Abstract
Major depressive disorder (MDD) is a very prevalent mental disorder that imposes an enormous burden on individuals, society, and health care systems. Most patients benefit from commonly used treatment methods such as pharmacotherapy, psychotherapy, electroconvulsive therapy (ECT), and repetitive transcranial magnetic stimulation (rTMS). However, the clinical decision on which treatment method to use remains generally informed and the individual clinical response is difficult to predict. Most likely, a combination of neural variability and heterogeneity in MDD still impedes a full understanding of the disorder, as well as influences treatment success in many cases. With the help of neuroimaging methods like functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), the brain can be understood as a modular set of functional and structural networks. In recent years, many studies have investigated baseline connectivity biomarkers of treatment response and the connectivity changes after successful treatment. Here, we systematically review the literature and summarize findings from longitudinal interventional studies investigating the functional and structural connectivity in MDD. By compiling and discussing these findings, we recommend the scientific and clinical community to deepen the systematization of findings to pave the way for future systems neuroscience roadmaps that include brain connectivity parameters as a possible precision component of the clinical evaluation and therapeutic decision.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is ranked as the leading cause of disability worldwide by the World Health Organization (WHO), with a lifetime prevalence of 4.4% in the general population [1]. The symptoms of MDD include depressed mood, anhedonia, i.e., diminished interest or pleasure, changes in appetite and sleep, fatigue, psychomotor agitation and retardation, feelings of worthlessness or guilt, diminished ability to concentrate, and suicidal thoughts. According to the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5), a diagnosis of MDD requires a combination of five or more of these symptoms being present for two weeks or more, most of the day, and nearly every day [2]. Thus, the diagnosis of MDD is purely based on symptoms, and the profile of manifested symptoms may vary from patient to patient, resulting in a heterogeneous group under the same diagnostic umbrella.
Supported by evidence-based medicine, the use of more effective therapies has advanced the medical field, but insufficient clinical improvement in MDD patients still poses a challenge. The first-line treatment for MDD is pharmacotherapy and psychotherapy, with the remission rates for both modalities around 50% [3, 4]. Without knowing a priori who might benefit or not, residual depressive symptoms and disease chronification occur. Following the encouraging results presented by electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS) has gained ground as an effective non-invasive stimulation method in the treatment of depression [5]. However, little is known about the use of neurobiological information that could potentially support more targeted clinical interventions in the future. In this context, connectivity has emerged as a very promising method for identifying and monitoring changes in the brain. Much expectation has been placed on understanding the functional, structural, or multimodal network changes associated with the alleviation of depressive symptoms. By understanding the effect of the treatment on the brain, one could improve the treatment effects, e.g., by better targeting the affected regions with a subject-tailored protocol that more precisely treat an individual presentation of MDD. Therefore, great importance is currently given to the possibility of monitoring patients with neuroimaging methods to quantify functional and structural brain networks for insights that may lead to more informed clinical decisions. However, the existing evidence is still quite fragmented.
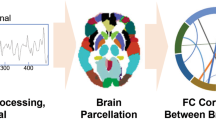
In this review, we have assembled the evidence available on the functional and structural brain connectivity predictors of treatment response and connectivity changes after treatment, to provide a broader view and new insights of neuroimaging contibutions to depression research. We also compiled the regions and connections reported in the included studies in figures to map and compare the findings of current treatment modalities. Finally, we have offered a critical perspective on how to improve and integrate the use of functional and structural connectivity data (Box 1 and 2) to potentially inform and guide the clinical interventions as a step to crystallize systems medicine in the future.
Materials and methods
Literature search
Studies were identified by searching with PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science (https://www.webofscience.com), and Cochrane Library (https://www.cochranelibrary.com), using the following keywords: (depression OR major depressive disorder) AND (functional connectivity OR structural connectivity OR diffusion tensor imaging) AND (pharmacotherapy OR medication OR antidepressant OR psychotherapy OR electroconvulsive therapy OR repetitive transcranial magnetic stimulation). The search process was conducted for articles published until 19 January 2023. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Study selection criteria
Inclusion criteria:
-
a.
Studies that have a sample group with a MDD diagnosis
-
b.
Longitudinal studies with one of the four treatment methods: pharmacotherapy, psychotherapy, ECT or rTMS
-
c.
Studies using resting-state functional magnetic resonance imaging (rsfMRI) functional connectivity (FC) and/or diffusion tensor imaging (DTI) structural connectivity (SC) analyses
Exclusion criteria
-
a.
Other neuropsychiatric diagnoses or comorbidities (except anxiety disorders)
-
b.
Sample size smaller than 20 patients for the MDD group
-
c.
Studies that used only graph theory, network control theory, or dynamic FC analyses
-
d.
Combination of different treatment methods without detailed description
-
e.
Studies focused on elderly, adolescent, remitted, first-episode or mild-moderate MDD
-
f.
Only females or males in the sample
-
g.
Review articles, meta-analyses, letters to editors, correspondences, not full-text articles
-
h.
Full text not in English
-
i.
Additional studies using previously published imaging data from identical samples with a similar analysis
Using the keywords, 7256 articles were identified by the search engines. After removing 957 duplicates, the remaining 6299 articles were screened by their titles and abstracts, and 6173 of them were excluded. The remaining 126 full-text articles were assessed for eligibility. Based on the selection criteria, 57 studies were included in the final review (see the PRISMA [6] flow diagram in Fig. 1).
Results
The 57 articles included in this review (Table 1) were organized into categories (Figs. 2,3) according to the primary treatment method (A. Pharmacotherapy, B. Psychotherapy, C. ECT, D. rTMS) and connectivity data presented (1. FC, or 2. SC).
