Abstract
The management of cytomegalovirus (CMV) infection was assessed with a survey performed in 2020 by the Infectious Diseases Working Party of European Society for Blood and Marrow Transplantation (EBMT). One-hundred-eighty of the 579 EBMT centres (31%) responded. CMV monitoring with quantitative PCR for CMV-DNAemia was used by 97% of centres while the duration of monitoring was variable according to the patient immune recovery and the ongoing immunosuppressive therapy. CMV prophylaxis for high-risk patients was used in 101 (56%) of centres: letermovir in 62 centres (61%), aciclovir/valaciclovir in 19 centres (19%), ganciclovir/valganciclovir in 17 centres (17%), foscarnet in 3 (3%). The most used trigger for pre-emptive therapy was a threshold of >103 copies/ml or >103 IU/ml. Ganciclovir/valganciclovir confirmed the preferred first line treatment both for pre-emptive and CMV disease therapy. CMV-cytotoxic T-cells were used mainly in the setting of relapsing/refractory CMV disease. Forty-eight centres reported CMV refractory/resistant infection due to mutated CMV strain.This survey showed that letermovir prophylaxis is adopted by more than half of centres using a prophylaxis approach for CMV infection. How letermovir prophylaxis will modify other important pillars of daily CMV management, such as frequency of CMV-DNAemia monitoring and preemptive therapy, remain a matter of investigation.
Similar content being viewed by others
Introduction
Human Cytomegalovirus (CMV), a double-stranded DNA virus belonging to the beta herpesvirus family, represents the most important cause of viral infection after allogeneic stem cell transplantation [1]. During the early infancy this virus determines a primary clinical or subclinical infection, remaining subsequently in a latent state in several types of leucocytes (lymphocytes, monocytes, dendritic cells) as well as CD34 + cells, under the control of T-cell immune effector cells [2]. The heavy immunosuppression associated with hematopoietic cell transplantation (HCT), characterized by severe and prolonged lymphocytopenia and T-cell function inhibition or dysfunction, may cause CMV reactivation, systemic viral infection and ultimately end-organ disease such as pneumonitis, colitis, and retinitis [3]. The main risk factors for CMV disease are the recipient CMV positive serology, in vivo or ex vivo T-cell depletion, the use of high dose steroids, the use of an HLA mismatched or an unrelated donor, the occurrence of graft-versus-host disease (GVHD), and cord blood as stem cell source [4]. Moreover, CMV infection can be an indirect cause of lower overall survival (OS) and non-relapse mortality (NRM) by producing cytokines such as interleukin-6 that enhances GVHD or by the undesired myelosuppressive effect of anti-CMV drug ganciclovir that predisposes to bacterial and fungal infection [5].
In the last 3 decades, the main strategy to prevent CMV diseases was the early treatment of CMV reactivation, named pre-emptive approach, that requires serial screening of blood for CMV viremia or antigenemia by PCR-CMV-DNA or pp65 protein detection, to start antiviral treatment upon detection of significant viremia or antigenemia. In comparison with a primary prophylaxis approach, the advantages of the pre-emptive strategy are the reduction of antiviral treatment duration, the containment of costs and days of hospitalization, the limitation of bone marrow suppression by ganciclovir/valganciclovir, and the facilitation of anti-CMV immunity restoration by allowing a mild-moderate CMV replication [6]. Until recently, CMV primary prophylaxis failed to demonstrate a significant effect on risk of death and OS in several trials with anti-CMV agents [7]. This scenario changed in 2017 with letermovir, a new anti-CMV drug. This drug has a new mechanism of action that is the inhibition of CMV viral terminase complex and in a randomized controlled trial was superior to pre-emptive therapy in reducing clinically significant CMV infections and improving 24-week OS [8]. After this trial, letermovir prophylaxis received the highest score of recommendation by the European Conference on Infections in Leukaemia, 8th edition (ECIL-8) [9]. The Infectious Diseases Working Party (IDWP) of European Society for Blood and Marrow Transplantation (EBMT) undertook in 2020 a survey to have an updated picture of the CMV management in the EBMT centres.
