Abstract
Repetitive transcranial magnetic stimulation (rTMS) is an effective treatment for individuals with major depressive disorder (MDD) who have not improved with standard therapies. However, only 30–45% of patients respond to rTMS. Predicting response to rTMS will benefit both patients and providers in terms of prescribing and targeting treatment for maximum efficacy and directing resources, as individuals with lower likelihood of response could be redirected to more suitable treatment alternatives. In this exploratory study, our goal was to use proton magnetic resonance spectroscopy to examine how glutamate (Glu), Glx, and total N-acetylaspartate (tNAA) predict post-rTMS changes in overall MDD severity and symptoms, and treatment response. Metabolites were measured in a right dorsal anterior cingulate cortex voxel prior to a standard course of 10 Hz rTMS to the left DLPFC in 25 individuals with MDD. MDD severity and symptoms were evaluated via the Inventory of Depression Symptomatology Self-Report (IDS-SR). rTMS response was defined as ≥50% change in full-scale IDS-SR scores post treatment. Percent change in IDS-SR symptom domains were evaluated using principal component analysis and established subscales. Generalized linear and logistic regression models were used to evaluate the relationship between baseline Glu, Glx, and tNAA and outcomes while controlling for age and sex. Participants with baseline Glu and Glx levels in the lower range had greater percent change in full scale IDS-SR scores post-treatment (p < 0.001), as did tNAA (p = 0.007). Low glutamatergic metabolite levels also predicted greater percent change in mood/cognition symptoms (p ≤ 0.001). Low-range Glu, Glx, and tNAA were associated with greater improvement on the immuno-metabolic subscale (p ≤ 0.003). Baseline Glu predicted rTMS responder status (p = 0.025) and had an area under the receiving operating characteristic curve of 0.81 (p = 0.009), demonstrating excellent discriminative ability. Baseline Glu, Glx, and tNAA significantly predicted MDD improvement after rTMS; preliminary evidence also demonstrates metabolite association with symptom subdomain improvement post-rTMS. This work provides feasibility for a personalized medicine approach to rTMS treatment selection, with individuals with Glu levels in the lower range potentially being the best candidates.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a devastating neuropsychiatric illness that affects approximately 4% of individuals around the globe annually [1]. It is associated with increased mortality [2], poor health outcomes [3, 4], economic burden [5], heightened disability [6, 7], and decreased life satisfaction [8]. Failure to benefit from standard antidepressant treatments, such as psychotherapy and psychiatric medication, is common and approximately 33% of treated MDD patients exhibit persistent symptoms after adequate pharmacotherapy [9], a syndrome referred to as treatment-resistant depression (TRD) [10, 11].
One promising alternative treatment for TRD is repetitive transcranial magnetic stimulation (rTMS), a type of noninvasive brain stimulation [12, 13]. rTMS has been widely adopted in clinical practice and has proven effective for MDD [14]. Large registry data indicates that while most patients benefit from rTMS, about one-third of those with MDD don’t respond (i.e., achieve ≥ 50% decrease in depression scores) [15, 16]. Furthermore, rTMS therapy is costly and time-intensive, with treatments 5 days a week for 4–6 weeks [12]. Thus, identifying individuals most likely to respond to rTMS prior to initiation of treatment via brain-based biomarkers would be time- and cost-effective to patients and providers.
Biomarkers guiding rTMS prescription must be sensitive, specific, and reliable to be clinically useful. Resting-state functional connectivity, structural imaging (cortical thickness), positron emission tomography (PET), single photon emission computed tomography (SPECT), and various electroencephalography (EEG) metrics have identified candidate neuroimaging-based biomarkers of rTMS outcomes [17,18,19,20,21]. Examples include high glucose metabolism in the frontal lobe [21] and anticorrelation between connectivity of the subgenual cingulate cortex and the left dorsolateral prefrontal cortex (DLPFC) [22]. While they have pointed to changes that correspond with successful treatment, none to date has proven sufficient for clinical application given the existing lack of standardization in data collection, preprocessing, and analysis, invasiveness (PET), duration of protocol, and variability in findings [23, 24].
One candidate method deserving of further investigation is proton magnetic resonance spectroscopy (MRS), an imaging technique that measures the concentration of biochemical compounds in the brain in vivo [25]. MRS measures total N-acetylaspartate (tNAA), a combination of N-acetylaspartate (NAA) and N-acetylaspartylglutamic acid (NAAG), and glutamatergic compounds (Glx), comprised of glutamate (Glu) and glutamine (Gln). In comparison to other techniques, MRS has several advantages: it is short in duration (~3 mins), has relatively standardized data collection and preprocessing techniques [26], reliably quantifies metabolites throughout the brain [27, 28], and provides an accurate, non-invasive measure of cellular metabolism [29], contributing substantive insight on depression neuropathology.
Glutamatergic and N-acetylated compounds have been associated with multiple neuronal and glial processes implicated in MDD [30,31,32,33,34]. Glu is the most abundant excitatory neurotransmitter in the brain and, along with Gln, is thought to reflect neuronal energy metabolism [35]. tNAA serves as a potential marker of neuronal health and integrity [36, 37] and may mitigate glutamatergic excitotoxicity [38]. Glutamatergic system dysfunction has been associated with heightened MDD symptomatology, as low tricarboxylic acid cycle activity may lead to aberrant excitatory neurotransmission within and between frontal and limbic structures [39,40,41].
In comparison to healthy controls, individuals with MDD often exhibit lower concentrations of Glx, Glu, and tNAA in the anterior cingulate cortex (ACC) and prefrontal cortex (PFC) [40, 42,43,44]; low metabolite levels are also associated with heightened depression severity [40, 43, 45,46,47]. Indeed, baseline glutamatergic metabolites may be biomarkers of rTMS outcomes: the majority of existing studies show individuals with low baseline concentrations of frontal lobe Glu and Glx have the greatest symptom improvement post-rTMS [41, 48,49,50], while others indicate higher concentrations of Glx and Glx/tCr predict greater reduction of depression symptoms [51, 52]. More research is required to reconcile the directionality of these findings. Though the relationship between baseline tNAA and depression improvement following rTMS was not significant in one study [51], others found lower levels of baseline tNAA in the ACC predicted MDD recovery following electroconvulsive therapy (ECT) [53, 54]. Thus, further exploration is needed to evaluate N-acetylated compounds as biomarkers of rTMS outcomes.
Although low Glu and tNAA have been significantly linked to specific MDD symptom domains including mood and cognition [55,56,57,58,59,60], studies have primarily examined the relationship between baseline metabolite levels and overall depression symptom scores following rTMS [41]. This is a limitation of the current literature, as rTMS has demonstrated the ability to ameliorate a broad spectrum of symptoms [61,62,63,64]. Characterizing the relationship between pre-treatment metabolite levels and improvement in specific symptoms clusters following rTMS may be useful for elucidating transdiagnostic pathological processes involved in neuropsychiatric disorders, thereby guiding a personalized medicine approach to rTMS therapy.
In this exploratory study, we evaluated baseline levels of Glx, Glu, and tNAA in the right dorsal anterior cingulate cortex (dACC) as predictors of rTMS treatment outcomes in patients with depression. We chose the dACC, a core node of the salience network and limbic system, as our region of interest because of its involvement in attentional control and affective evaluation [65,66,67]. Given its role in mood and cognition, hypoactivation of this structure has been associated with heightened symptoms in MDD [68]. rTMS-associated increases in glutamatergic metabolism may strengthen neurotransmission within the dACC and its connections to other frontolimbic structures, ultimately improving attentional control over negatively valanced self-referential thoughts and decreasing rumination and low mood in MDD. Though the DLPFC is traditionally used as a region of interest in predicting rTMS outcomes because it is typically the site of stimulation, imaging studies reveal significant relationships between metabolic [41] and functional signals [69, 70] in the dACC and post-rTMS outcomes in individuals with depression, providing further support for our voxel placement. Our selection of the right dACC was motivated by the demonstrated feasibility of our previous work [71] and that right dACC morphology at baseline is predictive of clinical response to intermittent theta burst stimulation [72]. Additionally, research suggests both the right and left dACC are functionally connected to the rTMS target (left DLPFC) in individuals with MDD [73], implicated in depression pathology [74], and may yield similar results.