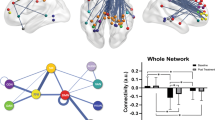
A Pharmacotherapy, B Psychotherapy, C Electroconvulsive therapy (ECT), and D Repetitive transcranial magnetic stimulation (rTMS). Color coding is red for baseline predictors of treatment response, blue for changes with treatment, and purple for predictors and changes. For simplicity, the hemisphere in the schemes does not always represent lateralization of the results.
A Pharmacotherapy, B Psychotherapy, C Electroconvulsive therapy (ECT), and D Repetitive transcranial magnetic stimulation (rTMS). Tracts in shades of red represent baseline predictors of treatment response, tracts in shades of blue represent changes with treatment, and tracts in shades of purple represent predictors and changes. For simplicity, the hemisphere in the schemes does not always represent lateralization of the results.
Pharmacotherapy and functional connectivity
Of the 13 studies that primarily focused on the association between baseline FC and response to selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), 11 studies reported increased FC within the brain that was associated with better treatment outcomes. Most of these results converged on brain regions that are part of the default mode network (DMN) as a potential biomarker of treatment response. Goldstein-Piekarski et al. (2018) reported that remitters had intact FC between the anterior and posterior parts of the DMN, compared to the hypoconnectivity seen in the non-remitters to SSRIs and SNRIs [7]. Ye et al. (2022) reported increased baseline right angular gyrus FC within the DMN in the remitted group compared with the non-remitted group, which was positively correlated with the reduction of depressive scores [8]. In addition to the increased FC within the DMN that was associated with better treatment outcomes [9,10,11], increased FC between DMN and frontoparietal network (FPN) [9, 10, 12], and somatomotor network (SMN) [10] was also reported. Additionally, treatment response was associated with increased FC between the right FPN and the posterior DMN, SMN, and somatosensory association cortex (SAC) by Martens et al. [13]. Increased FC between bilateral nucleus accumbens (NAcc) and rostral anterior cingulate cortex (rACC) was also associated with better treatment outcomes by Ang et al. (2020), but this effect was specific to Bupropion but not Sertraline [14]. Increased FC in other regions that were associated with better treatment outcomes was also reported, e.g., between the left hippocampus and left inferior frontal gyrus (IFG), and precuneus (PCu) [15]; and between left superior frontal gyrus (SFG) and thalamus [16]. Contrary to the aforementioned results, two studies reported decreased FC associated with better treatment outcomes, one within the orbitofrontal part of the DMN, including the subgenual anterior cingulate cortex (sgACC) [17], and one within and between the salience network (SN), FPN, and SMN [18]. Finally, van der Wijk et al. (2022) reported a mixed effect, i.e., higher FC in the posterior DMN and lower FC in the anterior DMN in early remitters compared to late- and non-remitter MDD [19]. In addition to these results that are limited to relatively short-term follow-ups, Ju et al. (2020) reported that connectome-based modeling could predict clinical improvement at 2 weeks, 1, 2, and 3 months after treatment [20].
Similar to the results for the biomarkers of treatment response, the change in FC with pharmacotherapy is still inconclusive, but the emphasis on the DMN is again evident. Cui et al. (2021) reported that the reduced FC in the anteromedial prefrontal cortex (amPFC) and posterior cingulate cortex (PCC) within the DMN was increased after treatment to levels comparable to those in the healthy controls (HC) [21]. In line with this, Yang et al., (2016) reported increased FC strength in the posterior DMN, including PCC, after treatment with Sertraline [22]. Fu et al. (2015) also found increased DMN FC after treatment, but only in the anterior components [17]. These results might suggest that DMN subnetworks can be affected differently by pharmacotherapy. This view is supported by Hsu et al. (2021), reporting that the FC between the medial temporal part of DMN and FPN was normalized after pharmacotherapy, while the core part of the DMN remained impaired [16]. The findings from Zhang et al. (2023) also support the idea that subnetworks of regions can be affected differently by treatment. They reported that only a subregion of the dorsal anterior cingulate (dACC) and rACC had increased FC with SN after treatment with SSRIs, while the FC of two other subregions of the anterior cingulate cortex (ACC) did not change after treatment [23]. Further emphasizing the importance of the DMN for the effect of pharmacotherapy on brain coupling, Ju et al. (2022) showed that only the decrease in DMN FC after pharmacotherapy distinguished remitters from non-remitters at 6 months, and stable from recurrent MDD during the 2-year follow-up [24]. In addition to the DMN, the reward system also appears to be important for the effect of pharmacotherapy, since increased FC between NAcc and dACC after treatment was associated with an improved physical quality of life [25]. Other networks were also reported to have decreased FC after pharmacotherapy, including SN, FPN, and SMN [18]. Contrary to these findings, Liu et al., (2021) reported that impaired FC in SMN, DMN, and dorsal attention network (DAN) in MDD was associated with the number of episodes and total illness duration, but FC did not change significantly when the patients achieved remission with Paroxetine [26].
Pharmacotherapy and structural connectivity
Of the seven studies that investigated the baseline SC as potential biomarkers of response to SSRIs and SNRIs, five of them focused only on the fractional anisotropy (FA) parameter. Three studies made predictions about patients’ clinical responses based on baseline FA parameters, reaching relatively high accuracies (62–88%). One of them predicted the status of remission with baseline FA in stria terminalis [27], another with stria terminalis and cingulum [28], and a third study with cingulum, superior fronto-occipital fasciculus (SFOF), and superior longitudinal fasciculus (SLF) [29]. Moreover, Zhou et al. (2011) found higher FA in the white matter tracts of the hippocampus bilaterally in treatment responders compared to non-responders at baseline [30]. Contrary to this finding, as well as to the authors’ hypotheses, Pillai et al. (2019) found lower FA in responders in the tracts between the raphe nucleus and amygdala compared to non-responders [31]. In addition to these studies focusing only on the FA parameter, two studies investigated four DTI parameters: Davis et al. (2019) reported that axial diffusivity (AD) in the external capsule and SLF was associated with clinical response [32], and Vieira et al. (2021) reported that higher FA and lower radial diffusivity (RD) in forceps minor and SLF was evident in responders compared to non-responders to Paroxetine [33].
