Abstract
Although altered reward sensitivity has been observed in individuals with bipolar disorder (BD), the brain function findings related to reward processing remain unexplored and inconsistent. This meta-analysis aimed to identify brain activation alterations underlying reward anticipation in BD. A systematic literature research was conducted to identify fMRI studies of reward-relevant tasks performed by BD individuals. Using Anisotropic Effect Size Signed Differential Mapping, whole-brain and ROI of the ventral striatum (VS) coordinate-based meta-analyses were performed to explore brain regions showing anomalous activation in individuals with BD compared to healthy controls (HC), respectively. A total of 21 studies were identified in the meta-analysis, 15 of which were included in the whole-brain meta-analysis and 17 in the ROI meta-analysis. The whole-brain meta-analysis revealed hypoactivation in the bilateral angular gyrus and right inferior frontal gyrus during reward anticipation in individuals with BD compared to HC. No significant activation differences were observed in bilateral VS between two groups by whole-brain or ROI-based meta-analysis. Individuals with BD type I and individuals with euthymic BD showed altered activation in prefrontal, angular, fusiform, middle occipital gyrus, and striatum. Hypoactivation in the right angular gyrus was positively correlated with the illness duration of BD. The present study reveals the potential neural mechanism underlying impairment in reward anticipation in BD. Some clinical features such as clinical subtype, mood state, and duration of illness confound the underlying neurobiological abnormality reward anticipation in BD. These findings may have implications for identifying clinically relevant biomarkers to guide intervention strategies for BD.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is a severe psychiatric disorder, characterized by depressive, manic, and mixed episodes with variable inter-episode remission. Individuals with BD often show excessive goal-directed and pleasure-seeking behavior during manic episodes while decreased hedonic capacity during depressive episodes, together with strong desires for goals and reward even in remission [1,2,3], suggesting impaired reward processing throughout BD. Moreover, altered reward processing is associated with the severity of clinical symptoms in BD [4, 5], and influences the development and course of BD [6,7,8]. According to the Behavioral Approach System (BAS) dysregulation model, reward hypersensitivity is related to both hypomanic/manic and depressive symptoms in individuals with BD when they respond to reward-relevant events [9, 10], and remains elevated in remission [11,12,13]. Hypersensitivity to reward-relevant stimuli may be a key component of emotional dysregulation, vulnerability, and affective lability in BD [14,15,16]. Exploring the neurobiological basis of impairments in reward processing in individuals with BD may thus be helpful to improve treatment and prevention.
Clinical research works have reported altered anticipatory processing in BD, which results in abnormalities in assigning the motivational value to anticipated outcomes and impaired decision-making strategies [17,18,19]. Reward anticipation is the initial prospect of a reward encountered during reward processing [20, 21], which motivates individuals to produce incentive motivation and make efforts to achieve goals [22]. In healthy individuals, reward anticipation processing such as signaling about anticipated reward levels [21, 23], activating under anticipated arousal and effort [24, 25], and processing outcome predictability [26,27,28] depends on the function of the ventral striatum (VS), the anterior cingulate cortex (ACC), and the parietal regions, respectively. Existing studies have proposed that aberrant responses in some reward-related brain regions, such as the ACC, the orbitofrontal cortex (OFC), and the striatum, confer risk for the development of bipolar spectrum disorders [29, 30]. Individuals with BD present abnormal activation of the cortical-striatal circuit during the performance of reward-relevant tasks [31,32,33]. For example, some whole-brain studies found hyperactivation in the prefrontal and cingulate cortex in euthymic individuals with BD during reward anticipation [34,35,36,37], while others found hypoactivation in the parietal lobe [31, 32].
The VS has been implicated as a key area coding reward anticipation [38, 39], which encompasses the ventral part of the caudate and the nucleus accumbens, and receives projections from dopaminergic cells respond to reward-predicting cues and top-down regulation from cortical regions [40,41,42]. An electroencephalography study provides evidence of a top-down regulation from the frontal area to VS during reward anticipation [40]. It is clear that the VS participates in several extended networks linked to a range of cognitive, affective, and social behaviors [43]. And resting-state functional connectivity study has reported this circuit-level alteration, which suggested attenuation of functional connectivity between the OFC and VS in BD [44]. However, a mixed pattern of VS activation was reported in response to reward in BD, with hyperactivation in the VS during reward anticipation in euthymic individuals with BD [34, 37] or hypoactivation of this region in manic individuals [45, 46]. From the above, there were no consistent findings regarding the neural activation alterations during reward anticipation in individuals with BD due to the small sample size, the heterogeneity in sample characteristics, and methodology. The heterogeneity reminds the necessity of further identifying specific neural mechanisms of reward anticipation in BD.
