Abstract
Background
The persistent high prevalence of anaemia among Indian women of reproductive age (WRA) despite aggressive long-term iron supplementation could be related to over-diagnosis from an inappropriately high haemoglobin (Hb) diagnostic cut-off. To develop an appropriate cut-off for Indian WRA, we hypothesized that during iron-folic acid (IFA) supplementation to a mixed (anaemic/non-anaemic) WRA population, the positive slope of the Hb-plasma ferritin (PF) response in anaemic women would inflect into a plateau (zero-response) as a non-anaemic status is reached. The 2.5th percentile of the Hb distribution at this inflection point will be the diagnostic Hb cut-off for iron-responsive anaemia.
Method
A hierarchical mixed effects model, with a polynomial mean and variance model to account for intraclass correlation due to repeated measures, was used to estimate the response curve of Hb to PF, or body iron stores, in anaemic and non-anaemic WRA (without inflammation), who were receiving a 90-day IFA supplementation.
Results
The Hb response curve at low PF values showed a steep increase, which inflected into a plateau at a PF of 10.1 µg/L and attained a steady state at a PF of 20.6 µg/L. The Hb distribution at the inflection was a normal probability distribution, with a mean of 12.3 g/dL. The 2.5th percentile value of this distribution, or the putative diagnostic Hb cut-off for anaemia, was 10.8 g/dL (~11 g/dL).
Conclusion
The derived Hb cut-off is lower than the current adult values of 12 g/dL and could partly explain the persistently high prevalence of anaemia.
Similar content being viewed by others
Introduction
The persistence of a high prevalence of anaemia among children and women of reproductive age (WRA) over several decades in India, despite many preventive and therapeutic policies, is somewhat a mystery [1, 2]. Potentially important causes for this are poor programmatic coverage and compliance to the iron folate (IFA) supplementation program, or persistent inflammation [3,4,5], or other technical causes like inappropriate capillary sampling of blood for Hb measurement or inaccurate measurements of Hb [6]. It is worth considering whether the high anaemia prevalence is an inflated value, or whether iron and/or folate deficiencies are not the primary aetiologies.
It is likely that the reported anaemia prevalence is inflated. The World Health Organization (WHO) haemoglobin (Hb) cut-off that is presently used to diagnose anaemia is statistically derived as the 2·5th percentile of the Hb distribution in a healthy Western population [7,8,9]. However, a recent Indian report showed that venous Hb distributions in healthy children (1–19 years) were left-shifted compared to the Hb distribution used by the WHO [10], with lower statistical Hb cut-offs for diagnosing anaemia.
It is also possible that most anaemia might not be only due to iron or folate deficiency. A national survey of Indian adolescents (10–19y old) showed that isolated iron deficiency (without inflammation) accounted for only 22.2% of anaemia, while the combination of iron deficiency with folate/vitamin B12 deficiency accounted for a further 19% [11]. The remaining proportion was due to other causes like folate or vitamin B12 deficiency (25.6%), hemoglobinopathies or unknown aetiologies (33.3%). The occurrence of the additional biochemical nutrient deficiencies could be coincidental since vitamin B12 supplementation along with IFA did not result in any additional Hb gain in anaemic Indian adolescents [12]. In support, systematic reviews have also indicated a marginal effect of multiple micronutrient supplements on Hb improvements, although the anaemia assessment methodologies may not have been completely reliable across the reviewed studies [13].
A better dynamic and functional approach to identify a Hb cut-off in a population might be based on the Hb relationship to body iron stores (plasma ferritin, PF; Body Iron Stores, BIS) in a population that is receiving IFA. In a population of both iron-deficient anaemic and non-anaemic individuals that are being supplemented with IFA, the Hb relation to PF or BIS during IFA supplementation should be bimodal; positive when iron deficiency is present, and flat when no deficiency is present. The positive Hb response to PF/BIS at low body iron status would inflect to a plateau as the body iron status became replete. The Hb distribution at this inflection point (or beyond) can be considered representative of normal healthy people without being affected by the most common causes of anaemia (iron and folate deficiency, active infections and chronic comorbidities), from which a population specific, statistical Hb diagnostic cut-off can be derived. A recent longitudinal intervention study in young Indian WRA offered such data for analysis, where sequential changes in venous blood Hb, iron store biomarkers like PF, soluble transferrin receptor (sTfR), BIS, and C-reactive protein (CRP) were measured during a 90-day IFA supplementation [14].