Davis et al. (2019) and Dong et al. (2020) investigated the effect of SSRIs and SNRIs on SC but found no significant changes after 8 weeks, and 6 months after treatment, respectively [32, 34]. Fan et al. (2020) found four SC alterations in patients with MDD compared to HC. After 6 months of Paroxetine, two of these alterations, between the right anterior and posterior temporal cortex, and between the left temporal cortex and the auditory cortex, reversed at remission, hinting towards state-dependent alterations in MDD [35].
Psychotherapy and functional connectivity
Although studies that investigated the relationship between brain connectivity and psychotherapy are scarce, Crowther et al. (2015) reported that the FC between the right insula and right middle temporal gyrus (MTG), and left intraparietal sulcus and orbitofrontal cortex (OFC) predicted response to behavioral activation therapy [36]. In addition, Späti et al. (2015) reported that higher FC between the frontal pole and dACC was related to higher levels of adaptive rumination and better response to cognitive behavioral therapy (CBT) [37]. The study by Dunlop et al., (2017), in which MDD patients were randomized to either CBT, Escitalopram, or Duloxetine, found that positive FC of sgACC with left ventrolateral prefrontal cortex (vlPFC), left ventromedial prefrontal cortex (vmPFC) and dorsal midbrain was associated with remission with CBT [38]. Conversely, negative FC of the same regions was associated with remission due to pharmacotherapy, suggesting different biomarkers of treatment response for different treatment methods.
Psychotherapy and structural connectivity
Wang et al. (2013) reported that the increased FA in the right thalamus in patients with MDD compared to HC did not change after guided imagery psychotherapy, but novel regions of increased FA were found in the left frontal lobe (supplementary motor area), and a decreased FA in the right angular gyrus white matter after the treatment [39]. Hence, psychotherapy might be exerting its clinical effects by changing the white matter integrity in additional regions, rather than recovering the aberrant SC profile evident in MDD. However, more studies are needed to confirm this hypothesis.
Electroconvulsive therapy and functional connectivity
Van der Waarde et al. (2015) focused on the predictors of ECT treatment response based on FC measures and reported that networks centered in the dorsomedial prefrontal cortex (dmPFC) and ACC could predict recovery with relatively high sensitivity (80 and 84%) and specificity (75 and 85%) [40]. According to Pang et al. (2022), baseline FC within the DMN and between the DMN and FPN could effectively predict the improvement of symptoms after ECT [41]. Contrary to these results, Chen et al. (2017) reported that the baseline FC was unrelated to the treatment response after ECT in their MDD patient sample [42].
Wei et al. (2018) and Mo et al. (2020) reported that the FC strength of the left angular gyrus increased after ECT [43, 44]. But in both of the studies, there was no difference in left angular gyrus FC between HC and patients with MDD in the baseline. Interestingly, the increased FC of the angular gyrus after ECT returned to HC levels one month after ECT [43]. Thus, the increased FC of the left angular gyrus in MDD seems to temporarily deviate from the HC levels with the intervention, eventually returning to normal levels after about a month. Wang et al. (2017) reported that there was increased FC between the left amygdala and left fusiform face area (FFA), and increased effective connectivity (EC) from FFA to the amygdala after ECT, which became closer to HC levels [45]. In line with this, Leaver et al. (2016) reported that the hyperconnectivity between ventral striatum (VS) and ventral DMN returned to normal levels after ECT, and the hypoconnectivity between the VS and anterior DMN modestly improved. However, the FC between the dmPFC and SN remained impaired after ECT [46]. Additionally, two studies found an association between change in FC after ECT and change in clinical measures. Pang et al. (2022) reported that the change in the FC between the medial prefrontal cortex (mPFC) and vlPFC was negatively correlated with symptom improvement [41]. Very similarly, Zhang et al. (2021) reported that the changed FC between dmPFC and dlPFC after ECT was associated with the amelioration of anhedonia [47].
Electroconvulsive therapy and structural connectivity
Although studies investigating baseline predictors of ECT response are very scarce, Repple et al. (2020) reported that baseline FA and mean diffusivity (MD), mainly in the corpus callosum (CC), internal capsule, and corona radiata, were positively and negatively associated with symptoms post-ECT, respectively [48].
Studies investigating changes in SC after ECT have also been scarce and their results are inconsistent. Lyden et al. (2014) found a decrease in RD and MD in the cingulum, forceps minor, left SLF, and anterior thalamic radiation (ATR), as well as increased FA in these tracts after ECT. The changes in the SC parameters of MDD were correlated with changes in the severity of depression, and were in the direction of HC values, suggesting a “normalization” [49]. Differently, Repple et al. (2020) found an increase in MD after ECT in the right hemisphere, mainly in the uncinate fasciculus, posterior limb of the internal capsule, inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFOF). But these changes deviated from HC values, and according to the authors, this discrepancy possibly reflects increased permeability of the blood-brain barrier after ECT, resulting in disturbed communication of fibers [48].