The primary aim of the current study was to explore the neural basis underlying impairment of reward anticipation in BD by whole-brain- and specific VS ROI-based meta-analyses. Furthermore, separate analyses were performed for individuals with different sub-types and clinical states of BD. Finally, the potential effects of clinical features on functional activation in BD were investigated using meta-regression analysis.
Materials and methods
Search strategies
A literature search of PubMed, Embase, ScienceDirect, and Web of Science databases was conducted to identify original fMRI studies of BD individuals performing reward-relevant tasks, which had been published in the English language in peer-reviewed journals up to June 2021. The search strategy included different combinations of the following terms: (‘bipolar disorder’ OR ‘manic depressive psychos*’ OR ‘mani*’ OR ‘bipolar depression’ OR ‘bipolar affective psychos*’) AND (reward* OR ‘risk’ OR ‘risk taking’) AND (‘magnetic resonance imaging’ OR MRI OR fMRI OR ‘functional magnetic resonance imaging’). The retrieved articles, including relevant reviews and meta-analyses, were searched to identify original studies that were potentially missed in the above searches.
Selection criteria
An fMRI study was retained if (1) a precise diagnosis of BD was made, (2) brain activation during reward anticipation was compared between individuals with BD and HC, (3) individuals were equal to or over 18 years old, (4) whole-brain and/or ROI analysis was used, and (5) stereotactic 3D coordinates of brain activation were reported.
Studies were excluded if (1) results were not based on the main effects of the group, (2) subjects were under 18 years old, (3) only small volume correction was used, and (4) the peak coordinates of effects were unavailable even after the authors were contacted via e-mail. If studies reported longitudinal experiments, only the baseline results were included.
Data extraction and quality assessment
This meta-analysis followed the guidelines for a Meta-analysis of Observational Studies in Epidemiology (MOOSE) (Supplementary Table S1). The following information was compiled for all the included studies: first author, year of publication, cohort size, age, sex, age at onset, illness duration, illness subtype, mood state, Hamilton Depression Scale (HAMD), Young Manic Rating Scale (YMRS), comorbidity, medication, task paradigm, imaging parameters (slice thickness, magnetic field strength, smoothing kernel, stereotactic template space, analysis software), and statistical threshold.
The peak coordinates and corresponding t statistics of significant differences in brain activation were extracted into a text file for each study. The results of studies using whole-brain and ROI-based analyses were retrieved and summarized, respectively. The literature search and data extraction were independently conducted by two authors (XPL and XLW). When extra information was required, a request was made to the corresponding author by e-mail.
In addition, a quality assessment score was computed according to the criteria modified from studies by Sanderson et al. [47] and Shepherd et al. [48]. The relevant checklist included 15 items relating to, for example, demographics, method of recruitment, task design, image acquisition and analysis, and consistency of the conclusions (Supplementary Table S2).
Coordinate-based meta-analysis
Anisotropic Effect-Size version of Seed-based Signed Differential Mapping (AES-SDM) software [49, 50], version 5.15 (https://www.sdmproject.com/), was used to investigate brain regions that potentially show consistent significant differences in brain activation between BD individuals and controls during reward anticipation.
Main meta-analysis
For the whole-brain meta-analysis, effect-size maps of differences between groups for each study were recreated to generate Monte Carlo brain maps by randomly permuting voxels from these brain maps. Then, estimated statistical maps were included in a random-effect meta-analytic model that weighted the contribution of each study according to its sample size. The threshold was set at p < 0.005 (voxel level), with SDM-z > 1 (peak height) and a cluster size ≥10 voxels, since it was found to be optimally balanced sensitivity and specificity in AES-SDM meta-analysis and was adopted by most of the previous AES-SDM studies [49, 51, 52].
For the ROI meta-analysis, we selected bilateral VS (including nucleus accumbens) as the ROIs, which were defined based on the Harvard-Oxford subcortical atlas [53]. We examined activation differences in left and right VS between BD and HC at p < 0.005 separately.