Methods
A 34-item questionnaire regarding CMV infection (incidence, diagnosis, monitoring, prophylaxis and therapy), previously approved by the scientific board of IDWP-EBMT was sent to 579 centres performing allogeneic HCT, with two rounds of invitations between January and March 2020. The questionnaire comprised 3 main sections: one to characterize the centre population (adult, paediatric, or mixed) and the annual transplant activity; one to know the diagnostic methods to detect and monitor CMV infection; and one to describe the strategies of prophylaxis, pre-emptive therapy of CMV infection and treatment of CMV disease (the questionnaire is available as supplementary file). The data collected concerned the year 2019. No specific demographic, clinical, virological, and transplant information was collected for each case of CMV infection reported by centres. CMV infection was considered as any episode of significant CMV replication determined by a PCR or an antigen test result above the reference threshold used by the centre in a patient with or without symptoms. The patient at high-risk of CMV reactivation was defined by the positive CMV serology in the recipient or in the donor or both [10]. Proven or probable CMV disease were defined according to published criteria [11] whereas the definition of possible CMV disease included all the remaining cases where CMV was retained responsible, but the episode did not match the proven or probable definitions. CMV drug resistance was defined as CMV-DNA mutations that confers a reduced susceptibility to antiviral drugs [12]. The graft source was defined manipulated when a procedure of ex-vivo T-cell depletion was performed.
Results
A reply to the questionnaire was received from 180 of the 579 mailed centres (31%). The responding centres were from all 5 continents, but prevalently from Europe, and included 40 countries (table S1). Among them, 104 (58%) were centres only for adult patients, 43 (24%) were centres only for paediatric patients, and 33 (18%) were centres with a joined adult and paediatric transplant program. In this last group, the same approach both for adult and paediatric patients for management of CMV infection was declared by 22 of 33 (67%) centres.
Overall, the responding centres performed in 2019 a median of 40 transplant procedures per centre (range 1- 247), with the median of 62 transplants (range 1-146) for adult/paediatric centres, of 43 transplants (range 5-247) for adult centres, and of 24 transplants (range 1-106) for paediatric centres. All centres performed both related and unrelated HCT. The main results of the survey are showed in Table 1.
CMV surveillance: 175 of 180 centres (97%) used PCR for CMV monitoring, 4 centres (2%) used serum pp65 antigenemia, and 1 centre used both PCR and pp65. Quantitative PCR was adopted in 173 of 175 centres (99%; 2 centres missing) using blood (122, 70%), plasma (44, 25%) or serum (6, 3%) while for 3 centres (2%) the data were missing. The quantitation of CMV load was expressed as CMV genomic copies/ml in 116 (66%), international unit/ml (IU) in 51 (29%), and both measures in 4 (2%) centres, respectively, while data were missing for 4 centres (2%). In 80 centres (44%), the determination of CMV-DNAemia in blood or plasma was also part of the pre-transplant work-up assessment.
CMV monitoring was performed for all types of allogeneic (allo)-HCT in 171 centres (95%), while in 7 centres (4%) CMV monitoring was limited to allo-HCT considered at higher risk of CMV infection such as unrelated HCT, related and unrelated cord blood (CB) HCT, T-cell replete or T-cell ex-vivo depleted haploidentical HCT, and positive CMV serology of the recipient or of the donor.
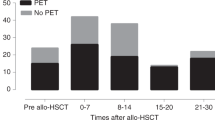
During the first 100 days post-allo-HCT, the frequency of surveillance was 1 time/week in 109 (61%), 2 times/week in 53 (29%), 2 times/week during the hospitalization for transplant followed by once a week after hospital discharge in 8 (4%), while the frequency was modulated according to immune recovery or recipient/donor CMV positivity in 4 centres (2%) and data were missing in 6 (3%).
