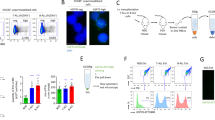
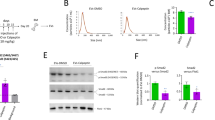
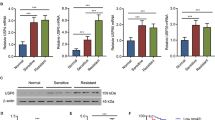
Abstract
microRNAs (miRNAs or miRs) can be delivered from acute myeloid leukemia (AML) cells to hematopoietic stem cells (HSCs) to regulate hematopoietic function via extracellular vesicles (EVs). In this study, we investigated the roles played by EVs that transport miR-548ac from AML cells in normal hematopoiesis. Bioinformatics analysis demonstrated that miR-548ac was highly expressed in AML-derived EVs. The expression of miR-548ac and TRIM28 and the targeting relationship were identified, and the results demonstrated that the expression of miR-548ac was upregulated in AML cell lines and AML cell-secreted EVs compared with CD34+ HSCs. AML-derived EVs targeted CD34+ HSCs to induce decreased expression of TRIM28 and downstream activation of STAT3. Exosomal miR-548ac was transferred into CD34+ HSCs to target TRIM28. Through gain- and loss-of-function assays, it was observed that the abrogated expression of miR-548ac or STAT3 promoted colony-forming units (CFU), whereas overexpressed miR-548ac repressed CFU, which was rescued by overexpression of TRIM28. Taken together, these results indicated that miR-548ac delivered by AML cell-derived EVs inhibits hematopoiesis via TRIM28-dependent STAT3 activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood 2016;127:42–52.
Lehrnbecher T, Sung L. Anti-infective prophylaxis in pediatric patients with acute myeloid leukemia. Expert Rev Hematol. 2014;7:819–30.
Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29:475–86.
Ye M, Zhang H, Yang H, Koche R, Staber PB, Cusan M, et al. Hematopoietic differentiation is required for initiation of acute myeloid leukemia. Cell Stem Cell. 2015;17:611–23.
Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–11.
Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139–43.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617–38.
Gargiulo E, Paggetti J, Moussay E. Hematological malignancy-derived small extracellular vesicles and tumor microenvironment: the art of turning foes into friends. Cells. 2019;8:511.
Marcucci G, Mrozek K, Radmacher MD, Garzon R, Bloomfield CD. The prognostic and functional role of microRNAs in acute myeloid leukemia. Blood 2011;117:1121–9.
Hornick NI, Doron B, Abdelhamed S, Huan J, Harrington CA, Shen R, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal. 2016;9:ra88.
Wolf D, Goff SP. TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 2007;131:46–57.
Kamitani S, Togi S, Ikeda O, Nakasuji M, Sakauchi A, Sekine Y, et al. Kruppel-associated box-associated protein 1 negatively regulates TNF-alpha-induced NF-kappaB transcriptional activity by influencing the interactions among STAT3, p300, and NF-kappaB/p65. J Immunol. 2011;187:2476–83.
King CA. Kaposi’s sarcoma-associated herpesvirus kaposin B induces unique monophosphorylation of STAT3 at serine 727 and MK2-mediated inactivation of the STAT3 transcriptional repressor TRIM28. J Virol. 2013;87:8779–91.
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood 2011;117:5701–9.
Kanna R, Choudhary G, Ramachandra N, Steidl U, Verma A, Shastri A. STAT3 inhibition as a therapeutic strategy for leukemia. Leuk Lymphoma. 2018;59:2068–74.
Wallace JA, O’Connell RM. MicroRNAs and acute myeloid leukemia: therapeutic implications and emerging concepts. Blood 2017;130:1290–301.
Huang Q, Wang L, Ran Q, Wang J, Wang C, He H, et al. Notopterol-induced apoptosis and differentiation in human acute myeloid leukemia HL-60 cells. Drug Des Dev Ther. 2019;13:1927–40.
Nikkhah H, Safarzadeh E, Shamsasenjan K, Yousefi M, Lotfinejad P, Talebi M, et al. The effect of bone marrow mesenchymal stem cells on the granulocytic differentiation of HL-60 cells. Turk J Hematol. 2018;35:42–48.
Bak RO, Dever DP, Porteus MH. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat Protoc. 2018;13:358–76.
Kornafel T, Tsao EY, Sabelhaus E, Surges L, Apkon SD. Physical and occupational therapy for a teenager with acute flaccid myelitis: a case report. Phys Occup Ther Pediatr. 2017;37:485–595.
