Abstract
Background
We sought to estimate the annual risk and 25-year cumulative risk of contralateral breast cancer among women with stage 0–III unilateral breast cancer.
Methods
We identified 812,851 women with unilateral breast cancer diagnosed between 1990 and 2015 in the SEER database and followed them for contralateral breast cancer for up to 25 years. Women with a known bilateral mastectomy were excluded. We calculated the annual risk of contralateral breast cancer by age at diagnosis, by time since diagnosis and by current age. We compared risks by ductal carcinoma in situ (DCIS) versus invasive disease, by race and by oestrogen receptor (ER) status of the first cancer.
Results
There were 25,958 cases of contralateral invasive breast cancer diagnosed (3.2% of all patients). The annual risk of contralateral breast cancer over the 25-year follow-up period was 0.37% and the 25-year actuarial risk of contralateral invasive breast cancer was 9.9%. The annual risk varied to a small degree by age of diagnosis, by time elapsed since diagnosis and by current age. The 25-year actuarial risk was similar for DCIS and invasive breast cancer patients (10.1 versus 9.9%). The 25-year actuarial risk was higher for black women (12.7%) than for white women (9.7%) and was lower for women with ER-positive breast cancer (9.5%) than for women with ER-negative breast cancer (11.2%).
Conclusions
Women with unilateral breast cancer experience an annual risk of contralateral breast cancer ~0.4% per year, which persists over the 25-year follow-up period.
Similar content being viewed by others
Background
A significant proportion of women with cancer in one breast, including women with ductal carcinoma in situ (DCIS),1 opt for bilateral mastectomy as their initial surgical treatment. Contralateral prophylactic mastectomy rates increased from 5 to 12% of all operations for unilateral breast cancer in the USA from 2004 to 2012.2 Amongst women having a mastectomy, contralateral prophylactic mastectomy rates increased from <5% pre-2000 to 30% in 2012 (USA)3 and 25% in 2013 (Canada).4 Preventive removal of the unaffected breast has been shown to reduce the incidence of second primary cancers, but not of breast cancer mortality in the general population.5,6 The anticipated benefit of a preventive contralateral mastectomy depends on the lifetime risk of contralateral breast cancer and is greater for younger women (who have a long life expectancy) than for older women.7,8 It is not clear to what extent the risk of contralateral breast cancer changes with time elapsed since the first cancer diagnosis or with attained age. To derive accurate estimates of contralateral risk by age at diagnosis, by time elapsed since diagnosis and by current age, it is necessary to follow a large cohort of breast cancer patients for 20 or more years after their first breast cancer.9 It is of interest to determine to what extent various host factors, tumour factors, and treatment impact on the risk of contralateral breast cancer and whether or not these factors should be considered when estimating the risk of contralateral breast cancer for an individual patient. Accurate and personalised knowledge of risk may facilitate counselling about the benefit of contralateral prophylactic mastectomy for women who are considering a bilateral mastectomy for the treatment of unilateral breast cancer.10 We studied a large US-based cohort of women with first primary stage 0–III breast cancer. As a primary objective, we estimated the annual and cumulative risks of contralateral breast cancer from diagnosis until 25 years post diagnosis. Our secondary objective was to identify host and tumour factors that modify these risks.
Methods
Using SEER*Stat statistical software version 8.3.6, we performed a case-listing session of women diagnosed with a first primary in situ or invasive breast cancer in the SEER registries, up to and including SEER 18 (November 2018 submission). Inclusion criteria include women with a breast cancer diagnosis (in situ or invasive) diagnosed between 1990 and 2015. We excluded women with a prior cancer, women diagnosed at age <30 or age 80 and older, had unknown laterality, undocumented nodal status, invasive cancers with histology not of ductal, lobular or mixed (ductal–lobular) subtype, in situ cancers with a histology not of ductal, discordant data entry in the SEER database (i.e. T stage classified as at least T1, but stage was classified as stage 0), missing follow-up, had bilateral breast cancer or stage IV disease. We excluded women who had no surgery and those who had a known bilateral mastectomy at diagnosis (Supplementary Table S1A). To avoid bias caused by increased surveillance at the time of treatment, and to avoid synchronous bilateral breast cancer patients, we began follow-up at 6 months post diagnosis,11 thereby excluding women with a contralateral in situ or invasive breast cancer diagnosed within 6 months of initial ipsilateral diagnosis. We also excluded women who had a bilateral mastectomy as a result of a new primary ipsilateral breast cancer within 6 months of initial diagnosis, women who developed a new (non-breast) primary malignancy within 6 months of initial breast cancer diagnosis, women who died within 6 months of initial diagnosis or women with only 6 months of follow-up available or less (Supplementary Table S1B).
Patient informed consent was not required. Thus, our study was exempted from review by the institutional review board of Women’s College Hospital. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies.12
For each case, we retrieved age at diagnosis, race, marital status and neighbourhood household income. We collected data on year of diagnosis, laterality, DCIS versus invasive disease, histologic subtype (ductal, lobular or mixed) among invasive cases, size (centimetres and T stage) and grade, nodal status (N stage), clinical stage, and biomarker status (oestrogen receptor (ER) and progesterone receptor (PR)). Treatments received include the type of breast surgery (lumpectomy and mastectomy), chemotherapy (yes, no and unknown) and radiotherapy (yes and no/unknown). Information on endocrine therapy was not available in the SEER*Stat database.
Patients were followed from 6 months after the diagnosis of their first breast cancer until the development of a contralateral invasive breast cancer, 25 years of follow-up, loss to follow-up, death or the end of the study period (December 2015).
