Abstract
Microglia shape the synaptic environment in health and disease, but synapses do not exist in a vacuum. Instead, pre- and postsynaptic terminals are surrounded by extracellular matrix (ECM), which together with glia comprise the four elements of the contemporary tetrapartite synapse model. While research in this area is still just beginning, accumulating evidence points toward a novel role for microglia in regulating the ECM during normal brain homeostasis, and such processes may, in turn, become dysfunctional in disease. As it relates to synapses, microglia are reported to modify the perisynaptic matrix, which is the diffuse matrix that surrounds dendritic and axonal terminals, as well as perineuronal nets (PNNs), specialized reticular formations of compact ECM that enwrap neuronal subsets and stabilize proximal synapses. The interconnected relationship between synapses and the ECM in which they are embedded suggests that alterations in one structure necessarily affect the dynamics of the other, and microglia may need to sculpt the matrix to modify the synapses within. Here, we provide an overview of the microglial regulation of synapses, perisynaptic matrix, and PNNs, propose candidate mechanisms by which these structures may be modified, and present the implications of such modifications in normal brain homeostasis and in disease.
Similar content being viewed by others
A small cell with many hats: the emerging complexity of microglia
Far from acting simply as a structural glue that holds neuronal networks together, as suggested by the Greek word from which the name “glia” is derived, it is now readily apparent that microglia and macroglia (astrocytes, oligodendrocytes) are important determinants of brain development and health [1]. Microglia in particular have been the focus of further reappraisal, as their functional repertoire has extended from the classically immune—detecting and resolving injury and invasive pathogens—to more non-immune roles in the homeostatic brain [2,3,4]. These findings have occurred across a backdrop of increasingly elegant methodological advances, including single-cell analyses [5,6,7], microglial ablation paradigms [8,9,10,11,12], and in vivo imaging techniques [13,14,15,16], that together have characterized the dynamic influence microglia have on virtually all major central nervous system (CNS) cell types over the lifespan of an organism. However, this increasing functional complexity suggests a greater opportunity for dysfunction and dyshomeostasis should microglia fail to properly perform their cellular roles at the appropriate times, as supported by a growing body of evidence implicating microglia as drivers of disease pathogenesis [17]. Therefore, taking stock of the homeostatic functions performed by these cells—at this critical juncture of glial biology research—may provide insight into what goes wrong in disease and how such deficits may be best targeted in the clinic.
Microglia and other resident tissue macrophages display considerable diversity across organs at the gene expression and chromatin levels [18], reflecting the varying developmental and functional roles they play in each tissue [19,20,21,22]. The recent wave of studies characterizing microglia at single-cell resolution indicate extensive transcriptional heterogeneity during development and disease, with a more homogeneous population evident in the homeostatic adult brain [5,6,7, 23, 24]. If an empty niche is available in the tissue myeloid compartment, cues from the local microenvironment can reprogram infiltrating bone marrow-derived monocytes or ontogenically foreign macrophages into microglia-like phenotypes [25,26,27,28,29,30]. The extent of transcriptional reprogramming appears to depend on the yolk-sac or hematopoietic origin of the cell in question [8, 26], at least in the CNS, where the adult resident macrophage population (e.g., microglia) derives from yolk-sac erythromyeloid progenitors [31,32,33,34]. The capacity for macrophage re-education, combined with the diverse transcriptional profiles of resident macrophages in different organs and stages of life, emphasizes the complexity of homeostatic functions performed by tissue myeloid cells, even in adults.
Research into the role of microglia in neurodevelopment has revealed numerous essential functions not directly related to immunity but essential for proper brain organization and tissue health [1, 21, 22]. Additional investigation into early microglial functions has drawn some to label these cells “architects” of the developing CNS [1, 35], with top-down roles in the spatial organization and survival of both neuronal and non-neuronal cell types. In the perinatal period, microglia orchestrate spatial patterning of neurons through a variety of regulatory mechanisms that dictate neuronal/neuronal precursor cell (NPC) survival, i.e., signaling through soluble/membrane-bound microglial factors (glutamate, reactive oxygen species, and TNF-α) that induce programmed cell death prior to microglial phagocytosis of debris, reported phagocytosis of live cells (“phagoptosis”), and secretion of pro-survival factors (e.g., insulin-like growth factor; IGF-1) [21]. Microglia continue to regulate neurogenesis in the adult brain by phagocytic maintenance of the hippocampal NPC pool [36]. A major re-evaluation of microglial effector functions in the brain was spurred by the discovery of synaptic engulfment and pruning by postnatal microglia that occurs in an activity- and complement-dependent manner to refine the neural circuitry [37,38,39] and continues to some extent in adults during memory processing [40].
Extensive work in recent years has elucidated the regulation of other glial cells by microglia, and vice versa, particularly in regard to astrocytes [41,42,43,44]. Microglia induce a neurotoxic phenotype in astrocytes (termed “A1” astrocytes) following LPS via release of microglial IL-1ɑ, TNF-ɑ, and C1q that promotes neuronal and oligodendrocyte death; loss of these factors (i.e., in Il1ɑ−/−Tnf−/−C1qɑ−/− triple knockout mice) or microglia themselves (Csf1r−/− mice) blocks A1 astrocyte formation [45]. Detrimental gain-of-function properties in neurotoxic astrocytes are accompanied by a loss of beneficial properties, including their support of neuronal outgrowth and synaptogenesis [45]. Subsequent studies determined that blocking these regulatory microglial factors by genetic or pharmacologic means ameliorates such neurotoxic astrocyte reactivity and/or neuronal death in models of amyotrophic lateral sclerosis (ALS) [46], Parkinson’s disease [47], optic nerve crush [48], glaucoma [48, 49], and aging [50]. It should be noted that the constitutive Il1ɑ−/−Tnf−/−C1qɑ−/− triple knockout mice utilized in many of the aforementioned studies on astrocyte reactivity in disease will have off-target effects in a number of pathways, given the importance of these three molecules in a broad array of signaling cascades and biological functions [39, 51,52,53]. Therefore, the in vivo findings generated from these mice should be validated with targeted in vitro approaches, as in [45, 46], or with the in vivo use of specific pharmacological agents (e.g., neutralizing antibodies to IL-1ɑ, TNF-ɑ, and C1q [48], or the glucagon-like peptide-1 receptor agonist NLY01 [47, 49]) and/or conditionally inducible knockout models.
In addition to affecting oligodendrocyte number via the cytotoxic effects of reactive astrocytes [45], microglia also choreograph oligodendrocyte patterning directly in disease and under homeostatic conditions [2, 54]. Postnatal microglia are required for early oligodendrocyte precursor cell (OPC) maintenance and maturation and continue to support the OPC pool in adulthood [55], and minocycline-based inhibition of postnatal microglial activation impairs oligodendrocyte differentiation [56]. Furthermore, early microglia promote developmental myelinogenesis [55] at least in part through the production of myelinogenic IGF-1 by a CD11c+ microglial subpopulation [57], and myelin in the adult brain is phagocytosed by microglia, where it accumulates with age [58, 59]. Interactions with oligodendrocytes in disease are multifaceted, such that microglia can support remyelination in certain cases, as in the secretion of activin A to promote OPC proliferation and differentiation [60], and persistently disrupt OPC/oligodendrocyte population dynamics and myelination in others [61].
Thus, despite traditional classifications of microglia as immune cells first, mounting data indicate that they are intricately entwined in the complex process of brain tissue development and adult brain homeostasis, interacting with virtually every cell type in the brain to orchestrate non-immune as well as classically immune-related processes. Microglial cellular functionality may be perturbed in one of two ways: through (1) toxic gain-of-function, as exemplified by chronically activated microglia that fail to resolve ongoing proinflammatory cytokine and neurotoxin production (e.g., plaque-associated microglia in Alzheimer’s disease [62]), or (2) loss of beneficial or protective function. An example of the latter may be found in the failure to appropriately prune neuronal synapses in development, which may contribute to autism spectrum disorders [63,64,65]. The extent to which these changes contribute to brain disease depends on a multitude of contextual factors, including brain age, existing disease pathology, and disease-relevant genetic and environmental risk factors, among others.
To gain insight into the roles they play, microglia may be depleted via toxin-, genetic-, and pharmacological-based methods, and we have recently reviewed the comparative advantages, disadvantages, and caveats of these different approaches [11]. To serve as an illustration, our lab has previously developed pharmacological paradigms of microglial depletion with inhibitors of colony-stimulating factor 1 receptor (CSF1R), such as PLX3397 and PLX5622, signaling through which microglia are dependent for survival [10, 66,67,68,69,70,71]. Inhibitor-induced depletion of microglia occurs in the absence of behavioral deficits, cytokine storm, brain pathology, or replacement by peripheral myeloid cells [10] and appears to be indefinitely maintainable as long as inhibition is continued (at least 6 months) [71]. Such approaches have been utilized to study the effects that microglia exert on not only neuronal and synaptic functions in health and disease [66, 67, 72] but also on other glial cells. For instance, astrocyte reactivity is detected in Huntington’s disease (HD) [45], and we reported resolution of disease-associated astrogliosis with microglial depletion via CSF1R inhibition in HD model mice [73] that is similar to the observed effects of microglial depletion on astrocyte reactivity in the context of methotrexate chemotherapy [61], consistent with the increasingly studied function of microglia as regulators of astrocyte responses.
Restoring CSF1R signaling following inhibitor treatment by inhibitor withdrawal induces full repopulation of the microglial niche via proliferation of surviving microglia [10, 74], which may be exploited to correct dysfunctional microglial phenotypes by replacing the old with new cells. This aspect of microglial biology has been utilized to promote brain recovery after traumatic brain injury (TBI) [75], neuronal lesion [76], and aging [77] by resolving chronically activated or otherwise dyshomeostatic microglia in vivo. When discussing CSF1R-based microglial depletion models, it should be noted as an experimental caveat that CSF1R is also expressed by peripheral myeloid cells, as well as microglia, and accordingly, a growing number of studies are examining the peripheral effects of CSF1R inhibition [71, 78,79,80,81,82,83,84]. However, such peripheral off-target effects in studies focused on microglial function may be controlled by utilizing subtypes or doses of CSF1R inhibitors that achieve little to no blood-brain barrier (BBB) penetrance in healthy adult mice [11], such as PLX73086 [85], Ki20227 [86], or PLX3397 at 75 ppm [10]. Ultimately, by eliminating microglia and observing the consequences of their absence and/or renewal on brain physiology in homeostasis and disease and whether these processes normalize injury- or disease-associated deficits, we may make inferences about their function in the brain.
In this review, we will discuss recent findings regarding microglial functionality in the developing and adult brain, with a special focus on the more nebulous non-immune roles these cells serve and how such functions may go wrong in disease. Microglia exert top-down influence on all major components of the modernly conceptualized tetrapartite synapse [87, 88], namely, pre- and postsynaptic compartments [37, 89], glia [45, 55], and the extracellular matrix (ECM) [72, 73, 90, 91]. The microglial modulation of astrocytes and oligodendrocytes has been reviewed eloquently elsewhere [22, 41,42,43,44, 54]. We will focus here on the interactions between microglia and ECM compartments, primarily regarding how the former influence the latter, and how this may relate to synaptic remodeling. Where appropriate, we will draw from our expertise in microglial ablation techniques to showcase the unique efficacy of these tools in elucidating general principles of microglial biology and new ways in which they can be utilized to drive novel discoveries in this field.