Based on the summarized findings, we anticipated that participants with lower levels of Glu, Glx, and tNAA would experience a greater reduction in overall depression severity following a standard course of rTMS. We also expected that lower baseline metabolite levels would predict improvement in mood and cognitive symptoms. This is based on prior work associating low Glu, Glx, and tNAA with greater symptom severity, and evidence indicating that rTMS appears to modulate neural activity in salience circuit nodes, such as the dACC, underlying depressed mood and cognitive dysfunction [75].
Materials and methods
Study Overview
Informed consent was obtained from all participants. Study participants completed an eligibility and enrollment interview, a baseline MRI scan, and self-report symptom assessment approximately two weeks prior to beginning rTMS. If the first rTMS session occurred more than two weeks after baseline procedures, depression severity was reassessed prior to rTMS. Participants then completed standard rTMS treatment (once-daily sessions for 6 weeks followed by 6 sessions tapered over 3 weeks (36 sessions); the number of sessions could be extended by up to 10 (46 maximum sessions); see the rTMS procedures section for further details). Post-rTMS symptom assessment was completed at the final rTMS session. All participants gave permission for research use of symptom data collected during routine rTMS care. See Fig. 1.
(1) The pre-rTMS MRI and assessment were completed approximately two weeks prior to the initiation of rTMS; (2) participants then underwent a full course of rTMS (an acute phase of 36 sessions with possible extension up to 46; see the rTMS procedures section and Table 1 for more details); (3) the post-rTMS assessment was completed on the last day of rTMS treatment. Created with biorender.com; rTMS: repetitive transcranial magnetic stimulation.
rTMS treatment and all clinical assessments took place at the Butler Hospital Transcranial Magnetic Stimulation (TMS) Clinic in Providence, RI, USA. MRI scanning took place at the Brown Magnetic Resonance Imaging Facility in Providence, RI, USA. rTMS treatments represented a course of naturalistic, insurance-covered care; the collection of MRI and additional symptom data was supported by an NIH grant (MH113929, PI Michael Fox, MD, PhD). Procedures were approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center, Butler Hospital, and the Mass General Brigham Human Research Committee of Brigham and Women’s Hospital.
Participants
Participants (n = 27) with a primary diagnosis of MDD [76] and a history of resistance or intolerance to standard antidepressant medication were recruited from the Butler Hospital TMS Clinic after it was determined they were approved for insurance-covered treatment. Treatment resistance (and hence eligibility for treatment and study participation) was variably defined by different medical insurance policies of the patients presenting to the TMS clinic, and generally required at least two unsuccessful antidepressant medication trials (i.e., an ineffective trial despite adequate dose and duration, or a trial terminated at subtherapeutic does/duration due to intolerable side effects); some policies also required a past trial of psychotherapy to be eligible for rTMS insurance coverage. Eligible participants (1) were able to read and speak English; (2) were 18-80 years old; (3) had undergone an extensive evaluation wherein a psychiatrist established a primary diagnosis of nonpsychotic, recurrent MDD and a treatment history of antidepressant medication resistance or intolerance; (4) met rTMS safety criteria and all other eligibility criteria for rTMS therapy according to their insurance; (5) had no prior history of rTMS treatment; (6) had not received ECT within the current depressive episode or within the past three months; and (7) met safety criteria for MRI scanning. Ineligible participants had any (1) comorbid (nonprimary) or past psychiatric disorder, that in the judgement of the investigators, had symptoms severe enough to interfere with the validity of the data collected; (2) significant current neurological illness (Parkinson’s disease, dementia, intracranial pressure, etc.); and (3) history of a seizure disorder.
Self-report assessments of MDD severity and symptom domains
MDD severity and symptom domains were measured using the Inventory of Depressive Symptomatology—Self Report (IDS-SR) scale [77], a standard 28-item measure [78] that has been consistently used to evaluate rTMS-associated outcomes [79, 80]. “Response” was categorically defined by 50% or greater reduction in IDS-SR total score from pre-treatment to post-rTMS (following the final session).
We analyzed the relationship between baseline metabolites and percent change from baseline to post-treatment in scores on three IDS-SR subscales developed by Han et al. (2021) [81]. These subscale domains represented “mood/cognition” (15 items; max score of 45), “somatic” (10 items related to bodily problems; max score of 30), and “immuno-metabolic” (5 items related to atypical/energy symptoms; max score of 15). Higher scores on the subscales indicated greater severity of symptoms in those dimensions. See [81] for further details regarding subscale construction.
As an exploratory analysis, we used principal component analysis (PCA) to reduce the dimensionality of the IDS-SR symptom domains (SPSS Statistics 28 (IBM, Armonk, NY, USA)). Because there is currently no expert consensus on which IDS-SR subscales best characterize MDD symptom domains, PCA demonstrated convergence between the data-driven grouping of the 30 individual items and the Han et al. (2021) subscales. We calculated percent change from baseline to post-treatment for the score on each IDS-SR item (((post – pre)/pre))*−100), and entered them into the PCA. 1 was added to each pre- and post-treatment score to avoid dividing by 0 in the percent change equation. An orthogonal varimax rotation with Kaiser normalization was applied during PCA. This yielded 30 factors, however, only the first two were utilized in subsequent analyses as they contributed to the majority of the variance (>10% each, Eigenvalue > 3). Items were retained for factor interpretation if the loading on the factor was >|0.3|.
rTMS procedures
Participants underwent standard-of-care rTMS treatment, i.e., a 6-week course of once-daily (5/week) sessions followed by 6 sessions tapered over 3 weeks. In cases (n = 5) where permitted by insurance, the acute course of 36 sessions was extended by up to 10 sessions when the patient had late onset of response or had not achieved remission (maximum of 46 sessions). The acute phase may have been truncated, and the taper phase started prior to session #30 for patients showing sustained remission before that point. A minority were not taking any psychiatric medications during the course of rTMS. The majority of rTMS patients continued stable doses of concurrent antidepressant medications or had minor dosage changes during the course. In rare cases (n = 2) it was medically necessary to start a new antidepressant medication. Concurrent psychiatric medications included antidepressants, stimulants, antipsychotics, anxiolytics, hypnotics, and mood stabilizers. rTMS patients in the clinic all initiate their first treatment series with the standard 10 Hz protocol (3000 pulses per session), applied with a figure-8 coil that targeted the left DLPFC with stimulation at an intensity of 120% relative to resting motor threshold. Standard clinical treatment protocol at the Butler Hospital TMS clinic was used to identify scalp location for coil placement over the DLPFC target (5 cm anterior to the hand representation of the motor cortex) including head measurements via flexible measuring tape and administration of single TMS pulses. Minor modifications to a patient’s stimulation protocol (i.e., adjusting the frequency and to which hemisphere the stimulation was applied) were made if deemed medically necessary by the treating physician to manage tolerability or optimize outcomes. Clinical and demographic features of the sample, along with rTMS treatment details, appear in Table 1; the protocol delivered during the majority of treatments is reported as the dominant protocol. Treatments were delivered using a NeuroStar TMS Therapy system (Neuronetics, Inc., Malvern, PA, USA) or a Nexstim NBS device (Nexstim Ltd., Helsinki, Finland).
MRI data collection procedures and analysis
Scanning was conducted on a Siemens (Erlangen, Germany) PRISMA 3 Tesla (T) MRI scanner (Siemens Medical Solutions, New York, NY, USA) and a 64-channel head coil.
Structural MRI
High resolution T1-weighted structural images were acquired from each participant via 3D-turbo field multiecho MEMPRAGE (slice thickness = 1 mm3, sagittal orientation, TR = 2530 ms, TE = 1.69 s, flip angle = 7°, in-plane matrix = 2562, slices = 176). Raw structural data was converted to the NIfTI-1 format using the Python-based program HeuDiConv (version 0.9.0) [82].
