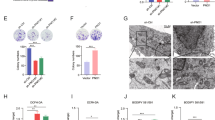
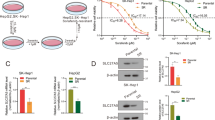
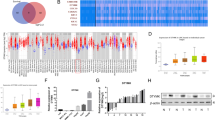
Abstract
DNA damaging agents are used as chemotherapeutics in many cancers, including hepatocellular carcinoma (HCC). However, they are associated with problems such as low sensitivity to chemotherapy and the induction of liver injury, underscoring the need to identify new therapies. Here, we investigated the differential regulatory effect of metabotropic glutamate receptor 5 (mGlu5) on chemosensitivity in HCC and chemotoxicity to the normal liver. The expression of mGlu5 was higher in HCC than in the normal liver, and correlated with poor prognosis according to The Cancer Genome Atlas database and Integrative Molecular Database of Hepatocellular Carcinoma. Cisplatin, oxaliplatin or methyl methanesulfonate (MMS) caused cell death by decreasing mGlu5 expression in HCC cells and increased mGlu5 expression in hepatic cells. In HCC cells, inhibition of mGlu5 aggravated MMS-induced DNA damage by increasing intracellular Ca2+ overload and mitogen-activated protein kinase (MAPK) activation, thereby promoting cell death, and activation of mGlu5 rescued the effect of MMS. However, in hepatic cells, mGlu5 inhibition alleviated MMS-induced DNA damage by downregulating Ca2+-derived MAPK pathways to advance hepatic cell survival. The opposite effects of mGlu5 overexpression or knockdown on MMS-induced DNA damage supported that cell death is a result of the differential regulation of mGlu5 expression. Inhibition of mGlu5 increased chemosensitivity and decreased chemotoxicity in a rat tumor model. This study suggests that mGlu5 inhibition could act synergistically with HCC chemotherapeutics with minimal side effects, which may improve the treatment of patients with HCC in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets and materials used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Shen J, Lin H, Li G, Jin RA, Shi L, Chen M, et al. TR4 nuclear receptor enhances the cisplatin chemo sensitivity via altering the ATF3 expression to better suppress HCC cell growth. Oncotarget. 2016;7:32088–99.
Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol. 2018;48:103–14.
Guan Y, Zhang Y, Hao L, Nie Z. CircRNA_102272 promotes Cisplatin-Resistance in hepatocellular carcinoma by decreasing MiR-326 targeting of RUNX2. Cancer Manag Res. 2020;12:12527–34.
Gold JM, Raja A. Cisplatin. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
Jiang Y, Shan S, Chi L, Zhang G, Gao X, Li H, et al. Methyl methanesulfonate induces necroptosis in human lung adenoma A549 cells through the PIG-3-reactive oxygen species pathway. Tumour Biol. 2016;37:3785–95.
Huang H, Chen J, Ding CM, Jin X, Jia ZM, Peng J. LncRNA NR2F1-AS1 regulates hepatocellular carcinoma oxaliplatin resistance by targeting ABCC1 via miR-363. J Cell Mol Med. 2018;22:3238–45.
Jiang Y, Shan S, Gan T, Zhang X, Lu X, Hu H, et al. Effects of cisplatin on the contractile function of thoracic aorta of Sprague-Dawley rats. Biomed Rep. 2014;2:893–7.
Milo-Cochavi S, Pareek M, Delulio G, Almog Y, Anand G, Ma LJ, et al. The response to the DNA damaging agent methyl methanesulfonate in a fungal plant pathogen. Fungal Biol. 2019;123:408–22.
Woynarowski JM, Faivre S, Herzig MC, Arnett B, Chapman WG, Trevino AV, et al. Oxaliplatin-induced damage of cellular DNA. Mol Pharm. 2000;58:920–7.
Wen L, Liang C, Chen E, Chen W, Liang F, Zhi X, et al. Regulation of multi-drug resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci Rep. 2016;6:23269.
Chung G, Kim CY, Yun YC, Yoon SH, Kim MH, Kim YK, et al. Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci Rep. 2017;7:9743.