Microglia and the extracellular matrix in health and disease
Perhaps more aptly considered the ‘glue’ of the nervous system than glia themselves, the ECM is a highly complex and dynamic molecular meshwork with roles in plasticity, biophysical protection, and cell signaling [87, 92,93,94]. While its role as an extracellular scaffold is well known, less appreciated is the ability of the ECM to limit, and thus functionally compartmentalize, the diffusion and localization of key molecules [87], including neurotransmitters [95, 96], ions [97,98,99], and membrane receptors [100,101,102]. Furthermore, matrix molecules such as the abundant chondroitin sulfate proteoglycans (CSPGs), which consist of glycosaminoglycan side chains attached to a core protein, may inhibit neurite and axon growth [103,104,105,106,107,108], myelination and remyelination by oligodendrocytes/OPCs [109,110,111], and neural stem cell migration [112]. This has been classically illustrated in astrocytic glial scars that form after injury (e.g., spinal cord injury) and serve as a physicochemical barrier to neuronal recovery and axonal regrowth, thought to be due in large part to the consequent deposition of matrix components such as CSPGs into the interstitial ECM by reactive astrocytes [111, 113,114,115,116]. This interpretation is complicated by the discovery that certain glial scar components (such as specific CSPG subtypes [117, 118]), and the glial scar as a whole [119,120,121], both appear to facilitate axon regeneration and neurite outgrowth. Additionally, disruption of injury-induced astrocyte responses upstream of scar formation impedes recovery by impairing injury containment, BBB repair, and inflammation resolution [121,122,123,124], and astrocytes themselves may adopt multiple reactive phenotypes that likely exist on a functional spectrum [44, 125] and that may, in turn, be influenced by proteoglycans [123, 126]. Whatever new findings future studies may hold, it is clear that the ECM has a profound influence on brain health and disease.
As in the case of astrocyte-mediated scar formation, the ECM and interstitial environment critically regulate neuroinflammation and the immune response. Structural components of the ECM and products of its degradation may serve as damage-associated molecular patterns (DAMPs) that induce or suppress microglial reactivity by signaling through pattern recognition receptors (e.g., Toll-like receptors), as reviewed elsewhere [127,128,129]. For instance, culturing microglia on a CSPG substrate in vitro induces microglial activation, proliferation, and the expression of IGF-1, MMP-2, and MMP-9, whereas pharmacological inhibition of CSPG production with xyloside following spinal cord injury differentially alters inflammation and cytokine production depending on the timing of treatment [130]. Alternatively, disaccharides generated from the degradation of CSPGs with the bacterial enzyme chondroitinase ABC (ChABC) confer an activated noncytotoxic microglial phenotype that is associated with protection in experimental autoimmune encephalomyelitis [131], spinal cord injury [130], and multiple models of neurotoxicity [132, 133]. This observation may serve as a confounding factor in studies on the effects of ECM on microglia and inflammation that utilize ChABC, and therefore should be kept in mind when designing such experiments. In addition to the activation state, ECM components may also affect microglial adhesion, migration, and morphology, as in the case of cell-matrix signaling mediated by transmembrane ECM receptors known as integrins [134,135,136]. However, rather than the autonomous effects of ECM on neuroimmunology, in this review we will focus on the perspective of top-down microglial influences on the matrix and how these processes relate to changes in the synaptic landscape, while keeping in mind the bidirectional nature of ECM−immune interactions.
In terms of composition, the brain ECM is made up largely of proteoglycans, such as CSPGs and heparan sulfate proteoglycans (HSPGs), glycoproteins, such as laminins and tenascins, and glycosaminoglycans, such as the abundant hyaluronan [87, 93], among other molecules (e.g., collagens) that together constitute approximately 20% of the overall brain volume [96, 137]. The structural molecules that constitute the brain ECM and the proteases that are secreted to remodel it are produced by neurons, astrocytes, microglia, and oligodendrocyte lineage cells with varying degrees of overlap in terms of cellular origin depending on the molecule in question [94, 111, 138, 139]. The ECM can be partitioned into structural subtypes based on organization and composition, which generally include (1) the basement membrane of the BBB, (2) the diffuse ECM found in interstitial and perisynaptic spaces, (3) the condensed, reticular ECM that ensheathes neuronal subsets and their perisomatic synapses to form structures known as perineuronal nets (PNNs), and (4) the perinodal ECM that surrounds nodes of Ranvier within axons and that displays compositional resemblance to PNNs [94, 111]. Along with synaptic terminals and glial cells, the diffuse perisynaptic matrix and the synaptic ECM of PNNs constitute the fourth compartment and most recent addition to the conventional model of synaptic function, the tetrapartite synapse [87, 93], and recent studies report that both structures are dynamically regulated by microglia in the homeostatic adult brain [72, 73, 90, 91].
Perineuronal nets
Discovered by Camillo Golgi and dismissed by Ramón y Cajal as a fixation artifact, perineuronal nets are specialized ECM structures that condense in a reticular fashion around the soma and proximal neurites (dendrites and axon initial segment) of neurons throughout the brain during development [94, 140]. Although PNNs are associated primarily with fast-spiking parvalbumin (PV)-expressing GABAergic interneurons, particularly in the cortex of the brain [141, 142], they are evident throughout the CNS and across a variety of neuronal subsets [92, 94, 142,143,144,145] and are generally (but not always) labeled with the lectin Wisteria floribunda agglutinin [94]. These formations serve as a molecular scaffold to stabilize and regulate the synapses they surround and reach adult levels during the closure of critical periods of neuroplasticity [92, 146], and the genetic or enzymatic removal of PNNs or their components are capable of reinstating critical period-like plasticity [146,147,148,149]. In line with their role as a synaptic scaffold, PNNs have been proposed as the molecular basis of long-term memory storage [150], and their experimental removal disrupts the consolidation [151, 152] and recall [152, 153] of various types of remote fear memories. Thus, the heightened plasticity afforded by PNN loss may impair long-term memory fidelity due to interference from new memory traces [92, 154, 155]. Physiologically, PNNs also protect host cells from neurotoxins such as Aβ1-42 and oxidative stress [156, 157], regulate neuronal excitability [158, 159], and augment neuronal firing by reducing membrane capacitance akin to myelin sheaths [160], thereby influencing excitatory-inhibitory balance. Furthermore, the removal of PNN components alters synaptic transmission [161], synaptic ion channel/neurotransmitter receptor localization [162], and synapse number [163, 164]. While future experiments will determine whether some of these effects at least partly result from changes to the perisynaptic matrix rather than the PNN proper, particularly in the case of brevican modifications [165], these findings taken together underscore the truly multimodal effects that the brain ECM in general, and PNNs specifically, exert on the cells they associate with and enwrap.
Previous studies have postulated that microglia may drive PNN loss in certain disease contexts due to their ability to secrete matrix-degrading enzymes (e.g., matrix metalloproteinases; MMPs) and/or their molecular activators or inhibitors [166,167,168,169]. Indeed, we have recently shown that CSF1R inhibitor-based microglial depletion prevents disease-associated PNN reductions in models of Huntington’s [73] and Alzheimer’s disease (AD) [90]. PNN components were evident in microglia in both AD mouse and human brain tissue, where they also colocalized with canonical dense-core plaques [90]. That we observed similar effects on PNN abundance in the relative absence of microglia across these models is striking, both due to their differential etiologies—intracellular vs. extracellular protein accumulation in the R6/2 HD [170] and 5xFAD model of AD [171], respectively—and the variable microglial phenotypes we observed, which resembled “classical activation” in 5xFAD [90] but not R6/2 brains [73], where they instead were associated with an interferon signature marked by enrichment of type I (IFNɑ, IFNβ) and type II (IFNγ) signaling pathways. In a similar vein, we extended these findings to Csf1r+/− mice, a neurodegenerative model of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) [172, 173], where early disease stage microglia that displayed homeostatic marker loss, but not proinflammatory gene upregulation, induced decreases in PNNs and presynaptic puncta (Arreola et al. [174]). In the same study, we confirmed these changes with inducible microglia-specific Csf1r haploinsufficiency (Cx3cr1+/CreERT2: Csf1r+/fl mice) and their normalization following CSF1R inhibitor-based depletion, validating the microglial origin of ALSP PNN and synaptic deficits. In addition, a recent study reported that microglial depletion with the specific CSF1R inhibitor PLX5622 prevents PNN loss induced by ketamine or 60 Hz light entrainment [175]. Therefore, it appears that microglia drive PNN loss in disease, as has been suggested based on temporal analysis of microglial activation and their accumulation of net material in prion disease [166, 176, 177] and following infection with human or simian immunodeficiency virus (HIV or SIV), which preferentially infect microglia and cause PNN degradation [168, 178, 179].
PNN deficits/decreases have also been observed across more diverse diseases, many if not all of which are also generally associated with microglial activation, including multiple sclerosis [169], stroke [180,181,182,183,184], traumatic brain injury [185, 186], spinal cord injury [187], epilepsy [188, 189], obesogenic high fat and high sugar diet consumption [190], glioma [160], Alzheimer’s disease [90, 191,192,193], and schizophrenia [167, 194,195,196]. The loss of protective PNNs leaves PV+ and other enwrapped neurons susceptible to injury [92, 156, 157], and accordingly, we found that PNN reductions preceded decreases in PV+ neurons in AD [90], where PV+ cells are particularly relevant to disease [197,198,199,200]. Indeed, PNN loss is associated with neuronal death and/or degeneration in a number of disease contexts [160, 169, 176, 180, 190, 193, 201]. It should be noted that several studies failed to detect differences in PNN abundance in clinical AD [202,203,204,205], and this may be partly attributable to the brain region under investigation or the method of PNN labeling used. While changes with non-diseased aging are less clear [90, 206,207,208], we and others have found age-related reductions in PNN density in older animals [90, 193, 208, 209], and discrepancies across studies may depend on the inclusion of both sexes and the brain region(s), mouse strain, and/or model organism being studied.
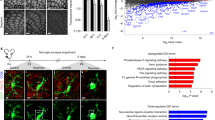
Even more striking, however, is the observation that PNN abundance is dramatically upregulated throughout the healthy adult brain following microglial depletion (Fig. 1) [72, 73, 90]. While neurons and glia may both express components that contribute to PNNs [94], neurons can express the core components of nets themselves and are capable of forming PNNs in vitro in the absence of glia [210]. For instance, neurons produce aggrecan [211], the inducible neuron-specific removal of which results in PNN ablation [147], as well as hyaluronan [212], which is continuously secreted and serves as a backbone by tethering associated CSPGs into PNNs with the aid of link proteins [213,214,215]. Therefore, our findings suggest that microglia basally regulate PNN density in the homeostatic brain, whether via direct or indirect enzymatic degradation and/or phagocytosis, such that their absence allows PNN components to accumulate. PNN enhancements induced by microglial depletion are also associated with increased excitatory and inhibitory synaptic connections to excitatory cortical neurons, as well as augmented neural activity in both cortical excitatory neurons and PV+ interneurons as assessed by in vivo calcium imaging [72]. Importantly, no overt changes in astrocytes were found in this study, as reported previously following pharmacological depletion in wild-type (WT) mice with either CSF1R inhibitor utilized [10, 71, 73]. Synaptic connectivity and neural activity are both normalized following microglial repopulation [72], which is consistent with the normalization of PNN densities we observed under similar conditions of inhibitor cessation following microglial elimination [216]. Loss of PNNs with disease thus likely reflects a toxic gain-of-function in microglia of this newly identified homeostatic role, whereby augmented or complementary PNN-degradative processes are activated, either via enhanced or alternative secretion of ECM-cleaving proteases or their modulators, and/or increased phagocytosis. Nonetheless, it remains possible that the effects reported here are mediated at least in part through associated downstream non-microglial pathways—such as astrocytes (e.g., via altered expression of gap junction channel subunit connexin 30, which facilitates PNN formation via inhibition of MMP9 expression [217]) or oligodendrocyte lineage cells [218], the population dynamics of which are altered by microglial depletion [55, 74]—and future studies should aim to resolve the distinct roles of glial and neuronal cells that may also be involved in PNN remodeling.