We calculated time from initial breast cancer diagnosis to a contralateral invasive breast cancer. We did not include contralateral in situ breast cancer events as an endpoint in our analysis. We also collected information on death from breast cancer or other causes (i.e. other cancer, heart disease, other diseases, unknown cause) in the follow-up period.
The age-specific annual incidence rates for contralateral breast cancer were calculated non-parametrically by dividing person-time and contralateral breast cancer events into the corresponding follow-up ages. We further calculated the annual risks of contralateral breast cancer for the 25-year period following a diagnosis of breast cancer for all women and for various subgroups, including DCIS versus invasive disease, and by ER status (among women with invasive breast cancer). Bootstrapping was performed to obtain 95% confidence limits for age-specific and annual rates of contralateral breast cancer. We performed 1000 bootstrap sampling iterations to generate these estimates. Due to the depletion of the cohort with follow-up time, we restricted some analyses to a 20-year follow-up to generate stable estimates where appropriate.
The cumulative (actuarial) risk of contralateral breast cancer in the 25-year period following a diagnosis of DCIS and invasive breast cancer was estimated using the Kaplan–Meier method. We estimated the 25-year cumulative incidence of contralateral invasive breast cancer by age at diagnosis, by DCIS versus invasive disease and for black versus white women.
We sought to identify predictors of contralateral breast cancer though a semi-parametric survival analysis. We estimated sub-distribution hazard ratios (sHRs) using the Fine and Gray proportional sub-hazards model to account for potential competing risks of death (i.e. death from breast cancer, other cancer, heart disease, other disease, unknown cause). Unadjusted and adjusted sHRs were estimated for subgroups defined by age at diagnosis, race, histological subtype, tumour grade, tumour size, nodal status, ER and PR status, and receipt of chemotherapy and radiotherapy. For the analysis of DCIS cases, nodal status and chemotherapy were not included in the model. 95% Confidence limits were generated for all HRs. P values were two-tailed with a level of significance set at <0.05. All statistical analyses were performed with SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC).
For each woman in the cohort, we estimated a 25-year risk of contralateral breast cancer given the woman’s risk factor profile. A 25-year risk of contralateral breast cancer probability was estimated using a Cox proportional hazards model with inputted covariates previously listed above.
Results
Among the 812,851 eligible women with breast cancer in our cohort, there were 659,639 cases of invasive breast cancer (81.2%) and 153,212 cases of DCIS (18.8%). Patient demographics, breast cancer characteristics and treatments received are presented for the entire cohort (Table 1), and separately for women with DCIS (Supplementary Table S2) and invasive breast cancer (Supplementary Table S3). Slightly more than half of the women had breast-conserving surgery (55.4%), 29.8% had a mastectomy and the remaining 14.7% had an unknown surgery type (women with a known bilateral mastectomy were excluded). The majority of women received radiotherapy (54.2%) and more than one-third received chemotherapy (34.1%). Information on anti-hormonal therapy and ovarian suppression was not available. Of the 812,851 women in the cohort, 70,068 (8.6%) died from breast cancer in the follow-up period. The 25-year actuarial breast cancer-specific mortality was 20.9% for invasive cases and was 4.9% for DCIS cases.
There were 25,958 cases of contralateral invasive breast cancer diagnosed in the cohort (3.2% of all patients). The mean time elapsed between the primary cancer and the contralateral cancer was 7.1 years. The annual risk of contralateral breast cancer for the entire cohort over the 25-year follow-up period was 0.37% and the 25-year cumulative incidence of contralateral invasive breast cancer was 9.9%.
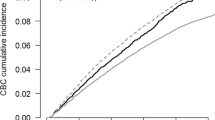
The 25-year cumulative risk of contralateral breast cancer was similar for women of all ages at diagnosis (Fig. 1). The risk ranged from 8% to 15% for the different ages (8–12% if we exclude the 33-year old patients). The risk was similar for women with DCIS and invasive breast cancer (Supplementary Figure S1).
The annual risks of contralateral invasive breast cancer for each year of follow-up in the 20 years following a diagnosis of breast cancer are presented in Fig. 2. For women with invasive breast cancer, the annual risk was relatively stable over the entire period, ranging from 0.3% to 0.5%. The risk did not appear to be in decline at the end of the follow-up period.
The rate of contralateral breast cancer for women with DCIS was similar to women with invasive breast cancer (Fig. 2). The rate was slightly higher for women with DCIS than for women with invasive breast cancer in the first 5 years post diagnosis, after which the risks were similar. The cumulative incidence curves of contralateral invasive breast cancer for women with DCIS and invasive primary breast cancer were nearly identical (Fig. 3). The 25-year actuarial risk of contralateral breast cancer was 10.1% for women with DCIS and 9.9% for women with invasive breast cancer.
Among women with invasive breast cancer, those with ER-positive tumours had a lower 25-year contralateral breast cancer risk than those with ER-negative tumours (9.5 versus 11.2%, P log-rank <0.0001). For the first 10 years, women with ER-negative invasive breast cancers have higher annual risks of contralateral invasive breast cancer than women with ER-positive invasive breast cancers (Fig. 4). After 10 years, the risks are similar for the two groups. Compared to women with invasive ER+/PR+ breast cancer, the adjusted sHR for contralateral breast cancer in the first 10 years for those with invasive ER+/ PR− breast cancer was 0.92 (95% confidence interval (CI) 0.87–0.97, P = 0.0036), for those with invasive ER−/PR+ breast cancer was 1.30 (95% CI 1.15–1.46, P < 0.0001) and for those with invasive ER−/PR− breast cancer was 1.40 (95% CI 1.33–1.46, P < 0.0001).
