Abstract
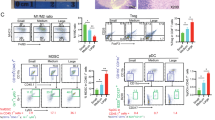
Local intratumor delivery with electroporation of low levels of plasmids encoding molecules, induces an antitumor effect without causing systemic toxicity. However, previous studies have predominately focused on the function of the delivered molecule encoded within the plasmid, and ignored the plasmid vector. In this study, we found vectors pUMVC3 and pVax1 induced upregulation of MHC class I (MHC-I) and PD-L1 on tumor cell surface. These molecules participate in a considerable number of immunoregulatory functions through their interactions with and activating inhibitory immune cell receptors. MHC molecules are well-known for their role in antigen (cross-) presentation, thereby functioning as key players in the communication between immune cells and tumor cells. Increased PD-L1 expression on tumor cells is an important monitor of tumor growth and the effectiveness of immune inhibitor therapy. Results from flow cytometry confirmed increased expression of MHC-I and PDL-1 on B16F10, 4T1, and KPC tumor cell lines. Preliminary animal data from tumor-bearing models, B16F10 melanoma, 4T1 breast cancer and KPC pancreatic cancer mouse models showed that tumor growth was attenuated after pUMVC3 intratumoral electroporation. Our data also documented that pSTAT1 signaling pathway might not be associated with plasmid vectors’ function of upregulating MHC-I, PD-L1 on tumor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data associated with this study are present in the paper or the Supplementary Materials. Materials are available upon request from the corresponding author.
References
Komel T, Bosnjak M, Kranjc Brezar S, De Robertis M, Mastrodonato M, Scillitani G, et al. Gene electrotransfer of IL-2 and IL-12 plasmids effectively eradicated murine B16.F10 melanoma. Bioelectrochemistry. 2021;141:107843.
Shi G, Edelblute C, Arpag S, Lundberg C, Heller R. IL-12 Gene electrotransfer triggers a change in immune response within mouse tumors. Cancers. 2018;10:498.
Fioretti D, Iurescia S, Fazio VM, Rinaldi M. In vivo DNA electrotransfer for immunotherapy of cancer and neurodegenerative diseases. Curr Drug Metab. 2013;14:279–90.
Shi G, Scott M, Mangiamele CG, Heller R. Modification of the tumor microenvironment enhances anti-PD-1 immunotherapy in metastatic melanoma. Pharmaceutics. 2022;14:2429.
Heller LC, Coppola D. Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Gene Ther 2002;9:1321–5.
Bosnjak M, Znidar K, Sales Conniff A, Jesenko T, Markelc B, Semenova N, et al. In vitro and in vivo correlation of skin and cellular responses to nucleic acid delivery. Biomed Pharmacother. 2022;150:113088.
Znidar K, Bosnjak M, Semenova N, Pakhomova O, Heller L, Cemazar M. Tumor cell death after electrotransfer of plasmid DNA is associated with cytosolic DNA sensor upregulation. Oncotarget. 2018;9:18665–81.
Znidar K, Bosnjak M, Jesenko T, Heller LC, Cemazar M. Upregulation of DNA sensors in B16.F10 melanoma spheroid cells after electrotransfer of pDNA. Technol Cancer Res Treat. 2018;17:1533033818780088.
Seliger B. Basis of PD1/PD-L1 Therapies. J Clin Med. 2019;8:2168.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208.
Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207.
Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–7.
Chew GL, Campbell AE, De Neef E, Sutliff NA, Shadle SC, Tapscott SJ, et al. DUX4 suppresses MHC class I to promote cancer immune evasion and resistance to checkpoint blockade. Dev Cell. 2019;50:658–71.e657.
Garrido C, Paco L, Romero I, Berruguilla E, Stefansky J, Collado A, et al. MHC class I molecules act as tumor suppressor genes regulating the cell cycle gene expression, invasion and intrinsic tumorigenicity of melanoma cells. Carcinogenesis. 2012;33:687–93.
Andersson E, Villabona L, Bergfeldt K, Carlson JW, Ferrone S, Kiessling R, et al. Correlation of HLA-A02* genotype and HLA class I antigen down-regulation with the prognosis of epithelial ovarian cancer. Cancer Immunol Immunother. 2012;61:1243–53.
Inoue M, Mimura K, Izawa S, Shiraishi K, Inoue A, Shiba S, et al. Expression of MHC Class I on breast cancer cells correlates inversely with HER2 expression. Oncoimmunology. 2012;1:1104–10.