MRS
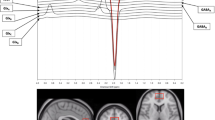
A single voxel was collected in the right dACC (15 x 15 x 10 mm3) using standard single voxel Point RESolved Spectroscopy (PRESS) (TE = 30 ms, TR = 3000 ms, averages = 64), which reliably allows for quantification of Glu, Glx, tNAA, Ins, and tCr at 3 T [83,84,85]. Guided by our group’s previous work quantifying Glu, Glx, and tNAA [71, 86], the voxel was placed and tangentially rotated to be anterior and parallel to the corpus callosum; the posterior portion of the voxel was placed in line with the frontal horns of the lateral ventricle; cerebrospinal fluid (CSF) contamination was minimalized along the longitudinal fissure. See Fig. 2A for example placement and 2B for a composite image of participants’ voxels. First-order auto-shimming and manual shimming were implemented.
A An example right dACC (15 x 15 x 10 mm3) voxel placed over its corresponding T1-weighted MEMPRAGE; B Composite right dACC voxel placement in all participants (n = 25), where the voxels shown are normalized to Montreal Neurological Institute (MNI)-152 space and the whiter the color, the greater percent of voxel overlap; C Average PRESS spectrum of all 25 participants, where the green line represents the average raw input data with no smoothing, the yellow line represents the average LCModel fit, and the gray shading represents standard deviation of the raw input data. The blue line at the top is a plot of the residual error, or the data minus the fit of the data, and the curved blue line underneath the spectra represents the baseline [85]. : L left, R right, Ins myoinositol, tCr total creatine, Glx glutamine and glutamate, Glu glutamate, tNAA total N-acetylaspartate.
PRESS data were processed and quantified using LCModel (version 6.3-1R) with a basis set provided by LCModel [85], accounting for scanner-specific timing and pulse sequences. Automatic zero- and first-order phase correction was done by LCModel, and only metabolites with a Cramer-Rao lower bound (CRLB), or estimate of LCModel fit, less than or equal to 20% SD were evaluated [71]. See Fig. 2C for the average spectrum across all participants. Using Gannet CoRegister (version 3.1), voxel masks were coregistered to individuals’ T1-weighted images [87, 88], providing percentages of white matter, grey matter, and CSF in the voxel. Participants with less than 60% gray matter were excluded from analyses for quality control purposes [71]. In accordance with expert consensus and recommendations for MRS [26], data were then corrected for water- and metabolite-specific T1 and T2 relaxation constants and water density [89], and for partial volume effects of CSF using the equation [*1/(1- CSF)] [88, 90]. This yielded water-referenced molal concentrations of Glu, Glx, tNAA, Ins, and tCr. To evaluate the specificity of Glu, Glx, and tNAA, we also analyzed Ins and tCr as comparator metabolites given the lack of evidence linking them to MDD pathoetiology or rTMS outcomes [41].
Statistical analysis
We excluded two participants with < 60% gray matter in the dACC voxel. Item-level IDS-SR data was unavailable for one participant. Thus, IDS-SR subscale and PCA analyses were based on n = 24 participants, whereas analyses involving overall IDS-SR scores had n = 25. We used Python 3 [91] or SPSS Statistics 28 for statistical analyses. We calculated descriptive statistics for baseline demographic and clinical data, rTMS treatment parameters (number of treatments, number of weeks receiving treatment, dominant rTMS treatment protocol), IDS-SR total and scores for the PCA symptom factors, as well as mood/cognition, somatic, and immuno-metabolic subscales. Descriptive statistics also characterized number of rTMS responders, IDS-SR percent change (total and subscale scores), and baseline metabolite levels.
We used Shapiro-Wilk tests to assess the normality of metabolite levels and depression score distribution. If distributions violated assumptions of normality (Shapiro-Wilk p < 0.05) they were sigmoidally transformed and the transformed variable was used in all subsequent analyses. Two-tailed Pearson correlations were used to evaluate CSF dependence via CSF voxel percentage and raw Glu, Glx, tNAA, Ins, and tCr molal concentrations (no tissue correction). Two-tailed paired t-tests compared pre- and post-rTMS IDS-SR scores. Independent samples t-tests and chi-square tests were conducted to compare responders versus non-responders on baseline IDS-SR full score, PCA and subscale scores, total number of rTMS sessions received, age, and sex. Independent samples t-tests were also used to test for baseline equivalence between response groups in the fraction of dACC voxel gray matter, white matter, and CSF.
Generalized linear regression models were used to test relationships between baseline metabolite levels and percent change in depression symptom severity (full IDS-SR) or change in specific symptom subdomains. Percent change scores were used to evaluate continuous rTMS outcomes and accounted for baseline MDD severity. This was done by subtracting the pre-treatment score from the post-treatment score, and then dividing that value by the pre-treatment score. The resultant decimal was multiplied by −100 to provide a value between −100 and 100, where positive numbers indicated symptom improvement, 0 indicated no change, and negative numbers indicated worsening of symptoms (equation: ((post– pre)/pre))*−100). This method is commonly used when evaluating the effects of metabolites on rTMS outcomes [50, 52, 92]. Separate models were created for each metabolite predicting percent change in score on six symptom scales (the IDS-SR full scale, 2 PCA-derived component scales, and the 3 Han et al. (2021) published subscales: mood/cognition, somatic, and immuno-metabolic) from pre-treatment metabolite level (continuous variables). We included age as a covariate in all models given the negative association of age with metabolite levels [93,94,95]. Sex was also included as a covariate in the models given reported sex differences in MDD etiology and symptom presentation [96], rTMS outcomes [97], and glutamatergic depression correlates [98]. In total, we created 18 generalized linear regression models for primary hypothesis testing, one for each metabolite (x3) and outcome (x6). Additional models for Ins and tCr were constructed in the same fashion to evaluate the specificity of hypothesized effects of interest. Bivariate Pearson’s correlations for Glu, Glx, and tNAA were plotted for illustrative purposes only as the resulting p-values are not independent [99].
Additional logistic regressions were conducted to evaluate baseline metabolites as predictors of binary outcomes, i.e., rTMS treatment response. There was a total of five models, i.e., 3 for the metabolites of interest and 2 metabolites for evaluating specificity. Age, sex, and baseline total IDS-SR scores were also included as covariates.
A Bonferroni correction (p ≤ 0.007) was applied for the seven outcomes of interest: change in IDS-SR total score, PCA Factor 1 score, PCA Factor 2 score, and scores on the mood/cognition, somatic, and immuno-metabolic subscales; and the dichotomous responder status. Following our previously published work, each metabolite was treated as its own family of tests [71]. Generalized linear regression and logistic regression outcomes reported in the Results are Bonferroni-corrected; any outcomes between p = 0.008-0.05 are considered to be marginally significant. Relationships between metabolites and outcomes were tested for influence of outliers via Cook’s D. Post-hoc sensitivity tests were conducted on models where change in psychiatric medication status (change=1, no change=0) and dominant rTMS protocol (excitatory (>1 Hz, over left DLPFC) = 1 or inhibitory (1 Hz over right DLPFC) = 0) were included as independent covariates in separate models to ensure they did not influence metabolite relationships with rTMS outcomes.
The area under the receiving operating characteristic (AUROC) curve [100] was computed for each metabolite (Glu, Glx, tNAA, Ins, and tCr) and the sensitivity (true positive rate) and specificity (true negative rate) were evaluated for prediction of responder status. We used the Hosmer-Lemeshow goodness of fit test to assess our models’ discriminative abilities [101]. The significance of the assessed performance predictions was not subjected to the Bonferroni correction.