Microglial depletion enhances perineuronal net abundance in the healthy adult brain. Immunohistochemically stained brain sections from wild-type male mice aged 3 months that were treated with vehicle (Control) or the CSF1R inhibitor PLX5622 at 1200 ppm for 10 days (Microglia-depleted). Brain sections were stained with antibodies against aggrecan (ACAN; AB1031, Millipore) and with the canonical PNN marker Wisteria floribunda agglutinin (WFA; B-1355, Vector Labs). Effects are displayed as A whole-brain stitched images or B 20× confocal images of the somatosensory cortex from the same brain sections (white boxes in (A)) together with IBA1 to show microglial depletion
Perisynaptic matrix
The vast majority (98%) of CSPGs within the CNS are found in the general diffuse ECM, including the perisynaptic matrix, as opposed to the highly specialized manifestations of the brain ECM that are PNNs [94]. Many key molecules coexist in both the perisynaptic matrix and in PNNs, so it is inherently difficult to tease apart the effects of their manipulations as resulting from changes to one or another ECM structure, particularly in the case of global genetic ECM knockout models. For instance, while aggrecan is a requisite component of the PNN backbone [147, 202, 219], the shorter CSPG brevican may be considered a reciprocally critical molecule to the perisynaptic ECM [165, 202, 215] and accumulates in synaptic fractions following biochemical fractionation of brain tissue [220, 221]. However, these CSPGs can be found across both structures, so additional research will likely be required to determine where and how they exert their effects on neuronal and synaptic physiology, and the extent to which interactions exist between ECM compartments. Interestingly, neuroglycan C (a.k.a. CSPG-5) appears to localize to perisynaptic regions of glutamatergic and GABAergic terminals and is often observed at the edges of PNNs [222], and its loss results in presynaptic functional deficits and premature elimination of synapses during development [223].
Several approaches exist to study the perisynaptic vs. perineuronal matrix. Mice deficient in link proteins HAPLN1 and HAPLN4, which serve to stabilize interactions between hyaluronan and CSPGs in PNNs [87], have overall unchanged CSPG levels but fail to incorporate these molecules into PNNs [94, 213, 224], and therefore may provide a means of studying the effects of disrupting these structures without affecting the perisynaptic matrix and diffuse ECM at large. Microinjections of ChABC near dendrites have also been successfully used to locally degrade perisynaptic CSPGs while leaving PNNs intact [225]. Alternatively, studies elucidating the function of the perisynaptic ECM could focus on regions that naturally lack PNNs [226], and vice versa in regions densely enriched with PNNs [227], although the effects of the perisynaptic matrix would not be entirely absent—just relatively minimized—in the latter case.
Research by several groups in the past decade has begun to shed light on the comparative composition of perisynaptic and PNN matrices in the CNS, as well as the organizational frameworks that distinguish them [161, 205, 215, 226,227,228,229,230]. The discovery of axonal coats (ACs) serves as one such example of a well-characterized perisynaptic matrix structure that exists as a separate entity from classical PNNs [230]. These round structures of aggrecan- and brevican-based ECM enwrap individual synaptic boutons contacting neuronal dendrites and somata and sometimes comingle with PNN components on associated neurons [215, 226, 230], with hypothesized roles at the synapse in restricting neurotransmitter spillover and receptor localization [87, 215, 227]. Although there is some degree of overlap, perisynaptic ECM can be found around neuronal subsets lacking PNNs [226, 230], as is the case for dopaminergic neurons and glutamatergic principal neurons in the substantia nigra and thalamus, respectively, on which presynaptic ACs make contact [230].
Additionally, activation of dopamine receptors and subsequent neuronal activity was shown in an elegant study to induce proteolysis of perisynaptic brevican and aggrecan in the ECM around excitatory synapses which, at least for brevican, was mediated by ADAMTS-4/5 [229]. It has also been shown that targeted perisynaptic matrix degradation induces structural plasticity of dendritic spines (e.g., enhanced spine motility and formation of spine head protrusions) [225] and similar structural changes are associated with increased functional plasticity as measured by LTP [231], and as such, CSPGs appear to restrict plasticity in either case. Thus, changes in upstream perisynaptic ECM could lead to downstream signaling-dependent changes in synaptic plasticity and further alterations in associated ECM in an increasingly complex, circuit-level process. Interestingly, we found elevated interstitial CSPG deposition in the brain parenchyma of both AD [90] and HD [73] mice, which may at least partially account for some of the beneficial effects of ChABC injections in related disease models that may have acted on the perisynaptic ECM rather than (or in addition to) PNNs [232,233,234].
Suggesting a direct role for microglia in the regulation of perisynaptic matrix-controlled synaptic plasticity, a recent study by Nguyen et al. determined that, in response to neuronal IL-33, microglia in the adult brain phagocytose and clear perisynaptic ECM components to promote dendritic spine formation, synaptic plasticity, and fear memory precision [91]. Importantly, they found that inhibition of this pathway decreased microglial engulfment of aggrecan and consequently enhanced aggrecan puncta density and deposition at the synapse, in addition to increasing total intact brevican while reducing levels of proteolyzed brevican. Thus, as in our work, loss of microglial function results in enhanced ECM deposition in the homeostatic brain. The occurrence of this phenomenon across multiple ECM compartments (i.e., the perisynaptic matrix [91] and perineuronal nets [73, 90]) together suggests a fundamental homeostatic role for microglia in ECM degradation and remodeling, which may be required for subsequent remodeling of synapses surrounded and stabilized by such ECM. These findings as they relate to PNNs are illustrated as a working model in Fig. 2. It should be noted that microglia may also act as a source of CSPGs and other ECM molecules in the interstitial matrix under certain conditions [139, 235], but this appears to represent a less prominent role compared to the negative regulatory influence they exert across ECM compartments.
Microglia regulate perineuronal net and synaptic integrity in health and disease. In this working model, microglia continuously maintain baseline PNN and perisynaptic extracellular matrix integrity in the healthy adult brain through the sustained release of proteases (or protease inhibitors/activators) and/or phagocytosis (not pictured). The absence of local microglia through experimental depletion enhances PNN deposition and density, in addition to synaptic number. In disease or injury, microglial activation or dyshomeostasis leads to upregulation of phagocytosis and/or protease secretion, resulting in PNN breakdown and excessive synaptic elimination, the latter of which may occur through related and/or unrelated cellular pathways
Potential mechanisms of microglial ECM regulation
Although microglia are linked to ECM remodeling in disease (i.e., PNN loss [73, 90, 166,167,168,169, 180, 181, 190]) and now also in the healthy homeostatic brain [72, 73, 90, 91], the molecular mechanism(s) by which this occurs are unclear. As observed with synapses during developmental pruning [37, 236], microglia may directly engulf and phagocytose ECM components. Indeed, aggrecan colocalizes with lysosomal CD68 in microglia, a marker of phagocytosis, and disrupting IL-33-based ECM engulfment by microglia reduces CD68+ lysosome number [91]. Furthermore, proteoglycans and PNN material can accumulate within disease-associated microglia/macrophages [90, 237] and in microglia following ketamine treatment [175], and phagocytic genes (e.g., Itgax, Clec7a, and Trem2) are upregulated in 5xFAD mice [67, 71], particularly in plaque-associated microglia [62], where we observed widespread PNN loss [90]. However, it is likely that microglial release of degradative enzymes is also involved in ECM turnover processes, especially as it applies to the remodeling of PNNs, in which CSPGs, tenascins, hyaluronan, and link proteins are more tightly woven together compared to the diffuse matrix [238].
Several proteases are immediately apparent candidates based on the capability of microglia to produce them and their ability, in turn, to degrade ECM components and core PNN molecules. These proteases primarily include MMPs, ADAMTS, and cathepsins. Microglia may also shape PNNs indirectly via modulators of protease activity, as in tissue inhibitors of metalloproteinases (TIMPs) [239,240,241], or by regulating protease or TIMP expression by other cells. For instance, it has been suggested [169] that glutamate released by activated microglia [242] could bind neuronal glutamate receptors and induce neuronal MMP expression [243]. While these are important and plausible mechanisms, for the purpose of conciseness, this review will focus on the direct action of microglia-sourced protease candidates.
MMPs are expressed at low to undetectable levels under homeostatic conditions in the adult brain and are upregulated in injury and disease; taken together, they have the capacity to degrade the entire gamut of ECM constituents [239]. Although not exclusively, MMP-2 and MMP-9 are secreted by microglia [244, 245] and act on a wide range of overlapping substrates, such as link proteins and aggrecan [239]. Substrate specificity is also evident in certain cases, as in the digestion of tenascin-C [246] and brevican [247] by MMP-2 but not MMP-9. MMP-2 and/or MMP-9 are upregulated by microglia in disorders where PNN breakdown occurs, such as stroke [248, 249], multiple sclerosis [250, 251], and glioma [252, 253], and pharmacological MMP blockade in glioma ameliorates enhanced MMP-2/9 activity and associated PNN loss [160]. While baseline PNNs are largely unchanged in MMP-9−/− mice, developmental monocular deprivation-induced PNN degradation is prevented, and ocular dominance (OD) plasticity is attenuated [254], findings mirrored in adult mice in the context of light reintroduction-induced plasticity following dark exposure [255]. Additionally, genetic reduction (e.g., haploinsufficiency) of MMP-9 [256] as well as MMP-2/9 inhibitor treatment [257] restores developmental PNN impairments in Fmr1 knockout mice, a model of Fragile X Syndrome (FXS); interestingly, MMP-2/9 inhibitor treatment also enhances WT PNN formation in the developing auditory cortex [257]. Of course, other MMPs may also play a role in brain ECM remodeling, as suggested by microglial Mmp14 upregulation following treatment with IL-33, which endogenously promotes ECM clearance and dendritic spine formation [91]. Corroborating this, we also independently identified Mmp14 upregulation in Csf1r+/− mice, a model of leukoencephalopathy and microglial dyshomeostasis [172, 173], and confirmed its capacity to degrade PNNs via in vivo injection of recombinant MMP-14 (K.N.G., unpublished data).
In addition to MMPs, microglia can also express ADAMTS-4 [91, 258, 259], which cleaves aggrecan [260] and brevican [247] at sites distinct from MMPs and, unlike MMPs, degrades CSPGs without affecting laminin [261]. Furthermore, the effects of MMP-2 and ADAMTS-4 are additive in degrading brevican [247], which may offer one plausible explanation for the ability of exogenous ADAMTS-4 to degrade PNNs in amyotrophic lateral sclerosis model SOD1G93A mice in which PNN breakdown had already occurred, but not WT mice [262, 263]. Microglial cathepsins also represent prime candidates in brain ECM turnover. Canonically localized to and functioning within the endolysosomal pathway to degrade proteins in bulk, several secreted cathepsins exist, including cathepsins S (CTSS) and B (CTSB) [244, 264]. CTSB is secreted by microglia following LPS activation [265], as is CTSS, which is also upregulated by brain lesion injury [266] and in bulk tissue of 5xFAD mice where we have reported PNN deficits (hippocampus, cortex) [71, 90]; it should be noted in the latter case that we did not observe significant upregulation of any Mmp genes in any regions examined [71]. We found that Ctss expression in the brain most closely follows the kinetics of microglial elimination and repopulation [74], which increases [72, 73, 90] and normalizes [216] PNN density, respectively, and indeed, its transcripts were consistently absent in microglia-depleted brains in our studies [71, 73, 74, 216]. CTSS is functionally stable at the neutral pH of the extracellular space, and under such conditions, it can efficiently degrade CSPGs neurocan and phosphacan, where CTSB at several-fold greater concentrations could not [266]. Further supporting the plausibility of CTSS-based ECM remodeling in particular, CTSS−/− mice display ameliorated tenascin-R reduction following facial nerve axotomy [267], which induces CTSS (but not CTSB) upregulation at the protein and mRNA levels, and incubation of mouse brain sections with CTSS eliminates WFA+ PNNs [268].
On the other hand, the upstream signals that trigger the clearance of ECM by microglia, whether by protease secretion and/or phagocytosis, and whether this differs across ECM compartments, together remain largely unknown. Neuronal IL-33 guides the engulfment of perisynaptic ECM by microglia in the homeostatic adult hippocampus [91], but it is unclear whether this pathway also guides the microglial regulation of PNNs. Experimental designs focused on the latter may benefit from targeting the expression of IL-33 or other candidate signaling molecules in PV+ interneurons (e.g., through a Cre-lox system), given the close association between this neuronal subtype and PNNs. As microglia appear to basally regulate PNN abundance, with their depletion enhancing PNN densities in the healthy brain [72, 73, 90], the signal(s) regulating this process should theoretically be homeostatically secreted. The CX3CL1-CX3CR1 axis is already well established as a major pathway of neuron-microglia communication that is involved in synaptic pruning and development [38, 269, 270] as well as microglial mobility, motility, and activation [270,271,272] and therefore seems to be a feasible candidate in the regulation of this process. However, PV+ neuron-associated PNN densities remain unchanged in Cx3cr1−/− mice [273], and thus, this pathway appears to be uninvolved in PNN remodeling by microglia.