Noblejas-López MDM, Nieto-Jiménez C, Morcillo García S, Pérez-Peña J, Nuncia-Cantarero M, Andrés-Pretel F, et al. Expression of MHC class I, HLA-A and HLA-B identifies immune-activated breast tumors with favorable outcome. Oncoimmunology. 2019;8:e1629780.
van Houdt IS, Sluijter BJ, Moesbergen LM, Vos WM, de Gruijl TD, Molenkamp BG, et al. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int J Cancer. 2008;123:609–15.
Leapman MS, Presley CJ, Zhu W, Soulos PR, Adelson KB, Miksad RA, et al. Association of programmed cell death ligand 1 expression status with receipt of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. JAMA Netw Open. 2020;3:e207205.
Sun JY, Zhang D, Wu S, Xu M, Zhou X, Lu XJ, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives, Biomarker. Research. 2020;8:35.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–51.
Möller K, Fraune C, Blessin NC, Lennartz M, Kluth M, Hube-Magg C, et al. Tumor cell PD-L1 expression is a strong predictor of unfavorable prognosis in immune checkpoint therapy-naive clear cell renal cell cancer. Int Urol Nephrol. 2021;53:2493–503.
Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Highly activated PD-1/PD-L1 pathway in gastric cancer with PD-L1 expression. Anticancer Res. 2018;38:107–12.
Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895–904.
Yu W, Hua Y, Qiu H, Hao J, Zou K, Li Z, et al. PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. 2020;11:506.
Kelany M, Barth TF, Salem D, Shakweer MM. Prevalence and prognostic implications of PD-L1 expression in soft tissue sarcomas. Pathol Oncol Res. 2021;27:1609804.
Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–30.
Deng C, Li Z, Guo S, Chen P, Chen X, Zhou Q, et al. Tumor PD-L1 expression is correlated with increased TILs and poor prognosis in penile squamous cell carcinoma. Oncoimmunology. 2017;6:e1269047.
Wells DJ. Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 2004;11:1363–9.
Gonçalves GAR, Paiva RMA. Gene therapy: advances, challenges and perspectives. Einstein. 2017;15:369–75.
Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012;3:287.
Brown BD, Lillicrap D. Dangerous liaisons: the role of “danger” signals in the immune response to gene therapy. Blood. 2002;100:1133–40.
Früh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11:76–81.
Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–201.
Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, Zhang P. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–93.
Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239–60.
Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, et al. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci USA. 1999;96:2285–90.
Bai H, Lester GMS, Petishnok LC, Dean DA. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci Rep. 2017;37:BSR20160616.
Rosazza C, Meglic SH, Zumbusch A, Rols MP, Miklavcic D. Gene electrotransfer: a mechanistic perspective. Curr Gene Ther. 2016;16:98–129.
Cannatà A, Ali H, Sinagra G, Giacca M. Gene therapy for the heart lessons learned and future perspectives. Circulation Res. 2020;126:1394–414.
Zundler S, Neurath MF. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–68.
Clanchy FI, Williams RO. Plasmid DNA as a safe gene delivery vehicle for treatment of chronic inflammatory disease. Expert Opin Biol Ther. 2008;8:1507–19.
Hicks KC, Chariou PL, Ozawa Y, Minnar CM, Knudson KM, Meyer TJ, et al. Tumor-targeted interleukin-12 and entinostat combination therapy improves cancer survival by reprogramming the tumour immune cell landscape. Nat Commun. 2021;12:5151.
Taniyama Y, Azuma J, Kunugiza Y, Iekushi K, Rakugi H, et al. Therapeutic option of plasmid-DNA based gene transfer. Curr Top Med Chem. 2012;12:1630–7.
Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J. et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–46.
Williams PD, Kingston PA. Plasmid-mediated gene therapy for cardiovascular disease. Cardiovascular Res. 2011;91:565–76.
Komel T, Omerzel M, Kamensek U, Znidar K, Lampreht Tratar U, Kranjc Brezar S, et al. Gene immunotherapy of colon carcinoma with IL-2 and IL-12 using gene electrotransfer. Int J Mol Sci. 2023;24:12900.
Greaney SK, Algazi AP, Tsai KK, Takamura KT, Chen L, Twitty CG, et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol Res. 2020;8:246–54.
Telli ML, Nagata H, Wapnir I, Acharya CR, Zablotsky K, Fox BA, et al. Intratumoral plasmid IL12 expands CD8(+) T cells and induces a CXCR3 gene signature in triple-negative breast tumors that sensitizes patients to aAnti-PD-1 therapy. Clin Cancer Res. 2021;27:2481–93.
