Abstract
Genetic alterations in COL4A2 are less common than those of COL4A1 and their fetal phenotype has not been described to date. We describe a three-generation family with an intragenic deletion in COL4A2 associated with a prenatal diagnosis of recurrent fetal intracerebral hemorrhage (ICH), and a myriad of cerebrovascular manifestations. Exome sequencing, co-segregation analysis, and imaging studies were conducted on eight family members including two fetuses with antenatal ICH. Histopathological evaluation was performed on the terminated fetuses. An intragenic heterozygous pathogenic in-frame deletion; COL4A2, c.4151_4168del, (p.Thr1384_Gly1389del) was identified in both fetuses, their father with hemiplegic cerebral palsy (CP), as well as other family members. Postmortem histopathological examination identified microscopic foci of heterotopias and polymicrogyria. The variant segregated in affected individuals demonstrating varying degrees of penetrance and a wide phenotypic spectrum including periventricular venous hemorrhagic infarction causing hemiplegic CP, polymicrogyria, leukoencephalopathy, and lacunar stroke. We present radiographic, pathological, and genetic evidence of prenatal ICH and show, for what we believe to be the first time, a human pathological proof of polymicrogyria and heterotopias in association with a COL4A2 disease-causing variant, while illustrating the variable phenotype and partial penetrance of this disease. We highlight the importance of genetic analysis in fetal ICH and hemiplegic CP.
Similar content being viewed by others
Introduction
Perinatal intracerebral hemorrhage (ICH) may be diagnosed antenatally or postnatally, resulting in various brain lesions depending upon the mechanism, vascular anatomy, and timing of the injury [1,2,3,4]. Fetal intraventricular hemorrhage (IVH) usually originates in the germinal matrix and may extend to the ventricles by a mechanism similar to that occurring in premature babies [5,6,7]. When they are extensive, germinal matrix hemorrhages (GMH) can lead to impaired venous drainage in the periventricular white matter (PVWM), causing periventricular venous hemorrhagic infarction (PVHI) that can subsequently evolve into a porencephalic cyst [5, 8, 9]. Diagnosis can be made by ultrasonography during pregnancy or by the appearance of early limb preference during infancy [9,10,11,12,13,14]. Prenatal GMH may result from acquired causes, such as fetal alloimmune thrombocytopenia or other coagulopathies, thrombophilia, antenatal trauma, or severe maternal disease, although the etiology is often not established [15,16,17]. Disease-causing variants in the collagen type IV alpha 1 (COL4A1) and the collagen type IV alpha 2 (COL4A2) genes are known genetic causes of antenatal/perinatal-onset [11, 12, 18,19,20,21,22,23,24].
COL4A1 and COL4A2 are abundant components of nearly all basement membranes (BMs), and therefore, as suggested by the evidence to date, both COL4A1 and COL4A2 are associated with systemic vascular BM disease resulting in a broad phenotypic spectrum, including neurological, renal, ophthalmological, muscular, and cardiac manifestations, starting in fetal life and extending until adulthood [10,11,12,13,14, 20, 23, 24]. The neurological phenotype associated with COL4A1 is expanding and includes small-vessel disease (SVD) of varying severity [13, 14, 21, 23, 25,26,27]. Fetal ICH with subsequent porencephalic cavitation has been described in association with COL4A1 and may result in destructive changes resembling hydranencephaly at the severe end [1, 11, 19, 28, 29]. Disease-causing variants in COL4A2 are rarer, and a frequency bias for COL4A1 over COL4A2 disease-causing variants has been recognized [30]. Although both sporadic and familial porencephaly are associated with COL4A2 and are considered to be caused by an antenatal/perinatal insult, they are usually discovered in infancy or childhood, commonly following an uneventful pregnancy and neonatal period [25, 28, 31,32,33,34]. To the best of our knowledge, the COL4A2-associated phenotype has not been documented in human fetal life. In the present study, we report six members from a three-generation family with a novel COL4A2 deletion that was identified following the diagnosis of fetal ICH in two consecutive pregnancies.
