Abstract
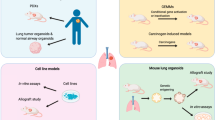
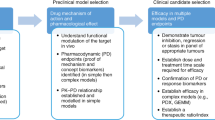
Esophageal cancer (EC) is the tenth most common cancer worldwide and has high morbidity and mortality. Its main subtypes include esophageal squamous cell carcinoma and esophageal adenocarcinoma, which are usually diagnosed during their advanced stages. The biological defects and inability of preclinical models to summarize completely the etiology of multiple factors, the complexity of the tumor microenvironment, and the genetic heterogeneity of tumors severely limit the clinical treatment of EC. Patient-derived models of EC not only retain the tissue structure, cell morphology, and differentiation characteristics of the original tumor, they also retain tumor heterogeneity. Therefore, compared with other preclinical models, they can better predict the efficacy of candidate drugs, explore novel biomarkers, combine with clinical trials, and effectively improve patient prognosis. This review discusses the methods and animals used to establish patient-derived models and genetically engineered mouse models, especially patient-derived xenograft models. It also discusses their advantages, applications, and limitations as preclinical experimental research tools to provide an important reference for the precise personalized treatment of EC and improve the prognosis of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Liu Z, Zhao Y, Kong P, Liu Y, Huang J, Xu E, et al. Integrated multi-omics profiling yields a clinically relevant molecular classification for esophageal squamous cell carcinoma. Cancer Cell. 2023;41:181–95.e9.
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–71.
Iriarte F, Su S, Petrov RV, Bakhos CT, Abbas AE. Surgical management of early esophageal cancer. Surg Clin North Am. 2021;101:427–41.
Lewis S, Lukovic J. Neoadjuvant therapy in esophageal cancer. Thorac Surg Clin. 2022;32:447–56.
Collins A, Miles GJ, Wood J, MacFarlane M, Pritchard C, Moss E. Patient-derived explants, xenografts and organoids: 3-dimensional patient-relevant pre-clinical models in endometrial cancer. Gynecol Oncol. 2020;156:251–9.
Van Nyen T, Moiola CP, Colas E, Annibali D, Amant F. Modeling endometrial cancer: past, present, and future. Int J Mol Sci. 2018;19:2348.
Jung J, Seol HS, Chang S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat. 2018;50:1–10.
Chen J, Liao S, Xiao Z, Pan Q, Wang X, Shen K, et al. The development and improvement of immunodeficient mice and humanized immune system mouse models. Front Immunol. 2022;13:1007579.
Giovanella BC, Fogh J. The nude mouse in cancer research. Adv Cancer Res. 1985;44:69–120.
Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019;8:889.
Forlani G, Shallak M, Accolla RS, Romanelli MG. HTLV-1 infection and pathogenesis: new insights from cellular and animal models. Int J Mol Sci. 2021;22:8001.
Chen H, Yang Y, Deng Y, Wei F, Zhao Q, Liu Y, et al. Delivery of CD47 blocker SIRPalpha-Fc by CAR-T cells enhances antitumor efficacy. J Immunother Cancer. 2022;10:e003737.
Belaid B, Lamara Mahammed L, Mohand Oussaid A, Migaud M, Khadri Y, Casanova JL, et al. Case report: interleukin-2 receptor common gamma chain defect presented as a hyper-IgE syndrome. Front Immunol. 2021;12:696350.
McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200.
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20:206.
Shin HY, Lee EJ, Yang W, Kim HS, Chung D, Cho H, et al. Identification of prognostic markers of gynecologic cancers utilizing patient-derived xenograft mouse models. Cancers. 2022;14:829.
Liu Z, Ahn MH, Kurokawa T, Ly A, Zhang G, Wang F, et al. A fast, simple, and cost-effective method of expanding patient-derived xenograft mouse models of pancreatic ductal adenocarcinoma. J Transl Med. 2020;18:255.
Pan B, Wei X, Xu X. Patient-derived xenograft models in hepatopancreatobiliary cancer. Cancer Cell Int. 2022;22:41.
Vaeteewoottacharn K, Pairojkul C, Kariya R, Muisuk K, Imtawil K, Chamgramol Y, et al. Establishment of Highly transplantable cholangiocarcinoma cell lines from a patient-derived xenograft mouse model. Cells. 2019;8:496.