Repetitive transcranial magnetic stimulation and functional connectivity
Much emphasis has been placed on the FC of sgACC as a potential predictor of rTMS treatment response. Baeken et al. (2014) reported that responders showed stronger anticorrelation between the sgACC and left dmPFC compared to non-responders [50], in line with the idea that clinical efficacy of rTMS related to sgACC-dlPFC anticorrelation [51]. Later, Weigand et al. (2018) also showed that rTMS efficacy was predicted by dlPFC stimulation sites that were more anterolateral and negatively correlated with sgACC [52], which is later replicated by further studies [53,54,55,56]. Contrary to these results, Hopman et al., (2021) did not evidence that baseline left dlPFC-sgACC FC was associated with treatment outcome [57]. Alternatively, Ge et al. (2020) reported that higher sgACC-dlPFC negative FC was associated with better response, but only on the right dlPFC, while the stimulation was delivered on the left dlPFC. The association with better rTMS treatment response was also evident in the rACC and left lateral parietal cortex [58]. In addition, Baeken et al. (2017) reported that sgACC FC with medial orbitofrontal cortex (mOFC) could distinguish responders and non-responders to rTMS treatment [59]. Furthermore, FC between the stimulation site in the dlPFC and other regions has also been reported to be associated with rTMS treatment response: Du et al. (2017) reported that increased negative FC between the stimulated dlPFC site and left NAcc could distinguish early improvers compared to non-improvers, and the FC strength negatively correlated with clinical efficacy [60]. Moreover, Kang et al. (2016) reported that decreased FC between the dlPFC and left caudate predicted clinical improvement after rTMS treatment [61]. Another region that was reported to predict rTMS treatment response was the anterior insula (AI). Iwabuchi et al. (2019) reported that fronto-insular EC and SN FC were positively correlated with early (1 month), but not sustained response (3 months) to rTMS [62]. Besides, Taylor et al. (2018) reported that FC between AI and PCC was lower in responders compared to non-responder to rTMS [63].
The change in the FC of the sgACC after rTMS treatment has also been studied extensively. Baeken et al. (2014) reported that the anticorrelated FC between the sgACC and the dmPFC was stronger in responders compared to non-responders at baseline. But after rTMS treatment, a positive correlation in FC between these regions was seen in responders compared to non-responders [50]. Later, Baeken et al. (2017) reported that baseline FC between sgACC and mOFC could distinguish responders and non-responders. They also reported that the FC between these regions increased after effective rTMS treatment, which was associated with a decrease in feelings of hopelessness [59]. Furthermore, Ge et al. (2020) reported that the hyperconnectivity between sgACC and visual cortex “normalized” after rTMS treatment [58]. Conversely, Iwabuchi et al. (2019) and Taylor et al. (2018) did not find any significant change in the FC profile of patients with MDD after active rTMS treatment compared to sham [62, 63]. But Taylor et al. (2018) further stated that sgACC FC to AN, DMN, and FPN decreased among patients who showed significant improvement after rTMS treatment, but not in non-responders [63], suggesting that such FC changes might be specific to the patients who show clinical improvement. Furthermore, Kang et al. (2016) reported that there was a greater reduction of FC strength between the dlPFC and left caudate after active rTMS compared to sham [61]. Change in the amygdala FC has also been reported by two studies. Chen et al. (2020) reported that the degree of “normalization” in FC between the left insula and amygdala was correlated with a change in depressive scores [64]. Finally, Eshel et al. (2020) showed that rTMS induced negative dlPFC-amygdala FC towards normative values, such that the dlPFC was better able to engage in top-down control of the amygdala [65].
Repetitive transcranial magnetic stimulation and structural connectivity
Studies investigating the relationship between rTMS and SC mainly focused on the connectivity of the left dlPFC stimulation site. Ning et al. (2022) reported that baseline SC of the vlPFC and dACC was correlated with changes in depressive scores [66]. Furthermore, Klooster et al. (2020) also reported that the indirect SC between the patient-specific stimulation site on the left dlPFC and the cingulate cortex has predictive potential for clinical response to rTMS treatment [67]. Two studies investigated functional as well as structural connectivity predictors of rTMS response: Fu et al. (2021) reported that functional and structural connectivity between the left dlPFC and insula [68], and Chen et al. (2022) reported that thalamo-prefrontal functional and structural connectivity predicted the efficacy of rTMS [69].
Ning et al. (2022) did not find any significant changes in the tracts connected to the stimulation targets on the left dlPFC after rTMS, but they found that rTMS increased FA and decreased RD in anteromedial prefrontal fiber bundles. The authors also report that the changes in the lateral prefrontal white matter tracts were significantly correlated with treatment response [66].
Discussion
In this article, we reviewed 57 longitudinal studies that investigated the functional and structural connectivity in patients with MDD undergoing four different treatment methods: Pharmacotherapy, Psychotherapy, ECT, and rTMS. We summarized the findings of the included studies for each treatment method (Table 1). Additionally, we displayed these findings on brain models (Figs. 2,3) to help visualize and compare the evidenced relationship between different treatment methods, and functional and structural connectivity. Finally, we made recommendations that seem crucial to us to overcome the limitations that still exist in this area.
For FC, we identified some regions that are commonly associated with different treatment methods. For instance, dlPFC and ACC seem to be common regions associated with pharmacotherapy [7, 12, 14, 15, 19, 23, 25, 70], as well as rTMS [50, 52, 57,58,59,60,61,62,63,64,65, 71]. Additionally, PCC has been reported more often in pharmacotherapy studies than in studies with other treatment methods [7, 19, 21, 22]. Noteworthy, the use of different classes of antidepressants in different studies might have caused variability in the results, which the available data do not allow us to evaluate with certainty. Compared to other treatment methods, dmPFC has been more frequently reported in ECT studies [40, 41, 47]. There are fewer studies on psychotherapy, but vlPFC and vmPFC have been commonly reported in the studies that used this treatment method [36,37,38]. Overall, the association of higher anticorrelation between the left dlPFC and the sgACC and better clinical efficacy after rTMS has been the finding that was replicated the most [54,55,56,57,58,59,60]. These promising findings support the personalization of rTMS stimulation sites on the left dlPFC based on patient-specific functional magnetic resonance imaging (fMRI) data using neuronavigation, with higher clinical success [72, 73]. A particularly promising aspect of rTMS, due to the stimulation focality of the figure-of-eight coil, is the possibility of personalizing treatment according to particular circuits implicated in symptoms manifested by the patient. The underlying functional and structural connections between the stimulation target, and the regions which may be directly implicated in different symptoms, such as psychomotor retardation, cognitive impairment, lack of emotion regulation, or somatic symptoms, can be useful. For instance, by using the connectivity information, the regions and tracts associated with the particular symptoms can possibly be modulated according to the symptom profile of the patient. Nevertheless, more prospective studies are needed to establish the validity of this precision approach.