Annual rates of contralateral breast cancer since diagnosis were calculated by ER status among women with invasive breast cancer. Women with ER-positive breast cancer had a lower annual rate of contralateral breast cancer in the first 10 years of follow-up, compared to women with ER-negative breast cancer. Beyond 10 years from diagnosis, the rate of contralateral breast cancer was similar in both groups.
We used the age-specific person-years accumulated in the cohort to calculate age-specific rates for contralateral breast cancer by current age, for each year from age 30 to 80 years. The age-specific annual rates of contralateral invasive breast cancer for the entire cohort are presented in Supplementary Figure S2. The rate was stable before age 55 years and rose slightly from age 55 to 75 years.
We also calculated the rate of a first primary breast cancer (in one breast) using age-specific incidence rates from the SEER registry (age-specific incidence divided by two). The annual risks of contralateral invasive breast cancer and of first primary breast cancer are compared in Supplementary Figure S2. The risk of first primary cancer rises steadily with age until age 70 years, whereas the annual risk of contralateral breast cancer is relatively stable. At age 35 years, the risk of contralateral breast cancer is ~16 times that of first primary cancer. At age 50 years, the annual risk of contralateral invasive breast cancer is ~2.6 times that of first cancer. At age 70 years, the annual risk of contralateral invasive breast cancer is ~1.8 times that of the first cancer.
We calculated the 20-year actuarial risk of contralateral breast cancer for women with invasive breast cancer (Table 2) and DCIS (Supplementary Table S4) for various subgroups and estimated sHRs for these. We chose a 20-year follow-up for the subgroup analysis because in several subgroups there were too few events after 20 years to generate a robust absolute risk estimate. Among women with invasive breast cancer, we observed an increased risk of contralateral breast cancer for those with mixed histology (ductal/lobular) versus ductal histology (sHR 1.28; 95% CI 1.22–1.35, P < 0.0001) but not for those with lobular histology versus ductal histology (sHR = 1.05; 95% CI 1.00–1.11, P = 0.0633). Women with tumours >3 cm in size were associated with an increased risk of contralateral invasive breast cancer, compared to women with smaller tumours. Women who received chemotherapy had a slightly decreased risk compared to those who did not have chemotherapy (sHR 0.92, 95% CI 0.89–0.95, P < 0.0001), whereas radiotherapy was associated with a slightly increased risk of contralateral invasive breast cancer (sHR 1.08, 95% CI 1.05–1.12, P < 0.0001). The sHR for radiotherapy was 1.04 (95% CI 0.98–1.10, P = 0.1972) for those treated from 1990 to 1999 and was 1.09 (95% CI 1.05–1.14, P < 0.0001) for those treated from 2000 to 2015. Relative rate estimates using Poisson regression are also reported for women with DCIS patients (Supplementary Table S5) and women with invasive breast cancer (Supplementary Table S6).
The 25-year cumulative incidence of contralateral invasive breast cancer for black women was 12.7% and for white women was 9.7% (Supplementary Figures S3 and S4). After adjustment for patient characteristics, tumour factors and competing risks of death, the sHR of contralateral breast cancer in black versus white women remained elevated (sHR 1.17; 95% CI 1.12–1.22, P < 0.0001) (Table 2).
We wished to assess the extent of variation in the (25-year) risk of contralateral breast cancer, using all the variables in a regression model. To do this, we used a Cox proportional hazards model with a Breslow estimator to calculate the predicted 25-year cumulative risk of contralateral breast cancer for each woman in the database. The frequency distribution of the 25-year cumulative risk of contralateral breast cancer is shown for all women in the cohort (Supplementary Figure S5), DCIS cases (Supplementary Figure S6) and invasive cases (Supplementary Figure S7). The mean predicted 25-year risk of contralateral breast cancer for the entire cohort was 10.2% and for 80% of the women in the cohort, the risk fell between 7.5 and 13%.
Discussion
Using a population-based observational cohort, we measured the risk of contralateral breast cancer for 812,851 women with stage 0–III breast cancer. The cumulative risk of contralateral breast cancer in the 25-year period following a first diagnosis was 9.9%, or 0.4% annually and the risk did not vary substantially by age at diagnosis, by years elapsed since diagnosis or by patient age. For the majority of women, the predicted 25-year risk fell within two percentage points of 10%.
Prior studies suggest that women diagnosed with breast cancer at a young age are at increased risk of contralateral breast cancer, compared to older women.8,12,13,14 In our study, the cumulative incidence of contralateral invasive breast cancers over a 25-year period was not higher for younger women than for older women. However, if the risk of contralateral breast cancer is constant at 0.4% per year, the risk of contralateral cancer to age 80 years for a woman diagnosed at age 35 years would be 18%, compared to 8% for a woman diagnosed at age 60 years. We did not observe an attenuation of risk with time since initial diagnosis; this suggests that it is reasonable to extrapolate the risk beyond the 20-year follow-up period.