Methods
We performed exome sequencing (ES) in fetal DNA of two consecutive pregnancies that were terminated because of extensive brain damage due to fetal IVH, their healthy mother, and their father who has hemiplegic CP. Following genetic diagnosis, additional family members were genetically and clinically evaluated.
Participants
The clinical phenotype of the proband fetuses and all the affected family members was obtained from clinical records and by clinical and neurological examinations when possible.
Genetic analysis
Genomic DNA was extracted from peripheral blood according to standard protocols. Fetal DNA was extracted from fetal material taken following the termination of pregnancy (TOP). ES was performed for both fetuses and their parents by Fulgent Diagnostics SEQ on Illumina HiSeq4000 using the Roche Nimblegen Protocol at a sequencing depth of 60X. Analysis of the raw data was performed with the Variantyx diagnostic platform (Variantyx Genomic Intelligence®) [35]. Nonsense, nonsynonymous, canonical splice sites, and indels in coding regions with a minor allelic frequency of <0.01 in public databases (gnomAD, ExAc, and 1000 genomes) were analyzed and interpreted according to the American College of Medical Genetics and Genomics (ACMG) guidelines [36].
Histopathological analysis
TOP was approved by the institutional committee according to the local law. A postmortem evaluation was performed on both fetuses at 26 weeks’ gestation after receiving parental consent. The autopsy procedures followed routine protocol [37]. Gross examination of the brain was performed by coronal sectioning of the cerebral hemispheres and mid-sagittal sectioning of the midbrain and cerebellum. Multiple tissue samples were submitted for histological processing. Four-μm-thick histological sections were prepared and stained with hematoxylin and eosin and examined along with the corresponding gross photographs. Immunohistochemical stains for GFAP, CD68, Collagen IV, CD 31, and CD34, PAS, and Prussian blue (Iron) were carried out. The immunohistochemical stains were carried out with a Ventana Benchmark XT immunostainer.
Results
Proband cases
Clinical findings
The clinical characteristics and pedigree of the study participants are summarized in Table 1 and Fig. 1, respectively. Case III-4 was a female fetus of a 32-year-old healthy mother. The pregnancy was spontaneous and uneventful, with normal prenatal sonographic scans until 25 weeks’ gestation, when a routine US examination revealed PVWM echogenicity and bilateral Grade II IVH. There was no history of maternal trauma, infection, or drug abuse. A neurosonographic examination at 26 weeks’ gestation showed ventriculomegaly with diffuse involvement of the brain parenchyma consistent with Grade III IVH with PVHI. Maternal serum did not demonstrate any platelet alloantibodies, and platelet genotyping did not reveal any human platelet antigen incompatibility, thus excluding neonatal alloimmune thrombocytopenia. TOP was performed at 26 weeks’ gestation.
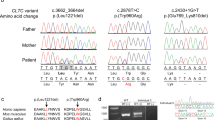
Co-segregation of the COL4A2 c.4151_4168del p.(Thr1384_Gly1389del) variant in the family pedigree with various degrees of CVD. Squares represent males, circles represent females, and triangles indicate fetuses. The crossing line denotes the termination of pregnancy. The filled symbols represent clinically affected cases. E (+/−/0) indicates whether genetic analysis was positive for the COL4A2 variant, negative, or not performed, respectively. The Arabic number is an identifier for each individual, whereas the generation is marked with a roman number.
Case III-5 was a female fetus conceived 15 months later. The prenatal follow-up was normal until 26 weeks’ gestation when the mother complained of reduced fetal movements. A repeat transvaginal US examination showed severe bilateral ventriculomegaly with right IVH together with extensive PVWM and caudate nucleus echogenicity, suggestive of deep venous hemorrhagic infractions (Fig. 2ii). A TOP was performed.