Results
Participant characteristics and clinical outcomes
Our sample (n = 25) was primarily Caucasian (96%, n = 24) and non-Hispanic (96%, n = 24), with an average age of 38 years (SD = 14.57); approximately 56% (n = 14) identified as females. Baseline IDS-SR scores were classified as “severe” with an average of 43.20 (SD = 6.22). Participants received an average of 37.48 (SD = 6.87) rTMS sessions over 9.75 (SD = 2.20) weeks. The average time between the MRI/self-report and rTMS start was 13.75 days (SD = 15.61). Seventeen (68%) were treated with predominantly left DLPFC 10 Hz stimulation, 2 (8%) with left DLPFC 5 Hz, 4 (16%) with right DLPFC 1 Hz, and 2 (8%) with mixed protocols, meaning they had 50% of one protocol and 50% of another. Following the final treatment session, 13 (52%) participants were classified as rTMS responders (Table 1).
The mean IDS-SR (full scale) score significantly decreased from baseline to treatment endpoint (p < 0.001), reflecting an average 46.35% decrease (SD = 32.66). Similarly, there were significant decreases in the mood/cognition, somatic, and immuno-metabolic subscales (p < 0.001). Shapiro-Wilk tests determined all percent change scores were normally distributed (W(24) = 0.92–94; p = 0.05–13). When comparing treatment responders and non-responders at baseline, there were no significant differences between mean IDS-SR scores (full-scale and subscales), mean number of rTMS sessions received, age, and sex (p > 0.05).
Baseline Metabolite Levels
Means and standard deviations for baseline Glu, Glx, tNAA, Ins, and tCr levels and their corresponding CRLBs are in Table 2. Shapiro-Wilk tests determined that all metabolite values other than tCr were normally distributed (W(25) = 0.93-0.98 ; p > 0.05). Originally, tCr had a mean of 6.06 institutional units (SD = 0.76) and was non-normally distributed W(24) = 0.72, p < 0.00001; following sigmoidal transformation, the mean was 1.00i.u. (SD = 0.00), and was normally distributed W(25) = 0.95 (p = 0.31). Tissue percentages of gray matter, white matter, and CSF are presented in Table 2. There were no significant relationships between raw metabolite concentrations and CSF dependence (all p > 0.05). All CRLB averages and full width half maximum (FWHM) values (Table 2) were within the standards for collecting high quality spectroscopy data [85]. There were no significant group differences in tissue percentages between rTMS responders and non-responders (p > 0.05).
Principal Component Analysis
The PCA (n = 24) yielded 2 factors meeting a priori thresholds. The first, which we call the “PCA mood & cognition factor,” had an Eigenvalue of 8.81 (29.35% of variance) and 22 item loadings. The second, which we refer to as the “PCA somatic factor,” had an Eigenvalue of 3.726 (12.42% of variance) and 16 item loadings. The factors were not significantly correlated (p > 0.05), justifying the orthogonal varimax rotation. See Table 3 for individual item loadings.
Predictors of Treatment Outcomes (Continuous Variables)
Percent Change in IDS-SR Total Score
As hypothesized, lower levels of glutamatergic and N-acetylated metabolites at baseline were associated with greater improvement in overall depression severity (Fig. 3A). We observed significant effects of Glu (B = −17.17, SE = 4.96, X2 = 11.97, p < 0.001), Glx (B = −15.51, SE = 4.46, X2 = 12.106, p < 0.001), and tNAA (B = −21.73, SE = 8.02, X2 = 7.34, p = 0.007). Covariate effects were not significant, save a marginal effect of age in the Glx model (B = 0.835, SE = 0.39, X2 = 4.59, p = 0.032). Effects of metabolite were non-significant in Ins and tCr models.
Scatter plots of bivariate Pearson correlations between baseline metabolites and continuous treatment outcomes. Panels show: A percent change in full scale IDS-SR score and Glu, Glx, and tNAA (n = 25); B PCA mood & cognition factor scores and Glu, Glx, and tNAA (n = 24); C percent change in the IDS-SR mood/cognition subscale and Glu, Glx, and tNAA (n = 24); and D percent change in the IDS-SR immuno-metabolic subscale and Glu, Glx, and tNAA (n = 24). Greater percent change indicates greater improvement in symptoms; p-values are not shown as noted in the text to avoid the “double-dipping” error of statistical significance. IDS-SR Inventory of Depression Symptomatology Self Report, Glu glutamate, Glx glutamine and glutamate, tNAA total N-acetylaspartate, Pre-Tx pre-rTMS treatment, PCA principal component analysis.
Percent change in PCA-derived mood/cognition and somatic component scales
Lower pre-rTMS concentrations of Glu (B = −0.55, SE = 0.17, X2 = 13.72, p < 0.001) and Glx (B = −0.51, SE = 0.13, X2 = 15.159, p < 0.001) were also associated with greater improvement in mood/cognitive symptoms (PCA Factor 1; Fig. 3B). The effect of age was marginally significant in the Glx model (B = 0.03, SE = 0.01, X2 = 5.76, p = 0.016). Lower tNAA concentrations were associated with greater mood/cognition symptom improvement at marginal significance (B = −0.62, SE = 0.25, X2 = 5.89, p = 0.015). Effects of metabolite on mood/cognition were non-significant for the remaining models. Aside from a marginally significant effect of age in the Glx model (B = −0.03, SE = 0.01, X2 = 4.896, p = 0.027), no metabolites were significant predictors of change in somatic symptoms on the PCA somatic scale.
Percent change in Han et al. [81] mood/cognition subscale
Low baseline Glu (B = −16.32, SE = 4.89, X2 = 11.15, p < 0.001) and Glx (B = −14.26, SE = 4.46, X2 = 10.25, p = 0.001) significantly predicted percent change in the published mood/cognition subscale (Fig. 3C). Lower tNAA concentrations were associated with greater mood/cognition symptom improvement at marginal significance (B = −18.61, SE = 8.09, X2 = 5.28, p = 0.022). Effects of metabolite on mood/cognition were non-significant for the remaining models.
Percent change in Han et al. [81] somatic subscale
Low Glu (B = −14.52, SE = 7.13, X2 = 4.14, p = 0.042), Glx (B = −14.21, SE = 6.31, X2 = 5.07, p = 0.024), and tNAA (B = −24.66, SE = 10.53, X2 = 5.48, p = 0.019), predicted percent change on the somatic IDS-SR subscale with marginal significance. Effects of metabolite on the somatic subscale improvement were not significant for the remaining models.
Percent change in Han et al. [81] immuno-metabolic subscale
Low Glu (B = −27.81, SE = 7.77, X2 = 12.87, p < 0.001), Glx (B = −22.26, SE = 7.38, X2 = 9.10, p = 0.003), and tNAA (B = −37.51, SE = 12.39, X2 = 9.17, p = 0.002) significantly predicted percent change in the immuno-metabolic subscale (Fig. 3D). Effects of metabolite on immuno-metabolic subscale percent change were not significant for the tCr and Ins models.
All relationships between metabolites and outcomes were not driven by outliers (D < 1). Post-hoc sensitivity analyses showed no significant effect of change in psychiatric medication status or dominant rTMS protocol on the relationship between metabolites and rTMS outcomes.
Predictors of rTMS response (Dichotomous outcome)
The effect of baseline Glu level in the prediction of rTMS treatment response was marginally significant (B = 1.55, SE = 0.69, X2 = 5.04, p = 0.025). Effects of metabolites on responder status were not significant for the remaining models. Post-hoc sensitivity analyses showed no significant effect of change in psychiatric medication on the relationship between metabolites and rTMS response. Dominant rTMS protocol was not significant in any models, however, when entered in the Glx model, Glx (B = 1.46, SE = 0.68, X2 = 4.60, p = 0.032) became marginally significant when predicting responder status.
The assessed performance prediction (Fig. 4) of the Glu model was excellent with an area under the curve (AUC) of 0.81 (SE = 0.09; p = 0.009). The performance for Glx and tNAA was acceptable, with AUC of 0.73 for Glx (SE = 0.10, p = 0.05) and AUC of 0.72 for tNAA (SE = 0.11, p = 0.064). Ins and tCr had no discriminative ability, with AUCs of 0.43 and 0.52 (SEs = 0.12, 0.12; p > 0.05).