Some breast cancer patients face higher risks of contralateral cancer than those reported here; however, variables in our model were restricted to those reported in SEER. In carriers of BRCA1 or BRCA2 mutations, the risk is ~2% per year and a younger age at diagnosis is associated with an increased risk of contralateral cancer.15,16,17,18,19,20 For other susceptibility genes, such as CHEK2, PALB2 and ATM, the risk of contralateral breast cancer is not as well characterised.21,22,23 The WECARE study demonstrated that a strong family history of breast cancer alone confers an elevated risk of contralateral breast cancer. Women with either a first-degree (RR 1.9, 95% CI 1.6–2.3) or second-degree (RR 1.4, 95% CI 1.2–1.7) relative with breast cancer had a 90% and 40% increased risk for contralateral breast cancer, respectively, compared to women without a family history. In a sub-analysis excluding women who were screened for and found to have a pathogenic variant in BRCA1, BRCA2, ATM, CHEK2*1100delC or PALB2, women with any affected first- or second-degree relative still had an elevated relative risk (RR 1.8, 95% CI 1.3–2.40).24 In an analysis of 78,775 women in a Swedish population cohort, we determined that the 15-year contralateral breast cancer risk was higher for women with a mother who had either unilateral (12%, 95% CI 11–13) or bilateral (13%, 95% CI 9.5–17) breast cancer compared to women with an unaffected mother (8.4%, 95% CI 8.1–8.7).25 These studies demonstrate the importance of family history on contralateral breast cancer risk, over and above genetic mutation status. However, the SEER database does not capture family history or genetic mutation status. SEER also does not capture information on commonly occurring single-nucleotide polymorphisms (SNPs) that have been reported to influence the risk for contralateral breast cancer.26,27 In a large case–control study conducted by the Breast Cancer Association Consortium, Kramer et al. reported on the impact of a personal risk score (PRS) based on 313 SNPs on the relative risk of developing metachronous contralateral breast cancer. Compared to women who scored at the 50th percentile, the odds ratios were 0.75 and 1.33 for those in the 10th percentile and 90th percentile, respectively.27 The authors did not calculate the actuarial risk of contralateral breast cancer in this case–control study, but if we assume a 25-year risk of 10%, then the great majority of women would fall within three percentage points of this mean. It is yet to be shown that providing a PRS for contralateral breast cancer will influence a woman’s surgical decision.
The WECARE investigators conducted a series of case–control studies with the goal of identifying predictors of contralateral breast cancer. WECARE determined that regular alcohol consumption (RR 1.3, 95% CI 1.0–1.6, P = 0.03) was associated with an increased risk of contralateral breast cancer.28 Subsequently, they found that women who smoked >10 cigarettes per day were also at increased risk (RR 1.50, 95% CI 1.08–2.08).29 The WECARE investigators also found that women who reached menarche before age 13 years were at an increased risk of contralateral breast cancer (RR 1.26, 95% CI 1.01–1.58), and increasing parity had a protective effect.30,31 They reported that obesity conferred an elevated contralateral risk in women who had an ER− first breast cancer only (RR 1.9, 95% CI 1.02–3.4).32 Other studies have reported a positive association between body mass index and contralateral risk.33 In women <45 years old, dense breasts (25–50% density) were associated with higher contralateral breast cancer risk compared to women with <25% mammographic density.34 These variables were not captured in the SEER database.
A previous SEER study reported a 0.6% annual risk for patients diagnosed between 1973 and 1996.35 A population-based study from the Netherlands of patients diagnosed between 2003 and 2008 also estimated the risk at 0.6% per year.36 A review of the MEDLINE database of studies published between 2000 and 2015 that evaluated risk of contralateral breast cancer cited the annual risk to be between 0.5 and 0.75%.37 Our mean annual risk of contralateral breast cancer of 0.4% is slightly lower than previous estimates. Increasing the use of systemic treatment for breast cancer may influence the current risk, as both chemotherapy and endocrine therapy lower the contralateral risk (our patients were diagnosed between 1990 and 2015).7,13,38 It is also possible that not all contralateral events were captured in the SEER database.
The risk of contralateral breast cancer following a diagnosis of DCIS was similar to the annual risk following invasive breast cancer. This is consistent with the results of two previous studies of the risk of contralateral invasive cancer post DCIS, one from Memorial-Sloan Kettering Cancer Centre (0.6% per year)39 and one from the Netherlands (0.45% per year) .40 In a previous analysis of SEER data, which included 1286 DCIS patients, the annual risk was 0.4% per year.41 In our study, there was a slightly greater risk with DCIS in the first 6 years compared to invasive disease. The transient difference might be related to the more extensive use of systemic therapy, including endocrine therapy, in invasive cancer patients than in DCIS patients.
We found that ER positivity was associated with a lower risk of contralateral breast cancer. Previous studies have shown that ER−/PR− tumours are associated with a relatively high risk of contralateral breast cancer.42 We believe this is a reflection of the fact that women with hormone-receptor-positive cancers often receive endocrine therapy, the effect of which on reducing contralateral breast cancer risk is well recognised.43,44,45 After a 5-year course of tamoxifen, women with hormone-receptor-positive breast cancer experience an increase in recurrences from years 5 to 20,46 and for many women, endocrine treatment is now extended to 10 years.47,48 As our cohort includes contemporary patients diagnosed up to 2015, this is consistent with our observation that the protective association with ER positivity was only seen in the first 10 years of follow-up. In a classification scheme based on ER and PR in combination, we found that the risk for ER+/PR− patients was similar to that of ER+/PR+ patients and the risk for ER−/PR+ patients was similar to that of ER−/PR− patients.
