort, the GFR in CKD-Epi passed on average from 80 mL/min/1.73 m2 at baseline to 55 mL/min/1.73 m2 on average at peak plasma creatinine concentration levels. The GFR improved at the end of the follow-up with a recovery of 15 mL /min/1.73 m2. This result is close to the effect of aging in the general population. The tolerance of the augmentation of plasma creatinine concentration was 50% during the first 6 months (the time needed to obtain a control of the ocular inflammation). At baseline, the mean plasma creatinine concentration was 82.1 ± 14.1 µmol/L, and the mean GFR was 82.5 ± 14.3 mL/min/1.73 m2. The most severe renal alteration during the CsA treatment occurred 2.6 ± 3.3 years after CsA initiation, with a mean peak plasma creatinine concentration of 124.0 ± 46.5 µmol/L, i.e., an increase by 42.0 ± 45.7 µmol/L (p < 0.001), and a mean GFR of 54.9 ± 19.4 mL/min, i.e., a decrease by 27.8 ± 19.7 mL/min/1.73 m2 (p < 0.001).
Mean time of 2.2 years after CsA discontinuation, patients experienced an improvement in renal function with a mean plasma creatinine concentration of 82.2 ± 14.2 µmol/L, and a mean GFR of 79.4 ± 13.9 mL/min/1.73 m2, with no significant difference with baseline data (respectively p = 0.91 and p = 0.09).
Long-term follow-up of plasma creatinine concentration in patients who continued CsA (n = 28) (Fig. 1d)
Twenty-eight patients were still treated with CsA at the end of the study. The steepest decline in renal function occurred 1.9 ± 2.3 years after CsA initiation, with a mean plasma creatinine concentration peak of 107.0 ± 25.4 µmol/L, i.e., an increase by 25.3 ± 23.1 µmol/L/1.73 m2 (p < 0.001), and a mean GFR of 54.7 ± 15.5 mL/min/1.73 m2, i.e., a decrease by 26.5 ± 16.6 mL/min/1.73 m2 (p < 0.001). A mean time of 3.6 ± 3.7 years after peak plasma creatinine concentration, corresponding to the more severe renal deterioration, patients showed an improvement, with a mean plasma creatinine concentration of 78.4 ± 17.1 µmol/L, i.e., a decrease by 28.9 ± 16.1 µmol/L (p < 0.001), and a mean GFR of 76.8 ± 15.5 mL/min/1.73 m2, i.e., an increase by 21.8 ± 14.6 mL/min/1.73 m2 (p < 0.001). Differences between baseline data and these best renal features corresponded to a non-significant increase in plasma creatinine concentration by 3.8 ± 13.3 µmol/L (p = 0.19), and a non-significant decrease in GFR by 5.2 ± 17.4 mL/min/1.73 m2 (p = 0.17). Finally, the mean plasma creatinine concentration at the end of the follow-up (6.5 ± 5.8 years after CsA initiation) was 88.3 ± 22.1 µmol/L, i.e., a non-significant increase by 6.0 ± 21.0 µmol/L (p = 0.15), and the mean GFR was 66.7 ± 18.5 mL/min/1.73 m2, i.e. a significant decrease by 14.2 ± 17.1 mL/min/1.73 m2 (p < 0.001) between CsA initiation and the end of the follow-up. In both groups (patients who continued or discontinued CsA at the end of the follow-up), subgroup analysis showed no significant difference between patients who received an initial CsA dose of 3 mg/kg per day and those who received a higher initial dose (5 mg/kg/d).
CsA trough concentrations (C0) (Table 2)
The target concentration used to adjust CsA dose was 100 ng/mL. There were differences in the change in C0 because the doses were adjusted to maintain the target CsA trough concentration within the first years to control inflammation. The difference in plasma creatinine concentration and in GFR between baseline and peak plasma creatinine concentration at about 2 years was significant (p < 0.001 for both). In contrast, the difference in C0 between baseline and peak plasma creatinine concentration was not significant (p = 0.14). The correlation between C0 and the GFR or the plasma creatinine concentration was not significant (p = 0.06 and p = 0.14, respectively).
