Abstract
For patients with acute myeloid and lymphoblastic leukaemia (AML/ALL) lacking a matched sibling or unrelated donor, haploidentical stem cell transplantation (HAPLO-SCT) is increasingly used. However, available data on the treatment of relapse after HAPLO-SCT, including feasibility and efficacy of a second HAPLO-SCT (HAPLO-SCT2), is scarce. Hence, adults with AML/ALL, that had undergone HAPLO-SCT2 without ex-vivo manipulation after haematologic relapse from HAPLO-SCT1 were selected for a retrospective registry analysis. Eighty-two patients (AML, n = 63, ALL, n = 19, median follow-up: 33 months) were identified. Engraftment rate was 87%. At day +180, cumulative incidences of acute GvHD II-IV°/chronic GvHD were 23.9%/22.6%, respectively. Two-year overall survival/leukaemia-free survival (OS/LFS) were 34.3%/25.4%; 2-year non-relapse mortality (NRM) and relapse incidence (RI) were 17.6% and 57%. Leukaemia was the most frequent cause of death. Separated by disease, 2-year OS/LFS/NRM/RI were 28.7%/22.3%/16.2%/61.6% in AML, and 55.3%/38.4%/23.5%/38.2% in ALL patients. In a risk-factor analysis among patients with AML, stage at HAPLO-SCT1 and HAPLO-SCT2, and interval from HAPLO-SCT1 to relapse significantly influenced outcome. Our data demonstrate that HAPLO-SCT2 is a viable option in acute leukaemia relapse after HAPLO-SCT1. Engraftment, toxicity, risk factors and long-term outcome are comparable to data reported after allo-SCT2 in a matched donor setting.
Similar content being viewed by others
Introduction
Acute myeloid and acute lymphoblastic leukaemia (AML/ALL) represent the most common indications for allogeneic haematopoietic stem cell transplantation (allo-SCT) in Europe [1]. The availability of HAPLO-SCT has expanded transplant options for patients lacking a human leucocyte antigen (HLA)-matched sibling or unrelated donor (MSD/MUD) [2,3,4,5] and is increasingly used [6]. Comparative analyses have shown that haploidentical (HAPLO) donor for first allo-SCT (allo-SCT1) in different stages of AML and ALL is a suitable alternative to MUD or MSD, associated with comparable results [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
For patients with acute leukaemia (AL) that relapse after allo-SCT1, a second allogeneic transplantation (allo-SCT2) represents a viable treatment option, especially in patients without relevant co-morbidities, who relapse >6 months from the first transplant. Historically, allo-SCT2 using either MSD or MUD after an allo-SCT1 from MSD/MUD could achieve long term survival independently of donor type for allo-SCT2 and donor change. Among others [24,25,26], this was reported by Christopeit et al. [27] who found 2-year overall survival (OS) and leukaemia-free survival (LFS) rates of 25% and 21%, after allo-SCT2 from MSD and MUD in a multicenter analysis including 179 patients. In the study of Kharfan-Dabaja et al., [28] 2- and 5-year OS after allo-SCT2 in 137 AML patients was 26%/19%, respectively. More recently, Nagler et al. [29] analysed the outcome of 245 ALL patients limited to MSD/MUD for allo-SCT2 and observed 2-year/5-year LFS rates of 20%/12%, and 2-year/5-year OS of 30%/14%.
With the increasing routine use of HAPLO-SCT, HLA-mismatched family donors were also used more frequently for second transplants [30]. Tischer et al. [31] reported on 20 consecutive patients with AL achieving 1-year OS/LFS of 45%/33% and non-relapse mortality (NRM) of 36% after sequential conditioning HAPLO-SCT2 for relapse following matched donor SCT1. Shimoni et al. [32] investigated the outcome of 556 patients after allo-SCT2 for AML relapse following matched SCT1 by dividing patients into three groups: same donor [n = 163, MSD/MSD-112, MUD/MUD-51], different matched donor [n = 305, MSD/different MSD-44, MSD/MUD-93, MUD/different MUD-168], or HAPLO [n = 88, MSD/HAPLO-45, MUD/HAPLO-43]. Two-year OS/LFS rates were 36.4%/23.5%, 28.7%/23.7%, and 23.3%21.8%, respectively, with no statistically significant differences among cohorts. However, on multivariate analysis, HAPLO-SCT2 was associated with higher NRM. In two further studies performed on behalf of the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT), Kharfan-Dabaja et al. reported comparable outcomes after allo-SCT2 from either MUD or HAPLO, both in AML [33] and ALL [34].
