Abstract
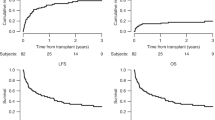
Although haploidentical stem cell transplantation (haplo-HSCT) offers almost all acute lymphoblastic leukaemia (ALL) patients an opportunity for immediate transplantation, it exhibits a higher incidence of graft failure and graft versus host disease (GVHD). Mesenchymal stem cells (MSCs) are characterised by their haematopoiesis-promoting and immunomodulatory capacity. Thus, we designed a combination of haplo-HSCT and MSCs for ALL patients. ALL patients (n = 110) were given haploidentical HSCs combined with allogenic MSCs, and ALL patients without MSC infusion (n = 56) were included as controls. The 100-day cumulative incidences of grade ≥2 acute GVHD (aGVHD) and grade ≥3 aGVHD were 40.00% and 9.09% compared to 42.32% (P = 0.79) and 22.79% (P = 0.03) in patients without MSC infusion, respectively. The 3-year cumulative incidences of chronic GVHD (cGVHD) and extensive cGVHD were 22.27% and 10.27% compared to 32.14% (P = 0.19) and 22.21% (P = 0.04) in patients without MSC infusion, respectively. No significant differences in the 3-year relapse incidence, nonrelapse mortality, leukaemia-free survival or overall survival in groups with and without MSC cotransplantation were observed. Multivariate analysis showed that MSC infusion contributed to a lower risk of developing extensive cGVHD. Our data suggested that haplo-HSCT combined with MSCs may provide an effective and safe treatment for ALL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Brown PA, Wieduwilt M, Logan A, DeAngelo DJ, Wang ES, Fathi A, et al. Guidelines insights: acute lymphoblastic leukemia, version 1.2019. J Natl Compr Canc Netw 2019;17:414–423.
Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet 2020;395:1146–1162.
Siegel SE, Stock W, Johnson RH, Advani A, Muffly L, Douer D, et al. Pediatric-inspired treatment regimens for adolescents and young adults with philadelphia chromosome-negative acute lymphoblastic leukemia: a review. JAMA Oncol 2018;4:725–734.
El Fakih R, Kharfan-Dabaja MA, Aljurf M. Refining the role of hematopoietic cell transplantation for acute lymphoblastic leukemia as novel therapies emerge. Biol Blood Marrow Transplant 2016;22:2126–2133.
Short NJ, Kantarjian H, Jabbour E. Optimizing the treatment of acute lymphoblastic leukemia in younger and older adults: new drugs and evolving paradigms. Leukemia 2021;35:3044–3058.
Hu GH, Zhao XY, Zuo YX, Chang YJ, Suo P, Wu J, et al. Unmanipulated haploidentical hematopoietic stem cell transplantation is an excellent option for children and young adult relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia after CAR-T-cell therapy. Leukemia 2021;35:3092–3100.
Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 2014;20:1573–1579.
Deng RX, Wu YJ, Xu LP, Liu KY, Huang XJ, Zhang XH. Clinical risk factors and prognostic model for idiopathic inflammatory demyelinating diseases after haploidentical hematopoietic stem cell transplantation in patients with hematological malignancies. Am J Hematol 2021;96:1407–1419.
Xue YJ, Suo P, Huang XJ, Lu AD, Wang Y, Zuo YX, et al. Superior survival of unmanipulated haploidentical haematopoietic stem cell transplantation compared with intensive chemotherapy as post-remission treatment for children with very high-risk philadelphia chromosome negative B-cell acute lymphoblastic leukaemia in first complete remission. Br J Haematol 2020;188:757–767.
Nagler A, Dholaria B, Labopin M, Savani BN, Angelucci E, Koc Y, et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia 2020;34:2766–2775.
Mancusi A, Ruggeri L, Velardi A. Haploidentical hematopoietic transplantation for the cure of leukemia: from its biology to clinical translation. Blood 2016;128:2616–2623.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol 2020;7:e157–e167.
Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol 2016;34:2843–2850.
Hashmi S, Ahmed M, Murad MH, Litzow MR, Adams RH, Ball LM, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol 2016;3(Jan):e45–52.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829–834.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327–334.
Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 2014;15:1009–1016.
Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol 2020;21:974–982.
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 2018;14:493–507.
Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, et al. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res 2005;15:539–547.
Reinisch A, Etchart N, Thomas D, Hofmann NA, Fruehwirth M, Sinha S, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 2015;125:249–260.
Crippa S, Rossella V, Aprile A, Silvestri L, Rivis S, Scaramuzza S, et al. Bone marrow stromal cells from beta-thalassemia patients have impaired hematopoietic supportive capacity. J Clin Invest 2019;129:1566–1580.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007;110:2764–2767.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLAidentical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005;11:389–398.
Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 2008;22:593–599.
Ji SQ, Chen HR, Wang HX, Yan HM, Zhu L, Liu J, et al. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: an excellent alternative for high-risk leukemia. Bone Marrow Transplant 2002;30:861–866.
Chen HR, Ji SQ, Wang HX, Yan HM, Zhu L, Liu J, et al. Humanized anti-CD25 monoclonal antibody for prophylaxis of graft-vs-host disease (GVHD) in haploidentical bone marrow transplantation without ex vivo T-cell depletion. Exp Hematol 2003;31:1019–1025.
Ji SQ, Chen HR, Yan HM, Wang HX, Liu J, Zhu PY, et al. Anti-CD25 monoclonal antibody (basiliximab) for prevention of graft-versus-host disease after haploidentical bone marrow transplantation for hematological malignancies. Bone Marrow Transplant 2005;36:349–354.