Area under the receiving operating characteristic (AUROC) curves for B Glu, C Glx, and D tNAA’s ability to predict rTMS responder status as a binary outcome (50% or greater improvement on full-scale IDS-SR score) from our generalized logistic regression analyses. Panel A shows how all three curves overlap with one another. The higher the AUC value, the greater the predictive ability of the baseline metabolite. IDS-SR Inventory of Depression Symptomatology Self Report, Glu glutamate, Glx glutamine and glutamate, tNAA total N-acetylaspartate.
Discussion
This study examined Glu, Glx, and tNAA as potential, prospective pre-treatment biomarkers of response and change in symptom domains in adults with primary MDD who received naturalistic rTMS treatment. We found at baseline, (1) low Glu, Glx, and tNAA were predictive of greater post-rTMS change in overall depression severity; (2) low Glu and Glx were predictive of greater change in the mood/cognition domain; (3) low Glu, Glx, and tNAA were predictive of greater change in the immuno-metabolic domain; and (4) lower concentrations of Glu were predictive of rTMS responder status. These relationships were largely independent of age and sex, aside from a marginally significant contribution of age in the models evaluating the impact of Glx on mood & cognition and full IDS-SR score. As hypothesized, relationships were specific to glutamatergic and N-acetylated compounds, as both Ins and tCr were not predictive of percent change in symptomatology or rTMS responder status. These results indicate that Glu, Glx, and tNAA could serve as specific biomarkers predictive of outcomes a patient might expect following a full course of rTMS. For example, individuals with MDD who have relatively lower levels of such metabolites, i.e., Glu, may serve as the best candidates for rTMS.
rTMS outcomes were superior for individuals with low concentrations of frontal Glu prior to treatment, suggesting rTMS may be most effective when upregulation of the Glu-Gln cycle can occur. The glutamate hypothesis of depression suggests low intracellular Glu contributes to MDD pathology [39]. In our working model of rTMS mechanisms, we propose frontal lobe Glu-Gln dynamics are key mediators of the therapeutic effects of rTMS via upregulation of low neuronal and astrocytic Glu via increased glucose utilization and glycolytic pathway and tricarbolic acid (TCA) cycle activity [41]. We hypothesize that MDD symptoms thus remit to the extent that rTMS modulates the Glu-Gln pathway [41]. Given our model and current findings, we hypothesize that individuals who present with depression and low right dACC Glu concentrations are able to achieve greater post-rTMS clinical outcomes because neurons can successfully upregulate Glu production via the Glu-Gln pathway. This in turn leads to Glu levels that are optimal for cellular metabolism, neuronal plasticity, homeostasis of ATP production, and prevention of hyperammonia [102, 103]. In contrast, individuals with higher Glu levels when depressed may not respond as well to rTMS because they are already at ceiling-level. In these individuals, homeostatic, endogenous neuroprotective mechanisms that are already engaged may prevent Glu levels from rising too high to halt excitotoxicity and dynamic circuit imbalance [104].
As predicted, we found relatively lower Glu and Glx concentrations related specifically to percent change (i.e., more improvement) in mood and cognition-related symptoms. This is consistent with prior literature indicating that dACC hypoactivity is predictive of improved cognitive control following treatment in individuals with MDD [105]. Interestingly, there were also significant associations between Glu, Glx, and tNAA and percent change in immuno-metabolic symptoms, which included oversleeping, overeating, weight gain, decreased energy level, and leaden paralysis. Such neurovegetative symptoms are also robustly associated with salience network activity involving connections within and between the ACC, amygdala, and insular cortex in individuals with MDD. Together, the major nodes of the salience network, the insula and ACC, integrate information regarding sleep, digestive system activity, and are significantly influenced by hunger-related hormones such as leptin and insulin [106], providing additional support for the patterns observed here. Our findings that dACC Glu, Glx, and tNAA concentrations were not significantly related to the somatic symptom domain is consistent with prior work, which indicates that somatic symptoms are primarily associated with abnormal connectivity between the insula and regions involved in somatosensation (such as the precuneus, midcingulate, and angular gyrus) [107].
Though lower baseline levels of both Glu and Glx were related to rTMS-associated symptom improvement, Glx did not have as strong of an effect as Glu in the generalized linear models, and did not predict rTMS response as a binary outcome. Because Glu comprises the majority of the Glx signal, the observed relationships may be driven by Glu rather than Gln concentrations. This suggests (1) Gln may not be as mechanistically involved in rTMS-related MDD symptom improvement as Glu, or (2) baseline Gln levels in MDD may be heightened, rather than low like Glu, altering the directionality of metabolite-symptom effects. Gln should be collected at a higher field strength, as 3 T does not allow for reliable Gln quantification. Age’s marginal significance in the Glx models of mood/cognition and overall depression symptom improvement could be explained through the positive relationship between Gln and age [108]. tNAA may not have had as strong as an effect as Glu considering it is a general marker for neuronal health and integrity and serves as a precursor to for both Glu and Gln [36].
There are several limitations to this study. 1H MRS data were only collected from the right dACC; thus our metabolic findings may not generalize to the left hemisphere or other cortical regions. Relationships between baseline Glu, Glx, and tNAA and treatment outcomes may reflect a general improvement in depression symptomatology rather than a specific effect of rTMS. This may also be true for other noninvasive brain stimulation interventions, such as ECT. However, it is noteworthy that our sample was characterized by individuals who had not improved with standard antidepressant medications. Because our study is limited by a small sample size (n = 25) and underpowered to detect medium-to-small effect sizes, our primary objective was to establish feasibility of using baseline metabolites to predict rTMS outcomes. Our results are therefore considered preliminary and set the stage for future studies with larger sample sizes to detect small effect sizes. Sample homogeneity with regards to race and ethnicity inhibits our ability to generalize results to Black, Hispanic, Asian, and Indigenous individuals, and reflects systemic barriers to healthcare access across the US [109]. Future studies need to collect more data to understand how comorbid diagnoses (i.e., post-traumatic stress disorder), transdiagnostic symptoms (i.e., anxiety), number and type of previous treatments, and recurrence and previous history of MDD influence baseline metabolite levels and their relationships with rTMS outcomes in individuals living with MDD. The absence of this information may skew results and hinder replicability.
Our study has several methodological and analytical strengths. Data were collected prospectively, and were not subject to recall or retrospective selection bias. By analyzing baseline tCr and Ins, we demonstrate the metabolic specificity of relationships, and show that our Glu, Glx, and tNAA findings are not a product of overall metabolic upregulation or MRS scanning artifacts, such as poor water-suppression. We also evaluated (1) adults aged across the lifespan with (2) relatively equal representation of both sexes in the sample, facilitating generalizability.
Importantly, this study used a sample of individuals receiving standard rTMS therapy, including high frequency stimulation to the left DLPFC and low frequency stimulation to the right DLPFC. Differences in stimulation type and laterality may limit our ability to isolate mechanisms specific to either technique. However, the heterogeneity of rTMS treatment increases the ecological validity of our findings considering both high and low frequency rTMS are commonly implemented in clinical practice [12] and generally demonstrate equal efficacy [110]. Glutamatergic and N-acetylated metabolites may be non-specific biomarkers of outcomes for all rTMS techniques. The lack of effect of stimulation type in our models suggests different modes of rTMS may work via a common mechanistic pathway between the bilateral DLPFC and right dACC. The DLPFC and dACC are functionally and structurally connected [111], and rTMS to the DLPFC restores aberrant hypoconnectivity between these regions in individuals with MDD [73]. rTMS-induced depolarizations in the bilateral DLPFC may influence metabolic activity in the right dACC via glutamatergic projections between these regions, ultimately improving depression symptoms. More controlled research will be needed to evaluate any relationships between baseline metabolites and differential effects of stimulation protocol on depression outcomes.