Thus, available data suggest comparable outcomes after allo-SCT2, regardless of donor type. However, in the vast majority, these analyses were based on patients that had received their first transplant from either a MSD or a MUD. Patients that had received two HAPLO-SCT represented a small minority at best, without a separate analysis. Hence, data supporting the feasibility and efficacy of HAPLO-SCT2 in patients who had developed leukaemia relapse after being transplanted from a HAPLO donor at allo-SCT1 is scarce [35, 36]. In this registry-based study we report on the outcome of 82 patients with AL that underwent HAPLO-SCT2 after relapse from HAPLO-SCT1 across EBMT centres.
Methods
Study population
This was a registry-based analysis of adults transplanted for AML and ALL. Eligible patients had to have received in-vivo T-cell replete HAPLO-SCT2 after haematologic relapse from a T-cell replete HAPLO-SCT1 between 2007 and 2021. Patients receiving HAPLO-SCT2 for other reasons such as graft failure were excluded, as were patients receiving ex-vivo T cell depleted grafts at either first or second HAPLO-SCT. Data were provided by the ALWP registry of the EBMT, which is a voluntary working society that collects data from more than 600 transplant centres. All participants are required to report all consecutive hematopoietic SCT including follow-up once a year. Regular audits are performed to check for data accuracy.
The protocol was approved by the ALWP general assembly and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients had provided written informed consent authorizing the use of their data for research purposes.
Statistics
Outcomes of interest were engraftment, OS, LFS, NRM, cumulative relapse incidence (RI), graft-versus-host disease (GvHD), and GvHD-free, relapse-free survival (GRFS). All outcomes were measured from the time of HAPLO-SCT2. Patient-, disease- and treatment-related characteristics at the time of HAPLO-SCT1, relapse and HAPLO-SCT2 were summarized using median and range for continuous, and frequency and percentage for categorical data. The Kaplan-Meier method was used to estimate OS, LFS and GRFS. Cumulative incidence functions were used for RI and NRM in a competing risk setting, while death and relapse were included as competing events when calculating the cumulative incidence of GvHD. All surviving patients were censored at the time of last documented contact. Univariate analyses were done using Gray’s test for cumulative incidence functions and the log-rank test for survival analyses. For univariate analyses, continuous variables were categorized and the median value was used as a cut-off point. The number of patients was not sufficient to allow for a reliable multivariate analysis which was therefore not performed. All p-values were two-sided, and values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS 25.0 (SPSS Inc, Chicago, IL, USA) and R 4.0.2 (R Core Team 2020).
Definitions
Complete remission (CR) was defined by bone marrow (BM) blasts <5%, absence of circulating blasts, absence of extramedullary disease, and haematologic recovery [37]. Relapse refers to BM blasts ≥5%, reappearance of blasts in peripheral blood or development of extramedullary disease [37]. Engraftment was defined as the first of three consecutive days with an absolute neutrophil count of ≥500 cells/μL. OS was defined as time from HAPLO-SCT2 to death. LFS was defined as time from HAPLO-SCT2 to either death or relapse/progression after HAPLO-SCT2. Following HAPLO-SCT2, NRM was defined as death without evidence of relapse/progression, GRFS was defined as alive status with neither grade III-IV acute GvHD, no-systemic therapy-requiring chronic GvHD, nor relapse or death [38]. The intensity of the conditioning regimen was classified based on established criteria [39]. Risk scoring of AML was performed according to the 2017 recommendations by the European Leukaemia Network (ELN) [37].