Participants and methods
Participants
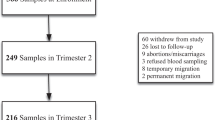
This longitudinal study was nested within a trial (CTRI No: 2019/02/017806, http://ctri.nic.in/) to evaluate the effect of IFA supplementation on the gut microbiome and Hb. The study details have been published earlier [14]. The study was conducted from February 2019 to February 2020, in WRA (n = 470), who were college undergraduates, residing in social welfare hostels in the suburbs of Hyderabad City, Telangana, India. The study was approved by the ethics committee of ICMR-National Institute of Nutrition (IEC: 06/II/2018). Prior informed consent from the participants and approval of the college authorities was taken for the study participation.
Initially, a total of 501 participants were screened, of which 12 were excluded (chronic morbidity, n = 3; refused to participate, n = 8; and planned to move out of study area, n = 1). IFA supplementation was therefore initiated in 489 participants. Of these, 97 participants reported gastrointestinal side effects (such as nausea, stomach pain, diarrhoea, dark stool, stomach pain and vomiting) in the first three weeks of supplementation. However, these symptoms subsided in many, and 80 participants continued taking the supplementation after counselling. The 17 participants who stopped consuming IFA tablets and 2 subjects who were not available for sample collection at the end of the study (90 days) were excluded. Data from the remaining 470 participants was included in the study analyses.
Non-anaemic participants (Hb≥12·0 g/dL) received a prophylactic dose of one IFA tablet (CyanoPharma Private Limited, Indore, Madhya Pradesh, India, 60 mg iron and 500 µg folic acid) once a week, while those with mild and moderate anaemia (Hb 8·0–11·9 g/dL) received a therapeutic dose of two IFA tablets, that is, a total of 120 mg iron and 1000 µg folic acid per day, for 90 days. Severely anaemic participants (Hb<8·0 g/dL) were initially excluded from the study and advised to avail clinic-based treatment as recommended by the Indian guidelines [15, 16]. However, because they were unwilling to visit a clinic, they were treated and monitored by a study-appointed clinician, with a higher IFA dose: two IFA tablets per day, with one containing 60mg iron and 500 µg folic acid and the other containing 100 mg iron and 500 µg folic acid (for a total of 160mg iron and 1000 µg folic acid per day) for 90 days, and subjects were followed up again at 180 and 365 days of post IFA supplementation as described previously for Hb and iron status measurements [14].
Blood analyses
Venous blood samples were collected at baseline, 45 and 90 days, and further at 180 and 365 days (although the intervention had stopped at 90 days). Hb, PF, sTfR, and CRP were estimated at all these time points, except that hepcidin was estimated only at baseline and 90days, and sTfR measures were not available at 45days time point. Hb concentrations were analysed by a calibrated autoanalyzer (Horiba ABX Micros 60 OT, Japan) on site, immediately after phlebotomy, as described previously [17], with an inter-assay CV of 1·2%. Since venous blood samples were used, with immediate analysis by the robust auto analyzer based method, potential errors related to sampling and Hb analysis were avoided. The PF (#FR248T, Calbiotech, USA), sTfR (# DY2474, R&D systems, USA), hepcidin (#DY8307-05, R&D systems, USA) and C-reactive protein (# DY1707, R&D systems, USA) measurements were performed using their respective ELISA kits, following manufacturer instructions as described elsewhere [14]. The CV of these assays was always <5%. BIS was calculated as the ratio of log sTfR and PF (R/F ratio) [18].