Anwer K, Kelly FJ, Chu C, Fewell JG, Lewis D, Alvarez RD. Phase I trial of a formulated IL-12 plasmid in combination with carboplatin and docetaxel chemotherapy in the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2013;131:169–73.
Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–903.
Semenova N, Bosnjak M, Markelc B, Znidar K, Cemazar M, Heller L. Multiple cytosolic DNA sensors bind plasmid DNA after transfection. Nucleic Acids Res. 2019;47:10235–46.
de Charette M, Marabelle A, Houot R. Turning tumour cells into antigen presenting cells: the next step to improve cancer immunotherapy? Eur J Cancer. 2016;68:134–47.
Bethune MT, Li XH, Yu J, McLaughlin J, Cheng D, Mathis C, et al. Isolation and characterization of NY-ESO-1-specific T cell receptors restricted on various MHC molecules. Proc Natl Acad Sci USA. 2018;115:E10702–11.
Leone P, Shin EC, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105:1172–87.
Koşaloğlu-Yalçın Z, Lanka M, Frentzen A, Logandha Ramamoorthy Premlal A, Sidney J, Vaughan K, et al. Predicting T cell recognition of MHC class I restricted neoepitopes. Oncoimmunology. 2018;7:e1492508.
Oner G, Önder S, Karatay H, Ak N, Tükenmez M, Müslümanoğlu M, et al. Clinical impact of PD-L1 expression in triple-negative breast cancer patients with residual tumor burden after neoadjuvant chemotherapy. World J Surg Oncol. 2021;19:264.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52.
Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–16.
Diskin B, Adam S, Cassini MF, Sanchez G, Liria M, Aykut B, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21:442–54.
DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400.
Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–70.
Rodríguez T, Méndez R, Del Campo A, Jiménez P, Aptsiauri N, Garrido F, et al. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7:34.
Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol Res. 2016;4:936–47.
Gong W, Song Q, Lu X, Gong W, Zhao J, Min P, et al. Paclitaxel induced B7-H1 expression in cancer cells via the MAPK pathway. J Chemother. 2011;23:295–9.
Muntjewerff EM, Meesters LD, van den Bogaart G, Revelo NH. Reverse signaling by MHC-I molecules in immune and non-immune cell types. Front Immunol. 2020;11:605958.
Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910–23.
Lv D, Shen Y, Peng Y, Liu J, Miao F, Zhang J. Neuronal MHC Class I Expression is regulated by activity driven calcium signaling. PloS One. 2015;10:e0135223.
Balan M, Mier Y Teran E, Waaga-Gasser AM, Gasser M, Choueiri TK, Freeman G, et al. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J Biol Chem. 2015;290:8110–20.
Miao J, Hsu PC, Yang YL, Xu Z, Dai Y, Wang Y, et al. YAP regulates PD-L1 expression in human NSCLC cells. Oncotarget. 2017;8:114576–87.
Munkonge FM, Dean DA, Hillery E, Griesenbach U, Alton EW. Emerging significance of plasmid DNA nuclear import in gene therapy. Adv Drug Deliv Rev. 2003;55:749–60.
Vaughan EE, DeGiulio JV, Dean DA. Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr Gene Ther. 2006;6:671–81.
Gasiorowski JZ, Dean DA. Intranuclear trafficking of episomal DNA is transcription-dependent. Mol Ther. 2007;15:2132–9.
Acknowledgements
This research was funded in part by the U.S. National Institutes of Health, R01 CA186730. The funders had no role in study design, collection of data, decision to publish, or in preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
RH conceived and supervised the study and aided in interpretation of data. GS and JS performed experiments. GS designed, interpreted, analyzed, and interpreted data.
Corresponding author
Ethics declarations
Competing interests
Dr. R. Heller is an inventor on patents which cover the technology that was used in the work reported in this manuscript. In addition, Dr. R. Heller owns stock in Inovio Pharmaceuticals, Inc.
Ethical approval
All animal experiments were approved by the University of South Florida Institutional Animal Care and Use Committee (IACUC; protocols #IS00007033, #IS00011648).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, G., Synowiec, J., Singh, J. et al. Modification of the tumor microenvironment enhances immunity with plasmid gene therapy. Cancer Gene Ther 31, 641–648 (2024). https://doi.org/10.1038/s41417-024-00728-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-024-00728-0