Subgroup meta-analysis
Subgroup meta-analyses were performed for studies that recruited individuals with BD type I (BD I) and individuals with euthymic BD, respectively.
Reliability analysis
In order to assess the effect of an individual study on the estimated pooled effect size, a whole-brain jack-knife sensitivity analysis was performed in which each study was discarded at successive repeat iterations in the meta-analysis.
Heterogeneity and publication bias analyses
The statistical heterogeneity of individual clusters was examined using a random-effect model with Q statistics (X2) distribution converted to z values and tested with a permutation approach (p < 0.005, uncorrected; peak height z = 1; cluster extent = 10). Publication bias was evaluated using Egger’s test (p < 0.05) [54].
Meta-regression analysis
The potential effects of average age, male percentage, age at onset, illness duration, HAMD score, YMRS score in the BD cohort, percentage of medication-free individuals, and quality score on the results were explored by meta-regression using a linear random-effect model. As in previous meta-analysis, in order to minimize the detection of spurious relationships, a threshold of p < 0.0005 was used and only brain regions with significant results in the main meta-analysis were considered [50, 52].
Results
Sample characteristics of studies included in the whole-brain meta-analysis
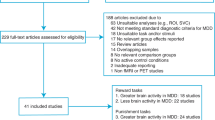
Fifteen studies (including 16 experiments) met the inclusion criteria for whole-brain-based meta-analysis, with a total of 372 BD individuals and 507 HC (Fig. 1). There were no significant differences in mean age between individuals with BD and HC (BD: 36.37 ± 9.73 years, HC: 34.76 ± 7.78 years, t = 0.60, p = 0.56). BD individuals showed a lower percentage of males than HC (BD: 159/372 = 42.74%, HC: 259/507 = 51.08%, χ2 = 5.99, p < 0.05).
The paradigms of reward-relevant tasks included Monetary Incentive Delay (eight studies), Card Guessing (five studies), Social Incentive Delay (three studies), and Lowa Gambling (one study). Twelve studies reported no significant difference in task performance or reaction time between BD individuals and HC, with three studies [32, 33, 55] providing no relevant information. The quality assessment scores ranged from 11.5 to 15 points with an average of 13.4 points. More detailed information about the demographic and clinical characteristics and the quality assessment score of all included studies are presented in Table 1. The details of the imaging parameters, statistical threshold, and results of the between-group analysis for each study are presented in Supplementary Table S3.
Whole-brain meta-analysis
Main meta-analysis
Compared with HC, BD individuals showed significant hypoactivation in the bilateral angular gyrus and right inferior frontal gyrus, with no brain region showing significant hyperactivation (Table 2 and Fig. 2a).
a Results of meta-analysis showed that BD individuals showed significant hypoactivation in the bilateral angular gyrus and the right inferior frontal gyrus. b Illness duration of individuals with BD is positively associated with activation of the right angular gyrus. Blue clusters represent hypoactivation in individuals with BD compared to healthy controls. B bilateral, BA brodmann area, IFG inferior frontal gyrus, R right.
Subgroup meta-analyses
The first subgroup analysis including 11 studies observed hyperactivation in the left anterior cingulate gyrus, and hypoactivation in the left middle occipital gyrus, right inferior frontal gyrus, and right angular gyrus in individuals with BD I compared to HC (Table 2 and Fig. 2b). The second subgroup analysis including eight studies observed hyperactivation in the left orbital frontal gyrus, left fusiform gyrus and left insula, and hypoactivation in the right inferior temporal gyrus and right striatum in individuals with euthymic BD compared to HC (Table 2 and Fig. 2c).
Reliability analyses
A whole-brain jack-knife sensitivity analysis of the main meta-analysis showed that hypoactivation of the bilateral angular gyrus was preserved in all 16 datasets, and the right inferior frontal gyrus remains significant in fourteen datasets (Supplementary Table S5).
Heterogeneity and publication bias analyses
There was no significant between-study heterogeneity in the results for the main and both subgroup meta-analyses. None of the clusters reported above showed significant publication bias based on Egger’s test (p > 0.05) in the main meta-analysis.