The homeostatic microglial receptor P2RY12 may instead serve to regulate microglial-PNN interactions, as blocking P2RY12 with the specific antagonist clopidogrel prevents ketamine-induced loss of PNNs in adult mice [175] and inhibits developmental ocular dominance plasticity [274], which is thought to be typically restricted by the formation of PNNs [146]; therefore, future studies may benefit from evaluating the functionality of P2RY12 in this regard. CSF-1/IL-34 could also represent putative regulatory molecules, as they are constantly produced in the brain at baseline and control microglial survival and cell densities by signaling through CSF1R [275]. We found that Csf1r haploinsufficiency (Csf1r+/−), as well as low-grade, brain-penetrant pharmacological CSF1R inhibition (150 ppm PLX5622), induced PNN deficits, which were rescued following microglial depletion with high doses of CSF1R inhibitors (Arreola et al., in press). Furthermore, alternative or complementary signaling pathways may be involved in the regulation of PNNs by microglia under conditions of dyshomeostasis and disease (e.g., via the detection of DAMPs by microglial TLRs [252]) which may, in turn, vary based on disease etiology and pathogenesis. Toxic gain- or loss-of-function in homeostatic signaling pathways regulating microglia-PNN interactions in the healthy brain may also occur in disease. Ultimately, the role of microglia as sculptors of PNNs and the ECM in general—particularly in the healthy brain—is just beginning to be elucidated, and as such, the signals promoting this process, as well as the downstream mechanisms mediating such sculpting, require further study.
The proteases proposed in this section are implicated in remodeling not only the ECM, but synapses as well [264, 276, 277]. This may underscore the functional relationship between the two structures—to sculpt synapses, an increasingly salient role of microglia, the matrix in which they are embedded would presumably also have to be restructured. Ongoing research continues to elucidate the bidirectional interactions between the ECM and synapses, but the involvement of microglia in this process has just begun to be examined [91]. Therefore, we will next discuss established findings on synaptic regulation by microglia in the context of specific and relevant ECM studies to shed light on putative mechanisms that may underlie the relationship between these components of the tetrapartite synapse.
Microglia at the synapse
Synaptic pruning and formation in development
Thorough monitoring of the CNS parenchyma by microglia [13] aptly positions these cells to respond rapidly to changes in the synaptic microenvironment. In the healthy brain, they interact with pre- and postsynaptic compartments, perisynaptic astrocytes, and the local extracellular milieu [88, 89, 278, 279]. This has thus far been best studied during development when microglia prune excess synapses [37, 38] to promote the removal of extranumerous or weak synapses in the refinement of neuronal networks [21, 35]. Accumulating evidence has implicated traditionally immune-associated molecules as critical elements in synaptic refinement. For example, complement cascade elements (e.g., C1q and C3) localize to synaptic compartments to tag synapses for elimination [39, 280, 281], inducing phagocytosis by complement receptor 3 (CR3)-expressing microglia in a neural activity-dependent manner [37]. On the other hand, genetic loss of CX3CR1, a receptor primarily expressed by microglia in the brain, is also associated with synaptic pruning deficits, resulting in an excess of dendritic spines, immature synapses, and immature brain circuitry in development [38, 269, 270] that persists as impaired synaptic transmission and functional brain connectivity in adults [64].
Microglia can also induce synapse formation, as shown by the addition of developing microglia to cultured hippocampal neurons in vitro, which increases dendritic spines and excitatory and inhibitory synapses via microglial IL-10 [282]. While this process did not require direct microglial contact, a recent study utilizing in vivo two-photon imaging of early postnatal (P8-P10) mouse brains observed microglial contact-induced filopodia formation on dendrites, which was reduced following minocycline treatment [16]. Decreased dendritic spine densities were observed in the same study following microglial depletion [16], which resembled the reduced spine formation reported by another group under similar circumstances [283]. However, caution must be taken regarding the interpretation of this result, as both studies utilized diphtheria toxin-based models of microglial ablation, which are associated with inflammation (e.g., upregulation of TNF-ɑ, IL-1β [8] or an interferon response [284]) that is not seen with genetic- or inhibitor-based models due to the manner in which microglial death is achieved [11]. Accordingly, IL-1β attenuates synaptic formation induced by IL-10 [282], and postnatal CSF1R inhibitor-based microglial depletion instead results in excess synapses [285] that are normalized following microglial repopulation [286]. Interestingly, loss of CSPG-5 (neuroglycan C), which normally localizes to the perisynaptic space [222], results in impaired presynaptic maturation as well as synaptic elimination that occurs earlier than normal in cerebellar Purkinje cells [223], which microglia survey and regulate [287,288,289,290]. As early developmental synaptic deficits are observed in other brain regions with CSPG-5 deficiency [291], together, this suggests a role for perisynaptic matrix remodeling during synaptic pruning and maturation.
It is perhaps no coincidence that PNNs begin forming in development soon after synaptic pruning is completed by microglia [38, 39], ~P14 in mouse cortex (finished by P40) and earlier in subcortical regions [213, 292], which would place them in ideal positions to guide PNN formation around newly refined synapses, e.g., through phagocytosis and/or controlled enzymatic degradation. MMP-2/9 inhibitor treatment enhances basal PNN density in postnatal mice, indicating that protease activity is indeed a limiting factor in their developmental construction [257]. Of note, MMP-2 and MMP-9 expression peaks in early postnatal development, where they codistribute with foci of proteolytic activity in neuropil, and with markers of synapses (PSD-95, synaptophysin) and growing axons, suggesting that these proteases actively shape the perisynaptic space associated with synapse formation [293]. Furthermore, the formation of adult levels of PNNs around visual cortical neurons by the end of the critical period restricts OD plasticity [146], such that their ablation in adults restores OD plasticity [146, 147, 149, 213], and the loss of microglial P2RY12 [274] (but not CX3CR1 [294]) or microglia themselves with CSF1R inhibition [295] prevents normal OD plasticity altogether.
Therefore, we postulate that the absence of OD plasticity following such microglial loss-of-function may be due to consequent failure to sculpt PNNs, which may form prematurely in these instances. Interestingly, synaptic elimination in the barrel cortex following developmental whisker trimming—which also specifically reduces barrel cortex PNNs [296]—requires CX3CR1 [297], while neither CX3CR1 [294] nor C1q [298] appear necessary for monocular deprivation-induced OD plasticity or related visual cortex synaptic remodeling. Thus, microglial mechanisms of synaptic sculpting are context-dependent, and this may also be the case for the regulation of nearby ECM. Future studies should investigate to what extent microglia and the microglial proteome are involved in regulating PNN formation during critical period closure and how this may relate to synaptic pruning. This could be explored via developmental or critical period CSF1R inhibition to determine whether PNNs appear earlier, and further delineated with a more specific approach (e.g., protease inhibitors) to determine exactly how microglia influence this process.
The pursuit of clarification regarding mechanisms that dictate which synapses are eliminated or spared has uncovered a delicate balance between ‘eat me’ and ‘do not eat me’ signals at the neuronal level. Phosphatidylserine (PS) localized to synaptic elements is one such molecule by which the CNS can modulate synaptic elimination [299, 300]. While initially recognized as a glycerophospholipid that is externalized on the cell membrane during the process of apoptosis to act as an ‘eat-me’ signal for phagocytes [301], synaptic elimination is partially abrogated in vitro by blocking PS via the addition of Annexin V or by culturing with microglia deficient for the phagocytic receptor TREM2 [300]. In vivo, synaptic PS exposure in the hippocampus and retinogeniculate areas parallels the temporal dynamics of microglial-mediated pruning, and C1q-deficient mice displayed increases in presynaptic PS exposure and reductions in PS phagocytosis by microglia, thereby implicating the complement system in PS-mediated synaptic pruning [300]. Aside from complement-related mechanisms, microglial loss of GPR56 decreased engulfment of PS+ synaptic inputs and consequently increased synapse number in the hippocampus and dorsal lateral geniculate nucleus [299], suggesting that multiple signaling pathways involved in the regulation of microglial pruning appear to converge on synaptic PS expression.
On the other hand, a recent study identified that CD47 signaling to the SIRPα receptor serves as a “do not eat me” signal that prevents excessive synaptic pruning in the retinogeniculate system during early development [236]. CD47 is enriched, and microglial expression of SIRPɑ is similarly increased during peak pruning, with CD47 localizing to more active synapses, and disruptions to either via knockout of CD47 or SIRPα increased microglial engulfment and reduced synapse number [236]. Studies such as these provide insight into the relationship between microglia and synaptic structures and, importantly, describe how the developing nervous system can exert spatiotemporal control over synapse elimination. Along these lines, a subpopulation of GABA-receptive microglia has recently been identified that specifically prunes inhibitory synapses in development [302]. Importantly, ablating the microglial GABAB receptor subunit GABAB1R to disrupt GABAB signaling in microglia, which mediates this effect, did not alter PNN densities [302]. Additionally, recent proteomic studies have identified a number of putative MMP-9 substrates, including nuclear, cytoplasmic, and extracellular proteins not solely involved in the ECM but that may have implications in synaptic plasticity, including Annexin V [303]. Intriguingly, it appears that MMP-based proteolytic cleavage of SIRPα in response to neuronal activity releases an extracellular SIRPα domain, which binds to presynaptic CD47 and promotes the maturation of presynaptic terminals [304]. ECM molecules may also interact with synaptic pruning pathway elements, as CSPGs bind and potentially inhibit C1q functional domains [305, 306], whereas loss of CD47 in glioblastoma cells enhances the expression of tenascin-C and consequent phagocytosis by tumor-associated macrophages [307].
While the prevailing theory is that microglia phagocytose whole synapses, recent advances in microscopy are facilitating greater direct imaging of microglial interactions with synapses at resolutions that may reveal more nuanced roles in synaptic remodeling. Such techniques have allowed researchers to observe microglia contacting presynaptic elements in hippocampal explants and subsequently phagocytose only fragments of the synapse in a process termed presynaptic trogocytosis [308], a phenomenon recently confirmed in vivo in Xenopus laevis tadpoles [309]. However, while Weinhard et al. found that complement signaling (specifically CR3-mediated) was not required for trogocytosis [308] as it is in developmental retinogeniculate pruning [37], complement signaling did regulate trogocytosis of retinal ganglion cell axons in Xenopus laevis [309]. In the latter case, neuronal overexpression of the synapse-associated amphibian regulator of complement activation 3 (aRCA3) inhibited trogocytosis and axonal pruning, whereas expression of axon membrane-bound complement C3 fusion protein enhanced axonal pruning [309]. Placing this in the context of the ECM, several studies suggest that perisynaptic axonal coats are synthesized by presynaptic neurons [205, 215, 226, 230] and thus may have particular relevance in the microglial trogocytosis of presynaptic components [308] in that this process might necessarily involve concurrent remodeling of the presynaptically generated ECM that supports synapses in these instances as well as a mechanism in place to allow such preferential targeting by microglia.
Synaptic elimination in the healthy adult brain and its dysregulation in disease
Although largely studied in the context of development thus far, growing research indicates that microglia maintain their roles as synaptic sculptors of the adult homeostatic brain, a function that may go awry in disease. Supporting this, we have demonstrated that elimination of microglia in healthy adult mice with CSF1R inhibitors increases the total density of hippocampal and visual cortex dendritic spines and PSD95 and synaptophysin immunolabeling in the hippocampus [66, 76]. Similarly, microglial elimination increases excitatory and inhibitory connections to visual cortex excitatory neurons and the neural activity of excitatory neurons and PV+ interneurons [72], in addition to enhancing PNN density [72, 73, 90], confirming that microglia serve as regulators of the synaptic and ECM landscape throughout adulthood. Indeed, as in development [37], hippocampal microglia continue to perform activity- and complement-dependent synaptic elimination to mediate normal memory turnover in the healthy adult brain, such that complement inhibition or microglial depletion prevents forgetting of contextual fear memories [40].