Simonyi A, Schachtman TR, Christoffersen GR. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug N Perspect. 2005;18:353–61.
Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharm Ther. 2003;98:325–54.
Storto M, de Grazia U, Battaglia G, Felli MP, Maroder M, Gulino A, et al. Expression of metabotropic glutamate receptors in murine thymocytes and thymic stromal cells. J Neuroimmunol. 2000;109:112–20.
Balakrishnan S. TPK, Paulose CS. Glutamate (mGluR-5) gene expression in brain regions of streptozotocin induced diabetic rats as a function of age: Role in regulation of calcium release from the pancreatic islets in vitro. J Biomed Sci. 2009;16:99.
Ferrigno A, Berardo C, Di Pasqua LG, Siciliano V, Richelmi P, Nicoletti F, et al. Selective blockade of the metabotropic glutamate receptor mGluR5 protects mouse livers in in vitro and ex vivo models of ischemia reperfusion injury. Int J Mol Sci. 2018;19:314.
Storto M, Battaglia G, Gradini R, Bruno V, Nicoletti F, Vairetti M. Mouse hepatocytes lacking mGlu5 metabotropic glutamate receptors are less sensitive to hypoxic damage. Eur J Pharm. 2004;497:25–7.
Jesse CR, Wilhelm EA, Bortolatto CF, Savegnago L, Nogueira CW. Selective blockade of mGlu5 metabotropic glutamate receptors is hepatoprotective against fulminant hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. J Appl Toxicol. 2009;29:323–9.
Storto M, Ngomba RT, Battaglia G, Freitas I, Griffini P, Richelmi P, et al. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against acetaminophen hepatotoxicity in mice. J Hepatol. 2003;38:179–87.
Martino JJ, Wall BA, Mastrantoni E, Wilimczyk BJ, La Cava SN, Degenhardt K, et al. Metabotropic glutamate receptor 1 (Grm1) is an oncogene in epithelial cells. Oncogene. 2013;32:4366–76.
Wen Y, Li J, Koo J, Shin SS, Lin Y, Jeong BS, et al. Activation of the glutamate receptor GRM1 enhances angiogenic signaling to drive melanoma progression. Cancer Res. 2014;74:2499–509.
Xi SS, Bai XX, Gu L, Bao LH, Yang HM, An W, et al. Metabotropic glutamate receptor 5 mediates the suppressive effect of 6-OHDA-induced model of Parkinson’s disease on liver cancer. Pharm Res. 2017;121:145–57.
Wu YL, Wang NN, Gu L, Yang HM, Xia N, Zhang H. The suppressive effect of metabotropic glutamate receptor 5 (mGlu5) inhibition on hepatocarcinogenesis. Biochimie. 2012;94:2366–75.
Marks CR, Shonesy BC, Wang X, Stephenson JR, Niswender CM, Colbran RJ. Activated CaMKIIalpha binds to the mGlu5 metabotropic glutamate receptor and modulates calcium mobilization. Mol Pharm. 2018;94:1352–62.
Chong ZZ, Li F, Maiese K. Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and beta-catenin during oxidative stress. Curr Neurovasc Res. 2006;3:107–17.
Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–52.
Jain R, Watson U, Vasudevan L, Saini DK. ERK activation pathways downstream of GPCRs. Int Rev Cell Mol Biol. 2018;338:79–109.
Gorvin CM. Insights into calcium-sensing receptor trafficking and biased signalling by studies of calcium homeostasis. J Mol Endocrinol. 2018;61:R1–R12.
Xia N, Zhang Q, Wang ST, Gu L, Yang HM, Liu L, et al. Blockade of metabotropic glutamate receptor 5 protects against DNA damage in a rotenone-induced Parkinson’s disease model. Free Radic Biol Med. 2015;89:567–80.
Jong YJ, Sergin I, Purgert CA, O’Malley KL. Location-dependent signaling of the group 1 metabotropic glutamate receptor mGlu5. Mol Pharm. 2014;86:774–85.
