Abstract
Understanding neurobiological characteristics of cognitive dysfunction in distinct psychiatric disorders remains challenging. In this secondary data analysis, we examined neurobiological differences in brain response during working memory updating among individuals with bipolar disorder (BD), those with unipolar depression (UD), and healthy controls (HC). Individuals between 18–45 years of age with BD (n = 100), UD (n = 109), and HC (n = 172) were scanned using fMRI while performing 0-back (easy) and 2-back (difficult) tasks with letters as the stimuli and happy, fearful, or neutral faces as distractors. The 2(n-back) × 3(groups) × 3(distractors) ANCOVA examined reaction time (RT), accuracy, and brain activation during the task. HC showed more accurate and faster responses than individuals with BD and UD. Difficulty-related activation in the prefrontal, posterior parietal, paracingulate cortices, striatal, lateral occipital, precuneus, and thalamic regions differed among groups. Individuals with BD showed significantly lower difficulty-related activation differences in the left lateral occipital and the right paracingulate cortices than those with UD. In individuals with BD, greater difficulty-related worsening in accuracy was associated with smaller activity changes in the right precuneus, while greater difficulty-related slowing in RT was associated with smaller activity changes in the prefrontal, frontal opercular, paracingulate, posterior parietal, and lateral occipital cortices. Measures of current depression and mania did not correlate with the difficulty-related brain activation differences in either group. Our findings suggest that the alterations in the working memory circuitry may be a trait characteristic of reduced working memory capacity in mood disorders. Aberrant patterns of activation in the left lateral occipital and paracingulate cortices may be specific to BD.
Similar content being viewed by others
Introduction
Highly disabling bipolar and major depressive (also called unipolar depression [UD]) disorders affect 60 million Americans and cost the United States more than $150 billion annually [1, 2]. Individuals suffering from these disorders spend half of their lives with depression and/or mania [3,4,5,6]. Depression is characterized by sad mood, anergia, sleep disturbances, and feelings of guilt and worthlessness. Mania is characterized by elated or irritable mood, excessive energy/activity, and impulsivity. These symptoms impair cognitive and emotional functioning and affect job performance, school work, and social relationships [3,4,5,6]. Understanding the distinct neurobiological characteristics of cognitive dysfunction in related psychiatric disorders remains challenging because multiple psychiatric disorders share common patterns of disruption in brain regions supporting cognitive functioning [7].
Working memory is involved in maintenance and real-time manipulation (i.e., updating) of information in a person’s mind [8]. It is critical for schooling, work, driving, and even social relationships. Difficult working memory tasks usually require more cognitive resources than easy tasks, so people perform these tasks more slowly and less accurately than easy tasks [9, 10]. Working memory updating can be measured using n-back tasks in which participants are required to match the stimuli they see on the screen with those they saw one, two, or three trials previously. Poorer working memory updating is related to poorer problem-solving ability [11], and elevated rumination characterizing depressive disorders [12]. Although disruption of working memory is a common cognitive deficit in Bipolar Disorder (BD) and UD [13,14,15,16,17], studies often report inconsistent findings and rarely include both diagnostic groups. For example, some studies reported that manic but not depressed individuals with BD had significant deficits in working memory [18], that euthymic individuals with BD did not differ from Healthy Controls (HC) [19], and that individuals with UD did not differ from HC [20]. However, other studies demonstrated that both individuals with BD and UD, compared to HC, showed slower and/or less accurate performance on working memory tasks [21, 22] with UD outperforming BD [22].
Difficult working memory tasks are associated with greater activation in prefrontal cortical (PFC), anterior cingulate, and posterior parietal regions than easy working memory tasks [23, 24]. Aberrant functioning of the PFC, anterior cingulate, and parietal cortices may underlie the working memory deficits in mood disorders [25,26,27,28,29,30] and even help distinguish BD from UD [31, 32]; however, controversies about specific alterations persist even within a single diagnostic category. Some fMRI studies report that compared to HC, individuals with mood disorders show greater activation in the PFC when the working memory task becomes more difficult [29, 30, 33], while other studies report opposite findings [34]. For example, individuals with BD, compared to HC, had smaller difficulty-related changes in the PFC activation [35]. Consistent with these findings, a recent meta-analysis examining neural substates of the n-back task reported reduced activation in lateral and medial PFC in individuals with BD compared to HC, but greater activation in the right posterior parietal cortex and superior frontal cortex in individuals with UD vs. HC [36]. However, other meta-analyses were unsuccessful in identifying reliable differences between individuals with mood disorders and HC. For example, one meta-analysis comparing brain activation patterns characterizing individuals with UD vs. HC found no significant differences between these two groups [37]. Likewise, a meta-analysis that examined the differences between euthymic individuals with BD vs. HC found no significant differences in brain activation during n-back tasks after family-wise error (FWE) correction [19].