Meta-regression analyses
The meta-regression analyses showed that the illness duration of BD individuals was positively correlated with hypoactivation in the right angular gyrus (MNI coordinates: x = 44, y = –60, z = 46; 19 voxels; SDM = –4.244; p < 0.001; Fig. 3). The meta-regression analysis showed no effect related to the average age, age at onset, HAMD score, YMRS score of the patient cohort, percentage of medication free individuals, or quality assessment score.
a Hyperactivation in left ACC and hypoactivation in the right angular gyrus, left middle occipital gyrus, and right inferior frontal gyrus were found in individuals with BD I relative to HC. b hyperactivation in the left OFC, left fusiform gyrus and insula, and hypoactivation in right temporal gyrus and striatum were found in individuals with euthymic BD relative to HC. Red and blue clusters represent hyperactivation and hypoactivation in individuals with BD compared to healthy controls, respectively. ACC anterior cingulate cortex, BD bipolar disorder, HC healthy controls, L left, OFC orbital frontal cortex, R right.
Sample characteristics of studies included in the ROI meta-analysis
Seventeen studies (eleven from whole-brain studies) comprising 18 samples were included in the VS ROI meta-analysis, with a total of 348 individuals with BD and 443 HC (Fig. 1). There were no significant differences in mean age (BD: 32.85 ± 6.51, HC: 33.44 ± 6.26, t = 0.68, p = 0.49) or male percentage (BD: 162/348 = 44.11%, HC: 204/443 = 51.08%, χ2 = 0.02, p = 0.89) between individuals with BD and HC.
The paradigms of reward-relevant tasks included Monetary Incentive Delay (ten studies), Card Guessing (four studies), Probabilistic Reversal Learning (one study), Social Incentive Delay (one study), and Incentivized Control Engagement (one study). Nine studies reported no significant difference in task performance or reaction time between BD individuals and HC, with one study providing no relevant information [55]. Four studies reported no significant difference in task performance between BD individuals and HC, and three studies reported lower accuracy [45, 46] and longer reaction times [56] in task performance in BD individuals than HC. The quality assessment scores ranged from 9 to 14 points with an average of 11.2 points. More detailed information about the demographic and clinical characteristics and the quality assessment score of all included studies are presented in Table 1. The details of the imaging parameters, statistical threshold, and results of the between-group analysis for each study are presented in Supplementary Table S3.
ROI-based meta-analysis
No significant activation difference was observed for bilateral VS ROIs between BD individuals and HC (effect size for left VS: 0.96; effect size for right VS: 0.97; p > 0.005).
Discussion
The current study revealed hypoactivation during reward anticipation in the bilateral angular gyrus and right inferior frontal gyrus in individuals with BD relative to HC by whole-brain meta-analysis, with no significant activation abnormality in bilateral VS by whole-brain or ROI analysis. Anomalous functioning of the frontal-parietal is the most significant finding in BD individuals. Hypoactivation in the right angular gyrus was positively correlated with illness duration in individuals with BD. Certain clinical characteristics such as illness subtype, mood state, and duration of illness influenced the brain activation alterations produced by BD during reward anticipation.
Hypoactivation of the prefrontal-parietal regions during reward anticipation
Our findings are consistent with other studies which reported hypoactivation in the right inferior frontal gyrus during reward processing by positron emission tomography [57], and decreased activity in the prefrontal cortex during Iowa Gambling Task by near-infrared spectroscopy [58] in individuals with BD relative to HC. Altered activation in the inferior frontal gyrus, an important part of the frontal-striatal circuit, is associated with reward hypersensitivity in BD [29, 30]. The inferior frontal gyrus is an important part of the lateral prefrontal cortex, which upregulates activity in the limbic and mesolimbic systems [59, 60]. Dysfunction of the inferior frontal gyrus has been observed in individuals with BD during executive control [61,62,63], and reward signals play a crucial role during this process [64,65,66]. The angular gyrus, a cross-modal integrative hub that converges multisensory information, can detect discrepancies between predicted and actual action consequences for multimodal feedback [67]. Activation in the angular gyrus during reward anticipation was correlated with nucleus accumbens dopamine release [68], which supports the hyperdopaminergia theory across mood states in BD [6]. The hyperdopaminergia theory suggests that an increase in striatal dopamine transporter levels may lead to a decrease in dopaminergic function and depression [69], while an increase in striatal dopamine receptor levels may lead to an increase in dopaminergic neurotransmission and mania [70, 71]. The disturbance in dopamine system homeostasis may be one of the pathophysiologies of BD, which has been tested to be a close connection with reward processing [72]. We also observed a positive correlation between hypoactivation of the angular gyrus and illness duration in BD individuals, which may suggest gradually impaired reward function during the development of illness [7].