For SC, results were vastly different for different treatment methods. In pharmacotherapy, cingulum [29, 32], forceps minor [33], SLF [29, 32, 33], SFOF [29], and white matter tracts that connect the limbic regions [27, 28, 30, 31] have been commonly reported as possible baseline predictors of treatment response. In the two rTMS studies, analysis of SC has been performed with seed-based methods, where the region of interest (ROI) selected was the stimulation target on the left dlPFC [66, 67]. This likely converged the results to particular white matter tracts that connect dlPFC to other regions. Nevertheless, considering the possibility of long-distance effects as suggested in FC, this may result from stronger focal intervention in rTMS in contrast to the effects of the other more systemic interventions. The results from the two ECT studies were widespread, but inconsistent, which makes it hard to make conclusions [48, 49]. Lastly, there is only one study investigating the effect of psychotherapy on SC, reporting results in frontal and parietal regions [39], which reinforces the need for more studies investigating this topic.
As the summary suggests, although there seems to be common regions reported in different FC and SC studies, the results remain quite inconsistent. To overcome this reproducibility problem and the difficulty of directly comparing results from different treatment modalities, we propose recommendations for future studies:
-
1.
Standardization of the analysis methods. The lack of standardization in the analysis methods hinders the comparability of the effects of different treatment modalities. For example, the choice of different seeds as ROI within or across different treatment modalities prevents the comparison between studies. In general, the selection of ROIs in a study invariably produces partial connectivity results, and such regions may not even be selected for evaluation in a second study. Therefore, it becomes impossible to compare studies if the raw data are not shared by the authors.
-
2.
Larger sample sizes for single-site studies and data sharing for multicenter analyses. One of the main reasons behind high variability and low reproducibility in the results is most likely the limited sample size, hence low statistical power and higher rates of false positives. When this aspect is combined with the heterogeneity in the MDD samples, it makes it very difficult to compare the effect of different treatment modalities or to conclude what the effect of each treatment method is. According to statistical power analyses; for a liberal threshold of 0.05, ~12 subjects are required to achieve 80% power at the single voxel level, and double the number of subjects are needed to maintain this level of power at more realistic thresholds correcting for multiple comparisons [74]. According to an evaluation, highly cited clinical fMRI studies published in high-impact journals between 1999–2018 had a median sample size of 14.5 subjects, which increased at a rate of 0.74 participant/year. Furthermore, only 9 out of 273 papers published in 2017 and 2018 had pre-study power calculations [75], suggesting that most neuroimaging studies might not have enough statistical power. This can contribute to the reporting of false positives and the reproducibility problem. Alarmingly, a recent article stated that reproducible brain-wide association studies (BWAS), studies investigating the associations between brain function or structure and complex cognitive or mental health phenotypes, require samples with thousands of individuals [76]. Therefore, we intend to raise the attention to the clinical and scientific communities to the importance of bigger sample sizes, pre-study power calculations, and sharing of clinical and imaging data of patients with MDD, which will allow more representative descriptions of the disorder on a global scale. This way, large-scale multicenter datasets analyzed via meta- and mega-analytical approaches can fill the existing gaps of knowledge about treatment modalities and related functional and/or structural connectivity.
-
3.
Longitudinal rather than cross-sectional study designs. Although longitudinal studies are harder to conduct due to higher costs, time, and drop-out rates, their importance was highlighted by the article that stated the importance of larger sample sizes for BWAS studies. They stated that: “For greater effect sizes and statistical power, neuroscience should focus on within-participant study designs over cross-sectional study designs, and on interventional (therapy, medications, brain stimulation, and surgery) over observational study designs” [76]. Especially, there is a lack of long-term follow-up of patients with neuroimaging. To our knowledge, there is only one long-term study where they followed up patients on pharmacotherapy every 6 months for 2 years with magnetic resonance imaging (MRI) [24]. These data are important to understand how long these connectivity changes may last after treatment, to show whether their patterns return to the diseased state over time and whether these patterns may be directly related to relapses. This feature becomes fundamental due to the high rate of recurrence of the disorder. In the future, this information could help in clinical decisions with more difficult cases, even before the constitution of an evident relapse is fully manifested by symptoms.
-
4.
Randomized studies comparing different treatment methods. These types of studies are needed for the validation of baseline biomarkers of response to specific treatment methods. Such studies are scarce, with only two comparative studies [38, 48] included in the review. Randomized studies like these can be very informative, aiming to help clinicians in their treatment choices in the future, stratifying the patient with the aid of neurobiological data.
-
5.
Analysis of multimodal data. Finally, we want to emphasize the importance of multimodal imaging for a more holistic understanding of the pathophysiology of MDD and the effects of its treatment. A coordinate-based meta-analysis of neuroimaging studies that examined the multimodal brain abnormalities in MDD identified spatially convergent structural and functional abnormalities in the sgACC, hippocampus, amygdala, and putamen [77], hinting towards commonalities in the functional and structural connectivity profiles, and the importance of multimodal studies in MDD research. Other promising approaches, such as structural covariance networks [78, 79], can add more layers of information about the organization of large-scale brain networks, and, therefore, should be incorporated in future analyses.
