Abstract
Background/Objectives
We examined the prevalence of anaemia, iron deficiency, and inflammation during pregnancy and their associations with adverse pregnancy and infant outcomes in India.
Subjects/Methods
Three hundred and sixty-six women participating in a randomised trial of vitamin B12 supplementation were monitored to assess haemoglobin (Hb), serum ferritin (SF), hepcidin, C-reactive protein (CRP), and alpha-1-acid glycoprotein (AGP) during pregnancy. Women received vitamin B12 supplementation (50 µg per day) or placebo daily; all women received daily prenatal iron–folic acid supplementation. Binomial and linear regression models were used to examine the associations of maternal iron biomarkers with pregnancy and infant outcomes.
Results
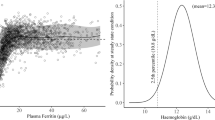
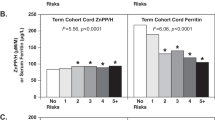
Thirty percent of women were anaemic (Hb < 11.0 g/dl), 48% were iron deficient (SF < 15.0 µg/l), and 23% had iron deficiency anaemia at their first prenatal visit. The prevalence of inflammation (CRP > 5.0 mg/l: 17%; AGP > 1.0 g/l: 11%) and anaemia of inflammation (Hb < 11.0 g/dl, SF > 15.0 µg/l, plus CRP > 5.0 mg/l or AGP > 1.0 g/l: 2%) were low. Infants born to anaemic women had a twofold higher risk of low birth weight (<2500 g; risk ratio [RR]: 2.15, 95%CI: 1.20–3.84, p = 0.01), preterm delivery (RR: 2.67 (1.43–5.00); p = 0.002), underweight (WAZ < −2; RR: 2.20, 95%CI: 1.16–4.15, p = 0.02), and lower MUAC (β(SE): −0.94 (0.45)cm, p = 0.03). Similarly, maternal Hb concentrations predicted higher infant birth weight (p = 0.02) and greater gestational age at delivery (β(SE): 0.28 (0.08) weeks, p = 0.001), lower risk of preterm delivery (<37 weeks; RR: 0.76, 95%CI: 0.66–86, p < 0.0001); and higher infant MUAC (β(SE): 0.36 (0.13) cm, p = 0.006). Maternal SF concentrations were associated with greater birth length (β(SE): 0.44 (0.20) cm, p < 0.03). Findings were similar after adjusting SF concentrations for inflammation. IDA was associated with higher risk of low birth weight (RR: 1.99 (1.08–3.68); p = 0.03) and preterm birth (RR: 3.46 (1.81–6.61); p = 0.0002); and lower birth weight (p = 0.02), gestational age at birth (p = 0.0002), and infant WAZ scores (p = 0.02).
Conclusions
The prevalence of anaemia and iron deficiency was high early in pregnancy and associated with increased risk of adverse pregnancy and infant outcomes. A comprehensive approach to prevent anaemia is needed in women of reproductive age, to enhance haematological status and improve maternal and child health outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
WHO The global prevalence of anaemia in 2011. Geneva: WHO; 2015.
Janbek J, Sarki M, Specht IO, Heitmann BL A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur J Clin Nutr. 2019.
McClung JP, Murray-Kolb LE. Iron nutrition and premenopausal women: effects of poor iron status on physical and neuropsychological performance. Annu Rev Nutr. 2013;33:271–88.
Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–87.
Haas JD, Brownlie Tt. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–88S.
Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res. 2009;130:627–33.
Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, et al. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016;8.
Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr. 2015;6:552–63.
Finkelstein JL, Kurpad AV, Thomas T, Srinivasan K, Duggan C. Vitamin B12 status in pregnant women and their infants in South India. Eur J Clin Nutr. 2017;71:1046–53.
Duggan C, Srinivasan K, Thomas T, Samuel T, Rajendran R, Muthayya S, et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr. 2014;144:758–64.
World Health Organization Training course on child growth assessment. Geneva: World Health Organization; 2008.
Gibson RS. Principles of nutritional assessment. 2nd ed. New York, NY: Oxford University Press, Inc; 2005.
Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level, 2nd ed. Geneva: World Health Organization; 2007.
Technical meeting: use and interpretation of haemoglobin concentrations for assessing anaemia status in individuals and populations. Geneva: World Health Organization; 2017.
Nemeth E, Ganz T. Anemia of inflammation. Hematol/Oncol Clin North Am. 2014;28:671–81.
WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization; 1995.
Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet 2014;384:857–68.
de Onis M, Blossner M. The World Health Organization Global Database on Child Growth and Malnutrition: methodology and applications. Int J Epidemiol. 2003;32:518–26.
WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: growth velocity based on weight, length and head circumference: methods and development. Geneva: World Health Organization; 2009.
WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: World Health Organization; 2006
Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55.
Thurnham DI, Northrop-Clewes CA, Knowles J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J Nutr. 2015;145:1137S–43S.
Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, et al. Adjusting ferritin concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr. 2017;106:359S–71S.
Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha SR, Rogers LM, et al. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr. 2017;106:1626S–33S.
Greenland S. Modeling and variable selection in epidemiologic analysis. Am J public health. 1989;79:340–9.
