Abstract
Objectives
To evaluate the therapeutic effect and safety profile of next generation mycophenolate sodium (MPS), which is different from mycophenolate mofetil with an enteric-coated formulation, in corticosteroid-refractory non-infectious inflammatory uveitis (CRU) patients.
Methods
Prospective, uncontrolled, open-label interventional case series. Forty consecutive patients at a tertiary uveitis referral centre received 6 months of oral MPS as the treatment regimen with follow-up 12 months. The main outcome measures were best-corrected visual acuity (BCVA), inflammatory index, steroid-sparing effect of tapering prednisone to ≤10 mg daily and side effects.
Results
Mean age of enroled patients was 49 (49 ± 13) years and 29 (72.5%) were female. Thirty-six (90.0%) had bilateral disease. There were 0 (0%) anterior uveitis, 2 (5.0%) intermediate uveitis, 22 (55.0%) posterior uveitis, and 16 (40.0%) panuveitis. Vogt-Koyanagi-Harada disease was the most common diagnosis (17/40, 42.5%), followed by idiopathic panuveitis (8/40, 20%) and idiopathic retinal vasculitis (5/40, 12.5%). LogMAR BCVA improved from 0.9 (SD = 0.09) to 0.31 (SD = 0.08) after 6 months of MPS with good steroid-sparing effect (p = 0.012). Further maintenance in LogMAR BCVA was evident after MPS discontinuation from 6th month to 12th month, from 0.31 (SD = 0.08) to 0.33 (SD = 0.07), respectively (p = 0.81). MPS was the only immunosuppressive drug needed to reach quiescent state in 29 patients (72.5%). The drug-related safety profile was satisfactory.
Conclusion
MPS is an effective steroid-sparing drug for the treatment of CRU. The effect seen was not only during the 6 months of therapy, but also extended to 12 months to maintain BCVA and inflammation control. The side effects were acceptable.
Similar content being viewed by others
Introduction
Uveitis is a potentially sight threatening intraocular inflammation and responsible for 10–15% of patients with blindness in the United States [1]. The inflammation may involve the iris, ciliary body, pars plana, vitreous body, retina, and choroid. The uncontrolled inflammation of uveitis can lead to several vision-threatening complications, including cataract, glaucoma, posterior synechiae, macular oedema, neovascularisation, retinal detachment, and optic neuropathy [1, 2]. The morbidity resulting from inadequately treated uveitis can therefore significantly worsen a patient’s quality of life.
Aetiology of uveitis may be infectious, non-infectious, trauma related or masquerades such as B-cell lymphoma. Non-infectious inflammatory uveitis, which accounts for 75% of our uveitis patient register database, is a presumed T-lymphocyte-mediated autoimmune disease characterised antigen-specific CD4+ by T-lymphocyte- and macrophage-induced eye damage [3, 4].
T-cell inhibiting drugs remain as the traditional mainstay of immunoregulatory treatment in non-infectious uveitis [5]. Cyclosporine exerts T-cell inhibitory actions and has been used as a steroid-sparing drug. Its use may be limited by side effects such as impairment of renal function, gastrointestinal complaints, and hypertension. Other treatment modalities focusing on two major different mechanistic pathways (T-cell inhibition and anti-TNF-α) are also available [5, 6]. Mycophenolate mofetil (MMF) is an anti-metabolite, which inhibits the replication of T- and B-cells, as well as interleukin-3 and other cytokines produced by macrophages [7]. Both mechanistic actions provide a rationale for treatment with MMF of patients with uveitis [8]. In prior studies for uveitis patients, MMF has been shown as an effective first-and second-line therapy [8,9,10,11]. Treatment dosage and duration, however, was not consistent in those patients. Furthermore, side effects can be seen in 10–30% patients of using MMF, including gastrointestinal (GI) discomfort, risk of opportunistic infections, and haematological disorders, thus limiting the use of MMF in the clinical setting [8,9,10].