The duration of CMV surveillance was reported as fixed until day +100 in 8 (4%) of centres while it was extended beyond day + 100 according the presence of the following criteria: active treatment for GVHD or immunosuppressive therapy or steroid therapy in 147 (82%), CD4 + count <0.25 × 109/L in 7 (4%), lymphocyte count <0.3 × 109/L) in 8 (4%), at least 6 months post-HCT in 3 (2%); data were missing for 7 centres (4%).
CMV prophylaxis: in high-risk patients (CMV seropositivity in the recipient or in the donor), drug prophylaxis was used in 101 (56%) with a higher prevalence in adult and adult/paediatric than paediatric centres (62% and 61% vs. 37%, p 0.002). The drugs used were: letermovir in 62 centres (61%), aciclovir/valaciclovir in 19 centres (19%), ganciclovir/valganciclovir in 17 centres (17%), foscarnet in 3 centres (3%). Among 62 centres that declared to use letermovir prophylaxis, 48 (77%) were adult HCT centres, 13 (21%) joined adult and paediatric centres and 1 (2%) paediatric centre; moreover, in the 13 centres with a joined adult and paediatric transplant program, 7 declared to adopt a common approach for CMV management both for adult and paediatric patients.
In addition to drug prophylaxis, the use of CMV-hyperimmune globulins and CMV cytotoxic T-lymphocytes (CMV-CTLs) in this setting were reported in 4 (4%) and 2 (2%) centres, respectively.
CMV infection and preemptive treatment: the median number of estimated preemptive treatments/years for CMV infection was 12 per centre (range 1-50).
The information on the drug used as first line preemptive therapy for unmanipulated allo-HCT were available for 175 centres: ganciclovir/valganciclovir in 168 centres (93%), foscarnet in 7 centres (4%); data were missing for 5 centres (3%). In addition to preemptive therapy, the use of CMV-hyperimmune globulins and CMV-CTLs in this setting were reported in 6 (3%) and 1 (1%) centre, respectively.
Considering ex-vivo T-cell depleted graft, the information on the first-line preemptive therapy was available for 119 centres: ganciclovir/valganciclovir in 111 centres (93%), foscarnet in 7 centres (6%), cidofovir in 1 centre (1%) while the data were missing in 61 centres (34%). CMV-hyperimmune globulins and CMV-CTLs were used together with preemptive drug therapy in 5 (3%) and 2 (1%) centres, respectively.
Table 2 shows the threshold used to start pre-emptive therapy in patients monitored with PCR for CMV and received an unmanipulated or an ex-vivo manipulated graft, respectively. A threshold >103 of copies/ml or IU/ml was the most used trigger to start preemptive therapy both in unmanipulated and in manipulated (ex-vivo T-depletion) grafts.
The criteria to establish the duration of pre-emptive treatment both for T-repleted and T-depleted allo-HCT was specified by 173 centres (96%), as follows: at least 2 weeks in 4 (2%), 2 consecutive CMV-DNAemia negative results in 138 (77%), until CMV-DNAemia gets down the threshold used to start treatment in 26 (14%), and until an adequate immune recovery is achieved in 5 (3%), not specified or missing in 7 (4%).
CMV disease and treatment: during the year 2019, 116 of 180 centres (64%) reported to have diagnosed ≥ 1 episode of CMV disease (median 2, range 1-50) for a total of 605 episodes. The episodes of CMV disease were classified as proven in 217 (36%), probable in 183 (30%) and possible in 205 (34%) while no information was given for the remaining 185 episodes.
The first-line treatment for CMV disease in unmanipulated allo-HCT was reported by 167 of 180 centres (93%): ganciclovir/valganciclovir in 158 (88%), foscarnet in 9 (5%), while data were missing for 13 centres (7%). CMV-hyperimmune globulin and CMV-CTLs were used in combination with drug therapy in 13 centres (7%) and 2 (1%). In manipulated HCT, the first-line therapy was reported in 113 centres (63%): ganciclovir/valganciclovir in 104 centres (92%) foscarnet in 8 (7%), cidofovir in 1 centre (1%) CMV-hyperimmune globulin and CMV-CTLs were used in combination with drug therapy in 10 (9%) and 4 (4%) centres, respectively.