While a previous study failed to show an influence of chemotherapy on the risk of contralateral breast cancer,49 we found that chemotherapy use was associated with a lower risk of contralateral breast cancer, consistent with the recent WECARE study45 and a population-based study from the Netherlands.50 In contrast, we found that radiation treatment was associated with a slightly increased risk of contralateral cancer, which has been related to scatter radiation to the contralateral breast during treatment. This was identified in prior SEER analyses of patients diagnosed from 1973 to 2007.35,51,52 The HR associated with radiotherapy did not show a decline with the calendar year. However, this small increase in contralateral breast cancer risk has not impacted radiotherapy treatment decisions.35
Black women had a higher cumulative incidence of contralateral breast cancer at 25 years than white women (12.7 versus 9.7%). This is consistent with a previous SEER analysis of cases diagnosed between 1973 and 1999.35 It is unclear if this disparity relates to inadequate screening after treatment of the first cancer or treatment compliance with endocrine therapy or is an inherent biologic phenomenon and this is an area for further research.53
We observed a rising risk of first primary breast cancer with age, but a relatively stable risk of contralateral breast cancer. This observation lends support to the theory first proposed by Peto and Mack in 2000 that cancer susceptibility is a dichotomous state and is subject to an on/off switch.54,55 It is reasonable to assume that for an untreated woman, at any time point, the risk of cancer in both breasts is the same. If a woman develops breast cancer at age 30 in her left breast, the risk to her right breast is 0.4% per year from then on. Under the assumption of symmetrical risk, we suppose the risk of cancer to her left breast was also 0.4% a year when she first developed breast cancer. This model implies that, for a woman of a given age, the risk of cancer in both breasts is equal, and is either negligible or is 0.4% annually. Under this paradigm, the increase in the risk of first primary cancer reflects an increase in the proportion of susceptible women with age. That is, the annual risk of cancer in the first breast is the product of the proportion of women who are susceptible and 0.4%. Under this model and based on the data in Supplementary Figure S2, we can infer that the proportion of women who are susceptible to breast cancer is 3% at age 30 years, increasing to 57% at age 70 years, after which the proportion no longer rises. The effect of family history on contralateral risk may be mediated through influencing this probability.54,55
Rates of contralateral prophylactic mastectomy and bilateral mastectomy are increasing among women with unilateral, sporadic breast cancer.2,3,4,6,8 This is occurring despite consensus guidelines discouraging the procedure in women of average risk.56,57,58,59 The literature suggests that many women who chose bilateral mastectomy overestimate their risk of developing a contralateral breast cancer.8,10,60,61,62 This emphasises the importance of physician counselling and improving patient education. Patients should be provided with accurate risk information before they make surgical decisions regarding contralateral prophylactic mastectomy. Despite the need, current risk calculators for contralateral breast cancer in the literature, including the Manchester tool,63 CBCRisk,64 and PredictCBC,65 have limited clinical use.66
In our study, we excluded 39,695 women with bilateral mastectomy because they were not at risk for the primary endpoint. However, based on our regression model, we can calculate the estimated risk of contralateral breast cancer for these women, assuming they had not had the contralateral mastectomy (Supplementary Figure S8). The mean risk at 25 years was 10.1% and was nearly the same as for those women who did not have a bilateral mastectomy (10.2%). This observation suggests that currently, decisions made about contralateral mastectomy are not driven entirely based on empiric risk. Ideally, we would have data on family history and other risk factors to fully evaluate this hypothesis.
Based on these data, we question that it is clinically useful to tailor the risk of contralateral breast cancer to individual women, based on their risk factor profile. Other than age, there was very little information in our model that could be useful to refine the risk. One could incorporate the risk factors under the model proposed here, but this would incur a degree of complexity. A simpler approach is to provide a woman with a general risk estimate of 10% over 25 years; ~80% of all patients would fall within two percentage points of this estimate. Exceptions would be made for carriers of pathogenic variants in BRCA1 and BRCA2 and those with a first-degree relative with breast cancer; these women would benefit from genetic counselling. It is of interest (and possibly counter-intuitive) that the risk of contralateral invasive breast cancer following a diagnosis of DCIS is the same as that following a diagnosis of invasive cancer. This challenges the opinion of those who consider a contralateral mastectomy to be an acceptable treatment for a woman with invasive cancer, but overtreatment for a woman with DCIS.
The strengths of our study were that we performed an analysis on the risks of contralateral breast cancer using a contemporary population-based cohort. This is the largest contemporary population-based study of contralateral breast cancer studied to date and patients were followed for an average of 8.7 years. We were able to examine the influence of patient, tumour and treatment factors on the risk.
There are several limitations. We did not have data on use of endocrine therapy, and this may have influenced the risks of contralateral cancer among ER+ breast cancer patients (invasive and DCIS). We did not have data on family history or BRCA1 or BRCA2 variant status and these women are at higher than average risk of contralateral breast cancer. We assumed that all contralateral cancer events were second primaries and not metastases. There may have been missed cases of contralateral breast cancer that were not captured in the follow-up and this would result in an underestimate of the risk for contralateral cancer. We did not consider contralateral mastectomies that were performed after the initial diagnosis and we did not censor patients at the time of contralateral mastectomy following an ipsilateral recurrence or a contralateral in situ breast cancer; however, we expect these cases to be few. We did not have screening histories and many of the contralateral cancers might have been the result of intensified screening efforts and this might help explain the ethnic disparities.
In summary, these data demonstrate that the risk of contralateral breast cancer in women with unilateral primary breast cancer is relatively constant throughout a patient’s lifetime and supports a model that breast cancer risk is determined by inherent susceptibility. The proportion of susceptible women in the population increases with age. To further examine this model, studies of the molecular signature which characterises the off/on susceptibility switch and which attempt to identify factors associated with acquired susceptibility should be pursued.
References
Zhang, B., Coopey, S. B., Gadd, M. A., Hughes, K. S., Chang, D. C. & Oseni, T. O. Trends in unilateral and contralateral prophylactic mastectomy use in ductal carcinoma in situ of the breast: patterns and predictors. Ann. Surg. Oncol. 26, 3863–3873 (2019).
Nash, R., Goodman, M., Lin, C. C., Freedman, R. A., Dominici, L. S., Ward, K. et al. State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004-2012. JAMA Surg. 152, 648–657 (2017).