Transvaginal neurosonographic examination of case III-4 (i) and III-5 (ii). (i) A modified coronal plane at 25w5d (A, B) showing echogenicity in the frontal PVWM bilaterally at different stages of evolution (arrows), and of IVH without ventriculomegaly (arrowhead, see also arrows in B). Parasagittal planes at 26w1d (C, D) demonstrating ventriculomegaly and a more extensive PVWM echogenicity (arrows) as well as blood clots within the dilated lateral ventricles. (ii) Transvaginal coronal plane US at 24 weeks’ gestation showing normal-appearing lateral ventricles and PVWM (A). B Transvaginal modified axial plane at 26 weeks’ gestation demonstrating bilateral lateral ventricle dilatation with extensive right IVH, and increased echogenicity suggestive of hemorrhagic infarction of the caudate.
Histopathological findings
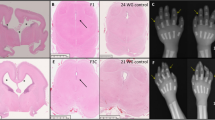
Postmortem examinations of cases III-4 and III-5 were performed at 26 weeks’ gestation and revealed fetal weight adequate for gestational age with normal head circumference. The gross and histological appearance of the internal organs and tissues, including kidneys and muscle, were normal in both autopsies. There was a transverse reduction of fingers 1, 3, 4, and 5 of the left hand in fetus III-4 (Fig. 3J).
A Gross image of a coronal section of the temporoparietal lobes showing bilateral IVH grade IV. Hemorrhagic contents in lateral ventricles, disintegrating the PVWM. B Disintegration of white matter with extravasation of erythrocytes, reactive astrocytes, and early axonal mineralization (H&E). C Prominent collagen IV in PVWM BM vasculature (arrows, collagen IV immunostain). D Marked irregular thickening of collagen IV in a PVWM-dilated vessel (collagen IV immunostain). E Thin delicate collagen IV in BM of a subcortical white matter vessel (collagen IV immunostain). F IVH grade IV, right temporoparietal lobe. The circles denote the location of histologic images G–I. G Dysplastic double cortex, left parietal lobe (asterisks, H&E) (H) White matter heterotopias, left temporal lobe (arrows, H&E) (I) polymicrogyria-like cortex, left Sylvian fissure (arrows, H&E) (J) Transverse reduction defect of fingers 1,3, 5, left hand.
Gross and histological examinations of the brains of both fetuses revealed partially resorbed and digested hemorrhagic content within the lateral ventricles with ependymal destruction as well as extensive damage to the PVWM with areas of focal necrosis, microscopic calcifications, and mineralization of the WM. Numerous microglia and reactive changes in astrocytes in both fetuses were demonstrated by CD68 and GFAP immunostains, respectively. The immunostains for collagen IV in the hemorrhagic PVWM revealed marked irregular thickening of vascular BM (Fig. 3C–E).
Focal cortical dysplasia, focal WM neuroblastic heterotopia associated with marked cortical thinning in the left anterior temporal lobe and 2-microscopic foci of polymicrogyria (PMG) in the left frontal and opercular areas (Fig. 3F–I) were noted in case III-5.
Genetic analysis
Molecular analysis revealed a novel intragenic heterozygous in-frame deletion of 18 base pairs in exon 44 of the COL4A2 gene; c.4151_4168del, (p.Thr1384_Gly1389del), (ENSG0000134871.19 ENST00000360467.7, ENSE00001468433) in the two fetuses (cases III-4, III-5), inherited from the father (individual II-2). The deletion causes a loss of six amino acids, including two glycine residues within the conserved area of the triple helical collagenous part of the protein. This deletion has not been reported in the population database (gnomAD, https://gnomad.broadinstitute.org) and segregated with other affected family members with various degrees of penetrance (Fig. 1). Therefore, it was defined as likely pathogenic based on ACMG criteria [36]. The variant and phenotype presented in the study was submitted to ClinVar (accession SCV001477492, URL: https://www.ncbi.nlm.nih.gov/clinvar/variation/7105/).