The duration of therapy for CMV disease was reported by 163 centres (91%) and resulted as follows: until clinical resolution of clinical symptoms and two consecutive CMV-DNAemia negative results in 124 (69%), until clinical resolution of symptoms and CMV-DNAemia gets down the threshold used to start treatment in 25 (14%), until an adequate immune recovery is achieved in 3 (2%), until 3-6 weeks of treatment is completed in 11 (6%), not specified or missing in 17 (9%).
Fifty centres (28%) declared also to use CMV-CTLs mainly in the context of the treatment of refractory or recurrent CMV disease or clinical trial. Lastly, in 48 of 180 centres (27%) a median of 2 (range 1-12) cases of CMV resistance (infection or disease) were reported.
Discussion
The use of antiviral drugs at onset of CMV antigenemia or viremia (preemptive therapy) represented the major advancement in the last 3 decades to reduce the incidence of CMV end-organ disease after HCT [13]. Using this strategy, the incidence of CMV disease is around 5% and 9% by day + 100 and one year after HCT, respectively. No significant overall differences in incidence are reported using pp65 antigenemia or PCR method except for a relative increase in gastrointestinal CMV disease compared to CMV pneumonia with the PCR method [13]. Despite the fact that the attributable mortality of CMV disease is now of approximately 1% with preemptive treatment, this strategy has some suboptimal characteristics such as the organ toxicity (myelotoxicity for ganciclovir/valganciclovir, nephrotoxicity for foscarnet or cidofovir), the requirement of frequent blood sampling for CMV load monitoring, especially in the first 3 months after HCT, and the fact that it exposes the patient to the negative impact of any significant CMV load viremia on the outcome. In fact, it has been shown that, in patients under CMV-PCR-based surveillance, a CMV load of 250 IU/ml or higher was associated with an increased risk of early mortality in the first 2 months post-HCT and of overall and non-relapse mortality in the first year after HCT [14]. Several attempts were performed in the last decade to find an effective anti-CMV prophylaxis regimen capable to prevent CMV infection in the early post-HCT phase and improve overall survival with new drugs such as maribavir or brincidofovir [15, 16], but only letermovir was shown to reduce clinically significant CMV infections and overall mortality by week 24 post-HCT; moreover, the safety analysis of study population showed no significant difference with placebo in the rate of any adverse effects, time to engraftment, incidence and severity of GVHD, and myelotoxicity [8]. Letermovir received the market authorization by Food and Drug Administration (FDA) on 8 November 2017 and by European Agency for Medicines (EMA) on 17 January 2018 for CMV seropositive patients older than 18 years who undergo HCT [17, 18] and its use was endorsed by several guidelines [9, 19, 20]. The use of letermovir is not approved for paediatric and adolescent patients yet but a paediatric investigational plan is underway to define the dosage for patients aged less than 18 years.