Statistics
The Hb response to PF or BIS of all participants, during and after IFA supplementation (i. e, data from all time points 0, 45, 90, 180 and 365 days), was plotted after excluding those with inflammation (high CRP). A hierarchical mixed effects polynomial regression of Hb on PF, with the suitable polynomial model of mean and variance together was used to account for intraclass correlation due to repeated measures. We initially assumed that the variance of Hb also changed along with its mean value over the change in PF. As a result, the model could estimate the parametric distribution of Hb at different PF concentrations. Since the Hb response to increasing PF/BIS would plateau at nutritional normality or ‘health’, there would be an inflection point beyond which the Hb would not respond to increases in PF/BIS, equivalent to ‘saturation’ of the Hb response. This inflection point was determined by equating the first order derivative of the Hb dose-response curve with PF or BIS, the latter as changes of -log (R/F) to zero. For the latter exploration, we relaxed the parametric polynomial assumption and assumed equal variance over the -log(R/F) ratio. A Gaussian generalized mixed effects additive model (GAMM) with cubic penalized spline was used, that estimated the degrees of freedom of the smoothed function of -log(R/F) by a Generalized Cross Validation (GCV) score. The first order derivatives of the smoothed line were derived by the finite difference method (by empirical definition of differentiation). The distribution of Hb at the identified inflection point was inspected to define its 2.5th percentile as the Hb cut-off to diagnose anaemia (this distribution would be the same at any point to the right of the inflection). The analyses were performed using statistical software R version 4.1.3 (R Core Team, 2022, Vienna, Austria).
Results
Participant characteristics
Data from 470 adolescent girls, without inflammation (CRP<5 mg/L), who took the prophylactic or therapeutic IFA supplementation for 90 days were available. Regardless of group, the mean ± SD age, body weight and BMI of all the subjects were 18·4 ± 1·0 years. 47·2 ± 10·4 kg, and 20·2 ± 3·9 kg/m2, respectively. Overall, the participants consumed ~76.4% of the prescribed dose during the 90-day intervention. At baseline, the mean Hb level was 10·8 g/dL (95% CI 10·6–10·9, n = 470) and the prevalence of anaemia (using the WHO cut-off of 12 g/dL) was 69·6% (95% CI 64·6–74·6). The mean baseline PF concentration was 12·0 µg/L (95% CI 11·5–12·6) with overall prevalence of ID of 68·7% (95% CI 63·1–74·3), using the present PF cut-off of 15 µg/L, the IDA prevalence was 62·4% (95% CI 56·3–68·5). With the IFA supplementation, mean Hb concentration significantly increased by 1·7 g/dL (95% CI 1·5–1·8) at 90 days. The mean (geometric) PF concentration also increased significantly at 90 days (by 11·6 µg/L, 95% CI 10·36–12·97). The mean plasma sTfR concentration decreased by 2·1 µg/mL (95% CI :1·73–2·35), while mean plasma hepcidin increased (by 14·9 ng/mL 95% CI 12·1–17·8), both significantly, at 90 days compared to baseline.
Hb dose-response curve and definition of Hb cut-off
When the paired and individual Hb values were plotted in relation to PF during the IFA supplementation, these mixed data fitted a 6th order polynomial for the mean model and 4th order polynomial for the variance model (the order of the polynomial was estimated by least Akaike Information Criteria (AIC) value). The Hb response showed a steep linear increase in relation to PF at low values of the latter, inflecting towards a plateau at a PF value of 10.1 µg/L, and subsequently attaining a steady state (plateau) after a PF value of 20.6 µg/L (Fig.1).
Paired data of Hb and PF was from 1705 observations; 383 at baseline, 373 at 45 days, 383 at 90 days, 312 at 180 days and 254 at 365 days. Estimated mean(bold line) and dispersion(shaded band) of Hb for a given PF level over the scatter diagram are depicted at left panel. Right panel depicts the estimated probability distribution of Hb at a state when Hb stops responding to PF (steady state).