The inferior frontal gyrus and angular gyrus are key components in the executive control network, which is assumed to modulate reward systems [73,74,75]. An intact executive functioning network may dampen an overactive reward system and therefore promote adaptive functioning [76]. Higher executive functioning was associated with increased activation in parietal areas during reward anticipation and increased limbic connectivity with frontal areas [77]. Decreased engagement of prefrontal-parietal regions may reflect difficulties in inhibiting excessive pleasure-seeking, increased impulsive behavior, and pronounced risk-taking tendencies in BD [78, 79].
Subgroup findings in BD
Individuals with BD I showed hyperactivation in the left dorsal ACC, and hypoactivation in the left middle occipital gyrus, right inferior frontal gyrus, and angular gyrus compared to HC. The ACC receives projections from the OFC, VS, and mesolimbic dopamine system, and is implicated in risk decision and uncertainty assessment [24, 80, 81]. The dorsal ACC plays a critical role in forming associations between rewards and actions [82]. Hyperactivation in the dorsal ACC may result in excessive stimulation of mesolimbic dopamine release, manifested as exaggerated hedonic responses and enhanced motivational drive [83]. Abnormalities of the occipital gyrus have been observed in spontaneous neural activities and emotional processing in BD [84,85,86]. The frontal-striatal and occipital regions are reliably activated during reward anticipation [87, 88]. Our results add to the evidence of functional impairment during reward anticipation in prefrontal and parietal-occipital regions in BD I.
Euthymic BD also showed hyperactivation in the left OFC, fusiform gyrus and insula, and hypoactivation in the right inferior temporal gyrus and striatum during reward anticipation. The OFC involves the first stage of cortical processing that represents reward value [89], and activation in this region updates rapidly when reward value changes and sends this information to the ACC for actions guided by outcomes [90]. The ventral temporal cortex is a key structure in high-level visual processing [91,92,93] and represents objects independently of their reward value [94]. Activation of the insula is correlated with subjective affective experience of rewards since the insula plays an important role in interoception [95]. The frontal-striatal circuit is a well-established neural pathway in the reward system, which involves dopaminergic projection from the midbrain nuclei to subcortical areas that are central to processing the reward properties of stimuli, and to cortical targets [96]. The nucleus accumbens, which is the center of VS and receives projections from the OFC, ACC, amygdala, and midbrain, can integrate incoming dopaminergic signals from cortical and limbic regions to guide decision making, track the outcomes of actions, and influence the direction of future ones [97]. These regions are involved in the valuation/motivation network [98] and salience/monitoring network [99, 100], which play an important role during reward anticipation in BD. These findings provide evidence that abnormal brain activation remains in frontal-temporal-striatal regions during reward anticipation in euthymic BD.
Null findings in VS
Despite negative findings during reward anticipation in BD by our whole-brain and ROI analyses, the VS has a relatively specific role in reward processing compared to other cognitive processes [101, 102]. It seems premature to draw the conclusion of no abnormal activation in VS in BD during reward processing, especially in reward anticipation. First, the heterogeneity of individuals in the included studies may bias the findings. For example, our subgroup analysis found significant hypoactivation in VS in individuals with euthymic BD. According to the BAS/reward hypersensitivity model of BD, the VS has been engaged contingent on mood, whereas elevated mood may increase the expected value and elicit VS activity but low mood may decrease the perceived value and dampen VS activity [103]. Second, the different processing types of the tasks also affected brain activation. Besides reward anticipation, VS was also found to have altered activation in reward receipt and loss anticipation in BD [12, 37, 104,105,106]. Moreover, striatal activation to reward cues is modulated by several factors involved in reward anticipation including the magnitude of the reward, the probability of reward receipt, the amount of time until the anticipated reward can be obtained, and the effort required to pursue the reward [107]. These studies suggest a complex role of VS in reward processing, suggesting the need for sophisticated fMRI protocols to separate them at the brain level.