Wang, T., Baskin, A. & Dossett, L. A. Deimplementation of the choosing wisely recommendations for low-value breast cancer surgery: a systematic review. JAMA Surg. 155, 759–770 (2020).
Findlay-Shirras, L., Lima, I., Smith, G., Clemons, M. & Arnaout, A. Canada follows the US in the rise of bilateral mastectomies for unilateral breast cancer: a 23-year population cohort study. Breast Cancer Res. Treat. 185, 517–525 (2021).
Carbine, N. E., Lostumbo, L., Wallace, J. & Ko, H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 4, CD002748 (2018).
Wong, S. M., Freedman, R. A., Sagara, Y., Aydogan, F., Barry, W. T. & Golshan, M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann. Surg. 265, 581–589 (2017).
Narod, S. A. Bilateral breast cancers. Nat. Rev. Clin. Oncol. 11, 157–166 (2014).
Lim, D. W., Metcalfe, K. A. & Narod, S. A. Bilateral mastectomy in women with unilateral breast cancer: a review. JAMA Surg. https://doi.org/10.1001/jamasurg.2020.6664 (2021).
Metcalfe, K., Gershman, S., Ghadirian, P., Lynch, H. T., Snyder, C., Tung, N. et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 348, g226 (2014).
Kaiser, K., Cameron, K. A., Beaumont, J., Garcia, S. F., Lacson, L., Moran, M. et al. What does risk of future cancer mean to breast cancer patients? Breast Cancer Res. Treat. 175, 579–584 (2019).
Cain, K. C., Harlow, S. D., Roderick, J. L., Nan, B., Yosef, M., Taffe, J. R. et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and diseases processes. Am. J. Epidemiol. 173, 1078–1084 (2011).
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gotzsche, P. C., Vandenbroucke, J. P. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457 (2007).
Chen, Y., Thompson, W., Semenciw, R. & Mao, Y. Epidemiology of contralateral breast cancer. Cancer Epidemiol. Biomark. Prev. 8, 855–861 (1999).
Yoon, T. I., Kwak, B. S., Yi, O. V., Kim, S., Um, E., Yun, K. W. et al. Age-related risk factors associated with primary contralateral breast cancer among younger women versus older women. Breast Cancer Res. Treat. 173, 657–665 (2019).
Metcalfe, K., Lynch, H. T., Ghadirian, P., Tung, N., Olivotto, I., Warner, E. et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 22, 2328–2335 (2004).
Basu, N. N., Ingham, S., Hodson, J., Lalloo, F., Bulman, M., Howell, A. et al. Risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a 30-year semi-prospective analysis. Fam. Cancer 14, 531–538 (2015).
Narod, S. A., Tung, N., Lubinski, J., Huzarski, T., Robson, M., Lynch, H. T. et al. A prior diagnosis of breast cancer is a risk factor for breast cancer in BRCA1 and BRCA2 carriers. Curr. Oncol. 21, 64–68 (2014).
Malone, K. E., Begg, C. B., Haile, R. W., Borg, A., Concannon, P., Tellhed, L. et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J. Clin. Oncol. 28, 2404–2410 (2010).
van den Broek, A. J., van’t Veer, L. J., Hooning, M. J., Cornelissen, S., Broeks, A., Rutgers, E. J. et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J. Clin. Oncol. 34, 409–418 (2016).
Su, L., Xu, Y., Ouyang, T., Li, J., Wang, T., Fan, Z. et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers in a large cohort of unselected Chinese breast cancer patients. Int. J. Cancer 146, 3335–3342 (2020).
Mellemkjaer, L., Dahl, C., Olsen, J. H., Bertelsen, L., Guldberg, P., Christensen, J. et al. Risk for contralateral breast cancer among carriers of the CHEK2*1100delC mutation in the WECARE study. Br. J. Cancer 98, 728–733 (2008).
National Comprehensive Cancer Network (NCCN). Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. V.2.2021. NCCN practice guidelines website. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (2021).
Couch, F. J., Shimelis, H., Hu, C., Hart, S. N., Polley, E. C., Na, J. et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 3, 1190–1196 (2017).
Reiner, A. S., Sisti, J., John, E. M., Lynch, C. F., Brooks, J. D., Mellemkjaer, L. et al. Breast cancer family history and contralateral breast cancer risk in young women: an update from the Women’s Environmental Cancer and Radiation Epidemiology Study. J. Clin. Oncol. 36, 1513–1520 (2018).
Narod, S. A., Kharazmi, E., Fallah, M., Sundquist, K. & Hemminki, K. The risk of contralateral breast cancer in daughters of women with and without breast cancer. Clin. Genet. 89, 332–335 (2016).
Robson, M. E., Reiner, A. S., Brooks, J. D., Concannon, P. J., John, E. M., Mellemkjaer, L. et al. Association of common genetic variants with contralateral breast cancer risk in the WECARE study. J. Natl Cancer Inst. 109, djx051 (2017).
Kramer, I., Hooning, M. J., Mavaddat, N., Hauptmann, M., Keeman, R., Steyerberg, E. W. et al. Breast cancer polygenic risk score and contralateral breast cancer risk. Am. J. Hum. Genet. 107, 837–848 (2020).
Knight, J. A., Bernstein, L., Largent, J., Capanu, M., Begg, C. B., Mellemkjaer, L. et al. Alcohol intake and cigarette smoking and risk of a contralateral breast cancer: the Women’s Environmental Cancer and Radiation Epidemiology study. Am. J. Epidemiol. 169, 962–968 (2009).