Collins AT, Lang SH. A systematic review of the validity of patient derived xenograft (PDX) models: the implications for translational research and personalised medicine. PeerJ. 2018;6:e5981.
Tanaka T, Nishie R, Ueda S, Miyamoto S, Hashida S, Konishi H, et al. Patient-derived xenograft models in cervical cancer: a systematic review. Int J Mol Sci. 2021;22:9369.
Lan T, Xue X, Dunmall LC, Miao J, Wang Y. Patient-derived xenograft: a developing tool for screening biomarkers and potential therapeutic targets for human esophageal cancers. Aging. 2021;13:12273–93.
Fujii E, Kato A, Suzuki M. Patient-derived xenograft (PDX) models: characteristics and points to consider for the process of establishment. J Toxicol Pathol. 2020;33:153–60.
Fujii E, Kato A, Chen YJ, Matsubara K, Ohnishi Y, Suzuki M. Characterization of EBV-related lymphoproliferative lesions arising in donor lymphocytes of transplanted human tumor tissues in the NOG mouse. Exp Anim. 2014;63:289–96.
Tse E, Kwong YL. Epstein Barr virus-associated lymphoproliferative diseases: the virus as a therapeutic target. Exp Mol Med. 2015;47:e136.
Nowalk A, Green M. Epstein-Barr virus. Microbiol Spectr. 2016;4. https://doi.org/10.1128/microbiolspec.
Corso S, Cargnelutti M, Durando S, Menegon S, Apicella M, Migliore C, et al. Rituximab treatment prevents lymphoma onset in gastric cancer patient-derived xenografts. Neoplasia. 2018;20:443–55.
Fujii E, Kato A, Chen YJ, Matsubara K, Ohnishi Y, Suzuki M. The status of donor cancer tissues affects the fate of patient-derived colorectal cancer xenografts in NOG mice. Exp Anim. 2015;64:181–90.
Veeranki OL, Tong Z, Mejia A, Verma A, Katkhuda R, Bassett R, et al. A novel patient-derived orthotopic xenograft model of esophageal adenocarcinoma provides a platform for translational discoveries. Dis Model Mech. 2019;12:dmm041004.
Read M, Liu D, Duong CP, Cullinane C, Murray WK, Fennell CM, et al. Intramuscular transplantation improves engraftment rates for esophageal patient-derived tumor xenografts. Ann Surg Oncol. 2016;23:305–11.
Okada S, Vaeteewoottacharn K, Kariya R. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine. Chem Pharm Bull. 2018;66:225–30.
Cutz JC, Guan J, Bayani J, Yoshimoto M, Xue H, Sutcliffe M, et al. Establishment in severe combined immunodeficiency mice of subrenal capsule xenografts and transplantable tumor lines from a variety of primary human lung cancers: potential models for studying tumor progression-related changes. Clin Cancer Res. 2006;12:4043–54.
Huang P, Westmoreland SV, Jain RK, Fukumura D. Spontaneous nonthymic tumors in SCID mice. Comp Med. 2011;61:227–34.
Santagostino SF, Arbona RJR, Nashat MA, White JR, Monette S. Pathology of aging in NOD scid gamma female mice. Vet Pathol. 2017;54:855–69.
van de Merbel AF, van der Horst G, van der Pluijm G. Patient-derived tumour models for personalized therapeutics in urological cancers. Nat Rev Urol. 2021;18:33–45.
Fujii E, Suzuki M, Matsubara K, Watanabe M, Chen YJ, Adachi K, et al. Establishment and characterization of in vivo human tumor models in the NOD/SCID/gamma(c)(null) mouse. Pathol Int. 2008;58:559–67.
Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–59.
Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–73.
Cho SY, Kang W, Han JY, Min S, Kang J, Lee A, et al. An integrative approach to precision cancer medicine using patient-derived xenografts. Mol Cells. 2016;39:77–86.
Ma F, Laster K, Nie W, Liu F, Kim DJ, Lee MH, et al. Heterogeneity analysis of esophageal squamous cell carcinoma in cell lines, tumor tissues and patient-derived xenografts. J Cancer. 2021;12:3930–44.
Sia D, Moeini A, Labgaa I, Villanueva A. The future of patient-derived tumor xenografts in cancer treatment. Pharmacogenomics. 2015;16:1671–83.
Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2:S134–9.
Liu J, Liu ZX, Wu QN, Lu YX, Wong CW, Miao L, et al. Long noncoding RNA AGPG regulates PFKFB3-mediated tumor glycolytic reprogramming. Nat Commun. 2020;11:1507.
Tsai MF, Chen SM, Ong AZ, Chung YH, Chen PN, Hsieh YH, et al. Shikonin induced program cell death through generation of reactive oxygen species in renal cancer cells. Antioxidants. 2021;10:1831.
Zhang Q, Liu Q, Zheng S, Liu T, Yang L, Han X, et al. Shikonin inhibits tumor growth of ESCC by suppressing PKM2 mediated aerobic glycolysis and STAT3 phosphorylation. J Cancer. 2021;12:4830–40.
Pang M, Xie X, Zhang Y, Laster KV, Liu K, Kim DJ. Ethyl ferulate suppresses esophageal squamous cell carcinoma tumor growth through inhibiting the mTOR signaling pathway. Front Oncol. 2021;11:780011.
Yang N, Lu X, Jiang Y, Zhao L, Wang D, Wei Y, et al. Arbidol inhibits human esophageal squamous cell carcinoma growth in vitro and in vivo through suppressing ataxia telangiectasia and Rad3-related protein kinase. Elife. 2022;11:e73953.
De Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57:1229–54.
Zou J, Li S, Chen Z, Lu Z, Gao J, Zou J, et al. A novel oral camptothecin analog, gimatecan, exhibits superior antitumor efficacy than irinotecan toward esophageal squamous cell carcinoma in vitro and in vivo. Cell Death Dis. 2018;9:661.
Kobayashi T, Makino T, Yamashita K, Saito T, Tanaka K, Takahashi T, et al. APR-246 induces apoptosis and enhances chemo-sensitivity via activation of ROS and TAp73-Noxa signal in oesophageal squamous cell cancer with TP53 missense mutation. Br J Cancer. 2021;125:1523–32.
Moy RH, Walch HS, Mattar M, Chatila WK, Molena D, Strong VE, et al. Defining and targeting esophagogastric cancer genomic subsets with patient-derived xenografts. JCO Precis Oncol. 2022;6:e2100242.
Su D, Zhang D, Jin J, Ying L, Han M, Chen K, et al. Identification of predictors of drug sensitivity using patient-derived models of esophageal squamous cell carcinoma. Nat Commun. 2019;10:5076.
Liu W, Miao C, Zhang S, Liu Y, Niu X, Xi Y, et al. VAV2 is required for DNA repair and implicated in cancer radiotherapy resistance. Signal Transduct Target Ther. 2021;6:322.
Roy S, Whitehead TD, Li S, Ademuyiwa FO, Wahl RL, Dehdashti F, et al. Co-clinical FDG-PET radiomic signature in predicting response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2022;49:550–62.
Nardella C, Lunardi A, Patnaik A, Cantley LC, Pandolfi PP. The APL paradigm and the "co-clinical trial" project. Cancer Discov. 2011;1:108–16.
Coussy F, Lavigne M, de Koning L, Botty RE, Nemati F, Naguez A, et al. Response to mTOR and PI3K inhibitors in enzalutamide-resistant luminal androgen receptor triple-negative breast cancer patient-derived xenografts. Theranostics. 2020;10:1531–43.
Zou J, Liu Y, Wang J, Liu Z, Lu Z, Chen Z, et al. Establishment and genomic characterizations of patient-derived esophageal squamous cell carcinoma xenograft models using biopsies for treatment optimization. J Transl Med. 2018;16:15.
Collins DC, Sundar R, Lim JSJ, Yap TA. Towards precision medicine in the clinic: from biomarker discovery to novel therapeutics. Trends Pharmacol Sci. 2017;38:25–40.
Bao Z, Li A, Lu X, Wang Z, Yu Y, Wu W, et al. Oxethazaine inhibits esophageal squamous cell carcinoma proliferation and metastasis by targeting aurora kinase A. Cell Death Dis. 2022;13:189.
Chulpanova DS, Kitaeva KV, Rutland CS, Rizvanov AA, Solovyeva VV. Mouse tumor models for advanced cancer immunotherapy. Int J Mol Sci. 2020;21:4118.