This survey, conducted in 2020, was developed to assess the management of CMV infection in the real world of EBMT centre practice one year after the market authorization of letermovir, i.e. in the year 2019 [9]. The first result of the survey is the confirmation that PCR monitoring is the preferred method to perform the CMV surveillance among EBMT centres. This method was adopted in 97% of centres and in almost all (95%) it was applied to all types of allogeneic HCT, irrespective of the type of donor source used. On the other hand, variability remains in other aspects of CMV monitoring such as the type of sample used (whole blood preferred over plasma or serum), the frequency of monitoring (twice a week preferred over once time a week in the early phase of allogeneic HCT when the patient is hospitalized), and duration of monitoring (preference for a reasoned decision based on the patient clinical immune status and ongoing immunosuppressive therapy than a fixed date, i.e. day + 100 post allo-HCT). This variability reflects financial, organizational, and technical differences between centres and the different attitude of transplant haematologists to personalize the path of patient care. As far as the use of prophylaxis for CMV infection in high-risk patients is concerned, it was reported only in 56% of centres. Letermovir and aciclovir/valaciclovir were the most used drugs by centres, in 61% and 19%, respectively. Letermovir received the market authorization by EMA in 2108 and a trend to use the prophylaxis with letermovir was already established in 2019, especially in adult patients, given that letermovir is not approved in paediatric patients yet. Actually, letermovir prohylaxis was used in 74% and 65% of centres caring for adult HCT. On the other hand, several factors may have slowed down this policy such as the variable time interval between EMA approval and the authorization of letermovir prescription at different European countries, the costs of the drug prophylaxis and the need for every allogeneic-HCT unit to reformulate the annual budget and approve the costs for letermovir, and the attitude of the centres and haematologists to transfer quickly in the routine practice the indications coming from a clinical trial. Retrospective data from “real world” use and cost-effectiveness model analysis, confirmed that anti-CMV primary prophylaxis with letermovir is associated with a reduction of CMV infections, shorter hospitalizations, reduced costs and improvement of haematological and renal parameters [21,22,23]. In this regard, our interpretation of existing data support that a broader use of letermovir primary prophylaxis in the next future may result into an improvement of the overall transplant outcomes, as suggested by a post-hoc analysis on overall mortality conducted on the patients recruited in the phase III trial of letermovir versus placebo [24]. Moreover, a retrospective single-centre study comparing the outcome of two adult cohorts of patients, transplanted before and after the introduction of letermovir prophylaxis, respectively, showed that the 1-year mortality of letermovir-treated cohort was significantly reduced compared with that of no-letermovir-treated cohort and superimposable to that of CMV negative recipient/donor pairs [25].
There was a large variability on the threshold of CMV-DNAemia used to start preemptive therapy although the preference was for a CMV load > 103 copies/ml or IU/ml both for unmanipulated and ex-vivo T-cell depleted HCT. This suggests that a common centre-approach in starting preemptive treatment irrespective of the type of transplant and the type of graft manipulation. On the other hand, the duration of preemptive treatment was not fixed but most of centres decided according to the virological response aiming at the complete clearance of CMV viremia or reduction to a lower level than the threshold used to start antiviral therapy. No new developments were reported concerning the choice of antiviral treatment both for preemptive therapy and the therapy of CMV disease. In fact, ganciclovir and valganciclovir remained the preferred first-line treatment of CMV infections, being used in 93% in the pre-emptive setting, and in 88-92% in the CMV disease setting [9, 26]. The use of passive immunization with CMV-hyperimmune globulins was very limited in prophylaxis and pre-emptive setting (3-4%) while 7-9% of centres declared to use them in the setting of treatment of CMV disease. This reflects the lack of controlled studies showing efficacy of this approach and the uncertain role of CMV-hyperimmune globulins and humoral immunity in preventing or controlling CMV infection in presence of deficient CMV-specific T-cell response [9, 27, 28]. The use of CMV-hyperimmune globulins is mainly reported as adjuvant therapy combined with antiviral drugs in the treatment of CMV disease, especially CMV pneumonia [29, 30]. However, a large retrospective analysis on cytomegalovirus pneumonia did not find any advantage on overall and 6-month CMV attributable mortality in using CMV-hyperimmune globulin as adjunct to antiviral therapy [31]. Whether addition of CMV-hyperimmune globulin would add benefit to other management strategies either given as prophylaxis, preemptive therapy, or for treatment of CMV disease should be studied in carefully designed prospective studies. Adoptive immunotherapy with CMV-CTLs was effective in the prophylaxis and therapy of CMV disease in several “proof of principle” academic or experimental studies conducted in the last two decades which demonstrated the feasibility and efficacy of ex vivo generated donor-derived or third-party-derived CMV-CTLs [32,33,34]. In this survey, the reported use of CMV-CTLs was very limited in the setting of prophylaxis or pre-emptive therapy while 28% of centres declared to use CMV-CTLs especially in the setting of CMV diseases refractory to antiviral treatment. Although there is strong rationale for adoptive immunotherapy in HCT patients and the preliminary studies showed a good safety profile and feasibility, more data are needed to define the efficacy of CMV-CTLs and durability of response, especially in the setting of unmanipulated transplant where patients receive immunosuppressive treatment. Lastly, 27% of centres reported one or more episodes of CMV infection due to drug resistant CMV strains. Drug resistance is usually observed in the setting of clinically refractory or relapsing CMV infection and it is caused by mutations in the CMV genes coding for UL97 protein kinase or UL54 DNA polymerase. Considered the limited therapeutic options, drug resistance is a risk factor for CMV diseases and mortality. In these context, maribavir demonstrated recently to be an useful option. In a phase III trial on hematopoietic-cell and solid-organ transplant patients with refractory or relapsing CMV infection, maribavir was superior to other antiviral treatments (ganciclovir/valganciclovir, foscarnet, cidofovir) in obtaining the virological and clinical clearance of CMV [35].