Neurogenic niches in the adult CNS, such as the olfactory bulb (OB) and dentate gyrus (DG) of the hippocampus, provide unique perspectives on synaptic modulation by microglia, as new neurons are continuously born, develop, and integrate into functional neuronal circuits in an already mature brain environment. Accordingly, elimination of microglia via CSF1R inhibition is reported to reduce the spine density of developing but not mature adult-born granule cells (abGCs), suggesting that microglia are necessary for the proper development of synapses in adult-born neurons [310]. This is mediated in part by CX3CR1 [310], as paralleled by a separate report on impaired synaptic integration and reduced spine density at the afferent level in adult-born granule neurons of the hippocampal DG in Cx3cr1−/− mice [273]. Another group found that microglial depletion with the same CSF1R inhibitor resulted in enhanced spine density on developing abGCs, but these spines were smaller and functionally immature; again, the effects were largely limited to young but not mature abGCs [311]. Taken together, these data suggest that the requirement of microglial CX3CR1 for the synaptic refinement of adult-born neurons in the OB [310] and DG [273] is a necessity only in their developmental stages, which could be determined with the use of inducible rather than constitutively deficient Cx3cr1−/− mice.
Interestingly, the absence of CX3CR1-mediated bidirectional communication between microglia and neurons in Cx3cr1−/− mice was also sufficient to enhance WFA+ and aggrecan deposition in the DG, where synaptic integration was impaired [273]. However, the authors found no difference in PNN density here, and the elevated proinflammatory profile of this region (e.g., TNF-ɑ, IL-6) [273] suggests that these changes could primarily be localized to the diffuse ECM via microglial activation of neurotoxic astrocytes [45] and their increased production of neurite-inhibitory CSPGs in turn [114]. In fact, activation of primary cortical microglia with polyinosinic-polycytidylic acid in vitro induced secretion of TNF-ɑ and IL-6 in the culture medium, in addition to several chemokines, and upregulated expression of Mmp2 and Mmp9, and treatment of hippocampal neurons with this microglial-conditioned medium impaired PNN structure [312]. Treatment of PNN-ensheathed neurons with this medium also led to a decrease in inhibitory vGAT presynaptic puncta but an increase in PSD-95 and gephyrin postsynaptic markers, whereas both inhibitory vGAT and excitatory vGlut presynaptic puncta were reduced while postsynaptic markers were unaffected in treated non-PNN-ensheathed neurons, underscoring the unique and complex role of PNNs in scaffolding and regulating embedded synapses [312]. In line with this complexity, others have reported that PNN disruption by genetic deletion of its components in primary hippocampal neurons in vitro transiently increases synaptic densities, only to later reduce them [164]. In vivo, the situation is likely different if not more complicated, as microglia can make direct contact with PNNs and synapses, potentially remodeling these structures via phagocytosis [40] as well as through secretion of proteolytic factors.
While synaptic elimination is known to be a normal process in brain development and homeostasis, the dysregulation of this process is recognized as an early feature of neurodegeneration [313,314,315]. Synaptic loss, as opposed to neuronal loss, serves as the most accurate indicator of cognitive decline [313, 316, 317]. Under neurodegenerative conditions, microglial-induced synapse loss may be viewed as a toxic gain-of-function with respect to normal synaptic-regulating processes [318], as in models of AD, where dysfunctional activation and upregulation of complement proteins C1q and C3 [319,320,321] or loss of microglial SIRPɑ [322] result in excessive phagocytosis of synaptic elements. Augmented complement-mediated synaptic loss also appears to occur in aging [323]. Microglia can also increase the expression of synaptotoxic factors such as TNF-ɑ in neurodegeneration, which produces synaptic deficits by inducing excitotoxicity [324, 325] or by promoting neurotoxic astrocyte reactivity [45]. Elimination of microglia or attenuation of microglial activation under neurodegenerative conditions or aging, however, leads to improved functional outcomes accompanied by restoration in spine number and synaptic surrogates [66, 67, 71, 77, 326, 327]. Thus, microglia play critical roles in the maintenance and pathological elimination of synaptic elements in disease (as reviewed previously [328]).
Few studies have explored the roles of microglia in the ECM as they relate to changes in synaptic health and number in neurodegenerative contexts. Microglial depletion prevents PNN loss in the 5xFAD hippocampus [90] and the downregulation of hippocampal synaptic genes at later time points [71], but further investigation is required to clarify whether these changes are occuring in the same neurons. Neurodevelopmental disorders may provide another avenue to investigate such processes, as in FXS, which is caused by genetic hypermethylation-induced loss of neurite-localized fragile X mental retardation protein (FMRP) [329]. Minocycline-based inhibition of MMP9 [330] or Mmp9 deficiency [331] rescues the immature dendritic spine phenotype in fragile X mouse hippocampal neurons in vitro, and pharmacological blockade [257] or genetic reduction [256] of MMP-9 restores in vivo cortical PNN density in FXS mice, as discussed earlier. Future studies should investigate how these ECM and synaptic effects may be related in neurons from the same brain region and under the same experimental conditions. Similarly, anomalous synaptic deficits are postulated to be important components of pathology associated with schizophrenia, as reduced dendritic spine densities [332,333,334] and disrupted PNNs in similar cortical regions (e.g., layer 3 of the prefrontal cortex [195]) have been reported, in addition to aberrant microglial elimination of synapses in schizophrenia patient-derived neural cultures [335] that is related in part to disease variants in complement component 4 (C4) [335, 336]. Given the increasingly reported roles microglia appear to play in ECM modulation, both at the level of PNNs and the perisynaptic matrix, it stands to reason that such synaptic, ECM, and microglial changes may be related in these situations as well. Overall, studies into neurodegenerative and neurodevelopmental disorders are increasingly alluding to complex interactions between glia and other elements of the tetrapartite synapse that may be determined to underlie major aspects of disease pathophysiology.
Concluding remarks
Altogether, the data thus far suggest that microglia serve a regulatory role in the modification of ECM and synaptic components; the goal now is to elucidate the dynamics of this relationship. Specifically, future studies should investigate how microglia mediate such ECM modifications (whether through protease secretion, phagocytosis, or a combination of both), how this process is resolved at the perisynaptic vs. PNN level, and how such alterations interface with synaptic function in CNS development, health, and disease. In terms of proteases, we propose MMPs, ADAMTS, and/or cathepsins as the most feasible mechanistic candidates given the current data, whether these molecules are directly expressed by microglia or are instead influenced by the secretion of other microglial factors in a more indirect manner.
At a more general level, it will be interesting to determine the extent to which PNN deficits are a common hallmark of neurodegenerative diseases, and furthermore how this relates to differential microglial phenotypes. It is also possible that some of the deleterious effects of diseased microglia on PNN integrity are mediated, if even only in part, by other glia (e.g., astrocytes [217] or oligodendrocytes [218]), which are often dysregulated concurrent with microglial dysfunction. However, the minimal changes in astrocytes evoked by microglial depletion in the homeostatic brain—particularly in comparison to the consequent dramatic and relatively ubiquitous upregulation of PNNs—together with the collective findings reviewed here suggest a central role for microglia in ECM and synaptic regulation. Thus, a novel role for microglia emerges in the basal regulation of PNNs and ECM in the healthy adult brain, and as with other microglial functions, this may serve as a valuable therapeutic target if, or when, it is pathogenically altered in disease.
References
Kierdorf K, Prinz M. Microglia in steady state. J Clin Investig. 2017;127:3201–9.
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225–42.
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311.
Salter MichaelW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24.
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271.e6.
Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron. 2019;101:207–23.e10.
Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–92.
Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43:92–106.
Han J, Harris RA, Zhang XM. An updated assessment of microglia depletion: current concepts and future directions. Mol Brain. 2017;10:25.
Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–97.
Green KN, Crapser JD, Hohsfield LA. To kill a microglia: a case for CSF1R inhibitors. Trends Immunol. 2020;41:771–84.
Han J, Zhu K, Zhang XM, Harris RA. Enforced microglial depletion and repopulation as a promising strategy for the treatment of neurological disorders. Glia. 2019;67:217–31.
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8.
Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci. 2019;22:1771–81.
Stowell RD, Sipe GO, Dawes RP, Batchelor HN, Lordy KA, Whitelaw BS, et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci. 2019;22:1782–92.
Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540.
Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27.
Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28.
Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–75.
Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974.
Schafer DP, Stevens B. Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol. 2015;7:a020545.
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2017;18:225–42.
Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6.
Tay TL, Sagar, Dautzenberg J, Grün D, Prinz M. Unique microglia recovery population revealed by single-cell RNAseq following neurodegeneration. Acta Neuropathol. Commun.2018;6:87
Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–26.
Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron. 2018;98:1170–1183.e8.
Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–49.
Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E, et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med. 2018;215:1627–47.
Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat Commun. 2018;9:4845.
Hohsfield LA, Najafi AR, Ghorbanian Y, Soni N, Hingco EE, Kim SJ, et al. Effects of long-term and brain-wide colonization of peripheral bone marrow-derived myeloid cells in the CNS. J Neuroinflammation. 2020;17:279.
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–51.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5.
Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–78.
Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–80.
Frost JL, Schafer DP. Microglia: architects of the developing nervous system. Trends Cell Biol. 2016;26:587–97.
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–95.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78.
Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020;367:688–94.
Han RT, Kim RD, Molofsky AV, Liddelow SA. Astrocyte-immune cell interactions in physiology and pathology. Immunity. 2021;54:211–24.
Liddelow SA, Marsh SE, Stevens B. Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol. 2020;41:820–35.
Vainchtein ID, Molofsky AV. Astrocytes and microglia: in sickness and in health. Trends Neurosci. 2020;43:144–54.
Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–67.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7.
Guttenplan KA, Weigel MK, Adler DI, Couthouis J, Liddelow SA, Gitler AD, et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat Commun. 2020;11:3753.
Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–8.
Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Münch AE, Weigel MK, et al. Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep. 2020;31:107776.
Sterling JK, Adetunji MO, Guttha S, Bargoud AR, Uyhazi KE, Ross AG, et al. GLP-1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. 2020;33:108271.
Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA. 2018;115:E1896–E1905.
McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45.
Benoit ME, Clarke EV, Morgado P, Fraser DA, Tenner AJ. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J Immunol. 2012;188:5682–93.
Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, et al. Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2021;20:102763.
Miron VE. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J Leukoc Biol. 2017;101:1103–8.
Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017;134:441–58.
Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34:2231–43.
Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 2017;36:3292–308.
Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19:995–8.
Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, et al. White matter aging drives microglial diversity. Neuron. 2021;109:1100–1117.e10.
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–8.
Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell. 2019;176:43–55. e13
Yin Z, Raj D, Saiepour N, Van Dam D, Brouwer N, Holtman IR, et al. Immune hyperreactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiol Aging. 2017;55:115–22.
Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry. 2017;22:1576–84.
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–6.
Xu Z-X, Kim GH, Tan JW, Riso AE, Sun Y, Xu EY, et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat Commun. 2020;11:1797.
Rice RA, Spangenberg EE, Yamate-Morgan H, Lee RJ, Arora RP, Hernandez MX, et al. Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J Neurosci. 2015;35:9977–89.
Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain. 2016;139:1265–81.
Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6:e26317.
Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–20.
Rojo R, Raper A, Ozdemir DD, Lefevre L, Grabert K, Wollscheid-Lengeling E, et al. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat Commun. 2019;10:3215.
Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat Commun. 2019;10:3758.
Liu Y-J, Spangenberg EE, Tang B, Holmes TC, Green KN, Xu X. Microglia elimination increases neural circuit connectivity and activity in adult mouse cortex. J Neurosci. 2021;41:1274–87.
Crapser JD, Ochaba J, Soni N, Reidling JC, Thompson LM, Green KN. Microglial depletion prevents extracellular matrix changes and striatal volume reduction in a model of Huntington’s disease. Brain. 2020;143:266–88.