In addition, reward deficits in mood disorders were associated with altered connectivity between VS and large-scale functional networks [108]. Particularly, reward anticipation was characterized by dense connectivity in the frontal-parietal-temporal-striatal network in BD [109]. Some studies have shown that individuals with BD exhibited decreased dynamic functional connectivity in frontal areas and increased VS and OFC functional connectivity during reward processing [110, 111]. In this context, altered activation in a selected region cannot fully explain complex patterns of reward impairments in BD; therefore, it is important to examine the functioning at the level of the brain network in future studies.
Limitations
This meta-analysis has several limitations that should be acknowledged. First, the number of studies included in the meta-analyses was relatively small, which limited the statistical power, especially in the case of different clinical sub-types and mood states of BD. Second, the comparability of different task paradigms’ difficulty and discriminability is an important question that awaits further work. Third, this study focused on the brain activation alterations of the reward anticipation phase during reward processing in BD. Thus, the results do not represent the full range of reward processing. It will be an interesting topic to discover whether a functional abnormality in a particular brain region may underlie impairment in loss anticipation, reward/loss outcome, and prediction error in individuals with BD. Fourth, only VS ROI meta-analysis was performed in BD due to the limited number of studies. Finally, the possible effect of medication on the findings in BD cannot be totally ruled out. The potentially confounding effects of psychotropic medication in bipolar neuroimaging research have been discussed previously, which found no or limited impact on fMRI results [112, 113]. However, we cannot completely rule out specific medication effects considering the evidence that psychotropic medications might generally blunt neural responses to reward anticipation [34, 35]. Further studies recruiting unmedicated patients or studies with a longitudinal design controlling for medication are needed.
Conclusions
The present study revealed significantly altered brain activation in prefrontal and inferior parietal lobule regions during reward anticipation processing in BD, suggesting the potential neurobiological mechanism underlying impairment in reward anticipation in BD. The clinical features of individuals with BD may affect the neurobiological basis during reward anticipation. Future prospective studies, recruiting different subgroups of BD, focusing on other phases of reward processing such as loss anticipation, reward outcome and prediction error, and using multimodal neuroimaging, are needed to better understand the longitudinal neural trajectory underlying reward processing in BD.
References
Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65.
Van der Gucht E, Morriss R, Lancaster G, Kinderman P, Bentall RP. Psychological processes in bipolar affective disorder: negative cognitive style and reward processing. Br J Psychiatry. 2009;194:146–51.
Zald DH, Treadway MT. Reward processing, neuroeconomics, and psychopathology. Annu Rev Clin Psychol. 2017;13:471–95.
Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, et al. Progression along the bipolar spectrum: a longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J Abnorm Psychol. 2012;121:16–27.
Johnson SL. Mania and dysregulation in goal pursuit: a review. Clin Psychol Rev. 2005;25:241–62.
Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22:666–79.
Kwan JW, Bauer IE, Hautzinger M, Meyer TD. Reward sensitivity and the course of bipolar disorder: a survival analysis in a treatment seeking sample. J Affect Disord. 2020;261:126–30.
Nusslock R, Alloy LB. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J Affect Disord. 2017;216:3–16.
Alloy LB, Abramson LY. The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Curr Dir Psychol Sci. 2010;19:189–94.
Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, et al. High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. J Abnorm Psychol. 2012;121:339–51.
Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J Psychopathol Behav Assess. 2001;23:133–43.
Mason L, O’Sullivan N, Montaldi D, Bentall RP, El-Deredy W. Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. Brain. 2014;137:2346–55.
Applegate E, El-Deredy W, Bentall RP. Reward responsiveness in psychosis-prone groups: hypomania and negative schizotypy. Personal Individ Differences. 2009;47:452–6.
Urosević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clin Psychol Rev. 2008;28:1188–205.
Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12.
Alloy LB, Nusslock R, Boland EM. The development and course of bipolar spectrum disorders: an integrated reward and circadian rhythm dysregulation model. Annu Rev Clin Psychol. 2015;11:213–50.
Christodoulou T, Lewis M, Ploubidis GB, Frangou S. The relationship of impulsivity to response inhibition and decision-making in remitted patients with bipolar disorder. Eur Psychiatry. 2006;21:270–3.
Duek O, Osher Y, Belmaker RH, Bersudsky Y, Kofman O. Reward sensitivity and anger in euthymic bipolar disorder. Psychiatry Res. 2014;215:95–100.