The inconsistencies in the behavioral and neuroimaging findings reported by meta-analyses and individual studies of mood disorders could be explained by high heterogeneity in participant samples, small sample sizes of individuals studies (often less than 25 participants per group), and methodological differences across studies (e.g., different scanning sequences, cognitive tasks, and analysis pipelines). In this secondary analysis, we aimed to identify neurobiological differences between individuals with BD, UD, and HC using one of the largest datasets ever collected in a single laboratory using the same working memory task [25, 38], similar eligibility criteria, and similar clinical assessments across the studies. Working memory was examined using the n-back task with letters as the stimuli and happy, fearful, and neutral faces as distractors [25, 38]. The distractors were introduced to examine the participants’ ability to inhibit irrelevant emotionally salient (happy and fearful faces) and emotionally ambiguous (neutral faces) information. Previous research suggested that individuals with BD and UD differ in their response to emotional faces [39, 40] with the former showing reduced performance when processing positive emotional stimuli and the latter showing reduced performance when processing negative emotional stimuli [40]. The differences in emotion processing could differentially affect the ability of individuals with BD and UD to resolve interference from emotional stimuli especially during a difficult working memory task. Considering these differences as well as the evidence for more severe cognitive deficit in BD than UD [22, 41] and smaller difficulty-related activation changes in working memory regions in BD versus HC [35], we hypothesized that the PFC, anterior cingulate, and parietal brain regions would show smaller difficulty-related activation changes in individuals with BD versus UD and HC, especially in the presence of happy faces. However, based on the meta-analytical findings [37], we did not expect to find differences between individuals with UD and HC.
Method
Participants
In this study, we performed secondary data analysis that combined n-back behavioral and neuroimaging data collected in three previous studies in the same laboratory (Table 1). All studies were approved by the University of Pittsburgh Human Resource Protection Office. Participants were recruited from community, universities, counseling, and medical centers through referrals and advertisements between 2009 and 2018. All participants signed informed consent to participate in the study. Table 1 describes the studies, participants, and the neuroimaging data acquisition parameters.
All participants were fluent in English and the majority were right-handed. HC had no personal or family history of psychiatric disorders. Symptomatic individuals met DSM-IV or DSM-5 criteria for either BD or UD. To be included into the studies, participants had to be within the age range appropriate for the study (Table 1) and be free of neurological, endocrine, and other systemic illnesses at the time of scan. Exclusion criteria applied to all participants included history of severe head trauma, systemic medical illness that could impact fMRI measures of cerebral blood flow, standard exclusion criteria for MRI scanning (e.g., claustrophobia, surgically implanted ferromagnetic devices and objects in/on the body, weight > 300 lbs), current pregnancy, premorbid IQ < 85 per the National Adult Reading Test (NART) [42], substance use disorder in the past 6 months (lifetime for HC) or current use of illicit substances as determined by the Structured Clinical Interview (SCID) and pre-scan saliva alcohol and urine drug screens, inability to understand or speak English, visual disturbance (<20/40 acuity in the Snellen test), and borderline personality disorder per SCID-II. Individuals with BD were excluded if they met criteria for a current manic/hypomanic episode.
The original samples included 168 participants from the BPA2 study, 253 participants from the DIAMOND study, and 54 participants from the COBY study. The quality assurance (image quality, excessive motion during scan, poor n-back task performance, less than 2 usable runs, or any combination of the above reasons) was passed by 136 participants from the BPA2 study (BD = 62, HC = 35, UD = 39), 38 participants from the COBY study (BD = 33, HC = 5), and 207 participants from the DIAMOND study (BD = 5, HC = 132, UD = 70). Thus, a total of 381 participants (BD = 100, UD = 109, HC = 172) were included in the data analyses. Table 2 reports demographic and clinical characteristics of the samples.
Clinical assessments
All participants were administered the SCID for DSM-IV or DSM-5 disorders [43, 44]; the Hamilton Depression Rating Scale (HAMD-17) [45] to assess the severity of depression during the last 1 week; Young Mania Rating Scale (YMRS) [46] to assess the severity of mania during the last 1 week; and State and Trait Anxiety Inventory (STAIY1, STAIY2) [47] to assess the severity of state and trait anxiety.
Behavioral assessment
Experimental paradigm
All participants performed the emotional faces n-back task [25, 38] in which they were presented with a sequence of letters. In the 0-back condition, participants had to press the response button when the letter ‘M’ appeared on the screen. In the 2-back condition, they had to press the response button when they saw a stimulus that was the same as the stimulus presented two trials ago (Fig. 1). Each block of n-back was presented either without distractors, or with happy, fearful, or neutral face distractors taken from the NimStim dataset [48]. The distractors were presented on the right and left sides of the letter. Participants were asked to ignore the faces because they were irrelevant to the n-back task. Participants completed 3 runs of the task in the BPA2 study and 2 runs in the COBY and DIAMOND studies. Each run consisted of eight blocks of trials (0-/2-back × no face/happy/fearful/neutral faces). Each block was preceded by a 4000-ms instruction screen informing about either 0-back or 2-back task conditions. A block included twelve 500-msec trials that were separated by jittered inter-trial intervals whose mean duration was 3500 msec. Participants were instructed to respond as quickly and accurately as possible whenever a target stimulus appeared on the screen.