The enteric-coated formulation of mycophenolate sodium (MPS, Myfortic®, Novartis, Basel, Switzerland) was developed to overcome some of these MMF side effects. MMF has to be metabolised to mycophenolate acid (MPA) to become active [7], while MPS is the active form of the drug. The pharmacokinetic properties of MPA are similar between MMF and MPS, with MPS possessing delayed release characteristics as expected [12]. MMF is used and proven effective in patients with organ transplants and inflammatory diseases [7, 13], and is also proven effective and safe in renal transplant patients [14]. Although MMF and MPS have similar metabolites, they frequently have different clinical conclusions in usage [15, 16]. This prospective study was designed to evaluate the efficacy and side effects of MPS as a steroid-sparing treatment in patients with corticosteroid-refractory non-infectious inflammatory uveitis (CRU). Compared with prior retrospective studies using MMF or MPS, the MySTRI study offers a well-scheduled documentation of the therapeutic effect and a more detailed safety profile of using MPS in CRU patients in a prospective manner.
Methods
Forty consecutive patients were prospectively recruited with written informed consent from the Uveitis Service at Chang Gung Memorial Hospital between 2010 and 2014. Patients were selected strictly according to the Standardization of Uveitis Nomenclature (SUN) Working Group [17]. Institutional Review Board (IRB)/Ethics Committee approval was obtained with ethics approval number # NCT01261169.
Enroled patients were naïve of having been taking systemic immunomodulatory agents (cyclosporine, mycophenolate, tacrolimus, sirolimus and interferon alfa-2a), anti-metabolites, anti-TNF-α therapy or any combination of these for the treatment of their intraocular inflammatory disease. History with review of systems was obtained at the first visit, as well as complete ocular examination performed at each visit. Laboratory investigations, such as antinuclear antibody, HLA-B27 haplotype, angiotensin-converting enzyme levels, fluorescent treponemal antibody absorption test for syphilis, interferon gamma release assay for tuberculosis and toxoplasma titers, were performed as directed by the clinical presentation of each patient. When an infectious cause or malignant masquerade syndrome was suspected, and the laboratory tests returned negative, diagnostic vitrectomy was considered.
Inclusion criteria of the disease diagnosis included: Ocular sarcoidosis: a granulomatous uveitis with posterior inflammation in a patient in which sarcoidosis had been established by means of a biopsy, cytology, or a positive scan. Intermediate uveitis: patients with clinical features as defined by the International Uveitis Study Group [17] after exclusion of multiple sclerosis and systemic infection such as Lyme Borreliosis. All patients with intermediate uveitis had a negative MRI with gadolinium contrast, prior to being enroled. Behcet’s disease: as per the International Study Group for Behcet’s Disease [18]. Idiopathic Retinal Vasculitis: where systemic or infectious causes had been eliminated. In particular, patients did not have evidence of granulomatosis with polyangiitis, systemic lupus erythematosus, polyarteritis nodosa, polymyositis, dermatomyositis, or other systemic vasculitic disorders. Infectious aetiologies were ruled out by appropriate screening. For the purpose of this study, Eales’ disease was excluded from this group. Vogt-Koyanagi-Harada disease (VKH): as per the international workgroup definition of VKH [19]. Sympathetic ophthalmia: a granulomatous uveitis involving the choroid and retina associated with trauma or multiple prior ocular surgeries. Idiopathic panuveitis: all non-infectious panuveitides that were not related to any of the diseases mentioned above.
Drug administration
Intravenous high dose methylprednisolone (1 g daily for 3 days then oral prednisone 1 mg/kg/day totalling 2 weeks) or high dose oral prednisone (1 mg/kg/day) for 2 weeks was uniformly given as the initial therapy according to disease severity, with intravenous methylprednisolone offered to patients with LogMAR best-corrected visual acuity (BCVA) of 0.6 or worse. Oral prednisone was gradually tapered in patients who maintained or improved their inflammatory activity by reducing the dose by 10 mg per week until 20 mg daily, then 5 mg per week until 10 mg daily and finally 2.5 mg every 1–2 weeks until cessation. If there was disease relapse then doubling of the last steroid dose with frequent clinical monitoring approach was adopted. No local therapy was given in any patient in this study. CRU patient was defined as the patient who received steroid treatment but cannot lower the dosage of oral corticosteroid to 10 mg daily or less in 3 months due to the clinical condition, or unresponsive to the initial high dose corticosteroid.