Mason L, Trujillo-Barreto NJ, Bentall RP, El-Deredy W. Attentional bias predicts increased reward salience and risk taking in bipolar disorder. Biol Psychiatry. 2016;79:311–9.
Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–7.
Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:Rc159.
Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci 2010;4:17.
Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63.
Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–45.
Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–41.
Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–97.
Verney SP, Brown GG, Frank L, Paulus MP. Error-rate-related caudate and parietal cortex activation during decision making. Neuroreport. 2003;14:923–8.
Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–8.
Nusslock R, Young CB, Damme KSF. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: Assessment and treatment implications. Behav Res Ther. 2014;62:74–87.
Bart CP, Titone MK, Ng TH, Nusslock R, Alloy LB. Neural reward circuit dysfunction as a risk factor for bipolar spectrum disorders and substance use disorders: a review and integration. Clin Psychol Rev. 2021;87:102035.
Kirschner M, Cathomas F, Manoliu A, Habermeyer B, Simon JJ, Seifritz E, et al. Shared and dissociable features of apathy and reward system dysfunction in bipolar I disorder and schizophrenia. Psychol Med. 2020;50:936–47.
Schwarz K, Moessnang C, Schweiger JI, Baumeister S, Plichta MM, Brandeis D, et al. Transdiagnostic prediction of affective, cognitive, and social function through brain reward anticipation in schizophrenia, bipolar disorder, major depression, and autism spectrum diagnoses. Schizophr Bull. 2020;46:592–602.
Manelis A, Stiffler R, Lockovich JC, Almeida JRC, Aslam HA, Phillips ML. Longitudinal changes in brain activation during anticipation of monetary loss in bipolar disorder. Psychol Med. 2019;49:2781–8.
Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–41.
Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–54.
Jogia J, Dima D, Kumari V, Frangou S. Frontopolar cortical inefficiency may underpin reward and working memory dysfunction in bipolar disorder. World J Biol Psychiatry. 2012;13:605–15.
Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–60.
Burton AC, Nakamura K, Roesch MR. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol Learn Mem. 2015;117:51–9.
Daniel R, Pollmann S. A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem. 2014;114:90–100.
Plichta MM, Wolf I, Hohmann S, Baumeister S, Boecker R, Schwarz AJ, et al. Simultaneous EEG and fMRI reveals a causally connected subcortical-cortical network during reward anticipation. J Neurosci. 2013;33:14526–33.
Shohamy D. Learning and motivation in the human striatum. Curr Opin Neurobiol. 2011;21:408–14.
Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–10.
Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233.
Shi J, Guo H, Liu S, Xue W, Fan F, Fan H, et al. Resting-state functional connectivity of neural circuits associated with primary and secondary rewards in patients with bipolar disorder. Soc Cogn Affect Neurosci. 2020;15:755–63.
Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–27.
Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, et al. Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology. 2014;39:1914–23.
Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–76.
Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–84.
Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–11.
Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive–compulsive disorder. Br J Psychiatry. 2009;195:393–402.
Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8.
Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–33.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Sharma A, Satterthwaite TD, Vandekar L, Katchmar N, Daldal A, Ruparel K, et al. Divergent relationship of depression severity to social reward responses among patients with bipolar versus unipolar depression. Psychiatry Res Neuroimaging. 2016;254:18–25.
Kollmann B, Scholz V, Linke J, Kirsch P, Wessa M. Reward anticipation revisited- evidence from an fMRI study in euthymic bipolar I patients and healthy first-degree relatives. J Affect Disord. 2017;219:178–86.
Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, et al. Decision-making in mania: a PET study. Brain. 2001;124:2550–63.
Ono Y, Kikuchi M, Hirosawa T, Hino S, Nagasawa T, Hashimoto T, et al. Reduced prefrontal activation during performance of the Iowa Gambling Task in patients with bipolar disorder. Psychiatry Res: Neuroimaging. 2015;233:1–8.
Harvey PO, Armony J, Malla A, Lepage M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J Psychiatr Res. 2010;44:707–16.
Tupak SV, Dresler T, Guhn A, Ehlis AC, Fallgatter AJ, Pauli P. et al. Implicit emotion regulation in the presence of threat: neural and autonomic correlates. Neuroimage. 2014;85(Pt 1):372–9.
Pompei F, Jogia J, Tatarelli R, Girardi P, Rubia K, Kumari V, et al. Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. Neuroimage. 2011;56:1677–84.