Najafi AR, Crapser J, Jiang S, Ng W, Mortazavi A, West BL, et al. A limited capacity for microglial repopulation in the adult brain. Glia. 2018;66:2385–96.
Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, et al. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020;40:2960–74.
Rice RA, Pham J, Lee RJ, Najafi AR, West BL, Green KN. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia. 2017;65:931–44.
Elmore MRP, Hohsfield LA, Kramár EA, Soreq L, Lee RJ, Pham ST, et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 2018;17:e12832.
Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncol. 2015;18:557–64.
Shi Y, Manis M, Long J, Wang K, Sullivan PM, Remolina Serrano J, et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med. 2019;216:2546–61.
Lei F, Cui N, Zhou C, Chodosh J, Vavvas DG, Paschalis EI. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc Natl Acad Sci USA. 2020;117:23336–8.
Szalay G, Martinecz B, Lénárt N, Környei Z, Orsolits B, Judák L, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499.
Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–38.
Wheeler DL, Sariol A, Meyerholz DK, Perlman S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Investig. 2018;128:931–43.
Hilla AM, Diekmann H, Fischer D. Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci. 2017;37:6113–24.
Bellver-Landete, V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle ME, et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun. 2019;10:518.
Ohno H, Kubo K, Murooka H, Kobayashi Y, Nishitoba T, Shibuya M, et al. A c-fms tyrosine kinase inhibitor, Ki20227, suppresses osteoclast differentiation and osteolytic bone destruction in a bone metastasis model. Mol Cancer Ther. 2006;5:2634–43.
Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33:503–12.
Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36.
Tremblay, M-È, Lowery R L, Majewska A K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527.
Crapser JD, Spangenberg EE, Barahona RA, Arreola MA, Hohsfield LA, Green KN. Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine. 2020;58:102919.
Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182:388–403. e15
Reichelt AC, Hare DJ, Bussey TJ, Saksida LM. Perineuronal nets: plasticity, protection, and therapeutic potential. Trends Neurosci. 2019;42:458–70.
Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–68.
Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–65.
Bikbaev A, Frischknecht R, Heine M. Brain extracellular matrix retains connectivity in neuronal networks. Sci Rep. 2015;5:14527.
Nicholson C, Syková E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–15.
Hrabetová S, Masri D, Tao L, Xiao F, Nicholson C. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol. 2009;587:4029–49.
Morawski M, Reinert T, Meyer-Klaucke W, Wagner FE, Tröger W, Reinert A, et al. Ion exchanger in the brain: quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci Rep. 2015;5:16471.
Bekku Y, Vargová L, Goto Y, Vorísek I, Dmytrenko L, Narasaki M, et al. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci. 2010;30:3113–23.
Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904.
Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–62.
Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–75.
Ohtake Y, Wong D, Abdul-Muneer PM, Selzer ME, Li S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci Rep. 2016;6:37152.
Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci. 2005;21:378–90.
Pearson CS, Mencio CP, Barber AC, Martin KR, Geller HM. Identification of a critical sulfation in chondroitin that inhibits axonal regeneration. Elife. 2018;7:7.
Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–66.
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, et al. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–6.
Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–30.
Keough MB, Rogers JA, Zhang P, Jensen SK, Stephenson EL, Chen T, et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun. 2016;7:11312.
Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML. et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp Neurol. 2013;247:113–21.
Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722–9.
Galindo LT, Mundim M, Pinto AS, Chiarantin G, Almeida M, Lamers ML, et al. Chondroitin sulfate impairs neural stem cell migration through ROCK activation. Mol Neurobiol. 2018;55:3185–95.
Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–3.
Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56.
Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–27.
Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–22.
Schäfer MKE, Tegeder I. NG2/CSPG4 and progranulin in the posttraumatic glial scar. Matrix Biol. 2018;68-69:571–88.
Nakanishi K, Aono S, Hirano K, Kuroda Y, Ida M, Tokita Y, et al. Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. J Biol Chem. 2006;281:24970–8.
Liddelow SA, Barres BA. Not everything is scary about a glial scar. Nature. 2016;532:182–3.
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200.
Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–43.
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308.
Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55.
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410.
Silvestri L, Baker JR, Rodén L, Stroud RM. The C1q inhibitor in serum is a chondroitin 4-sulfate proteoglycan. J Biol Chem. 1981;256:7383–7.
Ghorbani, S, Yong, VW. The extracellular matrix as modifier of neuroinflammation and remyelination in multiple sclerosis. Brain 2021. https://doi.org/10.1093/brain/awab059.
Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol. 2014;258:24–34.
Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia. 2014;62:1895–904.
Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 2008;5:e171.
Rolls A, Cahalon L, Bakalash S, Avidan H, Lider O, Schwartz M. A sulfated disaccharide derived from chondroitin sulfate proteoglycan protects against inflammation-associated neurodegeneration. FASEB J. 2006;20:547–9.
Rolls A, Avidan H, Cahalon L, Schori H, Bakalash S, Litvak V, et al. A disaccharide derived from chondroitin sulphate proteoglycan promotes central nervous system repair in rats and mice. Eur J Neurosci. 2004;20:1973–83.
Ebert S, Schoeberl T, Walczak Y, Stoecker K, Stempfl T, Moehle C, et al. Chondroitin sulfate disaccharide stimulates microglia to adopt a novel regulatory phenotype. J Leukoc Biol. 2008;84:736–40.
Smolders SM, Kessels S, Vangansewinkel T, Rigo JM, Legendre P, Brône B. Microglia: brain cells on the move. Prog Neurobiol. 2019;178:101612.
Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the α6β1 integrin. J Neurosci. 2002;22:1562–72.
Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–8.
Syková E. The extracellular space in the CNS: its regulation, volume, and geometry in normal and pathological neuronal function. Neuroscientist. 1997;3:28–41.
Song I, Dityatev A. Crosstalk between glia, extracellular matrix, and neurons. Brain Res Bull. 2018;136:101–8.
Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci. 2002;22:4611–24.
Vitellaro-Zuccarello L, De Biasi S, Spreafico R.One hundred years of Golgi’s “perineuronal net”: history of a denied structure.Ital J Neurol Sci. 1998;19:249–53.
Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15, and Neprilysin. Mol Psychiatry. 2015;20:154–61.
Lensjø, KK, Christensen A C, Tennøe S, Fyhn M, Hafting T. Differential expression and cell-type specificity of perineuronal nets in hippocampus, medial entorhinal cortex, and visual cortex examined in the rat and mouse. eNeuro. 2017;4:ENEURO.0379-16.2017.
Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, Dudek SM. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J Neurosci. 2016;36:6312–20.
Morikawa S, Ikegaya Y, Narita M, Tamura H. Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval. Sci Rep. 2017;7:46024.
Wegner F, Härtig W, Bringmann A, Grosche J, Wohlfarth K, Zuschratter W, et al. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABAA receptor α1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184:705–14.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–51.
Rowlands D, Lensjø KK, Dinh T, Yang S, Andrews MR, Hafting T, et al. Aggrecan directs extracellular matrix-mediated neuronal plasticity. J Neurosci. 2018;38:10102–13.
Boggio EM, Ehlert EM, Lupori L, Moloney EB, De Winter F, Vander Kooi CW, et al. Inhibition of semaphorin3A promotes ocular dominance plasticity in the adult rat visual cortex. Mol Neurobiol. 2019;56:5987–97.
Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37:1269–83.
Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci. 2013;110:12456–61.
Banerjee SB, Gutzeit VA, Baman J, Aoued HS, Doshi NK, Liu RC, et al. Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron. 2017;95:169–179.e3.
Shi W, Wei X, Wang X, Du S, Liu W, Song J, et al. Perineuronal nets protect long-term memory by limiting activity-dependent inhibition from parvalbumin interneurons. Proc Natl Acad Sci. 2019;116:27063–27073.
Thompson EH, Lensjø KK, Wigestrand MB, Malthe-Sørenssen A, Hafting T, Fyhn M. Removal of perineuronal nets disrupts recall of a remote fear memory. Proc Natl Acad Sci USA. 2018;115:607–12.
Christensen AC, Lensjø KK, Lepperød ME, Dragly SA, Sutterud H, Blackstad JS, et al. Perineuronal nets stabilize the grid cell network. Nat Commun. 2021;12:253.
Carulli D, Broersen R, de Winter F, Muir EM, Mešković M, de Waal M, et al. Cerebellar plasticity and associative memories are controlled by perineuronal nets. Proc Natl Acad Sci USA. 2020;117:6855–65.
Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA. 2013;110:9130–5.
Miyata S, Nishimura Y, Nakashima T. Perineuronal nets protect against amyloid beta-protein neurotoxicity in cultured cortical neurons. Brain Res. 2007;1150:200–6.
Balmer, TS. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro 2016;3:ENEURO.0112-16.2016.
Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67:570–88.
Tewari BP, Chaunsali L, Campbell SL, Patel DC, Goode AE, Sontheimer H. Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat Commun. 2018;9:4724.
Blosa M, Sonntag M, Jäger C, Weigel S, Seeger J, Frischknecht R, et al. The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held. J Physiol. 2015;593:4341–60.
Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95:639–655.e10.
Gottschling C, Wegrzyn D, Denecke B, Faissner A. Elimination of the four extracellular matrix molecules tenascin-C, tenascin-R, brevican, and neurocan alters the ratio of excitatory and inhibitory synapses. Sci Rep. 2019;9:13939.
Geissler M, Gottschling C, Aguado A, Rauch U, Wetzel CH, Hatt H, et al. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci. 2013;33:7742–55.
Frischknecht R, Seidenbecher CI. Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol. 2012;44:1051–4.
Franklin SL, Love S, Greene JR, Betmouni S. Loss of perineuronal net in ME7 prion disease. J Neuropathol Exp Neurol. 2008;67:189–99.
Bitanihirwe BKY, Woo T-UW. Perineuronal nets and schizophrenia: the importance of neuronal coatings. Neurosci Biobehav Rev. 2014;45:85–99.
Belichenko PV, Miklossy J, Celio MR. HIV-I induced destruction of neocortical extracellular matrix components in AIDS victims. Neurobiol Dis. 1997;4:301–10.
Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated matrix metalloproteinase-9 and degradation of perineuronal nets in cerebrocortical multiple sclerosis plaques. J Neuropathol Exp Neurol. 2008;67:888–99.
Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506.
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–40.
Chitu V, Gokhan S, Gulinello M, Branch CA, Patil M, Basu R, et al. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP). Neurobiol Dis. 2015;74:219–28.
Biundo F, Chitu V, Shlager G, Park ES, Gulinello ME, Saha K, et al. Microglial reduction of colony stimulating factor-1 receptor expression is sufficient to confer adult onset leukodystrophy. Glia. 2021;69:779–91.
Arreola MA, Soni N, Crapser JD, Hohsfield LA, Elmore MRP, Matheos DP, et al. Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R+/− mouse model of ALSP which can be rescued via CSF1R inhibitors. Science Advances. In Press.
Venturino, A, Schulz R., De Jesús-Cortés H., Maes M.E., Nagy B., Reilly-Andújar F., et al. Microglia enable mature perineuronal nets disassembly upon anesthetic ketamine exposure or 60-Hz light entrainment in the healthy brain. Cell Rep. 2021;36:109313.
Belichenko PV, Miklossy J, Belser B, Budka H, Celio MR. Early destruction of the extracellular matrix around parvalbumin-immunoreactive interneurons in Creutzfeldt−Jakob disease. Neurobiol Dis. 1999;6:269–79.
Borner R, Bento-Torres J, Souza DR, Sadala DB, Trevia N, Farias JA, et al. Early behavioral changes and quantitative analysis of neuropathological features in murine prion disease: stereological analysis in the albino Swiss mice model. Prion. 2011;5:215–27.
Bozzelli PL, Caccavano A, Avdoshina V, Mocchetti I, Wu JY, Conant K. Increased matrix metalloproteinase levels and perineuronal net proteolysis in the HIV-infected brain; relevance to altered neuronal population dynamics. Exp Neurol. 2020;323:113077.