Behavioral data analysis
Participants’ RT and accuracy were averaged per participant, n-back and emotional condition and evaluated using Group (BD/UD/HC) x n-back (0-back/2-back) × emotion (happy/neutral/fearful) mixed effects models (R packages ‘lme4’ [49], ‘lmerTest’ [50], and ‘psycho’ [51]) with age, IQ, and sex as covariates and participant as a random effect. The means and contrasts between conditions of interest were estimated from the mixed effect models using the ‘modelbased’ package [52] in R. When appropriate, the p-values were corrected for multiple comparisons using Benjamin and Hochberg’s False Discovery Rate (FDR) [53].
Neuroimaging
Data acquisition
The acquisition details are described in Table 1.
Preprocessing
The Digital Imaging and Communications in Medicine (DICOM) images were converted to the Brain Imaging Data Structure (BIDS) with heudiconv [54] using ReproIn heuristic [55]. The neuroimaging data quality was examined using mriqc 0.15.1 [56]. The data were preprocessed using fmriprep 20.1.1 [57]. Preprocessing steps included skull-stripping of T1w images, brain surface reconstruction using recon-all (FreeSurfer 6.0.1) [58], and brain masks generation. For each BOLD image, we applied motion correction, spatiotemporal filtering using mcflirt [59], and slice-timing correction using 3dTshift [60]. Preprocessing also included automatic removal of motional artifacts using ICA-AROMA [61], spatial smoothing with an isotropic, Gaussian kernel of 6 mm FWHM (full-width half-maximum), the BOLD image registration to the MNI template, and regressing out non-steady state volumes. High-pass temporal filter with 100 s cutoff was applied on the fmriprep preprocessed files.
1st-level and 2nd-level analyses
In the 1st-level analysis, explanatory variables included the no face distractor, and happy, fearful, and neutral face distractor conditions in 0-back and 2-back tasks, the instruction screens, and the motor response to account for the fact that only a fraction of trials (30%) required a motor response. The contrasts for 2-back minus 0-back were calculated for each emotional condition to determine the differences in brain activation for difficult vs. easy working memory tasks. A hemodynamic response was modeled using a Gamma function. The mean difference in brain activation between 2-back and 0-back tasks for available runs was calculated for each participant/emotional condition during the 2nd-level analysis.
Group-level analysis
The 2-back minus 0-back contrasts computed for each participant and each emotional condition during the 2nd-level analysis were used as inputs to the Sandwich Estimator (swe) [62], the approach used for nonparametric permutation inference for longitudinal and repeated measures neuroimaging data. The swe estimated a 3 (groups: HC/BD/UD) by 3 (emotions: happy/fear/neutral) model with scanner, study, age, sex, and IQ as covariates. Considering that the group analysis combined participants from different studies that were scanned on 2 different scanners, we orthogonalized the design matrix using the package ‘matlib’ in R (https://github.com/friendly/matlib) with a QR decomposition by Gram-Schmidt orthonormalization. The matrix included the variables in the following order: scanner, study, age, sex, IQ, and the columns modeling diagnostic groups and emotional conditions. The n-back conditions without face distractors were modeled at the 1st-level analyses but were not included into the group analysis because the effect of specific emotional distractor on the group differences could be hindered by a strong effect of a face presence (vs. no face). Although the main effect of emotions was included into the model for completeness, it was outside the focus of this paper and, therefore, the results are reported in Supplemental Materials. The swe was conducted in the whole brain with Threshold-Free Cluster Enhancement correction (TFCE) [63] and 5000 permutations. The outcome variables were the three F-test maps (a main effect of group, a main effect of emotion and a group-by-emotion interaction). The FWE p-value threshold was set to p < 0.01 to account for the three F-tests using the Bonferroni correction (0.05/3 = 0.0167).
All further analyses were conducted in R (https://www.r-project.org) using percent signal changes extracted using featquery for each participant and task condition from the brain regions identified in the group analysis described above. The FDR correction for multiple tests [53] was applied when appropriate. We compared the 2-back-minus-0-back differences for BD vs. UD, BD vs. HC, and UD vs. HC groups using mixed effect models with the percent signal changes as a dependent variable, and Group as an explanatory variable. Cohen’s d or partial eta2 were calculated as appropriate. Also, we used the mixed effect models to predict the 2-back-minus-0-back differences in RT and accuracy (separately) from the interaction between BD/UD/HC diagnostic status and the 2-back-minus-0-back differences in brain activation in the regions identified in the swe analysis. In all models, age, IQ, and sex were covariates and participants were a random effect.
Exploratory analyses
The goal of exploratory analyses was to investigate whether the differences in clinical measures between BD and UD differentially affect behavioral and brain activation outcomes in these individuals. We examined the interaction effect between diagnostic status (BD/UD) and current depression (HAMD-17), mania (YMRS), and anxiety (STAIY) symptoms as well as illness duration, mood state, and a total psychotropic medication load on the 2-back-minus-0-back differences in RT, accuracy, and brain activation in individuals with mood disorders (BD/UD). Age, IQ, and sex were covariates and participants were a random effect. Missing values in the HAMD-17 and YMRS assessments (one value per assessment) were imputed using the ‘mice’ package [64] in R.