Sereti E, Karagianellou T, Kotsoni I, Magouliotis D, Kamposioras K, Ulukaya E, et al. Patient Derived Xenografts (PDX) for personalized treatment of pancreatic cancer: emerging allies in the war on a devastating cancer? J Proteom. 2018;188:107–18.
Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013.
Wu C, Wang X, Shang H, Wei H. Construction of a humanized PBMC-PDX model to study the efficacy of a bacterial marker in lung cancer immunotherapy. Dis Markers. 2022;2022:1479246.
Celik H, Krug E, Zhang CR, Han W, Issa N, Koh WK, et al. A humanized animal model predicts clonal evolution and therapeutic vulnerabilities in myeloproliferative neoplasms. Cancer Discov. 2021;11:3126–41.
Courtois J, Ritacco C, Dubois S, Canti L, Vandenhove B, Seidel L, et al. Itacitinib prevents xenogeneic GVHD in humanized mice. Bone Marrow Transpl. 2021;56:2672–81.
Kemper K, Krijgsman O, Cornelissen-Steijger P, Shahrabi A, Weeber F, Song JY, et al. Intra- and inter-tumor heterogeneity in a vemurafenib-resistant melanoma patient and derived xenografts. EMBO Mol Med. 2015;7:1104–18.
Wegner CS, Hauge A, Andersen LMK, Huang R, Simonsen TG, Gaustad JV, et al. Increasing aggressiveness of patient-derived xenograft models of cervix carcinoma during serial transplantation. Oncotarget. 2018;9:21036–51.
Pearson AT, Finkel KA, Warner KA, Nor F, Tice D, Martins MD, et al. Patient-derived xenograft (PDX) tumors increase growth rate with time. Oncotarget. 2016;7:7993–8005.
Ben-David U, Ha G, Tseng YY, Greenwald NF, Oh C, Shih J, et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet. 2017;49:1567–75.
Shi J, Li Y, Jia R, Fan X. The fidelity of cancer cells in PDX models: characteristics, mechanism and clinical significance. Int J Cancer. 2020;146:2078–88.
Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13:4.
Garcia PL, Miller AL, Yoon KJ. Patient-derived xenograft models of pancreatic cancer: overview and comparison with other types of models. Cancers. 2020;12:1327.
Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017;23:393–410.
Clinton J, McWilliams-Koeppen P. Initiation, expansion, and cryopreservation of human primary tissue-derived normal and diseased organoids in embedded three-dimensional culture. Curr Protoc Cell Biol. 2019;82:e66.
Li X, Francies HE, Secrier M, Perner J, Miremadi A, Galeano-Dalmau N, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun 2018;9:2983.
Karakasheva TA, Gabre JT, Sachdeva UM, Cruz-Acuna R, Lin EW, DeMarshall M, et al. Patient-derived organoids as a platform for modeling a patient’s response to chemoradiotherapy in esophageal cancer. Sci Rep. 2021;11:21304.
Dijkstra KK, Monkhorst K, Schipper LJ, Hartemink KJ, Smit EF, Kaing S, et al. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 2020;31:107588.
Karakasheva TA, Kijima T, Shimonosono M, Maekawa H, Sahu V, Gabre JT, et al. Generation and characterization of patient-derived head and neck, oral, and esophageal cancer organoids. Curr Protoc Stem Cell Biol. 2020;53:e109.
Sachdeva UM, Shimonosono M, Flashner S, Cruz-Acuna R, Gabre JT, Nakagawa H. Understanding the cellular origin and progression of esophageal cancer using esophageal organoids. Cancer Lett. 2021;509:39–52.
Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, Sharma M, et al. The esophageal organoid system reveals functional interplay between notch and cytokines in reactive epithelial changes. Cell Mol Gastroenterol Hepatol. 2018;5:333–52.
Jeon MJ, Haugen BR. Preclinical models of follicular cell-derived thyroid cancer: an overview from cancer cell lines to mouse models. Endocrinol Metab. 2022;37:830–8.
Tseng HC, Wu MR, Lee CH, Hsiao JK. Differentiation capacity of bone marrow-derived rat mesenchymal stem cells from DsRed and Cre transgenic Cre/loxP models. Cells. 2022;11:2769.
Le Magnen C, Dutta A, Abate-Shen C. Optimizing mouse models for precision cancer prevention. Nat Rev Cancer. 2016;16:187–96.
Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163:39–53.
Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 2006;66:3355–8. discussion 8-9
Singh M, Murriel CL, Johnson L. Genetically engineered mouse models: closing the gap between preclinical data and trial outcomes. Cancer Res. 2012;72:2695–700.
Mahmoudian RA, Farshchian M, Abbaszadegan MR. Genetically engineered mouse models of esophageal cancer. Exp Cell Res. 2021;406:112757.
Zhang Y, Bailey D, Yang P, Kim E, Que J. The development and stem cells of the esophagus. Development. 2021;148:dev193839.
Raad S, David A, Que J, Faure C. Genetic mouse models and induced pluripotent stem cells for studying tracheal-esophageal separation and esophageal development. Stem Cells Dev. 2020;29:953–66.
Tetreault MP. Esophageal cancer: insights from mouse models. Cancer Growth Metastasis. 2015;8:37–46.
Cai EY, Garcia J, Liu Y, Vakar-Lopez F, Arora S, Nguyen HM, et al. A bladder cancer patient-derived xenograft displays aggressive growth dynamics in vivo and in organoid culture. Sci Rep. 2021;11:4609.
Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180:188–204.e22.
Wunderlich M, Manning N, Sexton C, Sabulski A, Byerly L, O’Brien E, et al. Improved chemotherapy modeling with RAG-based immune deficient mice. PLoS One. 2019;14:e0225532.
Xing H, Gao M, Wang Y, Zhang X, Shi J, Wang X, et al. Genome-wide gain-of-function screening identifies EZH2 mediating resistance to PI3Kalpha inhibitors in oesophageal squamous cell carcinoma. Clin Transl Med. 2022;12:e835.
Luo XJ, He MM, Liu J, Zheng JB, Wu QN, Chen YX, et al. LncRNA TMPO-AS1 promotes esophageal squamous cell carcinoma progression by forming biomolecular condensates with FUS and p300 to regulate TMPO transcription. Exp Mol Med. 2022;54:834–47.
Li S, Hoefnagel SJM, Read M, Meijer S, van Berge Henegouwen MI, Gisbertz SS, et al. Selective targeting BMP2 and 4 in SMAD4 negative esophageal adenocarcinoma inhibits tumor growth and aggressiveness in preclinical models. Cell Oncol. 2022;45:639–58.
Ballout F, Lu H, Chen L, Sriramajayam K, Que J, Meng Z, et al. APE1 redox function is required for activation of Yes-associated protein 1 under reflux conditions in Barrett’s-associated esophageal adenocarcinomas. J Exp Clin Cancer Res. 2022;41:264.
Liu Z, Wu K, Gu S, Wang W, Xie S, Lu T, et al. A methyltransferase-like 14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer stem cell persistence and radioresistance via histone deacetylase 2-mediated epigenetic modulation in esophageal squamous cell carcinoma. Clin Transl Med. 2021;11:e545.
Xuan Y, Sheng Y, Zhang D, Zhang K, Zhang Z, Ping Y, et al. Targeting CD276 by CAR-T cells induces regression of esophagus squamous cell carcinoma in xenograft mouse models. Transl Oncol. 2021;14:101138.
Munekage E, Serada S, Tsujii S, Yokota K, Kiuchi K, Tominaga K, et al. A glypican-1-targeted antibody-drug conjugate exhibits potent tumor growth inhibition in glypican-1-positive pancreatic cancer and esophageal squamous cell carcinoma. Neoplasia. 2021;23:939–50.
Li MY, Fan LN, Han DH, Yu Z, Ma J, Liu YX, et al. Ribosomal S6 protein kinase 4 promotes radioresistance in esophageal squamous cell carcinoma. J Clin Invest. 2020;130:4301–19.
Teichman J, Dodbiba L, Thai H, Fleet A, Morey T, Liu L, et al. Hedgehog inhibition mediates radiation sensitivity in mouse xenograft models of human esophageal adenocarcinoma. PLoS One. 2018;13:e0194809.
Steins A, Klaassen R, Jacobs I, Schabel MC, van Lier M, Ebbing EA, et al. Rapid stromal remodeling by short-term VEGFR2 inhibition increases chemotherapy delivery in esophagogastric adenocarcinoma. Mol Oncol. 2020;14:704–20.