At the commencement of the plateau of the Hb response to changes in PF, the estimated distribution of Hb was a normal probability distribution with mean 12·3 g/dL and standard deviation 0·8 g/dL. The 2·5th percentile of the Hb distribution at this steady state condition was 10·8 g/dL. A similar analysis of the Hb dose-response for changes in BIS (R/F ratio) also reflected similar results. Here, the estimated distribution of Hb at the commencement of its steady state was also a normal probability distribution with mean 12·6 g/dL and standard deviation 1·0 g/dL. The 2·5th percentile of this Hb distribution was 10·7 g/dL. Both these calculations (based on exposure measured as PF or the R/F ratio) identified the 2·5th percentile of the Hb distribution at the commencement of the steady state at ~10·8 g/dL (Fig. 2).
Paired data of haemoglobin and BIS was from 1228 observations: 380 at baseline, 380 at 90 days, 234 at 180 days and 234 at 365 days. Estimated mean Hb with 95% confidence band obtained from penalized cubic spline fitting over Body Iron Stores (without constant terms) is depicted in left panel. Estimated slopes of the curve at varying level of BIS depicted in right panel to estimate the point of BIS level at steady state condition.
Based on the Hb cut-off that we found, the prevalence of anaemia in this study sample of young WRA was estimated as 45·3% (95% CI 40·8–49·8) and 9·4% (95% CI 6·7–12·0) at baseline and 90 days of IFA treatment respectively. In contrast, the anaemia prevalence at the same time points, using the current WHO cut-off, were 69.6% (95% CI: 64.6–74.6) and 29.8% (95% CI: 22.2–37.4) respectively.
Discussion
This analysis defined the Hb response curve to increasing exposures of body iron status (PF or BIS, assuming that folate status was normalized and replete) from a longitudinal analysis of a highly supervised IFA treatment of 90 days, that tested the efficacy of a ‘screen and treat’ program to achieve anaemia reduction [14, 17]. The motivation for the present analysis came from the finding that even though the IFA compliance was high (76%) in this captive study setting for 90 days, the supplementation could not mitigate anaemia completely among a mixed group of anaemic and non-anaemic WRA, in whom a significant proportion remained either mild (18.9%) or moderately (10.8%) anaemic [14]. This analysis was agnostic to the type of IFA supplementation, different as it was for anaemic and non-anaemic subjects due to ethical considerations for anaemia treatment/prevention norms [16], and relied on the change in PF or BIS as the key exposure for Hb change. There was also some amount of non-compliance to the supplementary IFA, resulting in a dose gap for some, but the change in Hb did not correlate with this dose gap, indicating that enough iron was consumed by the participants to reach their plateau Hb response (Fig. 3).
The other possibility, that the anaemia was due to other causes like inflammation, was ruled out since we excluded those with a high CRP. The seemingly normal hepcidin concentrations [19] at baseline and the subsequent change at 90 days (Fig. 4) also suggest improvements in iron status. If a ‘healthy population’ is those in whom the Hb response to an increase in iron stores is null, then, the 2·5th percentile of this Hb distribution would be the cut-off that could be used to diagnose anaemia. The 2·5th percentile of the Hb response to either PF or BIS was ~10·8 g/dL and 5th percentile was ~11 g/dL. The WHO Hb cut-off that is currently used to diagnose anaemia [9], also used this 2·5thpercentile of a distribution of Hb in a healthy Western population, across age and sex.