Patients with were treated with MPS at a dose of 720 mg daily for 6 months then followed up for 12 months. Baseline complete blood count, liver function tests, and renal function tests were performed before and during MPS therapy. After stopping MPS at 6 months patients were not on any anti-inflammatory drugs. For non-responders or intolerance to MPS a second immunomodulatory agent (cyclosporine, azathioprine, cyclophosphamide or tacrolimus) and/or continuation of oral corticosteroid were considered.
Efficacy evaluation
Primary endpoint is the change in LogMAR BCVA from baseline to sixth month followed-up. The secondary endpoints are (1) Inflammatory activity; (2) central foveal thickness (CFT); (3) daily oral corticosteroid dosage.
Safety evaluation
The safety assessment contains adverse events, laboratory tests, and physical examination and vital signs in the study period. Adverse events were recorded from screening visit (Month 0). The number of adverse events is summarised along with statistical assessments for the study period. The severity and causality to MPS adverse events was also summarised. The laboratory tests included haematology, chemistry, pregnancy test, chest x-ray and additional blood tests as required. All laboratory tests were performed at each visit (Month 0, 1, 3, 6, and 12). Pregnancy test and chest x-ray was performed at month 0.
Drug interruption
MPS was discontinued in cases of the following situations: (1) positive pregnancy; (2) severe or serious adverse event that is not compatible with MPS administration, including adverse events that required treatment with an unacceptable co-medication; (3) the onset of malignancy (except cutaneous basal cell carcinoma); (4) uncontrolled life-threatening infection. Treatment was discontinued for a given patient if, on balance, continuation would be detrimental to the patient’s well-being. This included abnormal laboratory values, abnormal test procedure results, unsatisfactory therapeutic effect and study protocol violation.
Statistical analysis
In this study, continuous variables were expressed as mean ± standard deviation (SD) and compared using t-tests. Categorical variables were expressed as frequency and/or percentages. A two-tailed p value < 0.05 was considered statistically significant.
This clinical trial is registered online at ClinicalTrials.gov with Identifier NCT01261169.
This study adhered to the tenets of the Declaration of Helsinki.
Results
Enrolment began in May 2010 and recruitment was completed in August 2014. The subjects’ demographic and baseline data are shown in Table 1. A total of 40 patients with CRU were enroled to receive MPS treatment after confirming the diagnosis of corticosteroid-refractory status and clinically necessary to escalate to immunomodulatory therapy. Subjects were primarily females (29 patients, 72.5%) and had a mean age of 49 years. Most patients (36, 90.0%) had bilateral disease. The ratio of uveitis classification was: 2 (5.0%) intermediate uveitis; 22 (55.0%) posterior uveitis; and 16 (40.0%) panuveitis. There were no cases of anterior uveitis enroled. Vogt-Koyanagi-Harada disease (17/40, 42.5%) was the most common aetiological diagnosis, followed by idiopathic panuveitis (8/40, 20%) and idiopathic retinal vasculitis (5/40, 12.5%). The profiles of disease aetiology are shown in Table 2. In 11 patients (27.5%) MPS treatment was discontinued because of possible drug-related side effects and protocol compliance, nevertheless all 40 subjects completed the follow-up of 12-months duration.