Future work should include additional validated measures of specific MDD symptoms, such as depressed mood, anhedonia, comorbid anxiety, insomnia, impaired cognition, suicidality, and psychomotor agitation, to illuminate how Glu, Glx, and tNAA predict domains of symptomatic response. Additionally, future work should collect metabolite levels over multiple time points during and following rTMS treatment, enabling clinicians and scientists to better understand how rTMS influences metabolites longitudinally, and how changes in these levels correspond with treatment outcomes. With larger sample sizes, increased number of voxels per participant, information on symptom-specific outcomes, and metabolite concentrations over time, machine learning techniques could be used to better predict which individuals and depression biotypes [112] most likely to respond to rTMS, paving the way for an MRS-driven personalized medicine approach to rTMS therapy for MDD.
In summary, we found that pre-treatment levels of glutamatergic and N-acetylated compounds in the right dACC may serve as potential biomarkers for MDD outcomes after a full course of rTMS. Among MDD patients, lower levels of Glu, Glx, and tNAA predicted greater improvement in full scale depression scores after rTMS. Low baseline glutamatergic metabolites also specifically predicted greater decreases on symptom-specific changes in mood and cognition. Similarly, low Glu, Glx, and tNAA predicted decreases in scores on the immuno-metabolic subscale. Low Glu uniquely predicted rTMS responder status as a binary outcome. Together, these preliminary results provide rationale for exploring the utility of dACC Glu, Glx, and tNAA as biomarkers of rTMS efficacy for MDD. Though our findings need to be replicated with a larger sample size, this study is an important first step in (1) understanding how MRS metabolites predict and relate to rTMS outcomes associated with specific MDD symptom domains and (2) using neuroimaging-based techniques for optimization of psychiatric treatment selection, benefitting both patients and providers.
Data availability
The data that support the findings of this study are available from the corresponding author, MAG, upon reasonable request.
References
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. https://doi.org/10.1016/S0140-6736(07)61415-9.
Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202:22–7. https://doi.org/10.1192/bjp.bp.112.112169.
Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35:1–14. https://doi.org/10.1016/j.psc.2011.11.005.
Raič M. Depression and heart diseases: leading health problems. Psychiatr Danub. 2017;29:770–7.
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155–62. https://doi.org/10.4088/JCP.14m09298.
Xiang X, An R, Kang SW, Stagg BC, Ehrlich JR. Disability type, depression, and antidepressants use among older adults in the United States. Aging Ment Health. 2020;24:27–34. https://doi.org/10.1080/13607863.2018.1509298.
Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21:10. https://doi.org/10.1007/s11920-019-0997-0.
Koivumaa-Honkanen H, Kaprio J, Honkanen R, Viinamäki H, Koskenvuo M. Life satisfaction and depression in a 15-year follow-up of healthy adults. Soc Psychiatry Psychiatr Epidemiol. 2004;39:994–9. https://doi.org/10.1007/s00127-004-0833-6.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. https://doi.org/10.1176/ajp.2006.163.11.1905.
Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67:16–22.
Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. https://doi.org/10.1016/S0006-3223(03)00231-2.
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J Clin Psychiatry. 2018;79. https://doi.org/10.4088/JCP.16cs10905.
O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol psychiatry. 2007;62:1208–16. https://doi.org/10.1016/j.biopsych.2007.01.018.
Gaynes BN. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75:477. https://doi.org/10.4088/JCP.13r08815.
Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–39. https://doi.org/10.1017/S0033291713000512.
Fitzgerald PB, Hoy KE, Anderson RJ, Daskalakis ZJ. A study of the pattern of response to rTMS treatment in depression. Depress Anxiety. 2016;33:746–53. https://doi.org/10.1002/da.22503.
Corlier J, Wilson A, Hunter AM, Vince-Cruz N, Krantz D, Levitt J, et al. Changes in functional connectivity predict outcome of repetitive transcranial magnetic stimulation treatment of major depressive disorder. Cereb Cortex. 2019;29:4958–67. https://doi.org/10.1093/cercor/bhz035.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. https://doi.org/10.1038/nm.4246.
Boes AD, Uitermarkt BD, Albazron FM, Lan MJ, Liston C, Pascual-Leone A, et al. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimul. 2018;11:575–81. https://doi.org/10.1016/j.brs.2018.01.029.
Jha S, Chadda RK, Kumar N, Bal CS. Brain SPECT guided repetitive transcranial magnetic stimulation (rTMS) in treatment resistant major depressive disorder. Asian J Psychiatry. 2016;21:1–6. https://doi.org/10.1016/j.ajp.2016.02.003.
Baeken C, De Raedt R, Van Hove C, Clerinx P, De Mey J, Bossuyt A. HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. CNS Spectr. 2009;14:439–48. https://doi.org/10.1017/S1092852900020411.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. https://doi.org/10.1016/j.biopsych.2012.04.028.
Garnaat SL, Fukuda AM, Yuan S, Carpenter LL. Identification of clinical features and biomarkers that may inform a personalized approach to rTMS for depression. Pers Med Psychiatry. 2019;17-18:4–16. https://doi.org/10.1016/j.pmip.2019.09.001.
Delorme A. EEG is better left alone. Sci Rep. 2023;13:2372. https://doi.org/10.1038/s41598-023-27528-0.
Burtscher IM, Holtås S. Proton MR spectroscopy in clinical routine. J Magn Reson Imaging. 2001;13:560–7. https://doi.org/10.1002/jmri.1079.
Near J, Harris AD, Juchem C, Kreis R, Marjańska M, Öz G, et al. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2021;34:e4257. https://doi.org/10.1002/nbm.4257.
Liu XL, Li L, Li JN, Rong JH, Liu B, Hu ZX. Reliability of Glutamate quantification in human nucleus accumbens using Proton Magnetic Resonance Spectroscopy at a 70-cm wide-bore clinical 3T MRI system. Front Neurosci. 2017;11:686. https://doi.org/10.3389/fnins.2017.00686.
Baeshen A, Wyss PO, Henning A, O’Gorman RL, Piccirelli M, Kollias S, et al. Test–retest reliability of the brain metabolites GABA and Glx With JPRESS, PRESS, and MEGA-PRESS MRS Sequences in vivo at 3T. J Magn Reson Imaging. 2020;51:1181–91. https://doi.org/10.1002/jmri.26921.
Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: A phantom study at 4Tesla. J Magn Reson. 2011;208:210–8. https://doi.org/10.1016/j.jmr.2010.11.003.
Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Res Neuroimaging. 2006;147:1–25. https://doi.org/10.1016/j.pscychresns.2005.12.004.
Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in Schizophrenia—A systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. https://doi.org/10.1016/j.biopsych.2010.10.004.
Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M. Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol. 2017;30:246–62.
Faghihi R, Zeinali-Rafsanjani B, Mosleh-Shirazi M-A, Saeedi-Moghadam M, Lotfi M, Jalli R, et al. Magnetic Resonance Spectroscopy and its clinical applications: a review. J Med Imaging Radiat Sci. 2017;48:233–53. https://doi.org/10.1016/j.jmir.2017.06.004.
Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36. https://doi.org/10.1007/s11064-013-1199-5.
Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. https://doi.org/10.1007/978-3-319-08894-5_2.
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. https://doi.org/10.1016/j.pneurobio.2006.12.003.
Xie X, Shi Y, Ma L, Yang W, Pu J, Shen Y, et al. Altered neurometabolite levels in the brains of patients with depression: A systematic analysis of magnetic resonance spectroscopy studies. J Affect Disord. 2023;328:95–102. https://doi.org/10.1016/j.jad.2022.12.020.
Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–12. https://doi.org/10.1016/j.mehy.2006.02.047.
Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. https://doi.org/10.1016/j.neuropharm.2011.07.036.
Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. https://doi.org/10.1016/S0006-3223(99)00159-6.