It is possible that some of the non-responders were not anaemic to begin with, due to an inappropriate Hb diagnostic cut-off. Determining the appropriate value for biomarkers of health or nutrient status is not trivial. For example, biomarkers of nutrient status are usually based on statistical distributions of their body fluid concentrations in so-called ‘healthy’ populations, who have no deficiencies, either because they had unfettered access to generous nutrient intakes, or because they were screened clinically and by other biomarkers to rule out as many deficiencies as possible. These statistical exercises have been limited to data from High Income Countries (HICs) for obvious reasons, but it can be argued that the distribution of these biomarker concentrations is not only a function of health, but also of the habitual nutrient intake, or ethnicity, as has been noted earlier for Hb [10, 20]. If so, these biomarker distributions should be measured contextually, in different populations and social contexts. An approach using physiological functionality, or the presence or risk of disease, is preferable, but these outcome definitions are still not universally available. Some examples are the use of nutrient-linked enzyme activity to define nutrient status, such as antioxidant enzymes for selenium status [21], or flavoprotein enzymes for riboflavin status [22], but it might be argued that these are relatively static, multi-aetiological and cross-sectional. The mixed approach used in the present study is probably the best way forward, using paired data from the response to an intervention, and hierarchical mixed effects polynomial regression, to define inflection on the Hb-PF/BIS response curve, from which an iron-replete population and a statistical Hb cut-off could be identified. One could argue that the IFA intervention period here was short, but this was probably adequate, as a meta-analysis of iron supplementation trials found no significant association between the duration of iron supplementation (when >2 months) and the Hb response [23]. Thus, the present dynamic and functional approach is probably most rigorous and relevant to functionally define an iron-replete population from which to define the Hb cut-off.
The lower Hb cut-off in this analysis is in line with recent reports from India, and other LMICs, which also indicate that there are ethnic differences in the healthy Hb distribution across age groups and physiological conditions like pregnancy [10, 20, 24, 25]. These Hb distributions are usually left-shifted, meaning that the use of the 2·5th percentile to define the Hb diagnostic cut-off, provides lower values with significant effects on measured anaemia prevalence. Thus, in a national survey from India [2] that used accurate venous blood sampling, and estimated an anaemia prevalence of 30%, the prevalence reduced by two-thirds to 10·8%, when local ethnic-specific Hb diagnostic cut-offs derived from a healthy Indian children population selected from that survey were used [10]. The present WHO one-size-fits-all diagnostic Hb cut-off unnecessarily assumes homogeneity in the Hb distribution of healthy populations worldwide.
Based on the Hb cut-off derived in this study, the prevalence of anaemia reduced to 9·4% (95% CI 6·7–12·0) at the end of the IFA intervention. Ideally, the endline anaemia prevalence should have been in the vicinity of 2.5%, in a perfectly nourished group. It is possible that there were participants with refractory mild anaemia, possibly due to other causes (additional nutrient deficiencies such as vitamin B12, or hemoglobinopathies). It would of course, be worthwhile to apply the specific inclusion and exclusion criteria that were used by the WHO-identified studies to define their standard for Hb distributions and subsequently their Hb cut-off, to identify a healthy population here, or indeed in any low-middle income country. This would allow for region specific Hb cut-offs to be considered, as has been done earlier for children in India [10]. However, it does not seem such a group of WRA can be easily found, in whom all individuals would have Hb>12g/dL, which is the present WHO diagnostic cut-off for anaemia. This study identifies an alternate route to get to such a population, even after they have been treated with an iron intervention.
There are some limitations to this analysis. An extensive diagnostic work-up was not available for all specific aetiologies for anaemia. However, the most common aetiologies of anaemia were accounted for, such as iron and folic acid deficiency, as well as the presence of inflammation [11]. The status of vitamin B12, an important erythropoietic micronutrient, was not measured here, but it has been shown that this deficiency is unlikely to be a major cause of anaemia [11], and vitamin B12 supplementation does not improve Hb concentration in Indian studies [12, 13]. Haemoglobinopathies were not excluded, and that is a true limitation. We also did not consider red cell indices like mean corpuscular volume or haemoglobin concentration, but it has been shown that changes in Hb are the most sensitive to supplementation [26], and there was a sufficient duration of intervention for the change to occur.
A practical Hb threshold for diagnosing anaemia in India could be a rounded value of 11 g/dL, which also aligns with the 5th percentile of the steady state cut-off. In addition, the variation of the analytical method (around 0.1–0.2 g/dL), when added to the threshold of 10.8 g/dL, would produce an individual threshold of 11 g/dL, which is important to avoid missing any anaemic woman. For populations, the "biological” cut-off of 10.8 g/dL may be appropriate, as the variation error is at both sides of that value. However, the generalizability of these cut-offs for programmatic implementation, including in India, must await robust validation from other settings.