Chang K, Roche KW. Structural and molecular determinants regulating mGluR5 surface expression. Neuropharmacology. 2017;115:10–9.
Zhang YL, Xue G, Miao H, Zhou CC, Sun SH, Zhang Y. Folic acid supplementation acts as a chemopreventive factor in tumorigenesis of hepatocellular carcinoma by inducing H3K9Me2-dependent transcriptional repression of LCN2. Oncotarget. 2021;12:366–78.
Yang B, Feng X, Liu H, Tong R, Wu J, Li C, et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene. 2020;39:6529–43.
Zhang WY, Cai N, Ye LH, Zhang XD. Transformation of human liver L-O2 cells mediated by stable HBx transfection. Acta Pharm Sin. 2009;30:1153–61.
Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79.
Wada F, Koga H, Akiba J, Niizeki T, Iwamoto H, Ikezono Y, et al. High expression of CD44v9 and xCT in chemoresistant hepatocellular carcinoma: potential targets by sulfasalazine. Cancer Sci. 2018;109:2801–10.
Bashraf O, Ali AS, Eweis H, Ali SS. Protective effects of low dose vorinostat on cisplatin-induced nephrotoxicity in rats. Curr Mol Pharm. 2021;14:635–45.
Abdel-Daim MM, Abushouk AI, Donia T, Alarifi S, Alkahtani S, Aleya L, et al. The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environ Sci Pollut Res Int. 2019;26:13502–9.
Unel CC, Erol K. The role of ionic homeostasis in Cisplatin-Induced neurotoxicity: a preliminary study. Eurasia J Med. 2018;50:81–5.
Yuan J, Adamski R, Chen J. Focus on histone variant H2AX: to be or not to be. Febs Lett. 2010;584:3717–24.
Slamenová D, Kováciková I, Horváthová E, Wsólová L, Navarová J. Carboxymethyl chitin-glucan (CM-CG) protects human HepG2 and HeLa cells against oxidative DNA lesions and stimulates DNA repair of lesions induced by alkylating agents. Toxicol Vitr. 2010;24:1986–92.
Rajan I, Jayasree PR, Kumar PR. Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells. Tumour Biol. 2015;36:8479–89.
Moore M, Thor H, Moore G, Nelson S, Moldeus P, Orrenius S. The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+. J Biol Chem. 1985;260:13035–40.
Grolla AA, Fakhfouri G, Balzaretti G, Marcello E, Gardoni F, Canonico PL, et al. Abeta leads to Ca(2)(+) signaling alterations and transcriptional changes in glial cells. Neurobiol Aging. 2013;34:511–22.
Levi M, DeRemer SJ, Dou C, Ensminger WD, Smith DE. Disposition of WR-1065 in the liver of tumor-bearing rats following regional vs systemic administration of amifostine. Biopharm Drug Dispos. 2004;25:27–35.
Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499–507.
Abd Rashid N, Hussan F, Hamid A, Adib Ridzuan NR, Halim SASA, Abdul Jalil NA, et al. Polygonum minus essential oil modulates cisplatin-induced hepatotoxicity through inflammatory and apoptotic pathways. Excli J. 2020;19:1246–65.
Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct–ligated mice. Hepatology. 2003;38:355–63.
Khan MW, Zhao P, Khan A, Raza F, Raza SM, Sarfraz M, et al. Synergism of cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles on hepatocellular carcinoma cells for enhanced apoptosis and reduced hepatotoxicity. Int J Nanomed. 2019;14:3753–71.
Lee JY, Choe ES, Yang CH, Choi KH, Cheong JH, Jang CG, et al. The mGluR5 antagonist MPEP suppresses the expression and reinstatement, but not the acquisition, of the ethanol-conditioned place preference in mice. Pharm Biochem Behav. 2016;140:33–8.
Sanderson BJ, Shield AJ. Mutagenic damage to mammalian cells by therapeutic alkylating agents. Mutat Res. 1996;355:41–57.
Lajous H, Lelièvre B, Vauléon E, Lecomte P, Garcion E. Rethinking Alkylating(-Like) agents for solid tumor management. Trends Pharm Sci. 2019;40:342–57.