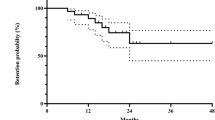
The efficacy outcomes, with the mean and SD of LogMAR BCVA at baseline and at every visit after the initiation of MPS, are shown in Fig. 1. There was a significant improvement in LogMAR BCVA from the baseline to sixth month, from 0.9 (SD = 0.09) to 0.31 (SD = 0.08), respectively (p = 0.012). Furthermore, the improvement of LogMAR BCVA was maintained even after the discontinuation of MPS, from 6th month to 12th month, from 0.31 (SD = 0.08) to 0.33 (SD = 0.07), respectively (p = 0.81).
Similarly, the improvement of the inflammatory activity from the baseline to the 12th month is shown in Fig. 2. With the initiation of intravenous methylprednisolone pulse therapy or high dose oral corticosteroids, the inflammatory cells in the anterior chamber and vitreous decreased significantly and this was maintained with MPS from the 1st month to the 6th month (p = 0.026). The therapeutic effect was extended to the 12th month, with no statistically significant difference in inflammatory activity between 6th and 12th month (p = 0.74). Overall, MPS was the only immunosuppressive drug needed to reach quiescent state in 29 patients (72.5%).
CFT showed corresponding significant decrease towards normalisation from the baseline to 6 months of MPS treatment, with mean CFT reducing from 369 μm (SD 19 μm) to 260 μm (SD 17 μm), respectively (p < 0.001), and remained stable till completion of follow-up time of 12 months (Fig. 3).
The steroid-sparing effect was also significant (Fig. 4). The steroid reduction while on MPS leading to BCVA improvement and CFT reduction were statistically significant, with p < 0.001 and p = 0.012, respectively. There were two subjects (5.0%) and one subject (2.5%), who had to keep the prednisone dose at more than 10 mg/day at 6th month and 12th month, respectively.
The safety profile of MPS is listed in Table 3. There were no ocular adverse events. Twelve patients experienced systemic side effects, with six patients experiencing two side effects and were of a mild nature. Table 3a shows the most common systemic side effects were GI disturbance (7.5%) and elevated liver enzymes (7.5%). These recovered rapidly after MPS cessation without permanent sequelae.
In total, 11 patients suspended MPS treatment. Five and two patients changed from MPS to a second immunomodulatory agent due to side effects (Table 3a) and lack of clinical response, respectively; two patients needed to undergo eye surgery were excluded from the study due to the confounding effect of perioperative steroid dosage alteration; and two patients were unable to return to the clinic as scheduled. Five patients required MPS treatment cessation, possibly due to drug-related causes of: elevated liver enzymes in two patients (5.0%), systemic infection in one patient (2.5%), psychologic depression in one patient (2.5%), and high creatinine in one patient (2.5%). This is summarised in Table 3b.
Overall, 11 out of 40 patients (27.5%) in our study required a second immunomodulatory agent to achieve adequate inflammation control. Of these, four patients had a second immunomodulatory agent in addition to MPS to control the inflammation. In these subjects, the ocular inflammation was controlled with the addition of cyclosporine in five patients (12.5%), azathioprine in four patients (10%), cyclophosphamide in one patient (2.5%) and tacrolimus in one patient (2.5%). This is displayed in Table 4.
Table 5 summarises the steroid-sparing effect of MPS. Ocular inflammation control was achieved with a steroid-sparing effect of 10 mg daily or less prednisone dose in MPS only and with a second immunomodulatory agent, in 27 patients (93.1%) and all patients (100%), respectively (Table 5).
Discussion
The MySTRI study, to our knowledge, is the first in the literature evaluating the treatment of all forms of CRU using a novel mycophenolate preparation, namely MPS. Previous studies had demonstrated the effectiveness of MPS in paediatric uveitis, intermediate uveitis, and birdshot chorioretinitis [20,21,22]. Our results demonstrated that MPS was highly effective. The primary endpoint analysis showed that BCVA and inflammation control improved significantly from baseline to 6 months, and, moreover, remained stable for at least a further 6 months until the end of the study at 12 months. This was not accounted for in a previous report of MMF study [11]. We also utilised a 6-month therapy of MPS due to funding restrictions which contrasted with the recent consensus statement by the Fundamentals Of Care for UveitiS (FOCUS) initiative, with one of our co-authors (Y.S.H.) as a collaborator, suggesting a longer 12 months non-corticosteroid therapy prior to cessation [23]. Our findings may indicate that a shorter course of therapy may achieve a similar effect and outcome provided adequate treatment is started in a timely manner [24]. Furthermore, the prospective nature of this study provides a stronger level of evidence to support the use of MPS.