In conclusion, the persistently high population prevalence of anaemia in Indian WRA (greater than 50%), is a cause for concern. Systematic biases in diagnosis must be considered, like the presently used diagnostic Hb cut-off. It is also worth noting that 60% of the anaemic WRA were ‘mildly’ anaemic, with Hb values close to the present cut-off, with high chance of being false positives. The one-size-fits-all Hb cut-off for anaemia may lead to overestimation of the problem and inappropriate use of economic resources. The use of ever-increasing doses of iron through supplementation and fortification without these considerations will have biological and economic consequences in low- and middle-income countries.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
International Institute for Population Sciences (IIPS). National Family Health Survey (NFHS-5), 20119-20: fact sheets: Key Indicators 22 States/UTs from phase I; 2020. http://rchiips.org/nfhs/factsheet_NFHS-5.shtml.
Ministry of Health and Family Welfare (MoHFW), Government of India, Comprehensive National Nutrition Survey (CNNS) National Report. New Delhi. 2019. https://nhm.gov.in/WriteReadData/l892s/1405796031571201348.pdf.
Pasricha SR, Armitage AE, Prentice AM, Drakesmith H. Reducing anaemia in low income countries: control of infection is essential. BMJ 2018;362:k3165.
Kulkarni B, Peter R, Ghosh S, Pullakhandam R, Thomas T, Reddy GB, et al. Prevalence of Iron Deficiency and its Sociodemographic Patterning in Indian Children and Adolescents: Findings from the Comprehensive National Nutrition Survey 2016-18. J Nutr. 2021;151:2422–34.
Nair K, Dripta R, Konapur A. Appropriate doses of iron for treatment of anemia amongst. Indian J Community Health 2018;30:39–53.
Neufeld LM, Larson LM, Kurpad A, Mburu S, Martorell R, Brown KH. Hemoglobin concentration and anemia diagnosis in venous and capillary blood: biological basis and policy implications. Ann N. Y Acad Sci. 2019;1450:172–89.
World Health Organization. Regional Office for Europe & United Nations Children’s Fund (UNICEF). (2001). Prevention and control of iron-deficiency anaemia in women and children : report of the UNICEF/WHO regional consultation, Geneva, Switzerland 3-5 February 1999. Copenhagen: WHO Regional Office for Europe. https://apps.who.int/iris/handle/10665/108513.
WHO. Hemoglobin concentrations for the diagnosis of anemia and assessment of severity (WHO/NMH/NHD/MNM/11.1). 2011. https://apps.who.int/iris/handle/10665/85839 (accessed 13 March 2023).
Garcia-Casal MN, Pasricha S-R, Sharma AJ, Peña-Rosas JP. Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Ann N. Y Acad Sci. 2019;1450:5.
Sachdev HS, Porwal A, Acharya R, Ashraf S, Ramesh S, Khan N, et al. Haemoglobin thresholds to define anaemia in a national sample of healthy children and adolescents aged 1-19 years in India: a population-based study. Lancet. Glob Health. 2021;9:e822–e31.
Sarna A, Porwal A, Ramesh S, Agrawal PK, Acharya R, Johnston R, et al. Characterisation of the types of anaemia prevalent among children and adolescents aged 1–19 years in India: a population-based study. Lancet Child Adolesc Health. 2020;4:515–25.
Gupta A, Kant S, Ramakrishnan L, Pandey RM, Khandelwal R, Kapil U, et al. Impact of daily-supervised administration of a package of iron and folic acid and vitamin B12 on hemoglobin levels among adolescent girls (12-19 years): a cluster randomized control trial. Eur J ClinNutr. 2021;75:1588–97.
Gera T, Sachdev H, Nestel P. Effect of combining multiple micronutrients with iron supplementation on Hb response in children: Systematic review of randomized controlled trials. Public Health Nutr. 2009;12:756–73.
