Abstract
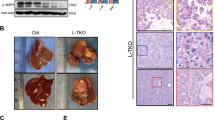
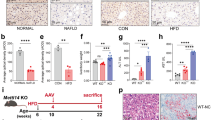
Macrophages plays a vital role in the development of non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC), but the polarization of macrophages was not consistent in previous reports and the contribution of hepatocytes to macrophage polarization is not clear. Here, we show that in clinical NASH and HCC samples, impaired Dicer activity was common and correlated with increased M1-like macrophages. Mice with Dicer deletion in hepatocytes could induce macrophages M1 polarization either in the development of NASH under high fat diet feeding, or in the carcinogenesis of HCC after DEN treatment. In hepatic cells, Dicer deletion delivered distinct lipid profile and increased lipid oxidation. Mechanically, Dicer deletion caused declined miR-192-3p and increased IGF2 in hepatocytes. Restoring miR-192-3p could suppress IGF2 and inhibit macrophage infiltration in the liver tissue, as well as reduce the lipid de novo synthesis and peroxidation. Overall, our data highlights the central role of Dicer-associated miR-192-3p in the etiopathogenesis of macrophage M1 polarization in NASH and HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data in this study is available upon request.
References
Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71:1851–64.
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–83.
Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
Kazankov K, Jorgensen SMD, Thomsen KL, Moller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59.
Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542–52.
Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y, et al. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304–15.e1-4.
Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98.
Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–83.
Sun LN, Xing C, Zhi Z, Liu Y, Chen LY, Shen T, et al. Dicer suppresses cytoskeleton remodeling and tumorigenesis of colorectal epithelium by miR-324-5p mediated suppression of HMGXB3 and WASF-2. Oncotarget. 2017;8:55776–89.
Liu Y, Song L, Ni H, Sun L, Jiao W, Chen L, et al. ERBB4 acts as a suppressor in the development of hepatocellular carcinoma. Carcinogenesis. 2017;38:465–73.
Liu Y, Song C, Ni H, Jiao W, Gan W, Dong X, et al. UBE2L3, a susceptibility gene that plays oncogenic role in hepatitis B-related hepatocellular carcinoma. J Viral Hepat. 2018;25:1363–71.
Gill RM, Kakar S. Nonalcoholic steatohepatitis: diagnostic challenges. Surg Pathol Clin. 2013;6:227–57.
Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878.
Ni H, Chen Y, Xia W, Wang C, Hu C, Sun L, et al. SATB2 defect promotes colitis and colitis-associated colorectal cancer by impairing Cl-/HCO3- exchange and homeostasis of gut microbiota. J Crohns Colitis. 2021;15:2088–102.
Sturm G, Finotello F, Petitprez F, Zhang JD, Baumbach J, Fridman WH, et al. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics. 2019;35:i436–45.
Lopez BG, Tsai MS, Baratta JL, Longmuir KJ, Robertson RT. Characterization of Kupffer cells in livers of developing mice. Comp Hepatol. 2011;10:2.
Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–405.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–42.
Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–32.
Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722–36.
Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–22.
Gu L, Zhu Y, Lin X, Lu B, Zhou X, Zhou F, et al. The IKKbeta-USP30-ACLY axis controls lipogenesis and tumorigenesis. Hepatology. 2021;73:160–74.
Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20:R321–39.
Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–8.
Bae MH, Lee MJ, Bae SK, Lee OH, Lee YM, Park BC, et al. Insulin-like growth factor II (IGF-II) secreted from HepG2 human hepatocellular carcinoma cells shows angiogenic activity. Cancer Lett. 1998;128:41–6.
Wang X, Lin L, Lan B, Wang Y, Du L, Chen X, et al. IGF2R-initiated proton rechanneling dictates an anti-inflammatory property in macrophages. Sci Adv. 2020;6:eabb7389.
Funding
This work was supported by the National Natural Science Foundation (No. 81972259, 31471189), the National Science Fund for Distinguished Young Scholars (No. 81525020), and the discipline construction and support project (No. XKTJ-JD202006) and the Hospital Youth aid project (No. SDFEYQN1901) of Second Affiliated Hospital of Soochow University. This work was also supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
YL and JL: study concept and design; CH, XL, YC, YZ, YY, QZ, HN, and WG: acquisition of data; YS, LS, and YL: analysis and interpretation of data; YL: drafting of the manuscript; CH and YL: statistical analysis; WG and LS: technical or material support; YL and JL: obtained funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, C., Li, X., Sui, Y. et al. Dicer deletion in hepatocytes promotes macrophages M1 polarization through dysregulated miR-192-3p/IGF2 in non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Gene Ther 29, 1252–1262 (2022). https://doi.org/10.1038/s41417-022-00432-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00432-x