Results
Clinical
A total of 381 participants passed f/MRI and behavioral data quality assurance. The missing IQ values for 10 participants were imputed as the sample mean. The results of the BD vs. UD vs. HC demographic and clinical characteristics comparisons are reported in Table 2. Compared to individuals with UD, individuals with BD were older, had earlier illness onset, longer illness duration, lower current depression scores based on HAMD-17 but higher current mania score based on YMRS. They also had higher state and trait anxiety, and higher total medication load. Based on the HAMD-17, 40% of those with BD were euthymic (score < 8), 36% mildly depressed (score 8–16), 14% moderately depressed (score 17–23), and 10% severely depressed (score > 24). was Among those with UD, 5% were euthymic (score < 8), 30% mildly depressed (score 8–16), 46% moderately depressed (score 17–23), and 19% severely depressed (score > 24).
Behavioral
Supplemental Figure S1 illustrates RT for correct responses and accuracy. There was a group-by-n-back interaction (F(2,1895) = 10.3, p < 0.001), as well as the main effects of n-back (F(1, 1895) = 195.8, p < 0.001) and group (F(2, 376) = 11.3, p < 0.001) on accuracy. More accurate responses were observed in younger participants (F(1,376) = 6.8, t = −2.6, p = 0.01) and those with higher IQ (F(1,376) = 18.8, t = 4.3, p < 0.001). There was no significant relationship of accuracy with sex (p = 0.83). Contrasts estimation with the FDR correction showed that HC were significantly more accurate than individuals with UD during 0-back (t = 2.4, p-FDR-corrected = 0.023), and more accurate than those with BD (t = 5.9, p-FDR-corrected < 0.001) and UD (t = 3.76, p-FDR-corrected < 0.001) during 2-back. Individuals with UD were more accurate than those with BD during 2-back (t = 2.16, p-FDR-corrected = 0.038). There was no significant main effect of distractor face emotions or interactions between emotion and other variables on accuracy.
There was a group-by-n-back interaction (F(2,1895) = 11.9, p < 0.001), as well as the main effects of n-back (F(1, 1895) = 864.9, p < 0.001) and group (F(2, 376) = 4.8, p = 0.008) on RT. Faster responses were observed in younger participants (F(1,376) = 21.0, t = 4.6, p < 0.001). There was no significant relationship of RT with sex (p = 0.97) or IQ (p = 0.06). Contrast estimation showed that the three groups did not differ from each other in their RT on the 0-back task. On the 2-back task, HC were significantly faster than individuals with BD (t = −3.9, p-FDR-corrected < 0.001) and UD (t = −3.5, p-FDR-corrected < 0.001) who did not differ from each other. There was no significant main effect of distractor face emotions or interactions between emotion and other variables on RT.
Neuroimaging
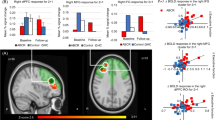
There were main effects of group (Figs. 2, 3, Table 3) and emotion (Supplemental Fig. S2, Supplemental Table S1) but no group-by-emotion interaction effect on the 2-back minus 0-back differences in brain activation. The regions identified in the whole brain analyses almost completely overlapped with those in the working memory circuitry derived by the NeuroSynth meta-analysis [65] from 1091 studies (Fig. 2), thus suggesting that the regions we identified pertain to the working memory circuitry.
The analysis of pairwise comparisons of BD vs. UD, BD vs. HC, and UD vs. HC showed that although the nominal differences at uncorrected p < 0.05 between BD and UD were observed in most of the regions, only the left lateral occipital cortex, superior division and the right paracingulate cortex survived the FDR correction with BD showing significantly less pronounced 2-back vs. 0-back differences than UD. Individuals with UD were not different from HC in any of the identified regions except for the right precuneus that deactivated for 2-back vs. 0-back and in which HC had greater deactivation than individuals with UD. Individuals with BD had smaller 2-back vs. 0-back activation differences than HC in the left putamen and inferior frontal gyrus, pars opercularis (LIFG), and the right caudate, frontal opercular cortex, precuneus, and thalamus. The effect sizes were small even for the comparisons with highly significant FDR-corrected p-values (Supplemental Table S2).
Participants’ age was negatively associated with the 2-back vs. 0-back activation differences in the LIFG (F(1,373) = 11.7, p < 0.001), left lateral occipital cortex (F(1,373) = 14.4, p < 0.001), left paracingulate cortex (F(1,373) = 12.5, p < 0.001), and bilateral posterior parietal cortices (left: F(1,373) = 18.7, p < 0.001, right: F(1,373) = 17.6, p < 0.001). Younger participants had greater activation differences between 2-back and 0-back. There was no significant group-by-age interaction effect nor significant effects of sex, IQ, group-by-sex, or group-by-IQ interaction effects on the 2-back vs. 0-back differences in any of the 16 brain regions (Supplemental Table S3).