In this study, ocular inflammation control was achieved with a steroid-sparing effect of 10 mg daily or less prednisone dose in MPS only and with a second immunomodulatory agent, in 93.1% and 100% of patients, respectively (Table 5). This was an excellent result and a pertinent finding, as the well-known side effects of the long-term corticosteroid therapy can be significantly minimised with this near-physiologic prednisone dose.
There were no serious systemic or ocular adverse events. Gastrointestinal disturbance was much less reported in this study than the prior study reports of using MMF [11], which may reflect the different coating design utilised in the active drug MPS. In our clinical trial, six (15%) patients ceased MPS therapy due to possible drug-related side effects. These subjects, however, had a fast recovery after the cessation of the drug, without permanent sequelae, and were able to be followed up to the conclusion of our study with either oral steroid as rescue therapy or another immunomodulatory agent if clinically indicated. Taking into account withdrawal from various reasons, MPS was the only immunosuppressive drug needed to reach quiescent state in 72.5% of total patients, which was still favourable.
Limitations of this study included: firstly, single centre with a small sample size; secondly, single arm with no control group to compare with other immunomodulatory drugs; thirdly, unique Taiwanese and ethnic Chinese population. Confounders for visual acuity, such as visually significant cataracts, were not able to be fully controlled. Finally, we did not possess the data to study the correlation of early timing of adequate treatment with the duration of therapy.
There remains to be great challenges in the management of CRU patients. The lack of randomised, prospective studies both in general and specific subsets of uveitis continue to create dilemmas to the clinician. As a result, it is often not possible to identify which treatment modality is appropriate for each individual patient. The MySTRI study has helped to provide further prospective data on one particular mycophenolate treatment preparation, namely MPS.
In conclusion, the MySTRI study demonstrated MPS achieved improved BCVA and satisfactory inflammation control in patients with CRU. The effects achieved were not only during the 6 months of MPS therapy, but also extended for at least a further 6 months upon cessation of the drug. The safety profile was highly acceptable. Further studies of head-to-head comparison by using MPS with other immunomodulatory drugs and of larger-scale prospective clinical trials are needed to elucidate the effect of MPS in each individual non-infectious uveitic entity. Research into the correlation of early timing of adequate treatment with the duration of therapy will also be invaluable to the management of complex uveitis patients.
Summary
What was known before
-
- Managing corticosteroid-refractory non-infectious inflammatory uveitis (CRU) appropriately remains a clinical challenge.
-
- Mycophenolate mofetil (MMF) has been shown as an effective first- and second-line therapy, however prospective data on the newer enteric-coated formulation of mycophenolate sodium (MPS), which was developed to overcome some of the MMF side effects, is lacking.
What this study adds
-
- The prospective MySTRI study shows MPS alone achieved improved vision and satisfactory inflammation control in 72.5% of CRU patients.
-
- MPS effects achieved were not only during the 6 months therapy, but also extended for at least a further 6 months upon cessation.
References
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6.
Jones NP. The Manchester Uveitis Clinic: the first 3000 patients, 2: Uveitis Manifestations, complications, medical and surgical management. Ocul Immunol Inflamm. 2015;23:127–34.
Hughes EH, Dick AD. The pathology and pathogenesis of retinal vasculitis. Neuropathol Appl Neurobiol. 2003;29:325–40.