Gonsalves MA, White TL, Barredo J, Fukuda AM, Joyce HE, Harris AD, et al. Repetitive transcranial magnetic stimulation-associated changes in neocortical metabolites in major depression: a systematic review. NeuroImage: Clin. 2022;35:103049. https://doi.org/10.1016/j.nicl.2022.103049.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced Prefrontal Glutamate/Glutamine and γ-Aminobutyric acid levels in major depression determined using proton magnetic resonance Spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. https://doi.org/10.1001/archpsyc.64.2.193.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24:952–64. https://doi.org/10.1038/s41380-018-0252-9.
Zhong S, Wang Y, Zhao G, Xiang Q, Ling X, Liu S, et al. Similarities of biochemical abnormalities between major depressive disorder and bipolar depression: A proton magnetic resonance spectroscopy study. J Affect Disord. 2014;168:380–6. https://doi.org/10.1016/j.jad.2014.07.024.
Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. https://doi.org/10.1016/j.biopsych.2008.10.025.
Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced Anterior Cingulate Cortex Glutamatergic Concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–8. https://doi.org/10.1097/00004583-200403000-00017.
Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28:720–5. https://doi.org/10.1038/sj.npp.1300085.
Luborzewski A, Schubert F, Seifert F, Danker-Hopfe H, Brakemeier E-L, Schlattmann P, et al. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. J Psychiatr Res. 2007;41:606–15. https://doi.org/10.1016/j.jpsychires.2006.02.003.
Yang X-R, Kirton A, Wilkes TC, Pradhan S, Liu I, Jaworska N, et al. Glutamate Alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ECT. 2014;30:242–7.
Godfrey KEM, Muthukumaraswamy SD, Stinear CM, Hoeh N. Effect of rTMS on GABA and glutamate levels in treatment-resistant depression: An MR spectroscopy study. Psychiatry Res: Neuroimaging. 2021;317:111377. https://doi.org/10.1016/j.pscychresns.2021.111377.
Baeken C, Lefaucheur JP, Van Schuerbeek P. The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: Insights from (1)H MR spectroscopy. Clin Neurophysiol. 2017;128:1664–72. https://doi.org/10.1016/j.clinph.2017.06.243.
Bhattacharyya P, Anand A, Lin J, Altinay M. Left Dorsolateral Prefrontal Cortex Glx/tCr predicts efficacy of high frequency 4- to 6-Week rTMS treatment and is associated with symptom improvement in adults with major depressive disorder: findings from a pilot study. Front Psychiatry. 2021;12:665347. https://doi.org/10.3389/fpsyt.2021.665347.
Merkl A, Schubert F, Quante A, Luborzewski A, Brakemeier E-L, Grimm S, et al. Abnormal Cingulate and Prefrontal Cortical neurochemistry in major depression after electroconvulsive therapy. Biol Psychiatry. 2011;69:772–9. https://doi.org/10.1016/j.biopsych.2010.08.009.
Njau S, Joshi SH, Espinoza R, Leaver AM, Vasavada M, Marquina A, et al. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J Psychiatry Neurosci. 2017;42:6–16. https://doi.org/10.1503/jpn.150177.
He J, Yan S, Song Z, Lu Q, Zhong S, Lai S, et al. Similarities and differences in working memory and neurometabolism of obsessive-compulsive disorder and major depressive disorder. J Affect Disord. 2022;311:556–64. https://doi.org/10.1016/j.jad.2022.05.069.
Shan Y, Jia Y, Zhong S, Li X, Zhao H, Chen J, et al. Correlations between working memory impairment and neurometabolites of prefrontal cortex and lenticular nucleus in patients with major depressive disorder. J Affect Disord. 2018;227:236–42. https://doi.org/10.1016/j.jad.2017.10.030.
Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience. 2017;346:284–97. https://doi.org/10.1016/j.neuroscience.2017.01.017.
Perlov E, Philipsen A, Maier S, Buechert M, Hesslinger B, Ebert D, et al. Depressive Cognitions May Affect Cingulate Neurochemistry in ADHD Patients. J Behav Brain Sci. 2011;1:6.
O’Connor RM, Finger BC, Flor PJ, Cryan JF. Metabotropic glutamate receptor 7: At the interface of cognition and emotion. Eur J Pharmacol. 2010;639:123–31. https://doi.org/10.1016/j.ejphar.2010.02.059.
Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–86. https://doi.org/10.1001/archgenpsychiatry.2009.39.
Zhang L, Zhu J, Zhang T, Jia Q, Hui L, Zhu H, et al. Comparative efficacy of add-on rTMS in treating the somatic and psychic anxiety symptoms of depression comorbid with anxiety in adolescents, adults, and elderly patients-A real-world clinical application. J Affect Disord. 2020;276:305–11. https://doi.org/10.1016/j.jad.2020.05.151.
Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress Anxiety. 2017;34:1029–39. https://doi.org/10.1002/da.22658.
Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. https://doi.org/10.1037//0022-3514.54.6.1063.
Lantrip C, Gunning FM, Flashman L, Roth RM, Holtzheimer PE. Effects of transcranial magnetic stimulation on the cognitive control of emotion: potential antidepressant mechanisms. J ECT. 2017;33:73–80.
Stevens FL, Hurley RA, Taber KH, Hurley RA, Hayman LA, Taber KH. Anterior Cingulate Cortex: Unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci. 2011;23:121–5. https://doi.org/10.1176/jnp.23.2.jnp121.
Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34:9–24. https://doi.org/10.1002/da.22556.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. https://doi.org/10.1016/j.tics.2010.11.004.
Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74. https://doi.org/10.1146/annurev.psych.53.100901.135148.
Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. https://doi.org/10.1038/npp.2010.166.
Ge R, Blumberger DM, Downar J, Daskalakis ZJ, Dipinto AA, Tham JCW, et al. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: A pilot study. J Affect Disord. 2017;218:75–81. https://doi.org/10.1016/j.jad.2017.04.060.
White TL, Gonsalves MA, Cohen RA, Harris AD, Monnig MA, Walsh EG, et al. The neurobiology of wellness: 1H-MRS correlates of agency, flexibility and neuroaffective reserves in healthy young adults. NeuroImage. 2021;225:117509. https://doi.org/10.1016/j.neuroimage.2020.117509.
Baeken C, van Beek V, Vanderhasselt M-A, Duprat R, Klooster D. Cortical thickness in the right anterior cingulate cortex relates to clinical response to left prefrontal accelerated intermittent theta burst stimulation: an exploratory study. Neuromodulation. 2021;24:938–49. https://doi.org/10.1111/ner.13380.
Zhang Y, Shao J, Wang X, Chen Z, Liu H, Pei C, et al. Functional impairment-based segmentation of anterior cingulate cortex in depression and its relationship with treatment effects. Hum Brain Mapp. 2021;42:4035–47. https://doi.org/10.1002/hbm.25537.
Pannekoek JN, van der Werff SJ, Meens PH, van den Bulk BG, Jolles DD, Veer IM, et al. Aberrant resting-state functional connectivity in limbic and salience networks in treatment–naïve clinically depressed adolescents. J Child Psychol Psychiatry. 2014;55:1317–27. https://doi.org/10.1111/jcpp.12266.
Godfrey KEM, Muthukumaraswamy SD, Stinear CM, Hoeh N. Decreased salience network fMRI functional connectivity following a course of rTMS for treatment-resistant depression. J Affect Disord. 2022;300:235–42. https://doi.org/10.1016/j.jad.2021.12.129.
Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18:65–87. https://doi.org/10.1016/0165-1781(86)90060-0.
Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. https://doi.org/10.1017/S0033291700035558.
Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, et al. Transcranial magnetic stimulation (TMS) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–96. https://doi.org/10.1002/da.21969.
Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin psychiatry. 2014;75:12379.
Han LKM, Schnack HG, Brouwer RM, Veltman DJ, van der Wee NJA, van Tol M-J, et al. Contributing factors to advanced brain aging in depression and anxiety disorders. Transl Psychiatry. 2021;11:402. https://doi.org/10.1038/s41398-021-01524-2.