Blood pressure (Table 1, Supplementary Tables 1 and 2)
The mean systolic BP was 129.9 ± 15.8 mmHg, and the mean diastolic BP was 77.6 ± 11.6 mmHg. Twenty-three patients (17.8%) had a history of HBP prior to initiation of treatment (Table 1). The mean BP at the time of highest GFR levels was 128.7/77.5 mmHg, and 42 patients (32.6%) met criteria for HBP. The mean BP at the end of the follow-up was 131.9/77.8 mmHg, and 32 patients (28.8%) had HBP. BP long-term monitoring at different occasions is shown (Supplementary Table 1). Between baseline and peak plasma creatinine concentration, a significant increase in systolic BP, diastolic BP and number of patients meeting HBP criteria was shown in the total population and in patients who discontinued CsA, no significant difference in these criteria was found between baseline and the end of the follow-up (Supplementary Table 2).
Ophthalmologic efficacy (Table 3) (Supplementary Fig. 1)
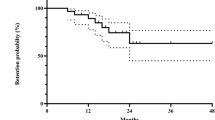
Complete ophthalmological data were available for 72 patients (50%) with a mean follow-up of 13.8 ± 6.8 years. Regarding CMO change after 6 months of treatment, 51 patients (70.8%) experienced a completely resorbed CMO in at least one eye (Supplementary Fig. 1), 6 patients (8.3%) experienced a partial response and 15 (20.8%) had no benefit of CsA treatment (Table 3). At 10 years 49 patients were ongoing the CsA treatment of which 35 patients (71.4%) showed a completely resorbed CMO in at least one eye and 7 patients (14.3 %) experienced a partial response. At 20 years CMO was completely resolved in at least one eye in 13 patients (54.2%) of 24 patients ongoing the CsA and in 45 patients (67.2%) of 67 patients at the end of the CsA treatment.
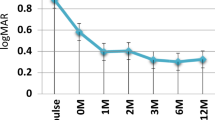
Vitreous inflammation assessed to ≤1+ according to Sun classification in at least one eye was shown in 17.8% of patients at inclusion (n = 72), in 82.1% of patients (n = 72) after 6 months, in 79.9% (n = 49) at 10 years, in 44.3% (n = 24) at 20 years showing statistical change when compared to initial time (p = 0.02). Log Mar BCVA were at initial time 0.51 ± 0.35 right eye (RE) and 0.54 ± 0.39 left eye (LE), at 6 months 0.41 ± 0.26 RE and 0.51 ± 0.2 LE, at 10 years 0.46 ± 0.28 RE and 0.50 ± 0.29 LE and at 20 years 0.41 ± 0.31 RE and 0.57 ± 0.29 LE (p = 0.29). At 20 years, BVCA dropped to less than +1 LogMar in 16 eyes of 11 patients of the 72 patients initially treated with CsA.
Cancer
Cancer screening protocol was set consisting of annual abdomino-renal echography, chest X-rays, mammography, vaginal swabs, and dermatologic examination. According to our data at the time of the last follow-up, a cancer was detected in 22 patients, including 7 breast cancers and 6 prostatic cancers. Four patients died: one from a breast cancer, one from a metastatic bladder cancer, and the aetiology was unknown in two patients. They received CsA for 21 years, 18 months, 4.6 years, and 4.2 years, respectively.
Discussion
To our knowledge, this is the first study showing efficacy and long-term CsA tolerance with an important CsA-IN reversibility with no significant difference 2 years after CsA discontinuation. The baseline was normal renal function in all patients. Moreover, the GFR decrease of less than 1 mL/min/year between baseline and the end of follow-up has been considered normal as reported in the literature [25]. CsA-IN was not supposed to be reversible, but this study proves that the CsA-IN is very important. Nephrotoxicity has two components, an “acute” reversible component related to the daily dose of CsA and a chronic reversible component related to exposure to CsA expressed by the area under the curve. In our study, CsA-IN was mainly reversible in patients with previously healthy kidneys, which corresponded to the normal decrease. Indeed, the decrease in GFR in CKD- Epi passing on average from 80 mL/min/1.73 m2 at baseline to 55 mL/min/1.73 m2 on average at peak plasma creatinine concentration to improve at the end of the follow-up with recovery of 15 mL/min/1.73 m2 This result is close to the effect of aging in the general population. CsA-IN resolved when CsA dose was decreased by the nephrologist due to an increase in plasma creatinine concentration and/or by the ophthalmologist when inflammation was controlled. Renal function continued to improve several years after CsA discontinuation, with no significant differences in GFR and plasma creatinine concentration between baseline and 2 years after CsA discontinuation, with an acceptable significant decrease compared with the “normal decrease” between baseline and the end of the follow-up more than a decade later. These results are in line with previous studies [21].