Results
Patients’ characteristics
A total of 82 patients (AML, n = 63; ALL, n = 19, median year of HAPLO-SCT2: 2018) were identified. The median age at HAPLO-SCT2 was 47.2 [range (r): 18.3–69.3] years for AML and 33.5 (r: 19.7–58.2) years for ALL. The median interval from HAPLO-SCT1 to relapse was 7.5 months (r: 0.8–59.4). A change of donor between HAPLO-SCT1 and HAPLO-SCT2 was chosen in 35 patients (63% of informative cases) with AML and 17 (90%) of patients with ALL. At start of conditioning for HAPLO-SCT2, 42 (67%) of AML patients and 7 (37%) of ALL patients had active disease. Myeloablative/reduced intensity conditioning (MAC/RIC) was used for HAPLO-SCT2 in 34 (43%) and 45 (57%) of patients, and post-transplant cyclophosphamide (PTCy) was the most common basis for GvHD prophylaxis (n = 54, 82%). See Table 1 for detailed patient-, disease- and treatment-related characteristics of HAPLO-SCT1 and 2.
Engraftment and GvHD rates
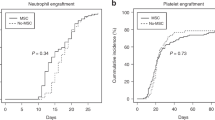
Overall, 87% of patients engrafted. Median time to engraftment was 18 days; engraftment rate by day +60 was 86% (CI 95%: 75.2 - 92). Cumulative incidences of acute GvHD grades II-IV and III-IV by day +180 were 23.9% (CI 95%: 14.7–34.4) and 15.3% (CI 95%: 8.1–24.6), respectively. The cumulative incidences of chronic GvHD and extensive chronic GvHD at 2 years were 22.6% (CI 95%: 13.6–32.9) and 11.2% (CI 95%: 5.2–19.8), respectively.
Outcome
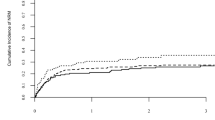
Median follow-up after HAPLO-SCT2, was 38 months (95% CI: 24.9–56.3) for AML and 19 months (95% CI: 7.1–48.1) for ALL patients. Kaplan-Meier estimates showed a 2-year OS and LFS for the entire cohort of 34.3% (CI 95%: 23.3–45.6) and 25.4% (CI 95%: 16–35.9), respectively. Two-year NRM and RI rates were 17.6% (CI 95%: 10.1–26.8) and 57% (CI 95%: 44.7–7.5) respectively (Fig. 1). The 2-year GRFS was 15.1% (CI 95%: 8–24.4).
When analysed separately by diagnosis, 2-year OS/LFS were 28.7% (CI 95%: 17.5–41)/ 22.3% (CI 95%: 12.5–33.8) for AML and 55.3% (CI 95%: 26.3–76.9)/ 38.4% (CI 95%: 16.2–60.4) for ALL, respectively. Two-year NRM for AML was 16.2% (CI 95%: 8.2–6.5) and 23.5% (CI 95%: 6.4–46.7) for ALL patients, while 2-year RI was 61.6% (CI 95%: 47.4–73) for AML and 38.2% (CI 95%: 16.1–60.2) for ALL patients. Consequently, leukaemia was the most frequent cause of death in both disease subgroups (n = 33, 62.3%). Other causes of death included infection in 12 (22.6%) and GvHD in 3 (5.7%) patients, whereas one patient each died from haemorrhage, graft failure, veno-occlusive disease, and post-transplant lymphoproliferative disease.