Halchenko Y, Goncalves M, Castello MVDO, T SG, Salo A, Hanke M, et al. nipy/heudiconv v0.9.0 (v0.9.0). Zonodo. 2020. https://doi.org/10.5281/zenodo.4390433.
Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horská A. Regional apparent metabolite concentrations in young adult brain measured by 1H MR spectroscopy at 3 Tesla. J Magn Reson Imaging. 2008;27:489–99. https://doi.org/10.1002/jmri.21285.
Geurts JJG, Barkhof F, Castelijns JA, Uitdehaag BMJ, Polman CH, Pouwels PJW. Quantitative 1H-MRS of healthy human cortex, hippocampus, and thalamus: Metabolite concentrations, quantification precision, and reproducibility. J Magn Reson Imaging. 2004;20:366–71. https://doi.org/10.1002/jmri.20138.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. https://doi.org/10.1002/mrm.1910300604.
White TL, Monnig MA, Walsh EG, Nitenson AZ, Harris AD, Cohen RA, et al. Psychostimulant drug effects on glutamate, Glx, and creatine in the anterior cingulate cortex and subjective response in healthy humans. Neuropsychopharmacology. 2018;43:1498–509. https://doi.org/10.1038/s41386-018-0027-7.
Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–52.
Harris AD, Puts NA, Edden RA. Tissue correction for GABA‐edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42:1431–40.
Gasparovic C, Chen H, Mullins PG. Errors in 1H-MRS estimates of brain metabolite concentrations caused by failing to take into account tissue-specific signal relaxation. NMR Biomed. 2018;31:e3914. https://doi.org/10.1002/nbm.3914.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Van Rossum G, Drake FL. Python 3 Reference Manual. Scotts Valley, CA: CreateSpace; 2009.
Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KAB, Kang G, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2016;41:E37–E45. https://doi.org/10.1503/jpn.150223.
Jayaweera HK, Lagopoulos J, Duffy SL, Lewis SJG, Hermens DF, Norrie L, et al. Spectroscopic markers of memory impairment, symptom severity and age of onset in older people with lifetime depression: Discrete roles of N-acetyl aspartate and glutamate. J Affect Disord. 2015;183:31–8. https://doi.org/10.1016/j.jad.2015.04.023.
Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–72. https://doi.org/10.1016/j.neurobiolaging.2004.07.001.
Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–5. https://doi.org/10.1016/j.mri.2008.06.002.
Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological sex differences in depression: a systematic review. Biol Res Nurs. 2018;20:383–92. https://doi.org/10.1177/1099800418776082.
Sackeim HA, Aaronson ST, Carpenter LL, Hutton TM, Mina M, Pages K, et al. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation. J Affect Disord. 2020;277:65–74. https://doi.org/10.1016/j.jad.2020.08.005.
Wickens MM, Bangasser DA, Briand LA. Sex differences in psychiatric disease: a focus on the glutamate system. Front Mol Neurosci. 2018;11. https://doi.org/10.3389/fnmol.2018.00197.
Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40. https://doi.org/10.1038/nn.2303.
Bewick V, Cheek L, Ball J. Statistics review 14: Logistic regression. Crit Care. 2005;9:112–8. https://doi.org/10.1186/cc3045.
Hosmer Jr DW, Lemeshow S, Sturdivant RX. Applied logistic regression: John Wiley & Sons; 2013.
Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm. 2014;121:799–817. https://doi.org/10.1007/s00702-014-1180-8.
Cooper AJL, Jeitner TM. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules. 2016;6:16. https://doi.org/10.3390/biom6020016.
Takahashi K, Foster JB, Lin C-LG. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci. 2015;72:3489–506. https://doi.org/10.1007/s00018-015-1937-8.
Crane NA, Jenkins LM, Bhaumik R, Dion C, Gowins JR, Mickey BJ, et al. Multidimensional prediction of treatment response to antidepressants with cognitive control and functional MRI. Brain. 2017;140:472–86. https://doi.org/10.1093/brain/aww326.
Toenders YJ, Schmaal L, Harrison BJ, Dinga R, Berk M, Davey CG. Neurovegetative symptom subtypes in young people with major depressive disorder and their structural brain correlates. Transl Psychiatry. 2020;10:108. https://doi.org/10.1038/s41398-020-0787-9.
Yan R, Geng JT, Huang YH, Zou HW, Wang XM, Xia Y, et al. Aberrant functional connectivity in insular subregions in somatic depression: a resting-state fMRI study. BMC Psychiatry. 2022;22:146. https://doi.org/10.1186/s12888-022-03795-5.
Hädel S, Wirth C, Rapp M, Gallinat J, Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Reson Imaging. 2013;38:1480–7. https://doi.org/10.1002/jmri.24123.
Simpson SM, Krishnan LL, Kunik ME, Ruiz P. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatr Q. 2007;78:3–14. https://doi.org/10.1007/s11126-006-9022-y.
Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74:143–52. https://doi.org/10.1001/jamapsychiatry.2016.3644.
Lichenstein SD, Verstynen T, Forbes EE. Adolescent brain development and depression: A case for the importance of connectivity of the anterior cingulate cortex. Neurosci Biobehav Rev. 2016;70:271–87. https://doi.org/10.1016/j.neubiorev.2016.07.024.
Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–80. https://doi.org/10.1016/S2215-0366(15)00579-9.
Acknowledgements
We would like to thank Polly Asi Gobin, Lauren Hindley, Fabienne McEleney, Lynn Fanella, Eric Tirrell, Edward Walsh, Michael Worden, Isabel Restrepo, Hannah Swearingen, Julie Joyce, Tim Souza, Megan Vigne, Jamie Kweon, and E. Frances Kronenberg for their help with data collection, preprocessing, and analysis. Data collection was supported by 1R01MH113929 to Michael Fox, MD (with subaward for Project at Butler Hospital to LLC). MAG’s effort was supported by the Hanlon Foundation (award to TLW), a Carney Institute Graduate Award in Brain Science (funded by the Dr. Daniel C. Cooper Graduate Award and the Charles A. Dana Graduate Fellowship Fund), and a Sigma Xi Grants in Aid of Research Award. TLW’s effort was supported by the Carney Institute for Brain Science Zimmerman Innovation Award and the Hanlon Foundation. JB’s effort was supported in part by grants from VA Clinical Science Research and Development (IK2 CX001824). ADH holds a Canada Research Chair in Magnetic Resonance Spectroscopy in Brain Injury. Research reported in this publication was supported in part by the research cores of the Center for Biomedical Research Excellence (COBRE) Center for Neuromodulation at Butler Hospital (P20GM130452; National Institute of General Medical Sciences). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the VA.
Author information
Authors and Affiliations
Contributions
MAG, JB, TLW, and LLC contributed to the overall conceptualization of this study. LLC (and the Butler TMS Clinic team) provided rTMS patient care and collection of self-report data. MAG led MRS data collection and conducted data quality control analyses with the assistance of MMD and ADH; TLW provided supervision of MRS data collection. MAG performed data analyses with input from JB. MAG drafted the manuscript and made revisions with edits and contributions from all coauthors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
MAG, JB, ADH, ED and MMD report no relevant financial disclosures. TLW has served as scientific advisor and consultant to Strategic Aid Partners Inc. In the past 2 years, LLC has served as a paid scientific advisor or consultant to Magnus Medical, Neuronetics Inc, Nexstim PLC, Neurolief LTD, Sage Therapeutics, Otsuka, Sunovion, and Janssen Pharmaceuticals Inc. LLC and her Butler Hospital research staff have received support through clinical trials contracts with Neuronetics Inc, Neurolief Inc, and Janssen Pharmaceuticals Inc for investigations that are unrelated to the subject of this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonsalves, M.A., White, T.L., Barredo, J. et al. Cortical glutamate, Glx, and total N-acetylaspartate: potential biomarkers of repetitive transcranial magnetic stimulation treatment response and outcomes in major depression. Transl Psychiatry 14, 5 (2024). https://doi.org/10.1038/s41398-023-02715-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02715-9