Abstract
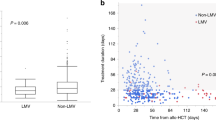
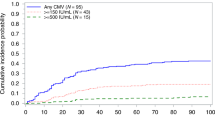
Letermovir prophylaxis revolutionized the approach to Cytomegalovirus infection in adult hematopoietic stem cell transplant (HCT), while data in pediatric setting are still lacking. We retrospectively analyzed 87 HCT children transplanted in 11 AIEOP centers receiving letermovir as off-label indication between January 2020 and November 2022. Letermovir was used as primary, secondary prophylaxis or CMV treatment in 39, 26 and 22 cases, respectively; no discontinuation due to toxicity was reported. Median duration was 100 days (14-256) for primary and 96 days (8-271) for secondary prophylaxis, respectively. None of the patients experienced CMV-clinically significant reactivation during Letermovir primary prophylaxis; one patient developed breakthrough infection during secondary prophylaxis, and 10 and 1 patient experienced asymptomatic CMV-reactivation and CMV-primary infection after drug discontinuation, respectively. Median duration of letermovir in CMV treatment was 40 days (7-134), with 4/22 patients suffering CMV-pneumonia, with an overall response rate of 86.4%. With a median follow-up of 10.7 months (8.2-11.8), estimated 1-year overall survival was 86%; no CMV-related deaths were reported in prophylaxis groups. This is the largest report on Letermovir use in pediatric HCT; real-life data confirm an excellent toxicity profile, with high efficacy as CMV prophylaxis; results in CMV-infection treatment should be investigated in larger, prospective trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3:e119–27.
Cantoni N, Hirsch HH, Khanna N, Gerull S, Buser A, Bucher C, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transpl. 2010;16:1309–14.
Schmidt-Hieber M, Tridello G, Ljungman P, Mikulska M, Knelange N, Blaise D, et al. The prognostic impact of the cytomegalovirus serostatus in patients with chronic hematological malignancies after allogeneic hematopoietic stem cell transplantation: a report from the Infectious Diseases Working Party of EBMT. Ann Hematol. 2019;98:1755–63.
Jakharia N, Howard D, Riedel DJ. CMV infection in hematopoietic stem cell transplantation: prevention and treatment strategies. Curr Treat Options Infect Dis. 2021;13:123–40.
Heston SM, Young RR, Tanaka JS, Jenkins K, Vinesett R, Saccoccio FM, et al. Risk factors for CMV viremia and treatment-associated adverse events among pediatric hematopoietic stem cell transplant recipients. Open Forum Infect Dis. 2022;9:ofab639.
Thomas G, Guy H, Nicole E, Helga R-S, Holger Z, Peter L. The Novel Anticytomegalovirus Compound AIC246 (Letermovir) Inhibits Human Cytomegalovirus Replication through a Specific Antiviral Mechanism That Involves the Viral Terminase. J Virol. 2011;85:10884–93.
Griffiths P, Lumley S. Cytomegalovirus. Curr Opin Infect Dis. 2014;27:554–9.
Derigs P, Radujkovic A, Schubert M-L, Schnitzler P, Schöning T, Müller-Tidow C, et al. Letermovir prophylaxis is effective in preventing cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation: single-center real-world data. Ann Hematol. 2021;100:2087–93.
Chemaly RF, Ullmann AJ, Ehninger G. CMV prophylaxis in hematopoietic-cell transplantation. N. Engl J Med. 2014;371:576–7.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl J Med. 2017;377:2433–44.
Mori Y, Jinnouchi F, Takenaka K, Aoki T, Kuriyama T, Kadowaki M, et al. Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transpl. 2021;56:853–62.
Cesaro S, Ljungman P, Tridello G, Mikulska M, Wendel L, Styczynski J, et al. New trends in the management of cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a survey of the Infectious Diseases Working Pary of EBMT. Bone Marrow Transpl. 2023;58:203–8.
Lin A, Maloy M, Su Y, Bhatt V, DeRespiris L, Griffin M, et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: Real-world experience. Transpl Infect Dis J Transpl Soc. 2019;21:e13187.
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e260–e272.
Styczyński J, Tridello G, Xhaard A, Medinger M, Mielke S, Taskinen M, et al. Use of letermovir in off-label indications: Infectious Diseases Working Party of European Society of Blood and Marrow Transplantation retrospective study. Bone Marrow Transpl. 2021;56:1171–9.