In conclusion, to overcome the reproducibility problem and to conduct studies and analyses that would be impactful for the patient, scientific and clinical communities, we reinforce the need of standardizing and systematizing the collection, curation, analysis, and sharing of long-term multimodal data from larger patient samples. There is hope that this will allow more representative descriptions of the disorder on a global scale for proper real-world classificatory validations at the subject level. On the verge of providing subject-based information that may assist rational decision-making in the future, for example, as part of “precision boards”—a term analogous to the successful name “tumor boards” currently implemented in multidisciplinary oncology teams—brain connectivity measures can add a unique neurobiological precision component to the evaluation of treatment modalities in complex MDD cases. But first, we need to overcome the existing limitations in the field with consideration of the above-mentioned recommendations to be able to embark on the systems medicine era.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. The reports of the included and excluded articles are available from the corresponding author upon request.
Change history
17 July 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41398-023-02543-x
References
Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association: Washington DC; 2013.
Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59.
Connolly KR, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an Academic Medical Center. J Clin Psychiatry. 2012;73:e567–e573.
Lefaucheur J-P, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150–206.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Goldstein-Piekarski AN, Staveland BR, Ball TM, Yesavage J, Korgaonkar MS, Williams LM. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry. 2018;8:57.
Ye Y, Wang C, Lan X, Li W, Fu L, Zhang F, et al. Baseline patterns of resting functional connectivity within posterior default-mode intranetwork associated with remission to antidepressants in major depressive disorder. NeuroImage: Clin. 2022;36:103230.
Chin Fatt CR, Jha MK, Cooper CM, Fonzo G, South C, Grannemann B, et al. Effect of intrinsic patterns of functional brain connectivity in moderating antidepressant treatment response in major depression. Am J Psychiatry. 2020;177:143–54.
Korgaonkar MS, Goldstein-Piekarski AN, Fornito A, Williams LM. Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder. Mol Psychiatry. 2020;25:1537–49.
DeMaster D, Godlewska BR, Liang M, Vannucci M, Bockmann T, Cao B, et al. Effective connectivity between resting-state networks in depression. J Affect Disord. 2022;307:79–86.
Wu H, Liu R, Zhou J, Feng L, Wang Y, Chen X, et al. Prediction of remission among patients with a major depressive disorder based on the resting-state functional connectivity of emotion regulation networks. Transl Psychiatry. 2022;12:391.
Martens MAG, Filippini N, Harmer CJ, Godlewska BR. Resting state functional connectivity patterns as biomarkers of treatment response to escitalopram in patients with major depressive disorder. Psychopharmacology. 2022;239:3447–60.
Ang Y-S, Kaiser R, Deckersbach T, Almeida J, Phillips ML, Chase HW, et al. Pretreatment reward sensitivity and frontostriatal resting-state functional connectivity are associated with response to bupropion after sertraline nonresponse. Biol Psychiatry. 2020;88:657–67.
Xiao H, Yuan M, Li H, Li S, Du Y, Wang M, et al. Functional connectivity of the hippocampus in predicting early antidepressant efficacy in patients with major depressive disorder. J Affect Disord. 2021;291:315–21.
Hsu L-M, Lane TJ, Wu CW, Lin C-Y, Yeh C-B, Kao H-W, et al. Spontaneous thought-related network connectivity predicts sertraline effect on major depressive disorder. Brain Imaging Behav. 2021;15:1705–17.
Fu CH, Costafreda SG, Sankar A, Adams TM, Rasenick MM, Liu P, et al. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. 2015;15:82.
Braund TA, Breukelaar IA, Griffiths K, Tillman G, Palmer DM, Bryant R, et al. Intrinsic functional connectomes characterize neuroticism in major depressive disorder and predict antidepressant treatment outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:276–84.
van der Wijk G, Harris JK, Hassel S, Davis AD, Zamyadi M, Arnott SR, et al. Baseline functional connectivity in resting state networks associated with depression and remission status after 16 weeks of pharmacotherapy: a CAN-BIND report. Cereb Cortex. 2022;32:1223–43.
Ju Y, Horien C, Chen W, Guo W, Lu X, Sun J, et al. Connectome-based models can predict early symptom improvement in major depressive disorder. J Affect Disord. 2020;273:442–52.
Cui J, Wang Y, Liu R, Chen X, Zhang Z, Feng Y, et al. Effects of escitalopram therapy on resting-state functional connectivity of subsystems of the default mode network in unmedicated patients with major depressive disorder. Transl Psychiatry. 2021;11:634.
Yang R, Gao C, Wu X, Yang J, Li S, Cheng H. Decreased functional connectivity to posterior cingulate cortex in major depressive disorder. Psychiatry Res Neuroimaging. 2016;255:15–23.
Zhang Y, Shao J, Wang X, Pei C, Zhang S, Yao Z, et al. Partly recovery and compensation in anterior cingulate cortex after SSRI treatment—evidence from multi-voxel pattern analysis over resting state fMRI in depression. J Affect Disord. 2023;320:404–12.
Ju Y, Wang M, Liu J, Liu B, Yan D, Lu X, et al. Modulation of resting-state functional connectivity in default mode network is associated with the long-term treatment outcome in major depressive disorder. Psychol Med. 2022;27:1–13.
Fischer AS, Holt-Gosselin B, Fleming SL, Hack LM, Ball TM, Schatzberg AF, et al. Intrinsic reward circuit connectivity profiles underlying symptom and quality of life outcomes following antidepressant medication: a report from the iSPOT-D trial. Neuropsychopharmacol. 2021;46:809–19.
Liu J, Fan Y, Ling-Li Z, Liu B, Ju Y, Wang M, et al. The neuroprogressive nature of major depressive disorder: evidence from an intrinsic connectome analysis. Transl Psychiatry. 2021;11:102.
Grieve SM, Korgaonkar MS, Gordon E, Williams LM, Rush AJ. Prediction of nonremission to antidepressant therapy using diffusion tensor imaging. J Clin Psychiatry. 2016;77:e436–e443.
