Abstract
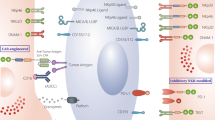
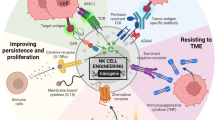
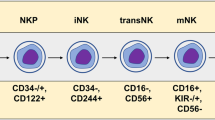
In recent years, immune cell-based cancer therapeutics have been utilized broadly in the clinic. Through advances in cellular engineering, chimeric antigen receptor (CAR) T-cell therapies have demonstrated substantial success in treating hematological tumors and have become the most prominent cell-based therapy with three commercialized products in the market. However, T-cell-based immunotherapies have certain limitations, including a restriction to autologous cell sources to avoid severe side-effects caused by human leukocyte antigen (HLA) mismatch. This necessity for personalized treatment inevitably results in tremendous manufacturing and time costs, reducing accessibility for many patients. As an alternative strategy, natural killer (NK) cells have emerged as potential candidates for improved cell-based immunotherapies. NK cells are capable of killing cancer cells directly without requiring HLA matching. Furthermore, NK cell-based therapies can use various allogeneic cell sources, allowing for the possibility of “off-the-shelf” immunotherapies with reduced side-effects and shortened manufacturing times. Here we provide an overview of the use of NK cells in cancer immunotherapy, their current status in clinical trials, as well as the design and implementation of delivery technologies—including viral, non-viral, and nanoparticle-based approaches—for engineering NK cell-based immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol. 2019;10:1–16.
Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacol Ther 2020;205:107419.
Badrinath N, Yoo SY. Recent advances in cancer stem cell-targeted immunotherapy. Cancers (Basel). 2019;11. https://doi.org/10.3390/cancers11030310.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96.
June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7:1–9.
Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics. 2016;3:16006.
Riddell shivani S, SR. CAR T cell therapy: challenges to bench-to-bedside efficacy. Physiol Behav. 2017;176:139–48.
Rose S. First-ever CAR T-cell therapy approved in U.S. Cancer Discov. 2017;7:OF1.
Calmes-Miller J. FDA approves second CAR T-cell therapy. Cancer Discov. 2018;8:5–6.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42.
Wei J, Han X, Bo J, Han W. Target selection for CAR-T therapy. J Hematol Oncol 2019;12:62.
Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a ‘safety switch’ to limit on-target, off-tumor toxicities of chimeric antigen receptor T-cells. Front Pharmacol. 2014;5. https://doi.org/10.3389/fphar.2014.00235.
Graham C, Jozwik A, Pepper A, Benjamin R. Allogeneic CAR-T cells. More than ease of access?. Cells. 2018;7:155.
Pfefferle A, Huntington ND. You have got a fast car: chimeric antigen receptor nk cells in cancer therapy. Cancers (Basel). 2020;12:1–23.
Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. 2015;5:1–13.
Li YEE, Hermanson D, Moriarity B, Bjordahl R, Mahmood S, Valamehr B, et al. Engineering human induced pluripotent stem cells with novel chimeric antigen receptors to generate natural killer (NK) cell cancer immunotherapies with targeted anti-tumor activity. Blood. 2017;130:1905–1905.
Liu E, Marin D, Banerjee P, MacApinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–53.
Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–31.
Esser R, Müller T, Stefes D, Kloess S, Seidel D, Gillies SD, et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16:569–81.
Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 2014. https://doi.org/10.1016/j.molonc.2013.12.001.
Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181.e5.
Burger MC, Zhang C, Harter PN, Romanski A, Strassheimer F, Senft C, et al. CAR-engineered NK cells for the treatment of glioblastoma: turning innate effectors into precision tools for cancer immunotherapy. Front Immunol. 2019;10:1–16.
Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–301.
Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8.
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 2020;59. https://doi.org/10.1016/j.ebiom.2020.102975.
Shaffer BC, Le Luduec JB, Forlenza C, Jakubowski AA, Perales MA, Young JW, et al. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:705–9.
Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–44.
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223.
Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42.
Damele L, Ottonello S, Mingari MC, Pietra G, Vitale C. Targeted therapies: friends or foes for patient’s NK cell-mediated tumor immune-surveillance? Cancers (Basel). 2020;12:774.
Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, et al. Markers and function of human NK cells in normal and pathological conditions. Cytom Part B Clin Cytom. 2017;92:100–14.
Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19.
Zhang J, Zheng H, Diao Y. Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20020317.
Lupo KB, Matosevic S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers (Basel) 2019;11. https://doi.org/10.3390/cancers11060769.
Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol. 2017;8. https://doi.org/10.3389/fimmu.2017.00631.
Baggio L, Laureano ÁM, Silla LM, da R, Lee DA. Natural killer cell adoptive immunotherapy: coming of age. Clin Immunol. 2017;177:3–11.
Yang S, Wen J, Li H, Xu L, Liu Y, Zhao N, et al. Aptamer-engineered natural killer cells for cell-specific adaptive immunotherapy. Small. 2019;15:1–11.
Screpanti V, Wallin RPA, Ljunggren H-G, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol. 2001;167:2068–73.
Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR. Trail-coated leukocytes that kill cancer cells in the circulation. Proc Natl Acad Sci USA. 2014;111:930–5.
Mitchell MJ, Webster J, Chung A, Guimarães PPG, Khan OF, Langer R. Polymeric mechanical amplifiers of immune cytokine-mediated apoptosis. Nat Commun. 2017;8:1–13.
Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29.
MacKay M, Afshinnekoo E, Rub J, Hassan C, Khunte M, Baskaran N, et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat Biotechnol. 2020;38:233–44.
Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:1–12.
Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Opin Immunol 2018;51:146–53.
Allife Medical Science and Technology, Co., Ltd. Study of anti-CD19 CAR NK cells in relapsed and refractory B cell lymphoma. Allife Medical Science and Technology. 2018.
Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - Advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:1–7.
Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32.
Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–70.
Yan Y, Steinherz P, Klingemann HG, Dennig D, Childs BH, McGuirk J, et al. Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res. 1998;4:2859–68.
Klingemann HG, Wong E, Maki G. A cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from blood. Biol Blood Marrow Transpl. 1996;2:68–75.
Jochems C, Hodge JW, Fantini M, Fujii R, Morillon YM, Greiner JW, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–73.
Liu H, Yang B, Sun T, Lin L, Hu Y, Deng M, et al. Specific growth inhibition of ErbB2-expressing human breast cancer cells by genetically modified NK-92 cells. Oncol Rep. 2015;33:95–102.
Chen X, Han J, Chu J, Zhang L, Zhang J, Chen C, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7:27764–77.
Pomeroy EJ, Hunzeker JT, Kluesner MG, Lahr WS, Smeester BA, Crosby MR, et al. A genetically engineered primary human natural killer cell platform for cancer immunotherapy. Mol Ther. 2019;28:52–63.
Yang Y, Lim O, Kim TM, Ahn YO, Choi H, Chung H, et al. Phase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 2016;4:215–24.
Orange J. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–26.
Herrera L, Santos S, Vesga MA, Anguita J, Martin-Ruiz I, Carrascosa T, et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep. 2019;9:1–10.
Luevano M, Daryouzeh M, Alnabhan R, Querol S, Khakoo S, Madrigal A, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol. 2012;73:248–57.
Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJN, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–83.
Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells. 2016;34:93–101.
Shankar K, Capitini CM, Saha K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res Ther. 2020. https://doi.org/10.1186/s13287-020-01741-4.
Kaufman DS, Bjordahl R, Zhu H, Blum R, Bahena A, Mahmood S, et al. Off-the-shelf natural killer cells with multi-functional engineering using a novel anti-CD19 chimeric antigen receptor combined with stabilized CD16 and IL15 expression to enhance directed anti-tumor activity. Blood. 2018;132:4541.
Cytovia Therapeutics. UPDATE - Cytovia therapeutics acquires worldwide rights to cytoImmune therapeutics’ first-in-class EGFR dual-targeting CAR for NK cell treatment of glioblastoma & other solid tumors. Cytovia Therapeutics; 2020.
Cytovia Therapeutics. Cytovia Therapeutics, Inc. appoints Dr. Wei Li as Chief Scientific Officer to accelerate the development of iPSC CAR-NK Cell Therapy for Cancer. GlobeNewswire. Cytovia Therapeutics; 2020.
Shimasaki N, Fujisaki H, Cho D, Masselli M, Lockey T, Eldridge P, et al. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy. 2012;14:830–40.