The 2-back minus 0-back differences in accuracy were explained by the interaction effect between Group and the 2-back minus 0-back activation differences in the right precuneus. This effect was driven by a significant negative association between the 2-back minus 0-back differences in brain activation and accuracy in the BD group (t = −3.5, p < 0.001). No such associations were observed in the UD (t = 0.9, p = 0.36) and HC (t = 0.6, p = 0.56) groups (Supplemental Table S4, Fig. S3).
The 2-back minus 0-back differences in RT were explained by the interaction effect between Group and the 2-back minus 0-back activation differences in the left lateral occipital cortex, left paracingulate cortex, right DLPFC, frontal opercular cortex, and right posterior parietal cortex. The effects were driven by a significant negative association between the 2-back vs. 0-back differences in brain activation and the 2-back vs. 0-back differences in RT in the BD group (left lateral occipital cortex: t = −3.5, p < 0.001; left paracingulate cortex: t = −4.0, p < 0.001; right DLPFC: t = −4.9, p < 0.001; frontal opercular cortex: t = −4.1, p < 0.001; right posterior parietal cortex: t = −2.7, p = 0.003). No such associations were observed in the UD (left lateral occipital cortex: t = 0.7, p = 0.5; left paracingulate cortex: t = 0.04, p = 0.97; right DLPFC: t = 0.2, p = 0.8; frontal opercular cortex: t = 0.3, p = 0.74; right posterior parietal cortex: t = 1.0, p = 0.3) and HC (left lateral occipital cortex: t = −1.3, p = 0.2; left paracingulate cortex: t = −0.02, p = 0.98; right DLPFC: t = −1.0, p = 0.3; frontal opercular cortex: −0.3, p = 0.76; right posterior parietal cortex: t = −0.9, p = 0.9) groups (Supplemental Table S5, Fig. S4).
Exploratory analyses
The exploratory analysis conducted across the BD and UD groups revealed no main effects of illness duration, mood state, HAMD-17, YMRS, state/trait anxiety, or total medication load as well as no interaction between these clinical variables and Group (BD/UD) on the 2-back minus 0-back differences in RT, accuracy, or activation differences in either brain region (Supplemental Tables S6, S7).
Discussion
In this study, we performed a secondary data analysis to examine working memory updating in a large sample of individuals with BD, UD, and HC across the three studies. Working memory updating was measured as the difference in behavioral (RT and accuracy) and brain responses in the 2-back (a difficult working memory task) compared to 0-back (an easy attentional task) tasks. Consistent with previous reports [66], HC were faster and more accurate than the individuals with BD or UD on the more difficult 2-back task, supporting a general deficit in working memory updating in mood disorders.
The three groups also differed from each other in the magnitude of brain activation changes between 2-back and 0-back in the bilateral PFC, posterior parietal, paracingulate, striatal regions as well as the left lateral occipital and the right precuneus and thalamic regions. Individuals with BD, compared to HC, showed smaller activation differences between 2-back and 0-back tasks in the bilateral striatum, LIFG, and the right frontal opercular cortex, thalamus and right precuneus. Individuals with BD, compared to those with UD, showed smaller activation differences in the left lateral occipital cortex and right paracingulate cortex. Individuals with UD, compared to HC, showed smaller activation differences in the right precuneus. Notably, greater reduction in accuracy for 2-back compared to 0-back was associated with smaller differences between 2-back and 0-back in the right precuneus, while greater worsening of RT for 2-back compared to 0-back was associated with smaller difference between 2-back and 0-back in the left lateral occipital cortex, left paracingulate cortex, right DLPFC, frontal opercular cortex, and right posterior parietal cortex in individuals with BD but not in those with UD or HC. Although the regions described above were identified in the whole brain analysis, they almost completely overlapped with the working memory circuitry determined by the NeuroSynth meta-analysis [65], thus suggesting that BD and UD affect functioning of the working memory circuitry critical for executive function and, specifically, working memory updating [8].
The findings of a negative association between the changes in behavioral performance and the changes in brain activation for 2-back, compared to 0-back, in BD suggest that worsening of accuracy and RT for a difficult working memory task might be related to inability to increase activation in the brain regions critical for working memory updating. In general, this idea is consistent with the previously proposed hypothesis that individuals with mood disorders, especially those with BD, have reduced working memory capacity compared to HC [35]. It is also consistent with the recently proposed model suggesting that patients with mood disorders may reach their maximum mental capacity at a lower load of cognitive tasks than HC [67].
Few studies compare neural correlates of working memory updating in individuals with BD, UD, and HC in the same study. Considering that these studies used different versions of the n-back task and had much smaller sample sizes, it is not surprising that our results were inconsistent with previous findings. For example, one study reported that individuals with UD had lower activation in the PFC compared to those with BD during the 1-back task [68]. Another study revealed that individuals with BD showed less deactivation in the medial frontal cortex than those with UD during the n-back task. Still another study suggested that the two groups of patients could be distinguished based on the activation differences for 2-back vs. 1-back tasks in the left DLPFC [32]. When comparing BD with HC, the results of our study were consistent with findings showing reduced difficulty-related activation changes in the PFC and parietal regions in individuals with BD compared to HC during the n-back task [26, 35, 69, 70], but inconsistent with the studies that either reported the opposite pattern of brain activation in BD [71] or no significant differences between these two groups [19]. Although a recent meta-analysis found no differences between individuals with UD and HC [37], our study, by contrast, found reduced difficulty-related difference in the right precuneus that showed greater deactivation in HC than UD. Our finding that both individuals with BD and UD deactivated right precuneus less than HC but did not differ from each other during 2-back vs. 0-back was inconsistent with the previous report of lower deactivation in BD than in UD in medial frontal cortex [72].