Richert-Przygonska M, Jaremek K, Debski R, Konieczek J, Lecka M, Dziedzic M, et al. Letermovir prophylaxis for cytomegalovirus infection in children after hematopoietic cell transplantation. Anticancer Res. 2022;42:3607–12.
Kuhn A, Puttkammer J, Madigan T, Dinnes L, Khan S, Ferdjallah A, et al. Letermovir as cytomegalovirus prophylaxis in a pediatric cohort: a retrospective analysis. Transpl Cell Ther. 2023;29:62.e1–62.e4.
Cheng C-N, Li S-S, Yeh Y-H, Shen C-F, Chen J-S. Letermovir prophylaxis for cytomegalovirus reactivation in children who underwent hematopoietic stem cell transplantation: A single-institute experience in Taiwan. J Microbiol Immunol Infect. 2022;55:323–7.
Daukshus N, Cirincione A, Siver M, Mathew S, Kunvarjee B, Chan A, et al. Letermovir for Cytomegalovirus Prevention in Adolescent Patients Following Hematopoietic Cell Transplantation. J Pediatric Infect Dis Soc. https://doi.org/10.1093/jpids/piac017. (2022).
Song J, Swallow E, Said Q, Peeples M, Meiselbach M, Signorovitch J, et al. Epilepsy treatment patterns among patients with tuberous sclerosis complex. J Neurol Sci. 2018;391:104–8.
Körholz KF, Füller MA, Hennies M, Holterhus M, Hagedorn S, Ahlmann M, et al. Letermovir for prophylaxis and pre-emptive therapy of cytomegalovirus infection in paediatric allogeneic haematopoietic cell transplant patients. Pediatr Drugs. 2023;25:225–32.
Chen TT, David AP, Barthelmess EK, MacBrayne CE. Letermovir for Cytomegalovirus prophylaxis in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2023;70:e30608.
Pérez Marín M, Decosterd LA, Andre P, Buclin T, Mercier T, Murray K, et al. Compassionate Use of Letermovir in a 2-Year-Old Immunocompromised Child with Resistant Cytomegalovirus Disease. J Pediatr Infect Dis Soc. 2019;9:96–99.
Chiereghin A, Belotti T, Borgatti EC, Fraccascia N, Piccirilli G, Fois M, et al. Off-label use of letermovir as preemptive anti-cytomegalovirus therapy in a pediatric allogeneic peripheral blood stem cell transplant. Infect Drug Resist. 2021;14:1185–90.
Kilgore JT, Becken B, Varga MG, Parikh S, Prasad V, Lugo D, et al. Use of letermovir for salvage therapy for resistant cytomegalovirus in a pediatric hematopoietic stem cell transplant recipient. J Pediatr Infect Dis Soc. 2020;9:486–9.
Strenger V, Sperl D, Kubesch K, Donnerer J, Schwinger W, Zach K, et al. Letermovir in paediatric HSCT recipients. J Antimicrob Chemother 2019;74:2820–1.
Carreras E, Dufour C, Mohty M, Kröger N The EBMT Handbook: Hematopoietic stem cell transplantation and cellular therapies. EBMT Handb Hematop Stem Cell Transplant Cell Ther: 2018;Vol III and Vol V:1–702.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91.
Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis Publ Infect Dis Soc Am. 2019;68:1420–6.
Liu LW, Yn A, Gao F, Olson M, Crain M, Abboud R, et al. Letermovir Discontinuation at Day 100 After Allogeneic Stem Cell Transplant Is Associated With Increased CMV-Related Mortality. Transpl Cell Ther. 2022;28:510.e1–510.e9.
Zamora D, Duke ER, Xie H, Edmison BC, Akoto B, Kiener R, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138:34–43.
Cassaniti I, Colombo AA, Bernasconi P, Malagola M, Russo D, Iori AP, et al. Positive HCMV DNAemia in stem cell recipients undergoing letermovir prophylaxis is expression of abortive infection. Am J Transpl J Am Soc Transpl Am Soc Transpl Surg. 2021;21:1622–8.
Robin C, Thiebaut A, Alain S, Sicre de Fontbrune F, Berceanu A, D’Aveni M, et al. Letermovir for Secondary Prophylaxis of Cytomegalovirus Infection and Disease after Allogeneic Hematopoietic Cell Transplantation: Results from the French Compassionate Program. Biol Blood Marrow Transpl. 2020;26:978–84.