Despite missing data, our results also suggested that monitoring CsA trough concentration is useful to manage CsA-IN in patients with an auto-immune disease, although no target concentration has been defined. We used the target of 100 ng/ml analogous to renal transplanted patients [23] and it appeared to be sufficient. These descriptive data of CsA trough concentration are the first published data for an auto-immune disease to our knowledge. The collaboration between the ophthalmologist and the nephrologist is very useful to adjust and monitor the daily CsA dose.
We have previously suggested that CsA can be used at the right dose from the onset of CPU with CMO even if plasma creatinine concentration increases. Indeed, we have shown that this early increase in plasma creatinine concentration was mainly reversible [21]. Moreover, the reversibility occurs after 2 years of CsA-adjusted treatment. Sight-threatening CMO requires prompt and effective CsA treatment in most cases for at least six months. The concomitant increase in plasma creatinine concentration, related to CsA daily dose, is reversible when properly monitored by a nephrologist.
Furthermore, after the first months, once intraocular inflammation is controlled, CsA daily dose should be tapered while controlling CPU activity. Indeed, the lowest area under the -curve (AUC) of the long-term exposure has been shown to correlate with long-term low-dose CsA-IN [21]. At CPU onset, the initial daily high dose of CsA correlated with plasma creatinine concentration increase is mainly reversible. While the CsA doses were tapered, the GFR improved in all patients, suggesting that renal function is related to the daily doses level too. After controlling CPU activity, the long-term daily dose of CsA must be as low as possible to reduce the long-term exposure to CsA, corresponding to the lowest AUC. This nephrotoxicity induced by a long-term exposure to CsA tends to be less reversible [21]. To minimize this chronic exposure, the doses of CsA were adjusted thanks to a dual ophthalmological and nephrological follow-up. CsA was effective in most CPU patients. Nowadays, various expensive systemic and local treatments are used in CPU, including biotherapies such as anti-TNF-alpha and Interferon alpha [6, 26]. CsA has been described to be effective in the treatment of uveitic macular oedema, It has been used widely since 30 years [27].
New CPU treatment algorithms have been suggested, including CsA [7], which appears to be useful in chronic diseases and requires successive therapeutic steps, in terms of financial approach, accessibility and galenic forms. Vitale et al. presented a cohort of 19 patients with a median follow-up of 36 months treated with low doses of 2.5 mg/kg, they noted regression of hyalitis in 88.5% of patients and improved visual acuity in 83,3% of patients [28]. Recently a cohort of 132 patients with Behcet’s disease was described with a follow-up of 25 years. Systemic corticosteroids therapy was used in 93% of patients and CsA in 74% of patients, with a long-term result that maintained visual acuity greater than 20/40 in more than half of patients [29]. Although adalimumab (ADA) is FDA and Europe approved for CPU, conventional immunosuppressive treatments such as CsA are still largely used as initial corticostroid-sparing because of their lower costs and sustained efficacy in CMO [27].
VISUAL 1, multinational double-masked, phase 3 trial, included patients with active refractory non-infectious intermediate, posterior, or panuveitis uveitis treated either with ADA or placebo, after a fixed common steroid tapering regimes [6]. The treatment failure was defined using a composite factor including new inflammatory lesions, aqueous humour cells, vitreous haze, BCVA. The time to treatment failure was showed doubled in ada group (24 weeks versus 13 weeks) however, chronic macular oedema rate was not found significantly different between the two groups. Visual III post hoc analysis showed that among patients with no macular oedema, the risk of development of new macular oedema was 67% lower with ADA than with placebo [6].
CsA-induced HBP tended to disappear after CsA dose reduction and discontinuation, with no significant difference between baseline and the end of the follow-up. Furthermore, HBP could be promoted by steroids that are often given in the early stages of the disease, especially because the middle-age onset of CPU is often concomitant with the prime occurrence of metabolic disorders. The adjustment of the doses of cyclosporine accompanied with control of hypertension and dyslipidaemia, made it possible to note the reversibility of nephrotoxicity in the current study.