Palika R, Dasi T, Ghosh S, Peter R, Parasannanavar DJ, Pradhan AS, et al. Efficacy of iron-folic acid treatment for reducing anemia prevalence and improving iron status in women of reproductive age: A one-year longitudinal study. ClinNutr ESPEN. 2022;49:390–7.
Kulkarni B, Augustine LF, Pullakhandam R, Pradhan AS, Dasi T, Palika R, et al. ‘Screen and Treat for Anaemia Reduction (STAR)’ strategy: study protocol of a cluster randomised trial in rural Telangana, India. BMJ Open. 2021;11:e052238.
MOHFW. Intensified National Iron Plus Initiative, Anaemia Mukt Bharat Operational Guidelines. Government of India; 2018. https://www.fitterfly.com/site/pdf/anemia-mukt-bharat.pdf.
Dasi T, Palika R, Pullakhandam R, Augustine LF, Boiroju NK, Prasannanavar DJ. et al. Point-of-care Hb measurement in pooled capillary blood by a portable autoanalyser: Comparison with venous blood Hb measured by reference methods in cross-sectional and longitudinal studies. Br J Nutr. 2022;128:1108–17.
Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64.
Mercade L, Metzger M, Haymann JP, Thervet E, Boffa J-J, Martin Flamant. et al. The Relation of Hepcidin to Iron Disorders, Inflammation and Hemoglobin in Chronic Kidney Disease. PLoS ONE. 2014;9:e99781.
Varghese JS, Thomas T, Kurpad AV. Evaluation of haemoglobin cut-off for mild anaemia in Asians - analysis of multiple rounds of two national nutrition surveys. Indian J Med Res. 2019;150:385–9.
Müller SM, Dawczynski C, Wiest J, Lorkowski S, Kipp AP, Schwerdtle T. Functional Biomarkers for the Selenium Status in a Human Nutritional Intervention Study. Nutrients 2020;12:676.
Reddi AS. Riboflavin nutritional status and flavoprotein enzymes in streptozotocin- diabetic rats. BiochimBiophysActa. 1986;882:71–76.
Gera T, Sachdev HP, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. J PediatrGastroenterolNutr. 2007;44:468–86.
Addo OY, Yu EX, Williams AM, Melissa FY, Andrea JS, Zuguo M, et al. Evaluation of hemoglobin cutoff levels to define anemia among healthy individuals. JAMA Netw Open. 2021;4:e2119123.
Ohuma EO, Young MF, Martorell R, Ismail LC, Peña-Rosas JP, Purwar M, et al. International values for haemoglobin distributions in healthy pregnant women. EClinical Med 2020;29e30:100660.
Sezgin G, Monagle P, Loh TP, Ignjatovic V, Hoq M, Pearce C, et al. Clinical thresholds for diagnosing iron deficiency: comparison of functional assessment of serum ferritin to population based centiles. Sci Rep. 2020;10:18233.
Funding
This secondary analysis was not funded. The human data came from a study that was funded by the Indian Council of Medical Research (ICMR), Government of India to BK, in turn nested in a larger study supported by a grant from the Systems Biology Research Initiative from the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) to RKV. The ICRISAT funding contributed to some activities related to data collection in the present study.
Author information
Authors and Affiliations
Contributions
SG and AVK wrote the first draft of the paper. All authors commented and edited draft versions and then approved the final version of the paper. SG, HSS, AVK and BK had primary responsibility for final content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
No separate ethical approval was required for this secondary analysis. The original study was nested within a trial (CTRI No: 2019/02/017806, http://ctri.nic.in/) to evaluate the effect of IFA supplementation on the gut microbiome and Hb. The original study was approved by the ethics committee of ICMR-National Institute of Nutrition (IEC: 06/II/2018). Prior informed consent from the participants and approval of the college authorities was taken for the study participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, S., Palika, R., Dasi, T. et al. Haemoglobin diagnostic cut-offs for anaemia in Indian women of reproductive age. Eur J Clin Nutr 77, 966–971 (2023). https://doi.org/10.1038/s41430-023-01308-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01308-5