Miettinen O. Theoretical Epidemiology. New York: John Wiley & Sons; 1985. 107
NFHS. National Family Health Survey-4: 2015–16. Government of India, 2016.
Ahankari AS, Myles PR, Dixit JV, Tata LJ, Fogarty AW. Risk factors for maternal anaemia and low birth weight in pregnant women living in rural India: a prospective cohort study. Public health. 2017;151:63–73.
Toteja GS, Singh P, Dhillon BS, Saxena BN, Ahmed FU, Singh RP, et al. Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull. 2006;27:311–5.
Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J Matern-fetal Neonatal Med. 2014;27:887–91.
Mohanty D, Gorakshakar AC, Colah RB, Patel RZ, Master DC, Mahanta J, et al. Interaction of iron deficiency anemia and hemoglobinopathies among college students and pregnant women: a multi center evaluation in India. Hemoglobin. 2014;38:252–7.
Hambidge KM, Westcott JE, Garces A, Figueroa L, Goudar SS, Dhaded SM, et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the women first trial. Am J Clin Nutr. 2019;109:457–69.
Baingana RK, Enyaru JK, Tjalsma H, Swinkels DW, Davidsson L. The aetiology of anaemia during pregnancy: a study to evaluate the contribution of iron deficiency and common infections in pregnant Ugandan women. Public health Nutr. 2015;18:1423–35.
van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72:247S–56S.
Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R. Overview of the biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Adv Nutr. 2016;7:349–56.
Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, Armitage AE, et al. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the Gambia. J Nutr. 2017;147:1131–7.
Shaw JG, Friedman JF. Iron deficiency anemia: focus on infectious diseases in lesser developed countries. Anemia. 2011;2011:260380:1–10.
Nair M, Choudhury MK, Choudhury SS, Kakoty SD, Sarma UC, Webster P, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob health. 2016;1:e000026.
Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495–504.
Sukrat B, Wilasrusmee C, Siribumrungwong B, McEvoy M, Okascharoen C, Attia J, et al. Hemoglobin concentration and pregnancy outcomes: a systematic review and meta-analysis. Biomed Res int. 2013;2013:769057:1–9.
Alwan NA, Cade JE, McArdle HJ, Greenwood DC, Hayes HE, Simpson NA. Maternal iron status in early pregnancy and birth outcomes: insights from the baby’s vascular health and iron in pregnancy study. Br J Nutr. 2015;113:1985–92.
Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol. 2015;52:339–47.
Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31:225–33.
Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106:1567S–74S.
Acknowledgements
We thank the mothers and children, and field teams, including physicians, nurses, midwives, research and laboratory, and administrative staff, who made this study possible; Tinu Samuel, Ramya Rajendran, Sumithra Muthayya, Ammu Lukose, Wafaie Fawzi, and Ron Bosch for their contributions to the parent randomised trial of vitamin B12 supplementation; and St. John’s Medical College, Bangalore, India, for its institutional support.
Author contributions
CD, JLF, KS, and AVK designed the research; all authors conducted the research; JLF conducted the data analysis and wrote the initial draft of the manuscript; BB conducted the laboratory analyses; JLF and CD had primary responsibility for final content. All authors contributed to the interpretation of data and in the development of this manuscript, read and approved the final version.
Funding
National Institutes of Health (NICHD R03HD054123 and K24DK104676); Indian Council of Medical Research (ICMR: 5/7/192/06-RHN); Rose Fellowship in Chronic Disease Epidemiology and Biostatistics; Harvard Institute for Global Health; Uwe Brinkmann Memorial Fellowship; Michael von Clemm Fellowship; South Asia Initiative Graduate Grant; Division of Nutritional Sciences, Cornell University
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The research protocols and study procedures were approved by the Institutional Ethical Board of St. John’s Medical College and the Harvard T.H. Chan School of Public Health Human Subjects Committee, and written informed consent was obtained from all participants. A Data Safety and Monitoring Board met twice annually during the course of the trial.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Finkelstein, J.L., Kurpad, A.V., Bose, B. et al. Anaemia and iron deficiency in pregnancy and adverse perinatal outcomes in Southern India. Eur J Clin Nutr 74, 112–125 (2020). https://doi.org/10.1038/s41430-019-0464-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0464-3
This article is cited by
-
Systemic inflammation, enteropathogenic E. Coli, and micronutrient insufficiencies in the first trimester as possible predictors of preterm birth in rural Bangladesh: a prospective study
BMC Pregnancy and Childbirth (2024)
-
Maternal low and high hemoglobin concentrations and associations with adverse maternal and infant health outcomes: an updated global systematic review and meta-analysis
BMC Pregnancy and Childbirth (2023)
-
The Interactive Approach of Rhizobacteria and l-tryptophan on Growth, Physiology, Tuber Characteristics, and Iron Concentration of Potato (Solanum tuberosum L.)
Journal of Plant Growth Regulation (2022)
-
Anemia and adverse outcomes in pregnancy: subgroup analysis of the CLIP cluster-randomized trial in India
BMC Pregnancy and Childbirth (2022)
-
High burden of anemia among pregnant women in Tanzania: a call to address its determinants
Nutrition Journal (2021)