Other family members
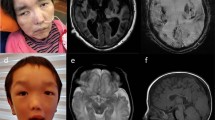
The parents of the 2 fetuses (II-2 and II-5, Fig. 1) are non-consanguineous Caucasians in their 30s. They have a 4-year-old healthy son. The mother (II-5) had an uneventful medical history apart from medically treated and well-controlled hypothyroidism. She had two prior spontaneous miscarriages at 6- and 8-weeks’ gestation and underwent an extensive workup for repeated miscarriages, including a thrombophilia workup, TORCH serology, and karyotyping, all of which were normal. The father (II-2), who had the same COL4A2 deletion, was born at term after an uneventful pregnancy as the second son to parents of Jewish-Moroccan ancestry. At 7 months of age, he was noted to have left-sided hemiparesis and later developed high myopia. Computed tomography revealed an enlarged right lateral ventricle (images not available). He was diagnosed with hemiplegic CP due to presumed perinatal injury, and no further workup was carried out. He had febrile seizures in early childhood, with no subsequent clinical seizures. He had learning difficulties and attended a special education school in early childhood with normal intellect in adult life. Following the diagnosis of the affected fetuses, he underwent brain magnetic resonance imaging (MRI) at 35 years of age, which revealed right-sided porencephaly, reduced volume of the right frontal WM, and an irregular, thickened cortex suggestive of focal opercular PMG (Fig. 4). An ophthalmological examination showed high myopia and tilted discs. His renal US, urinalysis, and creatine phosphokinase levels were normal.
Brain MRI of individuals II-2 at 35 years of age (A, C, E), III-6 at 10 months of age (B, D, F, G, H), II-3 at 32 years of age (I, J) and I-1 at age 65 years (K, L). Axial T2, coronal T1, and coronal T2 of individual II-2 (A, C) and III-6 (B, D) demonstrating frontal porencephalic dilation of the right lateral ventricle associated with loss of PVWM volume. Note the perisylvian cortical thickening-polymicrogyria (solid arrow). Wallerian degeneration is apparent through the corticospinal tracts in individuals (E, F). Axial T2-Flair of individual III-6 (G) illustrating a multifocal, predominantly posterior, PVWM hyperintensity, expanding into the centrum semiovale and associated with PVWM loss, sparing the cortex and basal ganglia, and ventricular dilatation suggesting periventricular venous infarction (PVI). Coronal susceptibility weighted image showing subtle linear hyperintensity lining the dilated frontal horn suggesting hemosiderin rim (H, black arrow). Axial T2-FLAIR MRI of individual II-3 (I, J) showing a peri-atrial left T2-weighted hyperintense lesion and a right remote frontal peri-insular focal cortical thickening suggestive of PMG (arrow). Axial T2-FLAIR and coronal T2 of individual I-1 (K, L) shows a remote hemorrhagic infarct in the left lentiform.
A clinical review of the extended paternal family revealed at least four other affected individuals, including his 65-year-old father (individual I-1), who has a history of diabetes mellitus, hypertension, and aortic stenosis but is neurologically intact. His brain MRI shows a remote hemorrhagic infarct in the left lentiform, with adjacent porencephalic dilatation of the lateral ventricle (Fig. 4). Remote lacunar infarcts in the left frontal area, and the deep WM as well as mild PVWM T2-hyperintesities are also evident.
The other affected family member is a 9-year old female (III-6), the niece of individual II-2 (Fig. 1) who was born at 37 + 6 gestational age following a normal pregnancy, with normal Apgar scores, birth weight, and head circumference. At the age of 6 months, she was diagnosed with right-sided mild hemiparetic CP. An MRI of the brain at age 10 months revealed left-sided porencephaly with bilateral signal abnormalities in the deep PVWM in both frontal and parietal regions, with loss of PVWM volume sparing of subcortical WM. SWI showed low-signal intensity on the ventricular margins suggestive of presumed perinatal GMH (Fig. 4). A thrombophilia workup revealed a maternally inherited heterozygous prothrombin variant (G21210A). She attended regular schools, had normal verbal and cognitive development, and had no clinical seizures. Her neurological examination showed right-sided spastic hemiparesis (GMFCS-2). She had no other systemic involvement.