Jia X, Wang P, Huang C, Zhao D, Wu Q, Lu B, et al. Toosendanin targeting eEF2 impedes Topoisomerase I & II protein translation to suppress esophageal squamous cell carcinoma growth. J Exp Clin Cancer Res. 2023;42:97.
Wu Q, Liu F, Ge M, Laster KV, Wei L, Du R, et al. BRD4 drives esophageal squamous cell carcinoma growth by promoting RCC2 expression. Oncogene. 2022;41:347–60.
Liu J, Liu ZX, Li JJ, Zeng ZL, Wang JH, Luo XJ, et al. The macrophage-associated lncRNA MALR facilitates ILF3 liquid-liquid phase separation to promote HIF1alpha signaling in esophageal cancer. Cancer Res. 2022;83:1476–89.
Liu F, Wu Q, Han W, Laster K, Hu Y, Ma F, et al. Targeting integrin alphavbeta3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin Transl Med. 2021;11:e548.
Wu X, Wang Z, Jiang Y, Zhou H, Li A, Wei Y, et al. Tegaserod maleate inhibits esophageal squamous cell carcinoma proliferation by suppressing the peroxisome pathway. Front Oncol. 2021;11:683241.
Jia X, Huang C, Hu Y, Wu Q, Liu F, Nie W, et al. Cirsiliol targets tyrosine kinase 2 to inhibit esophageal squamous cell carcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. 2021;40:105.
Zhang Y, Shi X, Xie X, Laster KV, Pang M, Liu K, et al. Harmaline isolated from Peganum harmala suppresses growth of esophageal squamous cell carcinoma through targeting mTOR. Phytother Res 2021;35:6377–88.
Zhao L, Zhang Y, Li A, Lu X, Li M, Yuan Q, et al. Azelnidipine inhibits esophageal squamous cell carcinoma proliferation in vivo and in vitro by targeting MEK1/2. Mol Ther Oncolytics. 2022;27:61–72.
Liu X, Jiang Y, Zhou H, Zhao X, Li M, Bao Z, et al. Dasabuvir suppresses esophageal squamous cell carcinoma growth in vitro and in vivo through targeting ROCK1. Cell Death Dis. 2023;14:118.
Shi X, Zhang Y, Xie X, Pang M, Laster K, Li J, et al. Ipriflavone suppresses growth of esophageal squamous cell carcinoma through inhibiting mTOR in vitro and in vivo. Front Oncol. 2021;11:648809.
Wang D, Zhang W, Zhang X, Li M, Wu Q, Li X, et al. Daurisoline suppresses esophageal squamous cell carcinoma growth in vitro and in vivo by targeting MEK1/2 kinase. Mol Carcinog 2023;62:517–31.
Zhou Y, He X, Jiang Y, Wang Z, Yu Y, Wu W, et al. Repurposed benzydamine targeting CDK2 suppresses the growth of esophageal squamous cell carcinoma. Front Med. 2022;290–303.
Ma F, Liu F, Nie W, Laster K, Tian X, Lu B, et al. 3,3’-Diindolylmethane plus eflornithine suppress DNA replication and cell cycle in esophageal squamous cell carcinoma in vivo. J Cancer. 2022;13:2607–19.
Kijima T, Nakagawa H, Shimonosono M, Chandramouleeswaran PM, Hara T, Sahu V, et al. Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 2019;7:73–91.
Derouet MF, Allen J, Wilson GW, Ng C, Radulovich N, Kalimuthu S, et al. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep. 2020;10:14514.
Acknowledgements
We would like to thank EnPapers (www.enpapers.com/) for English language editing.
Funding
This study was supported by the General Program of Henan Natural Science Foundation (No. 232300421166), Key Scientific and Technological Projects of Henan Province (No. 212102310634) and Key Scientific and Technological Project of Xinxiang City (No. GG2021008).
Author information
Authors and Affiliations
Contributions
ZJH and LF drafted the manuscript and completed the figures and tables; XHY and ZBY collected the references; CHW revised the manuscript; ZJH provided funding support. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Review Board of Xinxiang Medical University approved the study and all patients provided written informed consent prior to any study related activities.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, F., Xu, H., Cheng, H. et al. Patient-derived tumor models: a suitable tool for preclinical studies on esophageal cancer. Cancer Gene Ther 30, 1443–1455 (2023). https://doi.org/10.1038/s41417-023-00652-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00652-9