Cho FN, Chang TH, Shu CW, Ko MC, Liao SK, Wu KH, et al. Enhanced cytotoxicity of natural killer cells following the acquisition of chimeric antigen receptors through trogocytosis. PLoS ONE 2014;9. https://doi.org/10.1371/journal.pone.0109352.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–42.
Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–50.
Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J Immunol Res. 2018;2018. https://doi.org/10.1155/2018/4054815.
Littwitz E, Francois S, Gibbert K. Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection. 2013. https://doi.org/10.1186/1742-4690-10-127.
Streltsova MA, Barsov E, Erokhina SA, Kovalenko EI. Retroviral gene transfer into primary human NK cells activated by IL-2 and K562 feeder cells expressing membrane-bound IL-21. J Immunol Methods. 2017;450:90–94.
Guven H, Konstantinidis KV, Alici E, Aints A, Abedi-Valugerdi M, Christensson B, et al. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol. 2005;33:1320–8.
Lapteva N, Parihar R, Rollins LA, et al. Large-scale culture and genetic modification of human natural killer cells for cellular therapy. In: Methods in Molecular Biology. Humana; 2016. p. 195–202.
Davis HE, Morgan JR, Yarmush ML. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys Chem. 2002;97:159–72.
Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310.
Denning W, Das S, Guo S, Xu J, Kappes JC, Hel Z. Optimization of the transductional efficiency of lentiviral vectors: effect of sera and polycations. Mol Biotechnol. 2013;53:308–14.
Lowe E, Truscott LC, De Oliveira SN. In vitro generation of human NK cells expressing chimeric antigen receptor through differentiation of gene-modified hematopoietic stem cells. Methods Mol. Biol. 2016. https://doi.org/10.1007/978-1-4939-3684-7_20.
Carlsten M, Childs RW. Genetic manipulation of NK cells for cancer immunotherapy: Techniques and clinical implications. Front Immunol 2015;6:266.
Li L, Liu LN, Feller S, Allen C, Shivakumar R, Fratantoni J, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147–54.
Boissel L, Betancur M, Lu W, Wels WS, Marino T, Van Etten RA, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma. 2012;53:958–65.
Park H, Awasthi A, Ayello J, Chu Y, Riddell S, Rosenblum J, et al. ROR1-specific chimeric antigen receptor (CAR) NK cell immunotherapy for high risk neuroblastomas and sarcomas. Biol Blood Marrow Transplant. 2017;23:S136–S137.
Chen ZH, Yu YP, Zuo ZH, Nelson JB, Michalopoulos GK, Monga S, et al. Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene. Nat Biotechnol. 2017;35:543–50.
Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007;6:255–60.
Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol 2003;4:815.
Nakamura K, Nakayama M, Kawano M, Amagai R, Ishii T, Harigae H, et al. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc Natl Acad Sci USA. 2013;110:9421–6.
Somanchi SS, Somanchi A, Cooper LJN, Lee DA. Engineering lymph node homing of ex vivo-expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood. 2012;119:5164–72.
Somanchi A, Lee DA, Somanchi SS. Engineering receptor expression on natural killer cells through trogocytosis. In: Methods in Molecular Biology. Humana; 2016. p. 253–65.
Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–50.
Tong S, Moyo B, Lee CM, Leong K, Bao G. Engineered materials for in vivo delivery of genome-editing machinery. Nat Rev Mater. 2019. https://doi.org/10.1038/s41578-019-0145-9.
McKinlay CJ, Benner NL, Haabeth OA, Waymouth RM, Wender PA. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc Natl Acad Sci USA. 2018;115:E5859–E5866.
McKinlay CJ, Vargas JR, Blake TR, Hardy JW, Kanada M, Contag CH, et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc Natl Acad Sci USA. 2017;114:E448–E456.
Wilk AJ, Benner NL, Vergara R, Haabeth OAW, Levy R, Waymouth RM, et al. Charge-altering releasable transporters enable specific phenotypic manipulation of resting primary natural killer cells. bioRxiv:2020.02.28.970491 [Preprint] 2020. Available from: https://doi.org/10.1101/2020.02.28.970491.
Han X, Mitchell MJ, Nie G. Nanomaterials for therapeutic RNA delivery. Matter. 2020;3:1948–75.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2020. https://doi.org/10.1038/s41573-020-0090-8.
Mitchell MJ, Jain RK, Langer R. Engineering and physical sciences in oncology: challenges and opportunities. Nat Rev Cancer. 2017;17:659–75.