The mother of individual III-6 was 32-year-old healthy female (individual II-3) with normal perinatal history, development, and normal intellect. Her brain MRI showed a peri-atrial left T2-weighted hyperintense lesion as well as a right frontal peri-insular focal cortical thickening suggestive of PMG. A few punctuate hyperintense T2-lesions were also noted in the left frontal lobe, centrum semiovale, and the cerebellum. She had no other systemic involvement.
Discussion
Although reported for COL4A1 [11, 19], almost all evidence for an antenatal origin of ICH associated with COL4A2 has been demonstrated through postnatal imaging. In this report, we highlight the variable phenotypic spectrum of COL4A2-related disease throughout the life span in a three-generation pedigree. By providing antenatal human radiological and pathological evidence, we show, for what we believe is the first time, fetal origin of ICH, as well as isolated PMG and heterotopia due to a COL4A2 disease-causing variant. The detection of this COL4A2 variant in the two reported fetuses led to a diagnosis and an explanation for clinical features in other family members, highlighting a valuable role of genetic testing in fetal ICH.
In opposition to the reported understanding that COL4A2 variants are associated with milder phenotypes and later age at onset [30], we show that prenatal severe manifestations may occur as well. The wide phenotypic inter-familial variance, previously documented for both COL4A1 and COL4A2 [13, 34], suggests incomplete penetrance of the disease and the potential role of other modifying factors. The highly variable phenotypic spectrum of COL4A-related disease is a challenge in both diagnostic and genetic counseling perspectives. It emphasizes that the assessment of fetal ICH should include a multidisciplinary team approach involving gynecologists, pediatric neurologists, and geneticists. Importantly, asymptomatic carriers should also undergo MRI since they may harbor SVD or aneurysms even in early adulthood [13].
A review of the literature and the listed genetic variants to date do not report any other pathogenic in-frame deletions in the COL4A2 gene [30, 34]. Although the deletion reported here is an in-frame deletion, we show segregation of the variant throughout three generations and partial penetrance in extended family members. The possibility that another disease-causing variants arbitrarily segregating with affected family members without being detected in two WES analyses is ~3%. The deletion is at the distal end of the collagenous domain of the protein (position 1384-1389/1713 amino acids) in the triple helix domain. Numerous pathogenic missense variants located near the end of the triple helical part of COL4A2 have been reported, suggesting that this area is a “hot spot”. Although the deletion may cause an abnormal protein function by different pathogenic mechanism compared to the well-described G-Xaa-Yaa missense variants, a deletion may cause loss of COL4A2 chain synthesis from one allele, predicted to cause a loss of function of the heterotrimer. This, along with the segregation of the deletion with affected members, is highly supportive of its pathogenicity. Importantly, our results show that the location of the variant cannot be the sole factor for determining genotype–phenotype correlation in regard to the Cerebrovascular disease (CVD). Plausible explanations include genetic modifiers, mosaicism, or environmental influences. Notably, and contrary to what had previously been hypothesized about non-glycine variants being likely low-penetrance risk alleles for late-onset intracranial hemorrhage [33], we now show that COL4A2 deletions can cause a severe early-onset phenotype, similar to COL4A1. Of note, the prothrombin variant (G21210A) that had been identified in proband III-6 and individual II-3 was not found in any other family members. Given that thrombophilia is very uncommon in presumed perinatal stroke [38], and the phenotype was observed in family members that do not carry this variant, it is highly unlikely that this variant can serve to explain the neuroimaging findings identified in these two patients.