Univariate analyses of risk factors
An analysis of risk factors for OS, LFS, NRM and RI at 2 years was performed among the 63 patients with AML. Variables of significance for OS were stage at HAPLO-SCT1 (active disease vs CR; p = 0.008), stage at HAPLO-SCT2 (p = 0.047) and interval from HAPLO-SCT1 to relapse [≤ vs > median (8.74 months); p = 0.001]. The same variables were significant for LFS (see Table 2 for details). Myeloablative conditioning for HAPLO-SCT2 was of borderline significance for improved LFS compared to RIC (p = 0.053), without increasing NRM. Variables that reached significance for RI were stage at HAPLO-SCT2 (p = 0.003) and interval from HAPLO-SCT1 to relapse (p = 0.008), while MAC showed a trend towards lower RI as compared to RIC (p = 0.08). For NRM, advanced stage at HAPLO-SCT1 (p = 0.09) and donor change for HAPLO-SCT2 showed a trend towards higher NRM (p = 0.052). Beyond, the effect of donor change for HAPLO-SCT2 was investigated both within the entire group of patients with AML, and among patients with late relapse, i.e., beyond 6 months from HAPLO-SCT1. The latter cut off was chosen due to the increased frequency of HLA loss as basic mechanism of relapse reported among patients with late relapse, leading to most extensive loss of a graft-versus-leukaemia effect if the same donor is used for HAPLO-SCT2 [30, 36, 40]. However, there was no significant influence of donor change on outcome, neither among all AML patients, nor among those with late relapse (donor change, n = 37 vs same donor, n = 12). Detailed information of univariate analyses is shown in Table 2. We did not perform univariate analyses for ALL due to the limited number of patients in this cohort. Similarly, numbers of AML patients were considered insufficient for a reliable multivariate analysis.
Discussion
To our knowledge, this is the largest systematic analysis of patients with acute leukaemias undergoing HAPLO-SCT2 after relapse from a HAPLO-SCT1 reported so far. Nevertheless, over a period of 14 years, only 82 patients were identified within one of the largest available transplant registries. With the median year of HAPLO-SCT2 being 2018, this might be a consequence of less frequent use of HAPLO-SCT across Europe during the first years of our study period. The increasing use of HAPLO-SCT2 over time mirrors the gain of centres’ experience and optimization of supportive therapy in the setting of HAPLO-SCT. These aspects were reflected in the analyses from Shouval et al. [41] who recently reported a continuously improved outcome of HAPLO-SCT over the last two decades. However, the relatively low number of identified patients in our study might also reflect a general reluctance of transplanting physicians to expose their patients to a second HAPLO-SCT, most likely due to the expectation of increased toxicity or graft failure rates. Anyhow, according to our data, toxicity, GvHD rates as well as engraftment after HAPLO-SCT2 were comparable to results published after second transplants both from HLA matched donors and from HAPLO donors after matched SCT-1. In particular, the cumulative incidence of NRM was surprisingly low (17% at two years), underscoring the feasibility of a HAPLO-SCT2 after HAPLO-SCT1.
When analysed separately, outcome seemed to be better among patients suffering from ALL (2y-OS 55%, 2y-LFS 38%) as compared to AML. These results should however be interpreted with caution, as in our cohort, ALL patients were younger, more often received HAPLO-SCT2 in CR and from a new donor, and median follow-up was only 19 months. Nevertheless, the data are in concordance with the improvement over time of adult ALL patients after PTCy-based HAPLO-SCT1 [42] and HAPLO-SCT2 following matched-allo-SCT1 [34]. The integration of targeted therapies such as tyrosine kinase inhibitors, bispecific antibodies, or antibody-drug conjugates into both first line and salvage treatment of ALL might also have contributed to these recent improvements.
An analysis of possible risk factors for outcome was performed within the larger cohort of patients with AML. In univariate analyses, stage at HAPLO-SCT1 and HAPLO-SCT2, as well as the interval from HAPLO-SCT1 to relapse significantly influenced both OS and LFS, confirming data obtained after other treatments for AL relapse post-transplant [27,28,29, 32,33,34]. No significant influence could be detected for adverse ELN risk classification, conditioning intensity and donor change for HAPLO-SCT2. Although not significant for OS/LFS, a MAC for HAPLO-SCT2 rendered a trend towards significance for lower RI and improved LFS, while it was not associated with a higher NRM. One could argue that a MAC for HAPLO-SCT2 may confer a survival advantage in carefully selected patients that are expected to tolerate higher intensity conditioning regimes. For this purpose, the transplant conditioning intensity (TCI) score might be helpful to choose a suitable conditioning regimen for HAPLO-SCT2, since this tool showed an improved categorization of different regimens concerning both intensity and toxicity, and is regarded as a valid improvement of the RIC/MAC stratification system [43]. Unfortunately, the limited number of patients did not allow for a reliable multivariate analysis of risk factors to further validate the role of MAC conditioning.