Han G, Stern A, Su Y, Gyurkocza B, Sauter C, Shaffer B, et al. 595. Letermovir (LTV) for Secondary Cytomegalovirus (CMV) Prevention in High Risk Hematopoietic Cell Transplant (HCT) Recipients: Interim Results of a Single Center, Open Label Study. Open Forum Infect Dis. 2021;8:S401–S401.
Sassine J, Khawaja F, Shigle TL, Handy V, Foolad F, Aitken SL, et al. Refractory and Resistant Cytomegalovirus After Hematopoietic Cell Transplant in the Letermovir Primary Prophylaxis Era. Clin Infect Dis Publ Infect Dis Soc Am. 2021;73:1346–54.
Marty FM, Ljungman PT, Chemaly RF, Wan H, Teal VL, Butterton JR, et al. Outcomes of patients with detectable CMV DNA at randomization in the phase III trial of letermovir for the prevention of CMV infection in allogeneic hematopoietic cell transplantation. Am J Transpl J Am Soc Transpl Am Soc Transpl Surg. 2020;20:1703–11.
Royston L, Royston E, Masouridi-Levrat S, Chalandon Y, Van Delden C, Neofytos D. Predictors of breakthrough clinically significant cytomegalovirus infection during letermovir prophylaxis in high-risk hematopoietic cell transplant recipients. Immun, Inflamm Dis. 2021;9:771–6.
Knoll BM, Seiter K, Phillips A, Soave R. Breakthrough cytomegalovirus pneumonia in hematopoietic stem cell transplant recipient on letermovir prophylaxis. Bone Marrow Transpl. 2019;54:911–2.
Jung S, Michel M, Stamminger T, Michel D. Fast breakthrough of resistant cytomegalovirus during secondary letermovir prophylaxis in a hematopoietic stem cell transplant recipient. BMC Infect Dis. 2019;19:388.
Khawaja F, Batista MV, El Haddad L, Chemaly RF. Resistant or refractory cytomegalovirus infections after hematopoietic cell transplantation: diagnosis and management. Curr Opin Infect Dis. 2019;32:565–74.
Kim SJ, Huang YT, Foldi J, Lee YJ, Maloy M, Giralt SA, et al. Cytomegalovirus resistance in CD34+-selected hematopoietic cell transplant recipients. Transpl Infect Dis. 2018;20:1–9.
Reusser P, Einsele H, Lee J, Volin L, Rovira M, Engelhard D, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99:1159–64.
Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–9.
Avery RK, Arav-Boger R, Marr KA, Kraus E, Shoham S, Lees L, et al. Outcomes in Transplant Recipients Treated With Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation. 2016;100:e74–80.
Maffini E, Giaccone L, Festuccia M, Brunello L, Busca A, Bruno B. Treatment of CMV infection after allogeneic hematopoietic stem cell transplantation. Expert Rev Hematol. 2016;9:585–96.
Linder KA, Kovacs C, Mullane KM, Wolfe C, Clark NM, La Hoz RM, et al. Letermovir treatment of cytomegalovirus infection or disease in solid organ and hematopoietic cell transplant recipients. Transpl Infect Dis J Transpl Soc. 2021;23:e13687.
Acknowledgements
We thank all members of the Infectious Diseases Working Group (IDWG) of the Associazione Italiana di Oncologia e Ematologia Pediatrica (AIEOP) and their collaborators for their contribution to this report.
Funding
The authors declare no sources of funding.
Author information
Authors and Affiliations
Contributions
GF and BF contributed to design the study, collected data and wrote the paper; ZD collected data and revised the manuscript; MR, SE, MR, BF, CA, PG, DMR, CE, BA, PK, GP, OC, QF, and GA collected data; PD and LF supervised the project and revised the manuscript; CS conceived the study, collected the data, wrote, read, and supervised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galaverna, F., Baccelli, F., Zama, D. et al. Letermovir for Cytomegalovirus infection in pediatric patients undergoing allogenic hematopoietic stem cell transplantation: a real-life study by the Infectious Diseases Working Group of Italian Association of Pediatric Hematology-Oncology (AIEOP). Bone Marrow Transplant 59, 505–512 (2024). https://doi.org/10.1038/s41409-024-02209-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02209-2