Our exploratory analyses aimed to investigate whether the differences in clinical characteristics between individuals with BD and those with UD could explain the differences between these groups of individuals in behavioral and brain correlates of working memory. Consistent with some previous reports [72], we found no main effect of participants’ mood state, current depression and mania symptoms, state and trait anxiety, or psychotropic medications load. In addition, we found no interaction effects between these clinical characteristics and Group on the 2-back-minus-0-back differences in accuracy, RT, or brain activation. These results were inconsistent with previous studies showing that DLPFC inversely correlated with HAMD-17 scores in individuals with BD [26]. Although older individuals had smaller differences in LIFG, left lateral occipital cortex, left paracingulate cortex, and bilateral posterior parietal cortices activation for 2-back vs. 0-back, these effects did not depend on diagnostic status. This result was inconsistent with previous reports suggesting that participants’ age may contribute to depression-related working memory impairments [21]. Taken together, our findings support a previously proposed idea that the alterations in the working memory circuitry may be a trait characteristic of mood disorders [69].
It was proposed that individuals with depression have aberrant cognitive processing because of their inability to focus on the task and, at the same time, direct attention away from negative thoughts they experience [73]. Based on this, we expected that different emotional distractors would affect behavioral and brain response in the n-back task more in individuals with mood disorders than in HC. Inconsistent with these predictions, the effect of distractors was not sensitive to diagnostic status. Although the bilateral MFG, and the left juxtaposition and superior parietal lobules showed the main effect of emotions, there was no significant interaction effect between diagnostic status and emotion of the face distractor on either RT, accuracy, or brain activation. One explanation for the lack of the interaction effect is that the n-back task fully engaged participants’ attention, so the effect of distractors was diminished. The other explanation is that although faces are processed automatically [74], the effect of mood disorder diagnosis can only be observed when deep processing of emotional faces, including recognition of emotional expressions, is required, which was not the case in this study.
Although group differences were observed in multiple regions across the working memory circuitry, it appears that the difficulty-related changes in the left lateral occipital and right paracingulate (sometimes called dorsal anterior cingulate) cortices were specific to BD who showed the smaller difficulty-related changes in these regions than individuals with UD. In addition, the changes in the lateral occipital cortex were significantly associated with the difficulty-related changes in RT in BD. The lateral occipital cortex is important for object recognition [75], visual imagery [76], and response to emotional vs. neutral visual stimuli [77]. Recently, the occipital cortex started receiving attention in the context of BD. It was shown that individuals with BD have increased asymmetry [78], increased grey matter volume [79], and reduced ability to longitudinally adjust activation in these regions during anticipation of emotionally negative events [80]. The paracingulate cortex is critical for monitoring cognitive interference [81] and efficient target identification in the n-back tasks [82]. Previous studies revealed that this region had reduced cortical thickness in individuals with BD [83] and was important for BD/UD classification in the n-back task [32]. These results, taken together with the findings that individuals with BD had significantly lower accuracy in the 2-back task than those with UD, suggest that aberrant activation in the left lateral occipital and right paracingulate cortices may be a reason for diminished behavioral performance in the former. Specifically, individuals with BD may have lower working memory capacity than those with UD.
There were several limitations to this study. First, the experimental paradigm required participants to respond to targets only (which was only 30% of trials) thus limiting our ability to interpret incorrect responses as well as the processing of non-target items. Second, although the data were collected in the same laboratory using the same task, participants were scanned using two different scanners. We attempted to resolve this issue by orthogonalizing the design matrix to remove variance in the stepwise manner. Finally, results derived from secondary data analysis are limited by potential cohort effects within individual datasets.
In summary, our study has demonstrated that individuals with BD, UD, and HC differed in activation of the working memory circuitry during working memory updating. Difficulty-related activation changes in the left lateral occipital cortex and right paracingulate cortex were lower in individuals with BD, compared to those with UD. The left lateral occipital cortex function diminished with age and was associated with difficulty-related worsening of RT in BD but not in the other groups. Our findings are consistent with the proposal that the alterations in the working memory circuitry may be a trait characteristic of reduced working memory capacity in mood disorders, especially in BD. Aberrant patterns of difficulty-related response in the left lateral occipital and right paracingulate cortices could point to a specific marker of bipolar disorder.
References
Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States: 2009. J Affect Disord. 2011;129:79–83.
Greenberg PE, Fournier AA, Sisitsky T, Simes M, Berman R, Koenigsberg SH, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics 2021;39:653–65.
Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700.
Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–7.
Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108. https://doi.org/10.1016/j.jad.2007.06.014.
Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. Long-term implications of early onset in bipolar disorder: Data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry. 2004;55:875–81.
McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–85.
Baddeley A. Working memory. Curr Biol. 2010;20:R136–40.
Manelis A, Reder LM. He who is well prepared has half won the battle: An fMRI study of task preparation. Cereb Cortex. 2015;25:726–35.
Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage 2000;11:424–46.
Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17:172–9.
Meiran N, Diamond GM, Toder D, Nemets B. Cognitive rigidity in unipolar depression and obsessive compulsive disorder: Examination of task switching, Stroop, working memory updating and post-conflict adaptation. Psychiatry Res. 2011;185:149–56.
Brevik EJ, Eikeland RA, Lundervold AJ. Subthreshold depressive symptoms have a negative impact on cognitive functioning in middle-aged and older males. Front Psychol. 2013;4. https://doi.org/10.3389/fpsyg.2013.00309.
Ferrier IN, Stanton BR, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry. 1999;175:246–51.
Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2008;18:595–605.
Walshaw PD, Alloy LB, Sabb FW. Executive function in pediatric bipolar disorder and attention-deficit hyperactivity disorder: in search of distinct phenotypic profiles. Neuropsychol Rev. 2010;20:103–20.
Balanzá-Martínez V, Tabarés-Seisdedos R, Selva-Vera G, Martínez-Arán A, Torrent C, Salazar-Fraile J, et al. Persistent cognitive dysfunctions in bipolar I disorder and schizophrenic patients: a 3-year follow-up study. Psychother Psychosom. 2005;74:113–9.
Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–84.
Saldarini F, Gottlieb N, Stokes PRA. Neural correlates of working memory function in euthymic people with bipolar disorder compared to healthy controls: A systematic review and meta-analysis. J Affect Disord. 2022;297:610–22.
Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18.
Nikolin S, Tan YY, Schwaab A, Moffa A, Loo CK, Martin D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J Affect Disord. 2021;284:1–8.
Lee CY, Wang LJ, Lee Y, Hung CF, Huang YC, Lee MI, et al. Differentiating bipolar disorders from unipolar depression by applying the Brief Assessment of Cognition in Affective Disorders. Psychol Med. 2018;48:929–38.
Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59.
Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 2012;60:830–46.
Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JRC, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med. 2012;42:1417–28.
Fernández-Corcuera P, Salvador R, Monté GC, Salvador Sarró S, Goikolea JM, Amann B, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148:170–8.
Lagopoulos J, Ivanovski B, Malhi GS. An event-related functional MRI study of working memory in euthymic bipolar disorder. J Psychiatry Neurosci. 2007;32:174–84.
Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry. 2011;168:173–82.
Harvey PO, Fossati P, Pochon JB, Levy R, LeBastard G, Lehéricy S, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage 2005;26:860–9.
Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66.
Han KM, De Berardis D, Fornaro M, Kim YK. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;91:20–7.
Manelis A, Iyengar S, Swartz HA, Phillips ML. Prefrontal cortical activation during working memory task anticipation contributes to discrimination between bipolar and unipolar depression. Neuropsychopharmacology 2020;45:956–63.
Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29:490–501.
Pu S, Yamada T, Yokoyama K, Matsumura H, Kobayashi H, Sasaki N, et al. A multi-channel near-infrared spectroscopy study of prefrontal cortex activation during working memory task in major depressive disorder. Neurosci Res. 2011;70:91–7.
Macoveanu J, Kjærstad HL, Vinberg M, Harmer C, Fisher PMD, Knudsen GM, et al. Affective episodes in recently diagnosed patients with bipolar disorder associated with altered working memory-related prefrontal cortex activity: a longitudinal fMRI study. J Affect Disord. 2021;295:647–56.
Yaple ZA, Tolomeo S, Yu R. Mapping working memory-specific dysfunction using a transdiagnostic approach. NeuroImage Clin. 2021;31:102747.
Müller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA psychiatry. 2017;74:47–55.
Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion 2009;9:855–64.
Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, et al. Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:361–70.
Ruihua M, Meng Z, Nan C, Panqi L, Hua G, Sijia L, et al. Differences in facial expression recognition between unipolar and bipolar depression. Front Psychol. 2021;12:2929.
Lu Z, Wang Y, Xun G. Neurocognition function of patients with bipolar depression, unipolar depression, and depression with bipolarity. Front Psychiatry. 2021;12:1255.
Nelson HE. National Adult Reading Test (NART): For the assessment of premorbid intelligence in patients with dementia: Test manual. Windsor: NFER-Nelson; 1982.
First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5-Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015.
First MB Structured clinical interview for DSM-IV axis I disorders. Biometrics Res Dep. 1997;132.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35.
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983.
Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009. https://doi.org/10.1016/j.psychres.2008.05.006.
Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26.
Makowski D. The psycho Package: an Efficient and Publishing-Oriented Workflow for psychological science. J Open Source Softw. 2018;3:470.
