Abstract
Objectives
To evaluate the outcomes of adalimumab (ADA) treatment of patients with non-infectious uveitis and scleritis, focusing on efficacy, retention rate, and safety.
Methods
This retrospective, clinical cohort study included 62 patients (104 eyes) with active ocular inflammation treated with ADA. Primary outcomes were efficacy and cumulative drug retention rate (DRR) of ADA. The secondary outcomes included changes in ocular inflammatory parameters, changes in best-corrected visual acuity (BCVA) and central macular thickness (CMT), corticosteroid-sparing effect, impact of concomitant use of disease-modifying antirheumatic drug (DMARD) and ADA as first or ≥2nd biotherapy line on DRR, and adverse events.
Results
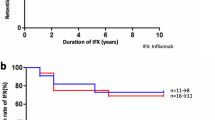
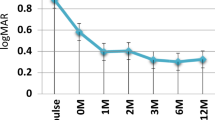
Forty-five patients (72.6%) achieved inactive disease at the end of follow-up. DRR at 6, 12, 24, and 48 months was 96.8%, 89.2%, 63.1%, and 63.1%, respectively. Of the 18 patients whose bi-weekly ADA treatment was escalated to weekly ADA due to primary or secondary inefficacy, 10 patients had inactive disease finally. BCVA improved (p < 0.001) and CMT decreased (p < 0.001) significantly at 6, 12, and 24 months after ADA therapy compared to baseline. Percentage of patients treated with ≥10 mg/day corticosteroid (61.3% vs. 6.4%) and DMARDs combined with ADA (46.8% vs. 37.1%) were lower at 6 months than at baseline. Concomitant DMARDs (p = 0.579) and use of ADA as first or ≥2nd biotherapy line (p = 0.527) had no significant effect on DRR. Most common adverse event was tuberculosis-related infections.
Conclusions
ADA seems to be effective and safe with good DRR to control ocular inflammation. Escalation to weekly ADA treatment may be an effective option in patients with primary or secondary inefficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the study findings are available from the corresponding author upon request.
References
Moudgil KD, Choubey D. Cytokines in autoimmunity: role in induction, regulation, and treatment. J Interferon Cytokine Res. 2011;31:695–703. https://doi.org/10.1089/jir.2011.0065.
Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P. et al. Adalimumab in Patients with Active Noninfectious Uveitis. N Engl J Med. 2016;375:932–43. https://doi.org/10.1056/NEJMoa1509852.
Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R. et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–5. https://doi.org/10.1159/000055677.
Sharma SM, Damato E, Hinchcliffe AE, Andrews CD, Myint K, Lee R. et al. Long-term efficacy and tolerability of TNFα inhibitors in the treatment of non-infectious ocular inflammation: an 8-year prospective surveillance study. Br J Ophthalmol. 2021;105:1256–62. https://doi.org/10.1136/bjophthalmol-2018-312767.
Durrani K, Kempen JH, Ying GS, Kacmaz RO, Artornsombudh P, Rosenbaum JT. et al. Adalimumab for Ocular Inflammation. Ocul Immunol Inflamm. 2017;25:405–12. https://doi.org/10.3109/09273948.2015.1134581.
Suhler EB, Lowder CY, Goldstein DA, Giles T, Lauer AK, Kurz PA. et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol. 2013;97:481–6. https://doi.org/10.1136/bjophthalmol-2012-302292.
Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–92. https://doi.org/10.1016/S0140-6736(16)31339-3.
Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121:785–96.e3. https://doi.org/10.1016/j.ophtha.2013.09.048.
Fabbroni M, Cantarini L, Caso F, Costa L, Pagano VA, Frediani B. et al. Drug retention rates and treatment discontinuation among anti-TNF-α agents in psoriatic arthritis and ankylosing spondylitis in clinical practice. Mediators Inflamm. 2014;2014:862969. https://doi.org/10.1155/2014/862969.
Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523–34. https://doi.org/10.1093/rheumatology/kev374.
Llorenç V, Cordero-Coma M, Blanco-Esteban A, Heras-Mulero H, Losada-Castillo MJ, Jovani-Casano V. et al. Drug Retention Rate and Causes of Discontinuation of Adalimumab in Uveitis: Real-World Data from the Biotherapies in Uveitis (BioÚvea) Study Group. Ophthalmology. 2020;127:814–25. https://doi.org/10.1016/j.ophtha.2019.11.024.
Fabiani C, Sota J, Vitale A, Rigante D, Emmi G, Vannozzi L, et al. Cumulative retention rate of adalimumab in patients with Behçet’s disease-related uveitis: a four-year follow-up study. Br J Ophthalmol. 2018;102:637–41. https://doi.org/10.1136/bjophthalmol-2017-310733.
Bitossi A, Bettiol A, Silvestri E, Di Scala G, Bacherini D, Lopalco G, et al. Adalimumab Accounts for Long-Term Control of Noninfectious Uveitis Also in the Absence of Concomitant DMARD Treatment: A Multicenter Retrospective Study. Mediators Inflamm. 2019;2019:1623847. https://doi.org/10.1155/2019/1623847.
Fabiani C, Vitale A, Emmi G, Bitossi A, Lopalco G, Sota J. et al. Long-term retention rates of adalimumab and infliximab in non-infectious intermediate, posterior, and panuveitis. Clin Rheumatol. 2019;38:63–70. https://doi.org/10.1007/s10067-018-4069-3.
Jabs DA, Nussenblatt RB, Rosenbaum JT.Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. https://doi.org/10.1016/j.ajo.2005.03.057.
Suhler EB, Adán A, Brézin AP, Fortin E, Goto H, Jaffe GJ. et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology. 2018;125:1075–87. https://doi.org/10.1016/j.ophtha.2017.12.039.