The cell source for HAPLO-SCT2 was BM in 18% of patients (36% at HAPLO-SCT1), which reflects the current trend towards PB as a stem cell source also for HAPLO-SCT in clinical practice due to easier harvesting, donor safety and comfort. Ruggeri et al. [44] had observed higher rates of acute GvHD in PB vs BM for HAPLO-SCT1 in AML/ALL, however, no difference in OS, LFS, NRM and GRFS were observed in this study. In contrast, Nagler et al. [45] showed higher GvHD and NRM rates, as well as inferior LFS and OS for HAPLO-SCT1 using PB compared to BM as the stem cell source in patients with ALL. In our study, there were no differences in 2-year NRM among recipients of BM and PB grafts after HAPLO-SCT2.
With respect to mechanisms behind post-transplant relapse after allo-SCT, loss of the mismatched HLA haplotype has been identified in about 1/3 of patients relapsing after HAPLO-SCT [40]. Obviously, HAPLO-SCT2 from the same donor as at HAPLO-SCT1 can be expected to be less effective after HLA loss. Since this phenomenon seems to occur more frequently among patients relapsing later than six months from HAPLO-SCT1 [36], we hypothesised that donor change might be advantageous among patients with late relapse, although data on HLA loss were not available in the patients analysed in this study. However, we were not able to detect an improved outcome after donor change among the 49 patients with a post-transplant remission of >6 months, which might be due to the low numbers as well as the fact that selection of a second HAPLO donor based on the investigation of HLA loss has only been introduced into clinical practice very recently.
Our study bears several limitations. First, the number of patients is limited despite covering a relatively long time period and having included all consecutive patients fulfilling the inclusion criteria. Besides the aspects discussed above, the low numbers might reflect a selection bias in our study population, including the risk not being representative of the entire population of patients relapsing after HAPLO-SCT. However, in general the patients investigated here represented a high-risk population with >1/3 of patients having suffered from early relapse, and 60% of patients having undergone HAPLO-SCT2 with an active disease. Hence, the cohort analysed here showed a disease-associated risk which was at least comparable to earlier studies on second SCT in other settings [27, 29, 32,33,34]. Nevertheless, the observed survival rates (2-year OS: 34%; 2-year LFS: 25%) are not inferior to those observed in these previous reports. Furthermore, we sought to report on patients and treatment characteristics with as many details as possible in order to allow for an exact comparison with published series as well as individual patients for whom HAPLO-SCT2 might be considered. Second, since PTCy was used for GvHD prophylaxis in 82% of HAPLO-SCT2, we were not able to separately study the influence of alternative strategies of GvHD prevention after HAPLO-SCT2, which, however, have been less frequently used across EBMT centres during recent years. Third, information on salvage treatment for relapse after HAPLO-SCT1 was not sufficient to include this aspect into the analysis. Recently, Piemontese et al. [46] described the overall treatment strategies applied for relapse after HAPLO-SCT1, but comparative analyses of the different salvage regimen were not possible even in this broader cohort. A thorough analysis of the short-term effects of the salvage treatment (remission, toxicity) and its influence in the outcome after HAPLO-SCT2 remains to be performed. Finally, as mentioned above, the limited number of patients did not allow for a reliable multivariate analysis of risk factors. Major risk factors already well established in other studies in different treatment settings were identified in our univariate analysis, suggesting that there are no substantial differences in the double HAPLO-SCT situation. Nevertheless, we might have missed less prominent factors influencing outcome, as well as the mutual dependence of the identified factors that might have been eliminated by multivariate testing.
Summarizing, within the limits of a retrospective registry-based analysis, our data show the feasibility of HAPLO-SCT2 after relapse post HAPLO-SCT1 with high engraftment rates and surprisingly low NRM rates. Outcome data as well as risk factors are comparable to results reported after allo-SCT2 in a matched donor setting. HAPLO-SCT2 is a viable option for AL patients relapsing after a HAPLO-SCT1. Nevertheless, there is still room for improvement. As discussed above, selection of a second HAPLO donor based on the presence or absence of HLA loss, as well as more efficient and less toxic strategies to achieve better disease control before HAPLO-SCT2, are among the options already available. Post-transplant maintenance strategies including novel drugs or additional cellular therapies should also be investigated after second transplantation including double HAPLO-SCT.