Regarding cancer occurrence, 22 patients were diagnosed with cancer (15.4%), while in the French general population, the overall cancer prevalence is 6.4% in men and 5.3% in women [30]. The distribution of cancer locations in our patients was similar to that of the general population.
The main limitation of our study that spanned nearly three decades is its retrospective design and the lack of data indeed. However, this study included a large cohort of patients with a rare disease, referred from the entire French territory to a national reference centre, assessed by a multidisciplinary team, with a very long follow-up, and our results are in line with those previously reported [21, 31]. This study provides an original in vivo analysis of CsA-IN in patients with initially healthy kidneys. Renal tolerance should be assessed closely specially in patients with an auto-immune disease.
Summary
What was known before
-
The long-term use of ciclosporin A (CsA) in the treatment of chronic posterior uveitis (CPU) macular oedema induce an irreversible nephrotoxicity in previously healthy kidneys.
What this study adds
-
The first long-term study of the safety and efficacy of ciclosporin A (CsA) in the treatment of chronic posterior uveitis (CPU) a rare sight threating disease.
-
Findings: In this non-interventional, longitudinal, retrospective follow-up cohort which span nearly three decades that included 143 patients with different CPU causes, mean creatinine level and mean GFR levels showed no significant difference after CsA discontinuation (mean time of 2.2 years after CsA withdrawal) with baseline data. A positive ophthalmological response was observed in 70.8% of patients after 6 months of treatment.
-
Meaning: The use of CsA in the treatment of macular oedema in CPU is safe and effective when monitored by a multidisciplinary team.
References
Minos E, Barry RJ, Southworth S, Folkard A, Murray PI, Duker JS, et al. Birdshot chorioretinopathy: current knowledge and new concepts in pathophysiology, diagnosis, monitoring and treatment. Orphanet J Rare Dis. 2016;11:61. https://doi.org/10.1186/s13023-016-0429-8
Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57. https://doi.org/10.1186/1750-1172-7-57
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6. https://doi.org/10.1136/bjo.80.4.332
Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group, Kempen JH, Altaweel MM, Holbrook JT, Sugar EA, Thorne JE, Jabs DA. Association between long-lasting intravitreous fluocinolone acetonide implant vs systemic anti-inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA. 2017;317:1993–2005. https://doi.org/10.1001/jama.2017.5103
Doycheva D, Zierhut M, Blumenstock G, Stuebiger N, Deuter C. Mycophenolate mofetil in the therapy of uveitic macular oedema-long-term results. Ocul Immunol Inflamm. 2012;20:203–11. https://doi.org/10.3109/09273948.2012.665562
Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–43. https://doi.org/10.1056/NEJMoa1509852
Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular oedema. Eye. 2016;30:1277–92. https://doi.org/10.1038/eye.2016.115
Massa H, Pipis SY, Adewoyin T, Vergados A, Patra S, Panos GD. Macular oedema associated with non-infectious uveitis: pathophysiology, etiology, prevalence, impact and management challenges. Clin Ophthalmol. 2019;13:1761–77. https://doi.org/10.2147/OPTH.S180580
Dawar FU, Xiong Y, Khattak MNK, Li J, Lin L, Mei J. Potential role of cyclophilin A in regulating cytokine secretion. J Leukoc Biol. 2017;102:989–92. https://doi.org/10.1189/jlb.3RU0317-090RR
Shihab FS, Yi H, Bennett WM, Andoh TF. Effect of nitric oxide modulation on TGF-beta1 and matrix proteins in chronic cyclosporine nephrotoxicity. Kidney Int. 2000;58:1174–85. https://doi.org/10.1046/j.1523-1755.2000.00273.x
Hošková L, Málek I, Kopkan L, Kautzner J. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiological Res. 2017;66:167–80. https://doi.org/10.33549/physiolres.933332
Gooch JL, King C, Francis CE, Garcia PS, Bai Y. Cyclosporine A alters expression of renal microRNAs: new insights into calcineurin inhibitor nephrotoxicity. PloS ONE. 2017;12:e0175242. https://doi.org/10.1371/journal.pone.0175242