Medina-Flores R, Wang G, Bissel SJ, Murphey-Corb M, Wiley CA. Destruction of extracellular matrix proteoglycans is pervasive in simian retroviral neuroinfection. Neurobiol Dis. 2004;16:604–16.
Hobohm C, Günther A, Grosche J, Rossner S, Schneider D, Brückner G. Decomposition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J Neurosci Res. 2005;80:539–48.
Quattromani MJ, Pruvost M, Guerreiro C, Backlund F, Englund E, Aspberg A, et al. Extracellular matrix modulation is driven by experience-dependent plasticity during stroke recovery. Mol Neurobiol. 2018;55:2196–213.
Härtig, W, Mages B., Aleithe S., Nitzsche B., Altmann S., Barthel H. et al. Damaged neocortical perineuronal nets due to experimental focal cerebral ischemia in mice, rats and sheep. Front Integr Neurosci. 2017;11:15.
Dzyubenko E, Manrique-Castano D, Kleinschnitz C, Faissner A, Hermann DM. Topological remodeling of cortical perineuronal nets in focal cerebral ischemia and mild hypoperfusion. Matrix Biol. 2018;74:121–32.
Karetko-Sysa M, Skangiel-Kramska J, Nowicka D. Disturbance of perineuronal nets in the perilesional area after photothrombosis is not associated with neuronal death. Exp Neurol. 2011;231:113–26.
Vita, SM., Grayson B E, Grill R J. Acute damage to the blood–brain barrier and perineuronal net integrity in a clinically-relevant rat model of traumatic brain injury. NeuroReport 2020;31:1167−1174.
Wiley CA, Bissel SJ, Lesniak A, Dixon CE, Franks J, Beer Stolz D, et al. Ultrastructure of diaschisis lesions after traumatic brain injury. J Neurotrauma. 2016;33:1866–82.
Sánchez-Ventura J, Giménez-Llort L, Penas C, Udina E. Voluntary wheel running preserves lumbar perineuronal nets, enhances motor functions, and prevents hyperreflexia after spinal cord injury. Exp Neurol. 2021;336:113533.
McRae PA, Baranov E, Rogers SL, Porter BE. Persistent decrease in multiple components of the perineuronal net following status epilepticus. Eur J Neurosci. 2012;36:3471–82.
Rankin-Gee EK, McRae PA, Baranov E, Rogers S, Wandrey L, Porter BE. Perineuronal net degradation in epilepsy. Epilepsia. 2015;56:1124–33.
Reichelt AC, Lemieux CA, Princz-Lebel O, Singh A, Bussey TJ, Saksida LM. Age-dependent and region-specific alteration of parvalbumin neurons, perineuronal nets, and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci Rep. 2021;11:5593.
Baig S, Wilcock GK, Love S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta Neuropathol. 2005;110:393–401.
Kobayashi K, Emson PC, Mountjoy CQ. Vicia villosa lectin-positive neurones in human cerebral cortex. Loss in Alzheimer-type dementia. Brain Res. 1989;498:170–4.
Cattaud V, Bezzina C, Rey CC, Lejards C, Dahan L, Verret L. Early disruption of parvalbumin expression and perineuronal nets in the hippocampus of the Tg2576 mouse model of Alzheimer’s disease can be rescued by enriched environment. Neurobiol Aging. 2018;72:147–58.
Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–66.
Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–35.
Pantazopoulos H, Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016:9847696.
Hijazi S, Heistek TS, Scheltens P, Neumann U, Shimshek DR, Mansvelder HD, et al. Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease. Mol Psychiatry. 2020;25:3380–98.
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–21.
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–5.
Ali F, Baringer SL, Neal A, Choi EY, Kwan AC. Parvalbumin-positive neuron loss and amyloid-β deposits in the frontal cortex of Alzheimer’s disease-related mice. J Alzheimer’s Dis. 2019;72:1323–39.
Guentchev M, Groschup MH, Kordek R, Liberski PP, Budka H. Severe, early and selective loss of a subpopulation of GABAergic inhibitory neurons in experimental transmissible spongiform encephalopathies. Brain Pathol. 1998;8:615–23.
Morawski M, Brückner G, Jäger C, Seeger G, Matthews RT, Arendt T. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol. 2012;22:547–61.
Morawski M, Brückner G, Jäger C, Seeger G, Arendt T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer’s disease. Neuroscience. 2010;169:1347–63.
Brückner G, Hausen D, Härtig W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience. 1999;92:791–805.
Lendvai D, Morawski M, Négyessy L, Gáti G, Jäger C, Baksa G, et al. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer’s disease. Acta Neuropathol. 2013;125:215–29.
Ueno H, Fujii K, Takao K, Suemitsu S, Murakami S, Kitamura N, et al. Alteration of parvalbumin expression and perineuronal nets formation in the cerebral cortex of aged mice. Mol Cell Neurosci. 2019;95:31–42.
Mafi AM, Hofer LN, Russ MG, Young JW, Mellott JG. The density of perineuronal nets increases with age in the inferior colliculus in the fischer brown norway rat. Front Aging Neurosci. 2020;12:27.
Hilbig H, Bidmon HJ, Steingrüber S, Reinke H, Dinse HR. Enriched environmental conditions reverse age-dependent gliosis and losses of neurofilaments and extracellular matrix components but do not alter lipofuscin accumulation in the hindlimb area of the aging rat brain. J Chem Neuroanat. 2002;23:199–209.
Brewton DH, Kokash J, Jimenez O, Pena ER, Razak KA. Age-related deterioration of perineuronal nets in the primary auditory cortex of mice. Front Aging Neurosci. 2016;8:270.
Miyata S, Nishimura Y, Hayashi N, Oohira A. Construction of perineuronal net-like structure by cortical neurons in culture. Neuroscience. 2005;136:95–104.
Lander C, Zhang H, Hockfield S. Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J Neurosci. 1998;18:174–83.
Fowke TM, Karunasinghe RN, Bai JZ, Jordan S, Gunn AJ, Dean JM. Hyaluronan synthesis by developing cortical neurons in vitro. Sci Rep. 2017;7:44135.
Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–47.
Kwok JC, Carulli D, Fawcett JW. In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J Neurochem. 2010;114:1447–59.
Jäger C, Lendvai D, Seeger G, Brückner G, Matthews RT, Arendt T, et al. Perineuronal and perisynaptic extracellular matrix in the human spinal cord. Neuroscience. 2013;238:168–84.
Hohsfield, LA et al. Subventricular zone/white matter microglia reconstitute the empty adult microglial niche in a dynamic wave. Preprint at bioRxiv https://doi.org/10.1101/2021.02.17.431594 (2021).
Ribot J, Breton R, Calvo CF, Moulard J, Ezan P, Zapata J, et al. Astrocytes close the mouse critical period for visual plasticity. Science. 2021;373:77–81.
Kohnke S, Buller S, Nuzzaci D, Ridley K, Lam B, Pivonkova H, et al. Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep. 2021;36:109362.
Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–47.
Li KW, Hornshaw MP, Van der Schors RC, Watson R, Tate S, Casetta B, et al. Proteomics analysis of rat brain postsynaptic density: implications of the diverse protein functional groups for the integration of synaptic physiology. J Biol Chem. 2004;279:987–1002.
Seidenbecher CI, Richter K, Rauch U, Fässler R, Garner CC, Gundelfinger ED.Brevican, a chondroitin sulfate proteoglycan of rat brain, occurs as secreted and cell surface glycosylphosphatidylinositol-anchored Isoforms.J Biol Chem.1995;270:27206–12.
Pintér A, Hevesi Z, Zahola P, Alpár A, Hanics J. Chondroitin sulfate proteoglycan-5 forms perisynaptic matrix assemblies in the adult rat cortex. Cell Signal. 2020;74:109710.
Jüttner R, Montag D, Craveiro RB, Babich A, Vetter P, Rathjen FG. Impaired presynaptic function and elimination of synapses at premature stages during postnatal development of the cerebellum in the absence of CALEB (CSPG5/neuroglycan C). Eur J Neurosci. 2013;38:3270–80.
Bekku Y, Saito M, Moser M, Fuchigami M, Maehara A, Nakayama M, et al. Bral2 is indispensable for the proper localization of brevican and the structural integrity of the perineuronal net in the brainstem and cerebellum. J Comp Neurol. 2012;520:1721–36.
Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci. 2012;32:18009–17.
Lendvai D, Morawski M, Brückner G, Négyessy L, Baksa G, Glasz T, et al. Perisynaptic aggrecan-based extracellular matrix coats in the human lateral geniculate body devoid of perineuronal nets. J Neurosci Res. 2012;90:376–87.
Blosa M, Sonntag M, Brückner G, Jäger C, Seeger G, Matthews RT, et al. Unique features of extracellular matrix in the mouse medial nucleus of trapezoid body—implications for physiological functions. Neuroscience. 2013;228:215–34.
Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, et al. Contributions of astrocytes to synapse formation and maturation potential functions of the perisynaptic extracellular matrix. Brain Res Rev. 2010;63:26–38.
Mitlöhner J, Kaushik R, Niekisch H, Blondiaux A, Gee C E, Happel M F K. et al. Dopamine receptor activation modulates the integrity of the perisynaptic extracellular matrix at excitatory synapses. Cells. 2020;9:260.
Brückner G, Morawski M, Arendt T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience. 2008;151:489–504.
de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, et al. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun. 2013;4:1484.
Stoyanov S, Sun W, Düsedau HP, Cangalaya C, Choi I, Mirzapourdelavar H, et al. Attenuation of the extracellular matrix restores microglial activity during the early stage of amyloidosis. Glia. 2021;69:182–200.
Végh MJ, Heldring CM, Kamphuis W, Hijazi S, Timmerman AJ, Li KW, et al. Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2014;2:76.
Howell MD, Bailey LA, Cozart MA, Gannon BM, Gottschall PE. Hippocampal administration of chondroitinase ABC increases plaque-adjacent synaptic marker and diminishes amyloid burden in aged APPswe/PS1dE9 mice. Acta Neuropathol Commun. 2015;3:54.
Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Döring A, Sloka S, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72:419–32.
Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100:120–134.e6.
Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–207.
Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–800.
Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–11.
Cross AK, Woodroofe MN. Chemokine modulation of matrix metalloproteinase and TIMP production in adult rat brain microglia and a human microglial cell line in vitro. Glia. 1999;28:183–9.
Welser-Alves JV, Crocker SJ, Milner R. A dual role for microglia in promoting tissue inhibitor of metalloproteinase (TIMP) expression in glial cells in response to neuroinflammatory stimuli. J Neuroinflammation. 2011;8:61.
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, et al. Tumor necrosis factor-α induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–8.
Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–30.
Nakanishi H. Microglial functions and proteases. Mol Neurobiol. 2003;27:163–76.
Könnecke H, Bechmann I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol. 2013;2013:914104
Siri A, Knäuper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270:8650–4.
Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, et al. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–90.
Planas AM, Solé S, Justicia C. Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8:834–46.
Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human. Stroke. 2006;37:1399–406.
Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–9.
Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5. J Immunol. 2007;178:8158–67.
Hu F, Ku MC, Markovic D, Dzaye O, Lehnardt S, Synowitz M, et al. Glioma-associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline. Int J Cancer. 2014;135:2569–78.
Markovic DS, Glass R, Synowitz M, Rooijen NV, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–62.
Kelly, E, Russo A S, Jackson C D, Lamantia C E, Majewska A K. Proteolytic regulation of synaptic plasticity in the mouse primary visual cortex: analysis of matrix metalloproteinase 9 deficient mice. Front Cell Neurosci. 2015;9:369.
Murase S, Lantz CL, Quinlan EM. Light reintroduction after dark exposure reactivates plasticity in adults via perisynaptic activation of MMP-9. eLife. 2017;6:e27345.
Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, et al. Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing Fmr1 knock-out mice. Cereb Cortex. 2018;28:3951–64.
Pirbhoy PS, Rais M, Lovelace JW, Woodard W, Razak KA, Binder DK, et al. Acute pharmacological inhibition of matrix metalloproteinase-9 activity during development restores perineuronal net formation and normalizes auditory processing in Fmr1 KO mice. J Neurochem. 2020;155:538–58.