Data availability
The authors declare that all data generated or analysed during this study will be available to any researcher wishing to use them for non-commercial purposes on reasonable request, without breaching participant confidentiality.
References
Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transpl. 2020;55:1604–13.
O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2002;8:377–86.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112:3574–81.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transpl. 2012;18:1859–66.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transpl. 2017;52:811–7.
Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transpl. 2014;20:1975–81.
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40.
Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1069–75.
Piemontese S, Ciceri F, Labopin M, Arcese W, Kyrcz-Krzemien S, Santarone S, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017;10:24.
Versluis J, Labopin M, Ruggeri A, Socie G, Wu D, Volin L, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017;1:477–85.
How J, Slade M, Vu K, DiPersio JF, Westervelt P, Uy GL, et al. T cell-replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide results in outcomes similar to transplantation from traditionally matched donors in active disease acute myeloid leukemia. Biol Blood Marrow Transpl. 2017;23:648–53.
Duléry R, Ménard AL, Chantepie S, El-Cheikh J, François S, Delage J, et al. Sequential conditioning with thiotepa in T cell- replete hematopoietic stem cell transplantation for the treatment of refractory hematologic malignancies: comparison with matched related, haplo-mismatched, and unrelated donors. Biol Blood Marrow Transpl. 2018;24:1013–21.
Doppelhammer M, Fraccaroli A, Prevalsek D, Bücklein V, Häbe S, Schulz C, et al. Comparable outcome after haploidentical and HLA-matched allogeneic stem cell transplantation for high-risk acute myeloid leukemia following sequential conditioning-a matched pair analysis. Ann Hematol. 2019;98:753–62.
Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104:524–32.
Al Malki MM, Yang D, Labopin M, Afanasyev B, Angelucci E, Bashey A, et al. Comparing transplant outcomes in ALL patients after haploidentical with PTCy or matched unrelated donor transplantation. Blood Adv. 2020;4:2073–83.
Brissot E, Labopin M, Russo D, Martin S, Schmid C, Glass B, et al. Alternative donors provide comparable results to matched unrelated donors in patients with acute lymphoblastic leukemia undergoing allogeneic stem cell transplantation in second complete remission: a report from the EBMT Acute Leukemia Working Party. Bone Marrow Transpl. 2020;55:1763–72.
Shem-Tov N, Peczynski C, Labopin M, Itälä-Remes M, Blaise D, Labussière-Wallet H, et al. Haploidentical vs. unrelated allogeneic stem cell transplantation for acute lymphoblastic leukemia in first complete remission: on behalf of the ALWP of the EBMT. Leukemia 2020;34:283–92.
Chang Y-J, Wang Y, Xu L-P, Zhang X-H, Chen H, Chen Y-H, et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13:27.
Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji R-M, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplanta. J Hematol Oncol. 2021;14:53.
Sanz J, Galimard J-E, Labopin M, Afanasyev B, Sergeevich MI, Angelucci E, et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J Hematol Oncol. 2021;14:84.
Wieduwilt MJ, Metheny L, Zhang MJ, Wang HL, Estrada-Merly N, Marks DI, et al. Haploidentical vs sibling, unrelated, or cord blood hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood Adv. 2022;6:339–57.
Nagler A, Labopin M, Swoboda R, Pioltelli P, Arat M, Yakoub-Agha I, et al. Haploidentical versus matched sibling donor hematopoietic stem cell transplantation for adult patients with relapsed/refractory acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Hemasphere. 2022;6:e790.
Yalniz FF, Saliba RM, Greenbaum U, Ramdial J, Popat U, Oran B, et al. Outcomes of second allogeneic hematopoietic cell transplantation for patients with acute myeloid leukemia. Transpl Cell Ther. 2021;27:689–95.
Shumilov E, Hasenkamp J, Maulhardt M, Mazzeo P, Schmidt N, Boyadzhiev H, et al. Outcomes of second allogeneic stem cell transplantation and anti-relapse strategies in patients with relapsed/refractory acute myeloid leukemia: a unicentric retrospective analysis. Hematol Oncol. 2022;40:763–76.