Verjans GM, van Hagen PM, van der Kooi A, Osterhaus AD, Baarsma GS. Vgamma9Vdelta2 T cells recovered from eyes of patients with Behcet’s disease recognize non-peptide prenyl pyrophosphate antigens. J Neuroimmunol. 2002;130:46–54.
Nussenblatt R. Treating intraocular inflammatory disease in the 21st century. Arch Ophthalmol. 2005;123:1000–1.
Atzeni F, Sarzi-Puttini P, Doria A, Iaccarino L, Capsoni F. Potential off-label use of infliximab in autoimmune and non-autoimmune diseases: a review. Autoimmun Rev. 2005;4:144–52.
Lipsky JJ. Mycophenolate mofetil. Lancet. 1996;348:1357–9.
Larkin G, Lightman S. Mycophenolate mofetil. A useful immunosuppressive in inflammatory eye disease. Ophthalmology. 1999;106:370–4.
Baltatzis S, Tufail F, Yu EN, Vredeveld CM, Foster CS. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 2003;110:1061–5.
Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112:1472–7.
Shen E, Rathinam SR, Babu M, Kanakath A, Thundikandy R, Lee SM, et al. Outcomes of Vogt-Koyanagi-Harada disease: a subanalysis from a randomized clinical trial of antimetabolite therapies. Am J Ophthalmol. 2016;168:279–86.
Budde K, Bauer S, Hambach P, Hahn U, Roblitz H, Mai I, et al. Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transpl. 2007;7:888–98.
Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66:507–15.
Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, et al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transpl. 2004;4:237–43.
Lee PC, Chang SS, Shieh SC, Wu ZC, Wang WM, Wang JD, et al. Cyclosporine or tacrolimus: which is the better partner for myfortic or cellcept? Transpl Proc. 2012;44:137–9.
Hummel M, Yonan N, Ross H, Miller LW, Sechaud R, Balez S, et al. Pharmacokinetics and variability of mycophenolic acid from enteric-coated mycophenolate sodium compared with mycophenolate mofetil in de novo heart transplant recipients. Clin Transpl. 2007;21:18–23.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
O’Neill TW, Rigby AS, Silman AJ, Barnes C. Validation of the International Study Group criteria for Behcet’s disease. Br J Rheumatol. 1994;33:115–7.
Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–52.
Doycheva D, Zierhut M, Blumenstock G, Sobolewska B, Voykov B, Hohmann J, et al. Mycophenolate sodium for the treatment of chronic non-infectious uveitis of childhood. Br J Ophthalmol. 2016;100:1071–5.
Deuter CME, Engelmann K, Heiligenhaus A, Lanzl I, Mackensen F, Ness T, et al. Enteric-coated mycophenolate sodium in the treatment of non-infectious intermediate uveitis: results of a prospective, controlled, randomised, open-label, early terminated multicentre trial. Br J Ophthalmol. 2018;102:647–53.
Doycheva D, Jagle H, Zierhut M, Deuter C, Blumenstock G, Schiefer U, et al. Mycophenolic acid in the treatment of birdshot chorioretinopathy: long-term follow-up. Br J Ophthalmol. 2015;99:87–91.
Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R Jr, Brezin AP, Chee SP, et al. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: Fundamentals Of Care for UveitiS (FOCUS) initiative. Ophthalmology. 2018;125:757–73.
Herbort CP Jr, Abu El Asrar AM, Takeuchi M, Pavésio CE, Couto C, Hedayatfar A, et al. Catching the therapeutic window of opportunity in early initial-onset Vogt-Koyanagi-Harada uveitis can cure the disease. Int Ophthalmol. 2019;39:1419–25.
Funding
CMRPG3B0441, CORPG3C0081.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Medical Ethics Committee approval # NCT01261169, Chang Gung Memorial Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, T.S., Tsang, W.M., Enkh-Amgalan, I. et al. Mycophenolate sodium in the treatment of corticosteroid-refractory non-infectious inflammatory uveitis (MySTRI study). Eye 34, 2098–2105 (2020). https://doi.org/10.1038/s41433-020-1066-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1066-y