Mukalel AJ, Riley RS, Zhang R, Mitchell MJ. Nanoparticles for nucleic acid delivery: Applications in cancer immunotherapy. Cancer Lett. 2019;458:102–12.
Granot Y, Peer D. Delivering the right message: challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—an innate immune system standpoint. Semin Immunol. 2017;34:68–77.
Allison SJ, Milner J. RNA interference by single- and double-stranded siRNA with a DNA extension containing a 3′ nuclease-resistant mini-hairpin structure. Mol Ther Nucleic Acids 2014;2. https://doi.org/10.1038/mtna.2013.68.
Gan Z, Lokugamage MP, Hatit MZC, Loughrey D, Paunovska K, Sato M, et al. Nanoparticles containing constrained phospholipids deliver mRNA to liver immune cells in vivo without targeting ligands. Bioeng Transl Med. 2020;5:e10161.
Shobaki N, Sato Y, Suzuki Y, Okabe N, Harashima H. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. J Control Release. 2020;325:235–48.
Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–89.
Meza Guzman LG, Keating N, Nicholson SE. Natural killer cells: tumor surveillance and signaling. Cancers (Basel) 2020;12:952.
Siegler EL, Kim YJ, Chen X, Siriwon N, Mac J, Rohrs JA, et al. Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers. Mol Ther. 2017;25:2607–19.
Wang X, Lang S, Tian Y, Zhang J, Yan X, Fang Z, et al. Glycoengineering of natural killer cells with CD22 ligands for enhanced anticancer immunotherapy. ACS Appl Mater Interfaces 2020;6:382–9.
Hong S, Yu C, Wang P, Shi Y, Cheng B, Chen M, et al. Glycoengineering of NK cells with glycan ligands of CD22 and selectins for B-cell lymphoma therapy. 2021;60:3603–10.
Zhang D, Zheng Y, Lin Z, Lan S, Zhang X, Zheng A, et al. Artificial engineered natural killer cells combined with antiheat endurance as a powerful strategy for enhancing photothermal-immunotherapy efficiency of solid tumors. Small. 2019;15:1–13.
Zu Y. Aptamer technology: a new approach to treat lymphoma? Blood Sci. 2020;2:11–15.
Guimarães PPG, Gaglione S, Sewastianik T, Carrasco RD, Langer R, Mitchell MJ. Nanoparticles for immune cytokine TRAIL-based cancer therapy. ACS Nano. 2018;12:912–31.
Wayne EC, Chandrasekaran S, Mitchell MJ, Chan MF, Lee RE, Schaffer CB, et al. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer. J Control Release. 2016;223:215–223.
Chandrasekaran S, Chan MF, Li J, King MR. Super natural killer cells that target metastases in the tumor draining lymph nodes. 2017;77:66–76.
Acknowledgements
M.J.M. acknowledges support from a U.S. National Institutes of Health (NIH) Director’s New Innovator Award (DP2 TR002776), a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), the National Institutes of Health (NCI R01 CA241661, NCI R37 CA244911, and NIDDK R01 DK123049), an Abramson Cancer Center (ACC)-School of Engineering and Applied Sciences (SEAS) Discovery Grant (P30 CA016520), and a 2018 AACR-Bayer Innovation and Discovery Grant, Grant Number 18-80- 44-MITC (to M.J.M.). A.G.H. is supported by a National Science Foundation (NSF) Graduate Research Fellowship (DGE 1845298).
Author information
Authors and Affiliations
Contributions
R.E., Z.Z., A.G.H., and M.J.M. conceived the ideas, researched the data for the manuscript, discussed the manuscript content, and wrote the manuscript. Z.Z. designed the display items. All authors reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Mayta, R., Zhang, Z., Hamilton, A.G. et al. Delivery technologies to engineer natural killer cells for cancer immunotherapy. Cancer Gene Ther 28, 947–959 (2021). https://doi.org/10.1038/s41417-021-00336-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00336-2
This article is cited by
-
Lipid nanoparticle-based mRNA delivery systems for cancer immunotherapy
Nano Convergence (2023)
-
Forks in the road for CAR T and CAR NK cell cancer therapies
Nature Immunology (2023)
-
NK cells direct the perspective approaches to cancer immunotherapy
Medical Oncology (2023)