Bill MA, Fuchs JR, Li C, Yui J, Bakan C, Benson DM Jr., et al. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with antitumor activity. Mol Cancer. 2010;9:165.
Hosoya T, Clifford M, Losson R, Tanabe O, Engel JD. TRIM28 is essential for erythroblast differentiation in the mouse. Blood 2013;122:3798–807.
Tsuruma R, Ohbayashi N, Kamitani S, Ikeda O, Sato N, Muromoto R, et al. Physical and functional interactions between STAT3 and KAP1. Oncogene 2008;27:3054–9.
Shastri A, Choudhary G, Teixeira M, Gordon-Mitchell S, Ramachandra N, Bernard L, et al. Antisense STAT3 inhibitor decreases viability of myelodysplastic and leukemic stem cells. J Clin Invest. 2018;128:5479–88.
De Kouchkovsky I, Abdul-Hay M. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. 2016;6:e441.
Duarte D, Hawkins ED, Akinduro O, Ang H, De Filippo K, Kong IY, et al. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell. 2018;22:64–77. e66
Litwinska Z, Luczkowska K, Machalinski B. Extracellular vesicles in hematological malignancies. Leuk Lymphoma. 2019;60:29–36.
Murillo OD, Thistlethwaite W, Rozowsky J, Subramanian SL, Lucero R, Shah N, et al. exRNA atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell 2019;177:463–77. e415
Boyiadzis M, Whiteside TL. Exosomes in acute myeloid leukemia inhibit hematopoiesis. Curr Opin Hematol. 2018;25:279–84.
Kotaki R, Koyama-Nasu R, Yamakawa N, Kotani A. miRNAs in normal and malignant hematopoiesis. Int J Mol Sci. 2017; 18:1495.
Zhao C, Du F, Zhao Y, Wang S, Qi L. Acute myeloid leukemia cells secrete microRNA-4532-containing exosomes to mediate normal hematopoiesis in hematopoietic stem cells by activating the LDOC1-dependent STAT3 signaling pathway. Stem Cell Res Ther. 2019;10:384.
Czerwinska P, Mazurek S, Wiznerowicz M. The complexity of TRIM28 contribution to cancer. J Biomed Sci. 2017;24:63.
Barde I, Rauwel B, Marin-Florez RM, Corsinotti A, Laurenti E, Verp S, et al. A KRAB/KAP1-miRNA cascade regulates erythropoiesis through stage-specific control of mitophagy. Science 2013;340:350–3.
Qi Z, Cai S, Cai J, Chen L, Yao Y, Chen L, et al. miR-491 regulates glioma cells proliferation by targeting TRIM28 in vitro. BMC Neurol. 2016;16:248.
Oh IH, Eaves CJ. Overexpression of a dominant negative form of STAT3 selectively impairs hematopoietic stem cell activity. Oncogene 2002;21:4778–87.
Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–67.
Ogura M, Uchida T, Terui Y, Hayakawa F, Kobayashi Y, Taniwaki M, et al. Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci. 2015;106:896–901.
Reilley MJ, McCoon P, Cook C, Lyne P, Kurzrock R, Kim Y, et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer. 2018;6:119.
Funding
This work was supported by the Foundation of Science and Technology Department of Jilin Province (20190101006JH, 20200404175YY), Foundation of the Education Department of Jilin Province (no. JJKH20200462KJ), Foundation of Health and Family Planning Commission of Jilin Province (no. 2019J066), Foundation of the Administration of Traditional Chinese Medicine of Jilin Province (no. 2019138), Foundation of Suzhou Institute of medical engineering, Chinese Academy of Sciences-Jilin Science and technology cooperation project (no. E0550101), Jilin Collaborative Innovation Center for Antibody Engineering, Jilin Medical University (no. 20180623045TC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, C., Zhao, Y., Zhao, J. et al. Acute myeloid leukemia cell-derived extracellular vesicles carrying microRNA-548ac regulate hematopoietic function via the TRIM28/STAT3 pathway. Cancer Gene Ther 29, 918–929 (2022). https://doi.org/10.1038/s41417-021-00378-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00378-6
This article is cited by
-
A novel therapeutic strategy: the significance of exosomal miRNAs in acute myeloid leukemia
Medical Oncology (2024)