Makowski D, Ben-Shachar M, Patil I, Lüdecke D Methods and Algorithms for Correlation Analysis in R. J Open Source Softw. 2020;5. https://doi.org/10.21105/joss.02306.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.
Halchenko Y, Goncalves M, Castello MV di O, Ghosh S, Hanke M, Dae, et al. nipy/heudiconv: v0.5.4 [0.5.4] - 2019-04-29. 2019. https://doi.org/10.5281/ZENODO.2653784.
Visconti di Oleggio Castello M, Dobson JE, Sackett T, Kodiweera C, Haxby JV, Goncalves M, et al. ReproNim/reproin 0.6.0. 2020. https://doi.org/10.5281/ZENODO.3625000.
Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0184661.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–94.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–41.
Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–8.
Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 2015. https://doi.org/10.1016/j.neuroimage.2015.02.064.
Guillaume B, Hua X, Thompson PM, Waldorp L, Nichols TE. Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage 2014;94:287–302.
Smith SM, Nichols TE Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009. https://doi.org/10.1016/j.neuroimage.2008.03.061.
van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
Breukelaar IA, Erlinger M, Harris A, Boyce P, Hazell P, Grieve SM, et al. Investigating the neural basis of cognitive control dysfunction in mood disorders. Bipolar Disord. 2020;22:286–95.
Petersen CS, Miskowiak KW. Toward a transdiagnostic neurocircuitry-based biomarker model for pro-cognitive effects: Challenges, opportunities, and next steps. CNS Spectr. 2021;26:333–7.
Zhu Y, Quan W, Wang H, Ma Y, Yan J, Zhang H, et al. Prefrontal activation during a working memory task differs between patients with unipolar and bipolar depression: a preliminary exploratory study. J Affect Disord. 2018. https://doi.org/10.1016/j.jad.2017.07.031.
Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. FMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res—Neuroimaging. 2010;182:22–9.
Brooks JO, Vizueta N, Penfold C, Townsend JD, Bookheimer SY, Altshuler LL. Prefrontal hypoactivation during working memory in bipolar II depression. Psychol Med. 2015. https://doi.org/10.1017/S0033291714002852.
Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–9.
Rodríguez-Cano E, Alonso-Lana S, Sarró S, Fernández-Corcuera P, Goikolea JM, Vieta E, et al. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017;19:386–95.
Christopher G, MacDonald J The impact of clinical depression on working memory. Cogn Neuropsychiatry 2005. https://doi.org/10.1080/13546800444000128.
Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia 2007;45:75–92.
Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vis Res. 2001;41:1409–22.
Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–42.
Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 1998;35:199–210.
Maller JJ, Anderson R, Thomson RH, Rosenfeld JV, Daskalakis ZJ, Fitzgerald PB. Occipital bending (Yakovlevian torque) in bipolar depression. Psychiatry Res - Neuroimaging. 2015;231:8–14.
Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: Evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–63.
Manelis A, Stiffler R, Lockovich JC, Almeida JRC, Aslam HA, Phillips ML. Longitudinal changes in brain activation during anticipation of monetary loss in bipolar disorder. Psychol Med. 2019. https://doi.org/10.1017/S0033291718003847.
Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60.
Li G, Chen Y, Le TM, Wang W, Tang X, Li CSR. Neural correlates of individual variation in two-back working memory and the relationship with fluid intelligence. Sci Rep. 2021;11:1–13.
Fornito A, Malhi GS, Lagopoulos J, Ivanovski B, Wood SJ, Saling MM, et al. Anatomical abnormalities of the anterior cingulate and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res—Neuroimaging. 2008;162:123–32.
Acknowledgements
This research was supported by grant from the National Institute of Health R03MH115894 to A.M. and in part by the University of Pittsburgh Center for Research Computing through the resources provided. Acquisition of the data used in this manuscript was supported by grants from the National Institute of Health R01MH076971, R01MH059929, R01MH100041. The authors thank participants for taking part in this research study.
Author information
Authors and Affiliations
Contributions
AM—obtained funding, organized and evaluated the data, analyzed and interpreted the data, drafted, and critically evaluated the manuscript. YOH—curated data organization and analyses, drafted and critically evaluated the manuscript. LB, RSS, GB—acquired and organized data, critically evaluated the manuscript. SS, RR—evaluated data quality, critically evaluated the manuscript. SI—curated data analyses, critically evaluated the manuscript. CDL, HAS—curated participants’ recruitment and study development, critically evaluated the manuscript MLP—obtained funding, developed the study, critically evaluated the manuscript All authors have read and approved the final version of the manuscript and agreed to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Competing interests
AM, YOH, LB, RSS, SS, RM, CDL, GB, SI, MLP declare no conflict of interest. HAS receives royalties from Wolters Kluwer, royalties and an editorial stipend from APA Press, and has served as a consultant for Intracellular Therapeutics, Physicians Postgraduate Press, and Medscape/WebMD.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manelis, A., Halchenko, Y.O., Bonar, L. et al. Working memory updating in individuals with bipolar and unipolar depression: fMRI study. Transl Psychiatry 12, 441 (2022). https://doi.org/10.1038/s41398-022-02211-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02211-6