Korgaonkar MS, Williams LM, Song YJ, Usherwood T, Grieve SM. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br J Psychiatry. 2014;205:321–8.
Korgaonkar MS, Rekshan W, Gordon E, Rush AJ, Williams LM, Blasey C, et al. Magnetic resonance imaging measures of brain structure to predict antidepressant treatment outcome in major depressive disorder. EBioMedicine. 2015;2:37–45.
Zhou Y, Qin L, Chen J, Qian L, Tao J, Fang Y, et al. Brain microstructural abnormalities revealed by diffusion tensor images in patients with treatment-resistant depression compared with major depressive disorder before treatment. Eur J Radiol. 2011;80:450–4.
Pillai RLI, Huang C, LaBella A, Zhang M, Yang J, Trivedi M, et al. Examining raphe-amygdala structural connectivity as a biological predictor of SSRI response. J Affect Disord. 2019;256:8–16.
Davis AD, Hassel S, Arnott SR, Harris J, Lam RW, Milev R, et al. White matter indices of medication response in major depression: a diffusion tensor imaging study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:913–24.
Vieira R, Coelho A, Reis J, Portugal-Nunes C, Magalhães R, Ferreira S, et al. White matter microstructure alterations associated with paroxetine treatment response in major depression. Front Behav Neurosci. 2021;15:693109.
Dong Q, Liu J, Zeng L, Fan Y, Lu X, Sun J, et al. State-independent microstructural white matter abnormalities in major depressive disorder. Front Psychiatry. 2020;11:431.
Fan Y, Liu J, Zeng L-L, Dong Q, Su J, Peng L, et al. State-independent and -dependent structural connectivity alterations in depression. Front Psychiatry. 2020;11:568717.
Crowther A, Smoski MJ, Minkel J, Moore T, Gibbs D, Petty C, et al. Resting-state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacol. 2015;40:1659–73.
Späti J, Hänggi J, Doerig N, Ernst J, Sambataro F, Brakowski J, et al. Prefrontal thinning affects functional connectivity and regional homogeneity of the anterior cingulate cortex in depression. Neuropsychopharmacol. 2015;40:1640–8.
Dunlop BW, Rajendra JK, Craighead WE, Kelley ME, McGrath CL, Choi KS, et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am J Psychiatry. 2017;174:533–45.
Wang T, Huang X, Huang P, Li D, Lv F, Zhang Y, et al. Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PLoS ONE. 2013;8:e63081.
van Waarde JA, Scholte HS, van Oudheusden LJB, Verwey B, Denys D, van Wingen GA. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry. 2015;20:609–14.
Pang Y, Wei Q, Zhao S, Li N, Li Z, Lu F, et al. Enhanced default mode network functional connectivity links with electroconvulsive therapy response in major depressive disorder. J Affect Disord. 2022;306:47–54.
Chen G-D, Ji F, Li G-Y, Lyu B-X, Hu W, Zhuo C-J. Antidepressant effects of electroconvulsive therapy unrelated to the brain’s functional network connectivity alterations at an individual level. Chin Med J. 2017;130:414–9.
Wei Q, Bai T, Chen Y, Ji G, Hu X, Xie W, et al. The changes of functional connectivity strength in electroconvulsive therapy for depression: a longitudinal study. Front Neurosci. 2018;12:661.
Mo Y, Wei Q, Bai T, Zhang T, Lv H, Zhang L, et al. Bifrontal electroconvulsive therapy changed regional homogeneity and functional connectivity of left angular gyrus in major depressive disorder. Psychiatry Res. 2020;294:113461.
Wang J, Wei Q, Bai T, Zhou X, Sun H, Becker B, et al. Electroconvulsive therapy selectively enhanced feedforward connectivity from fusiform face area to amygdala in major depressive disorder. Soc Cogn Affect Neurosci. 2017;12:1983–92.
Leaver AM, Espinoza R, Joshi SH, Vasavada M, Njau S, Woods RP, et al. Desynchronization and plasticity of striato-frontal connectivity in major depressive disorder. Cereb Cortex. 2016;26:4337–46.
Zhang T, He K, Bai T, Lv H, Xie X, Nie J, et al. Altered neural activity in the reward-related circuit and executive control network associated with amelioration of anhedonia in major depressive disorder by electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110193.
Repple J, Meinert S, Bollettini I, Grotegerd D, Redlich R, Zaremba D, et al. Influence of electroconvulsive therapy on white matter structure in a diffusion tensor imaging study. Psychol Med. 2020;50:849–56.
Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, et al. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry. 2014;4:e380–e380.
Baeken C, Marinazzo D, Wu G-R, Van Schuerbeek P, De Mey J, Marchetti I, et al. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15:286–97.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603.
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37.
Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry. 2019;86:e5–e7.
Siddiqi SH, Weigand A, Pascual-Leone A, Fox MD. Identification of personalized transcranial magnetic stimulation targets based on subgenual cingulate connectivity: an independent replication. Biol Psychiatry. 2021;90:e55–e56.
Kong G, Wei L, Wang J, Zhu C, Tang Y. The therapeutic potential of personalized connectivity-guided transcranial magnetic stimulation target over group-average target for depression. Brain Stimul. 2022;15:1063–4.
Rosen AC, Bhat JV, Cardenas VA, Ehrlich TJ, Horwege AM, Mathalon DH, et al. Targeting location relates to treatment response in active but not sham rTMS stimulation. Brain Stimul. 2021;14:703–9.
Hopman HJ, Chan SMS, Chu WCW, Lu H, Tse C-Y, Chau SWH, et al. Personalized prediction of transcranial magnetic stimulation clinical response in patients with treatment-refractory depression using neuroimaging biomarkers and machine learning. J Affect Disord. 2021;290:261–71.
Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul. 2020;13:206–14.
Baeken C, Duprat R, Wu G-R, De Raedt R, van Heeringen K. Subgenual anterior cingulate–medial orbitofrontal functional connectivity in medication-resistant major depression: a neurobiological marker for accelerated intermittent theta burst stimulation treatment? Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:556–65.
Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry. 2017;7:3.
Kang JI, Lee H, Jhung K, Kim KR, An SK, Yoon K-J, et al. Frontostriatal connectivity changes in major depressive disorder after repetitive transcranial magnetic stimulation: a randomized sham-controlled study. J Clin Psychiatry. 2016;77:e1137–43.
Iwabuchi SJ, Auer DP, Lankappa ST, Palaniyappan L. Baseline effective connectivity predicts response to repetitive transcranial magnetic stimulation in patients with treatment-resistant depression. Eur Neuropsychopharmacol. 2019;29:681–90.
Taylor SF, Ho SS, Abagis T, Angstadt M, Maixner DF, Welsh RC, et al. Changes in brain connectivity during a sham-controlled, transcranial magnetic stimulation trial for depression. J Affect Disord. 2018;232:143–51.
Chen F, Gu C, Zhai N, Duan H, Zhai A, Zhang X. Repetitive transcranial magnetic stimulation improves amygdale functional connectivity in major depressive disorder. Front Psychiatry. 2020;11:732.
Eshel N, Keller CJ, Wu W, Jiang J, Mills-Finnerty C, Huemer J, et al. Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacol. 2020;45:1018–25.
Ning L, Rathi Y, Barbour T, Makris N, Camprodon JA. White matter markers and predictors for subject-specific rTMS response in major depressive disorder. J Affect Disord. 2022;299:207–14.
Klooster DCW, Vos IN, Caeyenberghs K, Leemans A, David S, Besseling RMH, et al. Indirect frontocingulate structural connectivity predicts clinical response to accelerated rTMS in major depressive disorder. J Psychiatry Neurosci. 2020;45:243–52.
Fu Y, Long Z, Luo Q, Xu Z, Xiang Y, Du W, et al. Functional and structural connectivity between the left dorsolateral prefrontal cortex and insula could predict the antidepressant effects of repetitive transcranial magnetic stimulation. Front Neurosci. 2021;15:645936.
Chen D, Lei X, Du L, Long Z. Use of machine learning in predicting the efficacy of repetitive transcranial magnetic stimulation on treating depression based on functional and structural thalamo-prefrontal connectivity: a pilot study. J Psychiatr Res. 2022;148:88–94.
Wang L, An J, Gao H, Zhang P, Chen C, Li K, et al. Duloxetine effects on striatal resting‐state functional connectivity in patients with major depressive disorder. Hum Brain Mapp. 2019;40:3338–46.
Cash RFH, Cocchi L, Anderson R, Rogachov A, Kucyi A, Barnett AJ, et al. A multivariate neuroimaging biomarker of individual outcome to transcranial magnetic stimulation in depression. Hum Brain Mapp. 2019;40:4618–29.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179:132–41.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psyhiatry. 2020;177:716–26.
Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118:115–28.
Szucs D, Ioannidis JPA. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage. 2020;221:117164.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Gray JP, Müller VI, Eickhoff SB, Fox PT. Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am J Psychiatry. 2020;177:422–34.
Nestor SM, Mir-Moghtadaei A, Vila-Rodriguez F, Giacobbe P, Daskalakis ZJ, Blumberger DM, et al. Large-scale structural network change correlates with clinical response to rTMS in depression. Neuropsychopharmacol. 2022;47:1096–105.
Scheinost D, Holmes SE, DellaGioia N, Schleifer C, Matuskey D, Abdallah CG, et al. Multimodal investigation of network level effects using intrinsic functional connectivity, anatomical covariance, and structure-to-function correlations in unmedicated major depressive disorder. Neuropsychopharmacol. 2018;43:1119–27.
Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. 1994;2:56–78.
Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–41.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673.
McKeown MJ, Makeig S, Brown GG, Jung T-P, Kindermann SS, Bell AJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;160:29.
Seth AK. Causal connectivity of evolved neural networks during behavior. Network. 2005;16:35–54.
Chenevert TL, Brunberg JA, Pipe JG. Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology. 1990;177:401–5.
Hagmann P, Jonasson L, Maeder P, Thiran J-P, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. RadioGraphics. 2006;26:S205–S223.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–60.
Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43.
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74.
Northoff G. Psychopathology and pathophysiology of the self in depression — Neuropsychiatric hypothesis. J Affect Disord. 2007;104:1–14.
Chen G, Hu X, Li L, Huang X, Lui S, Kuang W, et al. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci Rep. 2016;6:21825.
Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Moood Anxiety Disord. 2011;1:3.
Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta- analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56.
van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2020;25:1511–25.
Acknowledgements
The work was supported by the German Federal Ministry of Education and Research (Bundesministerium fuer Bildung und Forschung, BMBF: 01 ZX 1507, “PreNeSt – e:Med”), the University Medical Center Göttingen, and the International Max Planck Research School for Neurosciences (www.gpneuro.uni-goettingen.de).
Author information
Authors and Affiliations
Contributions
AT: study conception and design, primary identification of articles, the decision of inclusion or exclusion of the articles, interpretation of the data, preparing figures/tables, manuscript writing, and final approval of the manuscript. RG-M: study conception and design, supervision, the decision of inclusion or exclusion of the articles, interpretation of the data, preparing figures/tables, manuscript writing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tura, A., Goya-Maldonado, R. Brain connectivity in major depressive disorder: a precision component of treatment modalities?. Transl Psychiatry 13, 196 (2023). https://doi.org/10.1038/s41398-023-02499-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02499-y