Bobadilla NA, Gamba G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. Am J Physiol Ren Physiol. 2007;293:F2–9. https://doi.org/10.1152/ajprenal.00072.2007
Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37:602–12. https://doi.org/10.1159/000351648
Le Hoang P, Girard B, Deray G, Le Minh H, De Kozak Y, Thillaye B, et al. Cyclosporine in the treatment of birdshot retinochoroidopathy. Transplant Proc. 1988;20:128–30. 3 Suppl 4
Vitale AT, Rodriguez A, Foster CS. Low-dose cyclosporine therapy in the treatment of birdshot retinochoroidopathy. Ophthalmology. 1994;101:822–31. https://doi.org/10.1016/s0161-6420(13)31254-8
Ryan C, Amor KT, Menter A. The use of cyclosporine in dermatology: part II. J Am Acad Dermatol. 2010;63:949–74. https://doi.org/10.1016/j.jaad.2010.02.062
Borigini MJ, Paulus HE. Innovative treatment approaches for rheumatoid arthritis. Combination therapy. Bailliere’s Clin Rheumatol. 1995;9:689–710. https://doi.org/10.1016/s0950-3579(05)80309-7
Griffiths B, Emery P. The treatment of lupus with cyclosporin A. Lupus. 2001;10:165–70. https://doi.org/10.1191/096120301672970034
Oh KT, Christmas NJ, Folk JC. Birdshot retinochoroiditis: long term follow-up of a chronically progressive disease. Am J Ophthalmol. 2002;133:622–9. https://doi.org/10.1016/s0002-9394(02)01350-8
Tostivint I, du Montcel ST, Jaudon MC, Mallet A, Le Hoang P, Bodaghi B, et al. Renal outcome after ciclosporin-induced nephrotoxicity. Nephrol Dialysis,Transplant. 2007;22:880–5. https://doi.org/10.1093/ndt/gfl634
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Burke JF Jr, Pirsch JD, Ramos EL, Salomon DR, Stablein DM, Van Buren DH, et al. Long-term efficacy and safety of cyclosporine in renal-transplant recipients. N Engl J Med. 1994;331:358–63. https://doi.org/10.1056/NEJM199408113310604
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. https://doi.org/10.1016/j.ajo.2005.03.057
Joubaud P. [Variations according to age and gender for creatinine clearance estimated with the Cockroft and Gault formula in a selected population of ambulatory adults]. Ann Biol Clin. 2004;62:547–54.
Fardeau C, Simon A, Rodde B, Viscogliosi F, Labalette P, Looten V, et al. Interferon-alpha2a and systemic corticosteroid in monotherapy in chronic uveitis: results of the randomized controlled BIRDFERON Study. Am J Ophthalmol. 2017;177:182–94. https://doi.org/10.1016/j.ajo.2017.03.001
Nussenblatt RB, de Smet MD, Rubin B, Freidlin V, Whitcup SM, Davis J, et al. A masked, randomized, dose-response study between cyclosporine A and G in the treatment of sight-threatening uveitis of noninfectious origin. Am J Ophthalmol. 1993;115:583–91. https://doi.org/10.1016/s0002-9394(14)71454-0
Vitale AT. Birdshot retinochoroidopathy. J Ophthalmic Vis Res. 2014;9:350–61. https://doi.org/10.4103/2008-322X.143376
Arevalo JF, Lasave AF, Al Jindan MY, Al Sabaani NA, Al-Mahmood AM, Al-Zahrani YA, et al. Uveitis in Behçet disease in a tertiary center over 25 years: the KKESH Uveitis Survey Study Group. Am J Ophthalmol. 2015;159:172–7. https://doi.org/10.1016/j.ajo.2014.10.013
Colonna M, Mitton N, Bossard N, Belot A, Grosclaude P, (FRANCIM) FN of CR. Total and partial cancer prevalence in the adult French population in 2008. BMC Cancer. 2015;15:153. https://doi.org/10.1186/s12885-015-1168-2
Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, Jouanneau C, Jaudon MC, Maksud P, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrology. 2002;13:2962–8. https://doi.org/10.1097/01.asn.0000034945.61533.26
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was partially supported by the Department of rare diseases of Novartis center Paris, France. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alafaleq, M., Freund, R., Penet, MA. et al. Ciclosporin A in bilateral auto-immune chronic posterior uveitis associated with macular oedema: a Long-term Observational Safety and Efficacy Study. Eye 36, 2144–2150 (2022). https://doi.org/10.1038/s41433-021-01829-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01829-y