Lemarchant S, Pruvost M, Montaner J, Emery E, Vivien D, Kanninen K, et al. ADAMTS proteoglycanases in the physiological and pathological central nervous system. J Neuroinflammation. 2013;10:133–133.
Hamel MG, Mayer J, Gottschall PE. Altered production and proteolytic processing of brevican by transforming growth factor β in cultured astrocytes. J Neurochem. 2005;93:1533–41.
Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–6.
Cua RC, Lau LW, Keough MB, Midha R, Apte SS, Yong VW. Overcoming neurite-inhibitory chondroitin sulfate proteoglycans in the astrocyte matrix. Glia. 2013;61:972–84.
Lemarchant S, Pomeshchik Y, Kidin I, Kärkkäinen V, Valonen P, Lehtonen S, et al. ADAMTS-4 promotes neurodegeneration in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2016;11:10.
Fang L, Teuchert M, Huber-Abel F, Schattauer D, Hendrich C, Dorst J, et al. MMP-2 and MMP-9 are elevated in spinal cord and skin in a mouse model of ALS. J Neurol Sci. 2010;294:51–6.
Nakanishi H. Cathepsin regulation on microglial function. Biochim Biophys Acta (BBA)—Proteins Proteom. 2020;1868:140465.
Ryan RE, Sloane BF, Sameni M, Wood PL. Microglial cathepsin B: an immunological examination of cellular and secreted species. J Neurochem. 1995;65:1035–45.
Petanceska S, Canoll P, Devi LA. Expression of rat cathepsin S in phagocytic cells (∗). J Biol Chem. 1996;271:4403–9.
Hao HP, Doh-Ura K, Nakanishi H. Impairment of microglial responses to facial nerve axotomy in cathepsin S–deficient mice. J Neurosci Res. 2007;85:2196–206.
Pantazopoulos, H, Gisabella B, Rexrode L, Benefield D, Yildiz E, Seltzer P, et al. Circadian rhythms of perineuronal net composition. eNeuro. 2020;7:ENEURO.0034-19.2020.
Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci. 2012;32:15106–11.
Basilico B, Pagani F, Grimaldi A, Cortese B, Di Angelantonio S, Weinhard L, et al. Microglia shape presynaptic properties at developing glutamatergic synapses. Glia. 2019;67:53–67.
Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, et al. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–51.
Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci. 1998;95:10896–901.
Bolós M, Perea JR, Terreros-Roncal J, Pallas-Bazarra N, Jurado-Arjona J, Ávila J, et al. Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav Immun. 2018;68:76–89.
Sipe GO, Lowery RL, Tremblay MÈ, Kelly EA, Lamantia CE, Majewska AK. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun. 2016;7:10905.
Easley-Neal C, Foreman O, Sharma N, Zarrin AA, Weimer RM. CSF1R ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions. Front Immunol. 2019;10:2199.
Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–57.
Gottschall PE, Howell MD. ADAMTS expression and function in central nervous system injury and disorders. Matrix Biol. 2015;44-46:70–76.
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80.
Tremblay MÈ, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60:541–58.
Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–82.
Linnartz B, Kopatz J, Tenner AJ, Neumann H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J Neurosci. 2012;32:946–52.
Lim S-H, Park E, You B, Jung Y, Park A-R, Park SG, et al. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One. 2013;8:e81218.
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609.
Rubino SJ, Mayo L, Wimmer I, Siedler V, Brunner F, Hametner S, et al. Acute microglia ablation induces neurodegeneration in the somatosensory system. Nat Commun. 2018;9:4578.
Milinkeviciute G, Henningfield CM, Muniak MA, Chokr SM, Green KN, Cramer KS. Microglia regulate pruning of specialized synapses in the auditory brainstem. Front Neural Circuits. 2019;13:55.
Milinkeviciute, G, Chokr S M, Crame K S. Auditory brainstem deficits from early treatment with a CSF1R inhibitor largely recover with microglial repopulation. eNeuro. 2021;8:ENEURO.0318-20.2021.
Stowell RD, Wong EL, Batchelor HN, Mendes MS, Lamantia CE, Whitelaw BS, et al. Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Dev Neurobiol. 2018;78:627–44.
Kana V, Desland FA, Casanova-Acebes M, Ayata P, Badimon A, Nabel E, et al. CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J Exp Med. 2019;216:2265–81.
Nakayama H, Abe M, Morimoto C, Iida T, Okabe S, Sakimura K, et al. Microglia permit climbing fiber elimination by promoting GABAergic inhibition in the developing cerebellum. Nat Commun. 2018;9:2830.
Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–47.
Ju¨ttner R, Moré MI, Das D, Babich A, Meier J, Henning M, et al. Impaired synapse function during postnatal development in the absence of CALEB, an EGF-like protein processed by neuronal activity. Neuron. 2005;46:233–45.
Brückner G, Grosche J, Schmidt S, Härtig W, Margolis RU, Delpech B, et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–29.
Aujla PK, Huntley GW. Early postnatal expression and localization of matrix metalloproteinases-2 and -9 during establishment of rat hippocampal synaptic circuitry. J Comp Neurol. 2014;522:1249–63.
Schecter RW, Maher EE, Welsh CA, Stevens B, Erisir A, Bear MF. Experience-dependent synaptic plasticity in V1 occurs without microglial CX3CR1. J Neuroscd. 2017;37:10541–53.
Ma X, Chen K, Cui Y, Huang G, Nehme A, Zhang L. et al.2020) Depletion of microglia in developing cortical circuits reveals its critical role in glutamatergic synapse development, functional connectivity, and critical period plasticity.J Neurosci Res.2020;98:1968–86.
McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation slters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–13.
Gunner G, Cheadle L, Johnson KM, Ayata P, Badimon A, Mondo E, et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat Neurosci. 2019;22:1075–88.
Welsh CA, Stephany CÉ, Sapp RW, Stevens B. Ocular dominance plasticity in binocular primary visual cortex does not require C1q. J Neurosci. 2020;40:769–83.
Li T, Chiou B, Gilman CK, Luo R, Koshi T, Yu D, et al. A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. EMBO J. 2020;39:e104136.
Scott-Hewitt N, Perrucci F, Morini R, Erreni M, Mahoney M, Witkowska A, et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020;39:e105380.
Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–27.
Favuzzi, E, Huang S, Saldi G A, Binan L, Ibrahim L A, Fernández-Otero M, et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell. 2021;184:4048−4063.e32.
Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, et al. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics. 2010;10:2214–23.
Toth AB, Terauchi A, Zhang LY, Johnson-Venkatesh EM, Larsen DJ, Sutton MA, et al. Synapse maturation by activity-dependent ectodomain shedding of SIRPα. Nat Neurosci. 2013;16:1417–25.
Lopez ME, Klein AD, Scott MP. Complement is dispensable for neurodegeneration in Niemann−Pick disease type C. J Neuroinflammation. 2012;9:216.
Kirschfink M, Blase L, Engelmann S, Schwartz-Albiez R. Secreted chondroitin sulfate proteoglycan of human B cell lines binds to the complement protein C1q and inhibits complex formation of C1. J Immunol. 1997;158:1324–31.
Ma D, Liu S, Lal B, Wei S, Wang S, Zhan D, et al. Extracellular matrix protein tenascin C increases phagocytosis mediated by CD47 loss of function in glioblastoma. Cancer Res. 2019;79:2697–708.
Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9:1228.
Lim TK, Ruthazer ES. Microglial trogocytosis and the complement system regulate axonal pruning in vivo. Elife. 2021;10:e62167.
Reshef R, Kudryavitskaya E, Shani-Narkiss H, Isaacson B, Rimmerman N, Mizrahi A, et al. The role of microglia and their CX3CR1 signaling in adult neurogenesis in the olfactory bulb. eLife. 2017;6,:e30809.
Wallace J, Lord J, Dissing-Olesen L, Stevens B, Murthy VN, et al. Microglial depletion disrupts normal functional development of adult-born neurons in the olfactory bulb. Elife. 2020;9:e50531.
Wegrzyn D, Freund N, Faissner A, Juckel G. Poly I:C activated microglia disrupt perineuronal nets and modulate synaptic balance in primary hippocampal neurons in vitro. Front Synaptic Neurosci. 2021;13:637549.
Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–46.
Henstridge CM, Pickett E, Spires-Jones TL. Synaptic pathology: a shared mechanism in neurological disease. Ageing Res Rev. 2016;28:72–84.
Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer’s disease: synapses gone cold. Mol Neurodegener. 2011;6:63.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80.
Henstridge CM, Sideris DI, Carroll E, Rotariu S, Salomonsson S, Tzioras M, et al. Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol. 2018;135:213–26.
Lee, E, Chung, W-S. Glial control of synapse number in healthy and diseased brain. Front Cell Neurosci. 2019;13:42.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–6.
Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med. 2017;9:eaaf6295.
Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2004;24:6457–65.
Ding X, Wang J, Huang M, Chen Z, Liu J, Zhang Q, et al. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat Commun. 2021;12:2030.
Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci. 2015;35:13029–42.
Socodato R, Portugal CC, Canedo T, Rodrigues A, Almeida TO, Henriques JF, et al. Microglia dysfunction caused by the loss of rhoa disrupts neuronal physiology and leads to neurodegeneration. Cell Rep. 2020;31:107796.
Cavanagh C, Tse YC, Nguyen HB, Krantic S, Breitner JC, Quirion R, et al. Inhibiting tumor necrosis factor-α before amyloidosis prevents synaptic deficits in an Alzheimer’s disease model. Neurobiol Aging. 2016;47:41–49.
Zhang D, Li S, Hou L, Jing L, Ruan Z, Peng B, et al. Microglial activation contributes to cognitive impairments in rotenone-induced mouse Parkinson’s disease model. J Neuroinflammation. 2021;18:4.
Azevedo EP, Ledo JH, Barbosa G, Sobrinho M, Diniz L, Fonseca AC, et al. Activated microglia mediate synapse loss and short-term memory deficits in a mouse model of transthyretin-related oculoleptomeningeal amyloidosis. Cell Death Dis. 2013;4:e789.
Wilton DK, Dissing-Olesen L, Stevens B. Neuron-glia signaling in synapse elimination. Annu Rev Neurosci. 2019;42:107–27.
Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14.
Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102.
Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci. 2014;34:9867–79.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73.
Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–89.
Stavisky SD, Willett FR, Wilson GH, Murphy BA, Rezaii P, Avansino DT, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–53.
Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–85.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) under awards R01NS083801 (NINDS), RF1AG056768 (NIA), RF1AG065329 (NIA), and U54 AG054349 (NIA Model Organism Development and Evaluation for Late-onset Alzheimer’s Disease (MODEL-AD)) to K.N.G., F31NS108611 (NINDS) to J.D.C., and F31NS111882 (NINDS) to M.A.A. We thank Rocio A. Barahona for providing whole-brain slidescan and 20× confocal images of microglia-depleted mouse brain sections immunohistochemically stained for perineuronal nets. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.D.C. and M.A.A. wrote the paper. J.D.C. created Fig. 1 and K.I.T. designed the schematic in Fig. 2. K.N.G. wrote and edited the paper and secured funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crapser, J.D., Arreola, M.A., Tsourmas, K.I. et al. Microglia as hackers of the matrix: sculpting synapses and the extracellular space. Cell Mol Immunol 18, 2472–2488 (2021). https://doi.org/10.1038/s41423-021-00751-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-021-00751-3
Keywords
This article is cited by
-
Preclinical translational platform of neuroinflammatory disease biology relevant to neurodegenerative disease
Journal of Neuroinflammation (2024)
-
Genetic contribution to microglial activation in schizophrenia
Molecular Psychiatry (2024)
-
Obesity affects brain cortex gene expression in an APOE genotype and sex dependent manner
International Journal of Obesity (2024)
-
Adolescent exposure to the Spice/K2 cannabinoid JWH-018 impairs sensorimotor gating and alters cortical perineuronal nets in a sex-dependent manner
Translational Psychiatry (2023)
-
Lipofuscin-like autofluorescence within microglia and its impact on studying microglial engulfment
Nature Communications (2023)