Knight, J. A., Fan, J., Malone, K. E., John, E. M., Lynch, C. F., Langballe, R. et al. Alcohol consumption and cigarette smoking in combination: a predictor of contralateral breast cancer risk in the WECARE study. Int. J. Cancer 141, 916–924 (2017).
Largent, J. A., Capanu, M., Bernstein, L., Langholz, B., Mellemkaer, L., Malone, K. E. et al. Reproductive history and risk of second primary breast cancer: the WECARE study. Cancer Epidemiol. Biomark. Prev. 16, 906–911 (2007).
Sisti, J. S., Bernstein, L., Lynch, C. F., Reiner, A. S., Mellemkjaer, L., Brooks, J. D. et al. Reproductive factors, tumor estrogen receptor status and contralateral breast cancer risk: results from the WECARE study. Springerplus 4, 825 (2015).
Brooks, J. D., John, E. M., Mellemkjaer, L., Lynch, C. F., Knight, J. A., Malone, K. E. et al. Body mass index, weight change, and risk of second primary breast cancer in the WECARE study: influence of estrogen receptor status of the first breast cancer. Cancer Med. 5, 3282–3291 (2016).
Akdeniz, D., Klaver, M. M., Smith, C. Z. A., Koppert, L. B. & Hooning, M. J. The impact of lifestyle and reproductive factors on the risk of a second new primary cancer in the contralateral breast: a systematic review and meta-analysis. Cancer Causes Control. 31, 403–416 (2020).
Knight, J. A., Blackmore, K. M., Fan, J., Malone, K. E., John, E. M., Lynch, C. F. et al. The association of mammographic density with risk of contralateral breast cancer and change in density with treatment in the WECARE study. Breast Cancer Res. 20, 23 (2018).
Gao, X., Fisher, S. G. & Emami, B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int. J. Radiat. Oncol. Biol. Phys. 56, 1038–1045 (2003).
Aalders, K. C., van Bommel, A. C. M., van Dalen, T., Sonke, G. S., van Diest, P. J., Boersma, L. J. et al. Contemporary risks of local and regional recurrence and contralateral breast cancer in patients treated for primary breast cancer. Eur. J. Cancer 63, 118–126 (2016).
Davies, K. R., Cantor, S. B. & Brewster, A. M. Better contralateral breast cancer risk estimation and alternative options to contralateral prophylactic mastectomy. Int. J. Women’s Health 7, 181–187 (2015).
Spronk, I., Schellevis, F. G., Burgers, J. S., de Bock, G. H. & Korevaar, J. C. Incidence of isolated local breast cancer recurrence and contralateral breast cancer: a systematic review. Breast 39, 70–79 (2018).
Miller, M. E., Muhsen, S., Olcese, C., Patil, S., Morrow, M. & Van Zee, K. J. Contralateral breast cancer risk in women with ductal carcinoma in situ: is it high enough to justify bilateral mastectomy? Ann. Surg. Oncol. 24, 2889–2897 (2017).
Elshof, L. E., Schaapveld, M., Schmidt, M. K., Rutgers, E. J., van Leeuwen, F. E. & Wesseling, J. Subsequent risk of ipsilateral and contralateral invasive breast cancer after treatment for ductal carcinoma in situ: incidence and the effect of radiotherapy in a population-based cohort of 10,090 women. Breast Cancer Res. Treat. 159, 553–563 (2016).
Ryser, M. D., Weaver, D. L., Zhao, F., Worni, M., Grimm, L. J., Gulati, R. et al. Cancer outcomes in DCIS patients without locoregional treatment. J. Natl Cancer Inst. 111, 952–960 (2019).
Reiner, A. S., Lynch, C. F., Sisti, J. S., John, E. M., Brooks, J. D., Bernstein, L. et al. Hormone receptor status of a first primary breast cancer predicts contralateral breast cancer risk in the WECARE study population. Breast Cancer Res. 19, 83 (2017).
Fisher, B., Costantino, J., Redmond, C., Poisson, R., Bowman, D., Couture, J. et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N. Engl. J. Med. 320, 479–484 (1989).
Cuzick, J., Sestak, I., Baum, M., Buzdar, A., Howell, A., Dowsett, M. et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 11, 1135–1141 (2010).
Langballe, R., Mellemkjaer, L., Mallone, K. E., Lynch, C. F., John, E. M., Knight, J. A. et al. Systemic therapy for breast cancer and risk of subsequent contralateral breast cancer in the WECARE study. Breast Cancer Res. 18, 65 (2016).
Pan, H., Gray, R., Braybrooke, J., Davies, C., Taylor, C., McGale, P. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Davies, C., Pan, H., Godwin, J., Gray, R., Arriagada, R., Raina, V. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Burstein, H. J., Lacchetti, C., Anderson, H., Buchholz, T. A., Davidson, N. E., Gelmon, K. A. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J. Clin. Oncol. 37, 423–438 (2019).
Hooning, M. J., Aleman, B. M. P., Hauptmann, M., Baaijens, M. H. A., Klijn, J. G. M., Noyon, R. et al. Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J. Clin. Oncol. 26, 5561–5568 (2008).
Kramer, I., Schaapveld, M., Oldenburg, H. S. A., Sonke, G. S., McCool, D., van Leeuwen, F. E. et al. The influence of adjuvant systemic regimens on contralateral breast cancer risk and receptor subtype. J. Natl Cancer Inst. 111, 709–718 (2019).
Neta, G., Anderson, W. F., Gilbert, E. & Berrington, A. Variation in the risk of radiation-related contralateral breast cancer by histology and estrogen receptor expression in SEER. Breast Cancer Res. Treat. 131, 1021–1027 (2012).