The modification of the BM structural properties due to variants in COL4A1/2 increases the fragility of the vessel wall when exposed to environmental factors. Although pathological changes in BM also occur in other tissues, the major site of vessel damage is the brain [39]. The hemorrhage observed in the fetal brain is thought to be caused by rupture of the weakened small vessels in the GM due to the altered BM. Brain MRIs for individuals II-2, II-3, and III-6 were performed later in life, challenging the exact timing of the insult. However, the PMG demonstrated in individuals II-2 and II-3, taken together with the lack of gliosis, clearly points to an early insult, probably before 24 weeks of gestation [1, 40]. Although malformations of cortical development were associated with COL4A1 and COL4A2 in both animal models and human [18, 20, 28, 41,42,43], isolated PMG not associated with schizencephaly, and heterotopias have never been associated with COL4A2. Although vascular injury may induce PMG [44, 45], the pathophysiology of migration anomalies in COL4A genes may not only be related to concrete vascular insult but can be explained by dysfunction of the glial-limiting membrane, disturbing the proper migration of neurons [31]. Of note, no other pathogenic variant associated with brain malformations was identified during the WES data annotation. Indeed, it is currently considered that hydranencephaly, schizencephaly, porencephaly, and PMG represent a continuum of brain injury, depending upon the timing and the severity of the insult [1, 40]. Thus, the family describe herein represents similar pathology occurring at different timelines of brain development.
The pathogenicity of COL4A1 and COL4A2 is not completely understood and can be related to both the reduction in extracellular-to-intracellular ratio and accumulation of mutant proteins, depending upon the specific variant. These may induce an intracellular cytotoxic effect and induce apoptosis [33, 34]. Currently, there is no targeted treatment for COL4A1/2-vasculopathy. Better understanding of the natural history as well as genotype-phenotype correlations and genetic-environmental interactions may eventually emerge, thus facilitating appropriate surveillance protocols and the development of targeted novel therapies. Performing an elective cesarean section is not sufficient to prevent brain trauma as antenatal hemorrhage may occur, even weeks before the anticipated delivery [25, 28]. Although optimal antenatal and perinatal management of COL4A-related disease has not been delineated and requires further investigation in humans, preventive measures might prevent additional injury and may also impact disease expression for late-onset disease. For adult-onset CVD, careful surveillance and management of modifiable environmental risk factors, including hypertension, diabetes, avoidance of risky physical activities, constipation and prolonged labor and even nutritional supplements (e.g., vitamin E, fish oil) which may increase the risk of bleeding, and the use of anticoagulants, should be discussed in counseling [26].
We suggest considering genetic testing for COL4A1 and COL4A2 in cases for which there is radiologic evidence of fetal/perinatal venous hemorrhagic infarctions with porencephaly, as well as when there is evidence of accompanying leukoencephalopathy, microhemorrhages or malformations of cortical development, particularly when associated with typical ocular or renal involvement. Since stroke is the most common cause of hemiparetic CP, patients with hemiparetic CP should undergo MRI with SWI sequence to detect remote bleeding, and genetic investigation should be considered in cases with the above-mentioned radiographic imaging patterns. In addition, testing for COL4A1/COL4A2 should be considered for patients with multiple lacunar infarcts or intracranial aneurysms.
Conclusions
Our study demonstrates, for what we believe to be the first time, a documentation of the antenatal occurrence of fetal autosomal dominant COL4A2-related ICH and provides histopathological human evidence of isolated PMG and heterotopia in association with COL4A2. This report extends and complements earlier studies on the COL4A2-associated phenotype. Our findings highlight the diverse clinical presentation of COL4A2 vasculopathy and emphasize the important role of genetic diagnosis in fetal ICH as well as in patients with hemiplegic CP, particularly those with a non-supporting perinatal history and characteristic MRI findings.
References
Govaert P. Prenatal stroke. Semin Fetal Neonatal Med. 2009;14:250–66.
Bruno CJ, Beslow LA, Witmer CM, Vossough A, Jordan LC, Zelonis S, et al. Haemorrhagic stroke in term and late preterm neonates. Arc Dis Child Fetal Neonatal Ed. 2014;99:48–53.
Jhawar BS, Ranger A, Steven DA, Del Maestro RF. A follow-up study of infants with intracranial hemorrhage at full-term. Can J Neurol Sci. 2005;32:332–9.