Tachibana T, Tanaka M, Hagihara M, Fujimaki K, Kanamori H, Nakajima H. Outcomes in patients with acute lymphoblastic leukemia who underwent second allogeneic hematopoietic cell transplantation for relapse after first transplantation. Int J Hematol. 2022;116:594–602.
Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhäuser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31:3259–71.
Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. 2018;4:1245–53.
Nagler A, Labopin M, Dholaria B, Finke J, Brecht A, Schanz U, et al. Second allogeneic stem cell transplantation in patients with acute lymphoblastic leukaemia: a study on behalf of the Acute Leukaemia Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2019;186:767–76.
Vago L, Ciceri F. Choosing the alternative. Biol Blood Marrow Transpl. 2017;23:1813–4.
Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transpl. 2014;49:895–901.
Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kröger N, et al. Donor selection for a second allogeneic stem cell transplantation in AML patients relapsing after a first transplant: a study of the Acute Leukemia Working Party of EBMT. Blood Cancer J. 2019;9:88.
Kharfan‐Dabaja MA, Labopin M, Brissot E, Kroger N, Finke J, Ciceri F, et al. Second allogeneic haematopoietic cell transplantation using HLA‐matched unrelated versus T‐cell replete haploidentical donor and survival in relapsed acute myeloid leukaemia. Br J Haematol. 2021;193:592–601.
Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Ciceri F, Finke J, Bruno B, et al. Comparing outcomes of a second allogeneic hematopoietic cell transplant using HLA-matched unrelated versus T-cell replete haploidentical donors in relapsed acute lymphoblastic leukemia: a study of the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2021;56:2194–202.
Imus PH, Blackford AL, Bettinotti M, Iglehart B, Dietrich A, Tucker N, et al. Major histocompatibility mismatch and donor choice for second allogeneic bone marrow transplantation. Biol Blood Marrow Transpl. 2017;23:1887–94.
Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia. 2015;29:1143–52.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33.
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–88.
Shouval R, Fein JA, Labopin M, Kröger N, Duarte RF, Bader P, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019;6:e573–e84.
Nagler A, Labopin M, Koc Y, Angelucci E, Tischer J, Arat M, et al. Outcome of T‐cell–replete haploidentical stem cell transplantation improves with time in adults with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2021;127:2507–14.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124:1428–37.
Nagler A, Dholaria B, Labopin M, Savani BN, Angelucci E, Koc Y, et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia. 2020;34:2766–75.
Piemontese S, Boumendil A, Labopin M, Schmid C, Ciceri F, Arcese W, et al. Leukemia relapse following unmanipulated haploidentical transplantation: a risk factor analysis on behalf of the ALWP of the EBMT. J Hematol Oncol. 2019;12:68.
Acknowledgements
The outstanding contribution of all EBMT centres whose patients could be included in this analysis is highly appreciated, as is the excellent work of the EBMT data managers.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
GFV was responsible for the literature research, writing the manuscript, interpreting results, and creating tables, and designed the work that led to submission. ML was responsible for the study designs, extracting and analysing of data, creating tables, interpreting results, provided feedback, revised, and corrected the manuscript, and approved the final version. JT, AMR, EA, AK, PG, AB, CEB, NK, MK and JLDM contributed to centre and patient enrolment, provided feedback, revised the manuscript, and approved the final version. AN, FC and MM, were responsible for study design, contributed to centre and patient enrolment, played a major role in interpreting the results, provided feedback, revised and corrected the manuscript, approved the final version. CS elaborated the scientific idea, contributed to centre and patient enrolment, conceived and designed the work that led to submission, was responsible for the study conduction, analysing data and interpreting results, revised, corrected and wrote the manuscript, approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Filippini Velázquez, G., Labopin, M., Tischer, J. et al. Second haploidentical stem cell transplantation (HAPLO-SCT2) after relapse from a first HAPLO-SCT in acute leukaemia—a study on behalf of the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 58, 907–915 (2023). https://doi.org/10.1038/s41409-023-01985-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01985-7