Berrington de Gonzalez, A., Curtis, R. E., Gilbert, E., Berg, C. D., Smith, S. A., Stovall, M. et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br. J. Cancer 102, 220–226 (2010).
Yedjou, C. G., Sims, J. N., Miele, L., Noubissi, F., Lowe, L., Fonseca, D. D. et al. Health and racial disparity in breast cancer. Adv. Exp. Med. Biol. 1152, 31–49 (2019).
Peto, J. & Mack, T. M. High constant incidence in twins and other relatives of women with breast cancer. Nat. Genet. 26, 411–414 (2000).
Easton, D. Breast cancer–not just whether but when? Nat. Genet. 26, 390–391 (2000).
Hunt, K. K., Euhus, D. M., Boughey, J. C., Chagpar, A. B., Feldman, S. M., Hansen, N. M. et al. Society of Surgical Oncology Breast Disease Working Group statement on prophylactic (risk-reducing) mastectomy. Ann. Surg. Oncol. 24, 375–397 (2017).
Wright, F. C., Look Hong, N. J., Quan, M. L., Beyfuss, K., Temple, S., Covelli, A. et al. Indications for contralateral prophylactic mastectomy: a consensus statement using modified Delphi methodology. Ann. Surg. 267, 271–279 (2018).
Boughey, J. C., Attai, D. J., Chen, S. L., Cody, H. S., Dietz, J. R., Feldman, S. M. et al. Contralateral prophylactic mastectomy (CPM) consensus statement from the American Society of Breast Surgeons: data on CPM outcomes and risks. Ann. Surg. Oncol. 23, 3100–3105 (2016).
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 1.2021. NCCN practice guidelines website. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2021).
Covelli, A. M., Baxter, N. N., Fitch, M. I., McCready, D. R. & Wright, F. C. ‘Taking control of cancer’: understanding women’s choice for mastectomy. Ann. Surg. Oncol. 22, 383–391 (2015).
Abbott, A., Rueth, N., Pappas-Varco, S., Kuntz, K., Kerr, E. & Tuttle, T. Perceptions of contralateral breast cancer: an overestimation of risk. Ann. Surg. Oncol. 18, 3129–3136 (2011).
Portschy, P. R., Abbott, A. M., Burke, E. E., Nzara, R., Marmor, S., Kuntz, K. M. et al. Perceptions of contralateral breast cancer risk: a prospective, longitudinal study. Ann. Surg. Oncol. 22, 3846–3852 (2015).
Basu, N. N., Ross, G. L., Evans, D. G. & Barr, L. The Manchester guidelines for contralateral risk-reducing mastectomy. World J. Surg. Oncol. 13, 237 (2015).
Chowdhury, M., Euhus, D., Arun, B., Umbricht, C., Biswas, S. & Choudhary, P. Validation of a personalized risk prediction model for contralateral breast cancer. Breast Cancer Res. Treat. 170, 415–423 (2018).
Giardiello, D., Steyerberg, E. W., Hauptmann, M., Adank, M. A., Akdeniz, D., Blomqvist, C. et al. Prediction and clinical utility of a contralateral breast cancer risk model. Breast Cancer Res. 21, 144 (2019).
Giardiello, D., Hauptmann, M., Steyerberg, E. W., Adank, M. A., Akdeniz, D., Blom, J. C. et al. Prediction of contralateral breast cancer: external validation of risk calculators in 20 international cohorts. Breast Cancer Res. Treat. 181, 423–434 (2020).
Author information
Authors and Affiliations
Contributions
Supervision: S.A.N. Conception and design: S.A.N. and V.G. Acquisition and analysis of data: V.G. Statistical analysis and critical review: S.A.N., V.G. and D.L. Interpretation of the results and writing of the original draft: S.A.N., V.G. and D.L. All authors revised the paper and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is exempt from informed patient consent and board approval by the Women’s College Hospital Ethics Board.
Data availability
All data relevant to the study are included in the article.
Competing interests
S.A.N. is an editorial board member of BJC. All other authors declare no competing interests.
Funding information
V.G. is supported by the Canadian Institutes of Health Research (CIHR) Frederick Banting & Charles Best Canada Graduate Scholarship Doctoral Research Award. D.W.L. is supported by the CIHR Fellowship, the Helen Marion Walker—Soroptimist Women’s Health Research Scholarship and the Canadian Cancer Society Chair in Breast Cancer Research at Women’s College Research Institute at Women’s College Hospital. S.A.N. is the recipient of the Tier I Canada Research Chair in Breast Cancer. This work was supported by the Peter Gilgan Centre for Women’s Cancers at Women’s College Hospital, in partnership with the Canadian Cancer Society.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Giannakeas, V., Lim, D.W. & Narod, S.A. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer 125, 601–610 (2021). https://doi.org/10.1038/s41416-021-01417-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01417-7
This article is cited by
-
Gynecologic Care of Black Breast Cancer Survivors
Current Breast Cancer Reports (2024)
-
Omission of axillary lymph node dissection in breast cancer patients with micrometastasis or isolated tumor cells in sentinel lymph nodes: a 12-year experience in a tertiary breast unit
Journal of Cancer Research and Clinical Oncology (2024)
-
Current status and challenges of breast cancer prevention~DNA methylation would lead to groundbreaking progress in breast cancer prevention~
Genes and Environment (2023)
-
Optimal adjuvant therapy in older (≥70 years of age) women with low-risk early-stage breast cancer
npj Breast Cancer (2023)
-
Fear of cancer recurrence in young women 5 years after diagnosis with a good-prognosis cancer: the VICAN-5 national survey
Journal of Cancer Survivorship (2023)