In conclusion, this survey showed that, after one year from market authorization letermovir, there was a trend among EBMT centres toward a prophylaxis approach. This trend may increase further as the use of the drug is approved by every national competent authority. This innovation, together with cellular therapy and more effective therapy for the refractory and relapsing CMV infection has the potential to reduce the negative impact of CMV seropositivity and infection on the transplant outcome.
Data availability
The results of the survey are available upon specific request to the corresponding author.
References
Giménez E, Torres I, Albert E, Piñana J-L, Hernández-Boluda J-C, Solano C, et al. Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): A systematic review, meta-analysis, and meta-regression analysis. Am J Transpl. 2019;19:2479–94.
Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. Table of Contents
de la Cámara R. CMV in hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis. 2016;8:e2016031.
Dziedzic M, Sadowska-Krawczenko I, Styczynski J. Risk factors for cytomegalovirus infection after allogeneic hematopoietic cell transplantation in malignancies: proposal for classification. Anticancer Res. 2017;37:6551–6.
Jakharia N, Howard D, Riedel DJ. CMV infection in hematopoietic stem cell transplantation: prevention and treatment strategies. Curr Treat Options Infect Dis. 2021;13:123–40.
Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–9.
Gagelmann N, Ljungman P, Styczynski J, Kröger N. Comparative efficacy and safety of different antiviral agents for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transpl. 2018;24:2101–9.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–44.
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e260–e272.
Ljungman P, Brand R, Hoek J, de la Camara R, Cordonnier C, Einsele H, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59:473–81.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91.
Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2019;68:1420–6.
Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2012;18:1687–99.
Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3:e119–127.
Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11:284–92.
Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2019;25:369–81.
FDA Approves PREVYMIS (Letermovir). https://www.accp1.org/ACCP1/5Publications_and_News/FDA_Approves_PREVYMIS__Letermovir_.aspx (accessed 1 May2022).
EMA. Prevymis. European Medicines Agency. 2018.https://www.ema.europa.eu/en/medicines/human/EPAR/prevymis (accessed 1 May2022).
Brissot E, Alsuliman T, Beauvais D, Bonnin A, Mear J-B, Souchet L, et al. [Antiviral prophylaxis for CMV, HSV/VZV and HBV in allogeneic hematopoietic cell transplantation in adult patients: Guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC)]. Bull Cancer. 2020;107:S1–S6.
Olson AL, Politikos I, Brunstein C, Milano F, Barker J, Hill JA, et al. Guidelines for infection prophylaxis, monitoring and therapy in cord blood. Transplant Transpl Cell Ther. 2021;27:359–62.
Royston L, Royston E, Masouridi-Levrat S, Vernaz N, Chalandon Y, Van Delden C, et al. Letermovir primary prophylaxis in high-risk hematopoietic cell transplant recipients: a matched cohort study. Vaccines (Basel). 2021;9:372.
Malagola M, Pollara C, Polverelli N, Zollner T, Bettoni D, Gandolfi L, et al. Advances in CMV management: a single center real-life experience. Front Cell Dev Biol. 2020;8:534268.