Singh RK, Kumar S, Prasad DN, Bhardwaj TR. Therapeutic journey of nitrogen mustard as alkylating anticancer agents: historic to future perspectives. Eur J Med Chem. 2018;151:401–33.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharm. 2014;740:364–78.
Mikula-Pietrasik J, Niklas A, Uruski P, Tykarski A, Ksiazek K. Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells. Cell Mol Life Sci. 2020;77:213–29.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95.
Abd-Elrahman KS, Hamilton A, Vasefi M, Ferguson SSG. Autophagy is increased following either pharmacological or genetic silencing of mGluR5 signaling in Alzheimer’s disease mouse models. Mol Brain. 2018;11:19.
Ribeiro FM, Vieira LB, Pires RG, Olmo RP, Ferguson SS. Metabotropic glutamate receptors and neurodegenerative diseases. Pharm Res. 2017;115:179–91.
Litim N, Morissette M, Di Paolo T. Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease: An update from the last 5 years of research. Neuropharmacology. 2017;115:166–79.
Yu LJ, Wall BA, Wangari-Talbot J, Chen S. Metabotropic glutamate receptors in cancer. Neuropharmacology. 2017;115:193–202.
Jin DZ, Guo ML, Xue B, Mao LM, Wang JQ. Differential regulation of CaMKIIα interactions with mGluR5 and NMDA receptors by Ca(2+) in neurons. J Neurochem. 2013;127:620–31.
Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets. 2010;9:574–95.
Dai SH, Qin N, Chen T, Luo P, Zhang L, Rao W, et al. Activation of mGluR5 attenuates NMDA-induced neurotoxicity through disruption of the NMDAR-PSD-95 complex and preservation of mitochondrial function in differentiated PC12 cells. Int J Mol Sci. 2014;15:10892–907.
Negri S, Faris P, Maniezzi C, Pellavio G, Spaiardi P, Botta L, et al. NMDA receptors elicit flux-independent intracellular Ca2+ signals via metabotropic glutamate receptors and flux-dependent nitric oxide release in human brain microvascular endothelial cells. Cell Calcium. 2021;99:102454.
Feng S, Wei Q, Hu Q, Huang X, Zhou X, Luo G, et al. Research progress on the relationship between acute pancreatitis and calcium overload in acinar cells. Dig Dis Sci. 2019;64:25–38.
Thiel A, Hamel A, Schaefer K, Cardoso R, Beilstein P. Slight hypercalcemia is not associated with positive responses in the Comet Assay in male rat liver. Mutat Res. 2017;820:26–30.
Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–95.
Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–97.
Calebiro D, Godbole A, Lyga S, Lohse MJ. Trafficking and function of GPCRs in the endosomal compartment. Methods Mol Biol. 2015;1234:197–211.
Sergin I, Jong YI, Harmon SK, Kumar V, O’Malley KL. Sequences within the C Terminus of the Metabotropic Glutamate Receptor 5 (mGluR5) Are Responsible for Inner Nuclear Membrane Localization. J Biol Chem. 2017;292:3637–55.
Acknowledgements
The authors thank all members in the group for helpful suggestions. We thank International Science Editing (http://www.internationalscienceediting.com, quotation number ISE 86005) for editing this manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (81972231, 81372587, and 81171886).
Author information
Authors and Affiliations
Contributions
HZ designed the research program and charged correspondence; HMY performed the most of experiments, analyzed the data and wrote the manuscript; TZH, YNZ and SDZ performed the part of experiments; YLW collected patient samples. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee of Capital Medical University (2019SY040). All experimental protocols involving animals were approved by the Committee on Animal Care and Usage of Capital Medical University (SCXK 2016-0006).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, HM., Hou, TZ., Zhang, YN. et al. Blocked metabotropic glutamate receptor 5 enhances chemosensitivity in hepatocellular carcinoma and attenuates chemotoxicity in the normal liver by regulating DNA damage. Cancer Gene Ther 29, 1487–1501 (2022). https://doi.org/10.1038/s41417-022-00465-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00465-2