Elchalal U, Yagel S, Gomori JM, Porat S, Beni-Adani L, Yanai N, et al. Fetal intracranial hemorrhage (fetal stroke): does grade matter? Ultrasound Obstet Gynecol. 2005;26:233–43.
de Vries LS, Roelants-van Rijn AM, Rademaker KJ, Van Haastert IC, Beek FJ, Groenendaal F. Unilateral parenchymal haemorrhagic infarction in the preterm infant. Eur J Paediatr Neurol. 2001;5:139–49.
Kocaman C, Yilmaz Y. Etiological analysis of presumed perinatal stroke. Brain Dev. 2012;34:133–9.
Takanashi J, Barkovich AJ, Ferriero DM, Suzuki H, Kohno Y. Widening spectrum of congenital hemiplegia: periventricular venous infarction in term neonates. Neurology. 2003;61:531–3.
Haddad J, Messer J, Aranda J. Periventricular haemorrhagic infarction associated with subependymal germinal matrix haemorrhage in the premature newborn. Report of two cases. Eur J Pediatr. 1992;151:63–5.
Kirton A, DeVebere G, Pontigon AM, Macgregor D, Shroff M. Presumed perinatal ischemic stroke: Vascular classification predicts outcomes. Ann Neuro. 2008;63:436–43.
Vermeulen RJ, Peeters-Scholte C, Van Vugt JJ, Barkhof F, Rizzu P, van der Schoor SR, et al. Fetal origin of brain damage in 2 infants with a COL4A1 mutation: fetal and neonatal MRI. Neuropediatrics. 2011;42:1–3.
de Vries LS, Koopman C, Groenendaal F, Van Schoonevel M, Verheijen FW, Verbeek E. et al. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann Neurol. 2009;65:12–8.
Lichtenbelt KD, Pistorius LR, De Tollenaer SM, Mancini GM, De Vries LS. Prenatal genetic confirmation of a COL4A1 mutation presenting with sonographic fetal intracranial hemorrhage. Ultrasound Obstet Gynecol. 2012;39:726–7.
Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, et al. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–5.
van der Knaap MS, Smit LM, Barkhof F, Pijnenburg YA, Zweegman S, Niessen HW, et al. Neonatal porencephaly and adult stroke related to mutations in collagen IV A1. Ann Neurol. 2006;59:504–11.
Dale ST, Coleman LT. Neonatal alloimmune thrombocytopenia: antenatal and postnatal imaging findings in the pediatric brain. AJNR. 2002;23:1457–65.
Sherer DM, Anyaegbunam A, Onyeije C. Antepartum fetal intracranial hemorrhage, predisposing factors and prenatal sonography: a review. Am J Perinatol. 1998;15:431–41.
Hayes B, Ryan S, Stephenson JB, King MD. Cerebral palsy after maternal trauma in pregnancy. Dev Med Child Neurol. 2007;49:700–6.
Yoneda Y, Haginoya K, Kato M, Osaka H, Yokochi K, Arai H, et al. Phenotypic spectrum of COL4A1 mutations: porencephaly to schizencephaly. Ann Neurol. 2013;73:48–57.
Meuwissen ME, de Vries LS, Verbeek HA, Lequin MH, Govaert PP, Schot R, et al. Sporadic COL4A1 mutations with extensive prenatal porencephaly resembling hydranencephaly. Neurology. 2011;76:844–6.
Meuwissen ME, Halley DJ, Smit LS, Lequin MH, Cobben JM, de Coo R, et al. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet Med. 2015;17:843–53.
Vahedi K, Alamowitch S. Clinical spectrum of type IV collagen (COL4A1) mutations: a novel genetic multisystem disease. Curr Opin Neurol. 2011;24:63e8.
Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer JM, et al. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem Cell Biol. 1995;104:267–75.
Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–95.
Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:97–110.
Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–96.
Shah S, Ellard S, Kneen R, Lim M, Osborne N, Rankin J, et al. Childhood presentation of COL4A1 mutations. Developmental Med Child Neurol. 2012;54:569–57.
Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, et al. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–4.
Gould DB, Campbell FP, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in Cola1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71.
Tonk M, Haan J. A review of genetic causes of ischemic and hemorrhagic stroke. J Neurol Sci. 2007;257:273–9.
Jeanne M, Gould DB. Genotype–phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol. 2017;57-58:29–44.
Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhäuser-Klaus A, Pretsch W, et al. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175:725–36.
Yoneda Y, Haginoya K, Arai H, Yamaoka S, Tsurusaki Y, Doi H, et al. De novo and inherited mutations in COL4A2, encoding the type IV collagen a2 chain cause porencephaly. Am J Hum Genet. 2012;90:86–90.
Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, et al. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90:91–101.
Verbeek E, Meuwissen ME, Verheijen FW, Govaert PP, Licht DJ, Kuo DS, et al. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur J Hum Genet. 2012;20:844–51.
Weisz-Hubshman M, Meirson H, Michaelson-Cohen R, Beeri R, Tzur S, Bormans C, et al. Novel WWOX deleterious variants cause early infantile epileptic encephalopathy, severe developmental delay and dysmorphism among Yemenite Jews. Eur J Paediatr Neurol. 2019;23:418–26.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med: Off J Am Coll Med Genet. 2015;17:405–24.
Bove KE. Practice guidelines for autopsy pathology: the perinatal and pediatric autopsy. Autopsy Committee of the College of American Pathologists. Arch Pathol Lab Med. 1997;121:368–76.
Curtis C, Mineyko A, Massicotte P, Leaker M, Jiang XY, Floer A, et al. Thrombophilia risk is not increased in children after perinatal stroke. Blood. 2017;129:2793–800.
Volonghi I, Pezzini A, Del Zotto E, Giossi A, Costa P, Ferrari D, et al. Role of COL4A1 in basement-membrane integrity and cerebral small-vessel disease. The COL4A1 stroke syndrome. Curr Med Chem. 2010;17:1317–24.
Yakovlev PI, Wadsworth RC. Schizencephalies; a study of the congenital clefts in the cerebral mantle; clefts with hydrocephalus and lips separated. J Neuropathol Exp Neurol. 1946;5:169–206.
Labelle-Dumais C, Dilworth DJ, Harrington EP, de Leau M, Lyons D, Kabaeva Z, et al. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 2011;7:e1002062.
Cavallin M, Mine M, Philbert M, Boddaert N, Lepage JM, Coste T, et al. Further refinement of COL4A1 and COL4A2 related cortical malformations. Eur J Med Genet. 2018;61:765–72.
Vitale G, Pichiecchio A, Ormitti F, Tonduti D, Asaro A, Farina L. Cortical malformations and COL4A1 mutation: three new cases. Eur J Paediatr Neurol. 2019;23:410–7.
Raets M, Dudink J, Raybaud C, Ramenghi L, Lequin M, Govaert P. Brain vein disorders in newborn infants. Dev Med Child Neurol. 2015;57:229–40.
Govaert P, Lequin M, Korsten A, Swarte R, Kroon A, Barkovich AJ. Postnatal onset cortical dysplasia associated with infarction of white matter. Brain Res. 2006;1121:250–5.
Acknowledgements
We are grateful to the patients for consenting to participate in this study. Variantyx Genomic Intelligence® is thanked for their assistance in the interpretation of the genetic analysis. Esther Eshkol is thanked for editorial assistance.
Funding
This study was funded by the Brain Center at Tel Aviv Sourasky Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All experiments were performed in accordance with relevant guidelines and regulations. The study protocols were reviewed and approved by the Institutional Review Board. Informed consent was obtained from all participants prior to genetic testing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hausman-Kedem, M., Ben-Sira, L., Kidron, D. et al. Deletion in COL4A2 is associated with a three-generation variable phenotype: from fetal to adult manifestations. Eur J Hum Genet 29, 1654–1662 (2021). https://doi.org/10.1038/s41431-021-00880-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00880-3