Wang HX, Yan HM, Duan LN, Wang ZD, Zhu L, Xue M, et al. Haploidentical hematopoietic stem cell transplantation in child hematologic malignancies with GCSF- mobilized marrow grafts without T-cell depletion: a single-center report of 45 cases. Pediatr Hematol Oncol 2009;26:119–128.
Wang HX, Yan HM, Liu J, Duan LN, Wang ZD, Zhu L, et al. Haploidentical hematopoietic stemcell transplantation for non-Hodgkin lymphoma with bone marrow involvement. Leuk Lymphoma 2009;50:1488–1493.
Wang HX, Yan HM, Wang ZD, Xue M, Liu J, Guo ZK. Haploidentical hematopoietic stem cell transplantation in hematologic malignancies with G-CSF mobilized bone marrow plus peripheral blood stem cells grafts without T cell depletion: a single center report of 29 cases. Leuk Lymphoma 2012;53:654–659.
Han DM, Zheng XL, Ding L, Yan HM, Wang ZD, Xue M, et al. Risk factors in patients undergoing haploidentical hematopoietic stem cell transplantation for high-risk childhood acute leukemia. Int J Hematol 2017;106:820–831.
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005;105:4120–4126.
Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 2010;5:550–560.
Zhu H, Yang F, Tang B, Li XM, Chu YN, Liu YL, et al. Mesenchymal stem cells attenuated PLGA-induced inflammatory responses by inhibiting host DC maturation and function. Biomaterials 2015;53:688–698.
Liu J, Han D, Wang Z, Xue M, Zhu L, Yan HM, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 2013;15:185–191.
Ding L, Han DM, Zheng XL, Yan HM, Xue M, Liu J, et al. A study of human leukocyte antigen-haploidentical hematopoietic stem cells transplantation combined with allogenic mesenchymal stem cell infusion for treatment of severe aplastic anemia in pediatric and adolescent patients. Stem Cells Transl Med 2021;10:291–302.
Ding L, Han DM, Zheng XL, Yan HM, Xue M, Liu J, et al. Infusion of haploidentical hematopoietic stem cells combined with mesenchymal stem cells for treatment of severe aplastic anemia in adult patients yields curative effects. Cytotherapy. 2021:24;205-12.
Han DM, Wang ZD, Zheng XL, Ding L, Yan HM, Xue M, et al. Co-infusion of mesenchymal stromal cells has no effect on relapse and infection in patients with leukemia undergoing haploidentical hematopoietic stem cell transplant. Leuk Lymphoma 2015;56:2965–2968.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995;15:825–828.
Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003;9:215–233.
Verma D, Zanetti C, Godavarthy PS, Kumar R, Minciacchi VR, Pfeiffer J, et al. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia 2020;34:1540–1552.
Chen YL, Tang C, Zhang MY, Huang WL, Xu Y, Sun HY, et al. Blocking ATM-dependent NF-kappaB pathway overcomes niche protection and improves chemotherapy response in acute lymphoblastic leukemia. Leukemia 2019;33:2365–2378.
Dotoli GM, Santis GC, Orellana MD, Prata KL, Caruso SR, Fernandes TR, et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant 2017;52:859–862.
Boberg E, Bahr L, Afram G, Lindström C, Ljungman P, Heldring N, et al. Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: a phase II study. Stem Cells Transl Med 2020;9:1190–1202.
Li H, Guo Z, Jiang X, Zhu H, Li XS, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells 2008;26:2531–2541.
Li H, Jiang Y, Jiang X, Guo XM, Ning HM, Li YH, et al. CCR7 guides migration of mesenchymal stem cell to secondary lymphoid organs: a novel approach to separate GvHD from GvL effect. Stem Cells 2014;32:1890–1903.
Li H, Guo ZK, Li XS, Hou CM, Tang PH, Mao N. Functional and phenotypic alteration of intrasplenic lymphocytes affected by mesenchymal stem cells in a murine allosplenocyte transfusion model. Cell Transplant 2007;16:85–95.
Lee MW, Ryu S, Kim DS, Lee JW, Sung KW, Koo HH, et al. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia 2019;33:597–611.
Acknowledgements
This work was supported by National Natural Sciences Grants China (No. 82172388, 81871771, 81500083 and 81572159), the Beijing Natural Sciences Foundation (No. 7192203, 7182123 and L212065) and other local Science Foundations (No. 21QNPY096, No.SYDW-2020-08).
Author information
Authors and Affiliations
Contributions
LD was responsible for the collection and/or assembly of data, data analysis, data interpretation, manuscript writing, financial support and final approval of the manuscript. DMH was responsible for the provision of study material or patients and the collection and/or assembly of data. HMY contributed to the collection and/or assembly of data, data analysis and data interpretation. JXZ and XLZZ contributed to the provision of study material or patients. MX, JL and LZ contributed to the collection and/or assembly of data. NM, ZKG and HMN contributed to the study conception and study design. HXW was responsible for the study conception, study design, data analysis, data interpretation and final approval of the manuscript. HZ was responsible for the study conception, study design, data analysis, data interpretation, manuscript writing, financial support and final approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ding, L., Han, DM., Yan, HM. et al. Infusion of haploidentical HSCs combined with allogenic MSCs for the treatment of ALL patients. Bone Marrow Transplant 57, 1086–1094 (2022). https://doi.org/10.1038/s41409-022-01688-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01688-5