Alsumali A, Chemaly RF, Graham J, Jiang Y, Merchant S, Miles L, et al. Cost-effectiveness analysis of cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplant recipients from a US payer perspective. J Med Virol. 2021;93:3786–94.
Ljungman P, Schmitt M, Marty FM, Maertens J, Chemaly RF, Kartsonis NA, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2020;70:1525–33.
Su Y, Stern A, Karantoni E, Nawar T, Han G, Zavras P et al. Impact of letermovir primary Cytomegalovirus (CMV) prophylaxis on 1-year mortality after allogeneic hematopoietic cell transplantation (HCT): a retrospective cohort study. Clin Infect Dis. 2022;: ciab1064.
Pande A, Dubberke ER. Cytomegalovirus infections of the stem cell transplant recipient and hematologic malignancy patient. Infect Dis Clin North Am. 2019;33:485–500.
Sokos DR, Berger M, Lazarus HM. Intravenous immunoglobulin: appropriate indications and uses in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2002;8:117–30.
Emery V, Zuckerman M, Jackson G, Aitken C, Osman H, Pagliuca A, et al. Management of cytomegalovirus infection in haemopoietic stem cell transplantation. Br J Haematol. 2013;162:25–39.
Alexander BT, Hladnik LM, Augustin KM, Casabar E, McKinnon PS, Reichley RM, et al. Use of cytomegalovirus intravenous immune globulin for the adjunctive treatment of cytomegalovirus in hematopoietic stem cell transplant recipients. Pharmacotherapy. 2010;30:554–61.
Alsuliman T, Kitel C, Dulery R, Guillaume T, Larosa F, Cornillon J, et al. Cytotect®CP as salvage therapy in patients with CMV infection following allogeneic hematopoietic cell transplantation: a multicenter retrospective study. Bone Marrow Transpl. 2018;53:1328–35.
Erard V, Guthrie KA, Seo S, Smith J, Huang M, Chien J, et al. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin Infect Dis. 2015;61:31–39.
Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, et al. Off-the-shelf virus-specific T cells to treat bk virus, human herpesvirus 6, cytomegalovirus, epstein-barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35:3547–57.
Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. 2018;78:1085–103.
Pei X-Y, Liu X-F, Zhao X-Y, Lv M, Mo X-D, Chang Y-J, et al. Comparable anti-CMV responses of transplant donor and third-party CMV-specific T cells for treatment of CMV infection after allogeneic stem cell transplantation. Cell Mol Immunol. 2022;19:482–91.
Avery RK, Alain S, Alexander BD, Blumberg EA, Chemaly RF, Cordonnier C et al. Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial. Clin Infect Dis. 2021: ciab988.
Acknowledgements
We thank all EBMT centres that contributed to the survey (see below) and the doctors and nurses at each EBMT centre for their dedication to patient care and health.
Author information
Authors and Affiliations
Contributions
SC designed the study; SC, GT and LW prepared the survey; LW collected the data; SC and GT analysed the data and prepared the final report; SC and GT wrote the manuscript; SC, PL, MM, JS, DA, and RdlC discussed the results and critically reviewed the manuscript; all authors approved the final version of the manuscript manuscript.
Corresponding author
Ethics declarations
Competing interests
PL declared the following interests: investigator, speaker with honoraria going to the employer, endpoint committee member for Merck Sharp Dohme; speaker with honoraria going to the employer, scientific expert, adjudication committee member for Takeda; scientific expert for AiCuris; speaker with honoraria going to the employer for Biotest. RdlC declared the following interests: speaker with honoraria for Merck Sharp Dohme, Astellas and Clinigen. The other authors declare to have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cesaro, S., Ljungman, P., Tridello, G. et al. New trends in the management of cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a survey of the Infectious Diseases Working Pary of EBMT. Bone Marrow Transplant 58, 203–208 (2023). https://doi.org/10.1038/s41409-022-01863-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01863-8