Eurelings LEM, Missotten TOAR, van Velthoven MEJ, van Daele PLA, van Laar JAM, van Hagen PM. et al. Long-Term Follow-up of Patients With Uveitis Treated With Adalimumab: Response Rates and Reasons for Discontinuation of Therapy. Am J Ophthalmol. 2022;240:194–204. https://doi.org/10.1016/j.ajo.2022.03.017.
Liberman P, Berkenstock MK, Burkholder BM, Chaon BC, Thorne JE. Escalation to Weekly Adalimumab for the Treatment of Ocular Inflammation. Ocul Immunol Inflamm. 2021;29:1564–8. https://doi.org/10.1080/09273948.2020.1749857.
Lee J, Koreishi AF, Zumpf KB, Minkus CL, Goldstein DA. Success of Weekly Adalimumab in Refractory Ocular Inflammatory Disease. Ophthalmology. 2020;127:1431–3. https://doi.org/10.1016/j.ophtha.2020.04.009.
Martín-Varillas JL, Calvo-Río V, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A. et al. Successful Optimization of Adalimumab Therapy in Refractory Uveitis Due to Behçet’s Disease. Ophthalmology. 2018;125:1444–51. https://doi.org/10.1016/j.ophtha.2018.02.020.
Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai WC, Al-Maini MH. et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: Results from a multinational, real-world clinical practice, non-interventional study. PLoS One. 2017;12:e0175207. https://doi.org/10.1371/journal.pone.0175207.
Cordero-Coma M, Calleja-Antolín S, Garzo-García I, Nuñez-Garnés AM, Álvarez-Castro C, Franco-Benito M. et al. Adalimumab for Treatment of Noninfectious Uveitis: Immunogenicity and Clinical Relevance of Measuring Serum Drug Levels and Antidrug Antibodies. Ophthalmology.2016;123:2618–25. https://doi.org/10.1016/j.ophtha.2016.08.025.
Ducourau E, Rispens T, Samain M, Dernis E, Le Guilchard F, Andras L. et al. Methotrexate effect on immunogenicity and long-term maintenance of adalimumab in axial spondyloarthritis: a multicentric randomised trial. RMD Open. 2020;6:e001047. https://doi.org/10.1136/rmdopen-2019-001047.
Leinonen ST, Aalto K, Kotaniemi KM, Kivelä TT. Anti-adalimumab antibodies in juvenile idiopathic arthritis-related uveitis. Clin Exp Rheumatol. 2017;35:1043–6.
Fabiani C, Vitale A, Emmi G, Vannozzi L, Lopalco G, Guerriero S. et al. Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017;36:183–9. https://doi.org/10.1007/s10067-016-3480-x.
Ming S, Xie K, He H, Li Y, Lei B. Efficacy and safety of adalimumab in the treatment of non-infectious uveitis: a meta-analysis and systematic review. Drug Des Devel Ther. 2018;12:2005–16. https://doi.org/10.2147/DDDT.S160431.
Li B, Li H, Zhang L, Zheng Y. Efficacy and Safety of Adalimumab in Noninfectious Uveitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pharmacol. 2021;12:673984. https://doi.org/10.3389/fphar.2021.673984.
Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, Cordero-Coma M, Ortega G, Ortego N. et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–81. https://doi.org/10.1016/j.ophtha.2012.02.018.
Dobner BC, Max R, Becker MD, Heinz C, Veltrup I, Heiligenhaus A. et al. A three-centre experience with adalimumab for the treatment of non-infectious uveitis. Br J Ophthalmol. 2013;97:134–8. https://doi.org/10.1136/bjophthalmol-2011-301401.
Park SE, Jun JW, Lee DH, Lee SC, Kim M. The Effect of Adalimumab in Korean Patients with Refractory Noninfectious Uveitis. Yonsei Med J. 2021;62:177–81. https://doi.org/10.3349/ymj.2021.62.2.177.
Lejoyeux R, Diwo E, Vallet H, Saadoun D, Tezenas du Montcel S, Bodaghi B. et al. INFLIXIMAB and ADALIMUMAB in Uveitic Macular Edema. Ocul Immunol Inflamm. 2018;26:991–6. https://doi.org/10.1080/09273948.2018.1498110.
Tang Lee Say TL, Yang V, Fingret JM, Zagora S, Symes R, Younan C. et al. Adalimumab in patients with vision-threatening uveitis: real-world clinical experience. BMJ Open Ophthalmol. 2021;6:e000819. https://doi.org/10.1136/bmjophth-2021-000819.
Çekiç C, Aslan F, Vatansever S, Topal F, Yüksel ES, Alper E, et al. Latent tuberculosis screening tests and active tuberculosis infection rates in Turkish inflammatory bowel disease patients under anti-tumor necrosis factor therapy. Ann Gastroenterol. 2015;28:241–6.
Acknowledgements
We would like to thank Elif Bağatur Vurgun, Department of Ophthalmology, Marmara University School of Medicine, Istanbul, Turkey, for assisting with the data collection.
Author information
Authors and Affiliations
Contributions
FÇ: Study supervision. Concept and study design. Data collection, interpretation, analysis, and statistics. Drafting, revision, and final approval of the manuscript. HC: Study supervision. Concept and study design. Data interpretation. Revision and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study protocol was approved by the Institutional Review Board of Marmara University School of Medicine Hospital.
Informed consent
The study was performed following the Declaration of Helsinki principles, and all patients provided written informed consent about having their medical information used in the study analysis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çam, F., Celiker, H. Efficacy, retention rate and safety of adalimumab treatment in patients with non-infectious uveitis and scleritis: a real-world, retrospective, single-centre study. Eye 38, 893–901 (2024). https://doi.org/10.1038/s41433-023-02800-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02800-9