Robinson JL, Monkul ES, Tordesillas-Gutiérrez D, Franklin C, Bearden CE, Fox PT, et al. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Res: Neuroimaging. 2008;164:106–13.
Mazzola-Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J Psychiatr Res. 2009;43:432–41.
Crone EA, Steinbeis N. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn Sci. 2017;21:205–15.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Curr Directions Psychological Sci. 2012;21:8–14.
van Kemenade BM, Arikan BE, Kircher T, Straube B. The angular gyrus is a supramodal comparator area in action-outcome monitoring. Brain Struct Funct. 2017;222:3691–703.
Weiland BJ, Heitzeg MM, Zald D, Cummiford C, Love T, Zucker RA, et al. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Res. 2014;223:244–52.
Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, et al. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disord. 2011;13:406–13.
Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52:471–7.
Wong DF, Pearlson GD, Tune LE, Young LT, Meltzer CC, Dannals RF, et al. Quantification of neuroreceptors in the living human brain: IV. Effect of aging and elevations of D2-like receptors in schizophrenia and bipolar illness. J Cereb Blood Flow Metab. 1997;17:331–42.
Weiland BJ, Heitzeg MM, Zald D, Cummiford C, Love T, Zucker RA, et al. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Res: Neuroimaging. 2014;223:244–52.
Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. 2015;66:83–113.
Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213.
Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–45.
Yee DM, Braver TS. Interactions of motivation and cognitive control. Curr Opin Behav Sci. 2018;19:83–90.
Kryza-Lacombe M, Christian IR, Liuzzi MT, Owen C, Hernandez B, Dougherty LR, et al. Executive functioning moderates neural reward processing in youth. Cogn Affect Behav Neurosci. 2021;21:105–18.
Cattarinussi G, Di Giorgio A, Wolf RC, Balestrieri M, Sambataro F. Neural signatures of the risk for bipolar disorder: a meta-analysis of structural and functional neuroimaging studies. Bipolar Disord. 2019;21:215–27.
Townsend JD, Bookheimer SY, Foland-Ross LC, Moody TD, Eisenberger NI, Fischer JS, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–50.
Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22.
Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. J Neurosci. 2010;30:3339–46.
Drevets WC, Ongür D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–6. 190–1
Shan X, Qiu Y, Pan P, Teng Z, Li S, Tang H, et al. Disrupted regional homogeneity in drug-naive patients with bipolar disorder. Front Psychiatry. 2020;11:825.
Cerullo MA, Eliassen JC, Smith CT, Fleck DE, Nelson EB, Strawn JR, et al. Bipolar I disorder and major depressive disorder show similar brain activation during depression. Bipolar Disord. 2014;16:703–12.
Jiang X, Wang X, Jia L, Sun T, Kang J, Zhou Y, et al. Structural and functional alterations in untreated patients with major depressive disorder and bipolar disorder experiencing first depressive episode: a magnetic resonance imaging study combined with follow-up. J Affect Disord. 2021;279:324–33.
Guo Z, Chen J, Liu S, Li Y, Sun B, Gao Z. Brain areas activated by uncertain reward-based decision-making in healthy volunteers. Neural Regen Res. 2013;8:3344–52.
Ivanov I, Liu X, Clerkin S, Schulz K, Friston K, Newcorn JH, et al. Effects of motivation on reward and attentional networks: an fMRI study. Brain Behav. 2012;2:741–53.
Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2:fcaa196.
Rolls ET. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019;224:3001–18.
Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302.
Wandell BA, Rauschecker AM, Yeatman JD. Learning to see words. Annu Rev Psychol. 2011;63:31–53.
Grill-Spector K, Weiner KS. The functional architecture of the ventral temporal cortex and its role in categorization. Nat Rev Neurosci. 2014;15:536–48.
Rolls ET. Invariant visual object and face recognition: neural and computational bases, and a model, VisNet. Front Comput Neurosci. 2012;6:35.
Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67.
Saint-Cyr JA. Frontal-striatal circuit functions: context, sequence, and consequence. J Int Neuropsychol Soc. 2003;9:103–27.
Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52.
Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213:135–41.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67.
Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–52.
Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–77.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
Mason L, Eldar E, Rutledge RB. Mood instability and reward dysregulation-a neurocomputational model of bipolar disorder. JAMA Psychiatry. 2017;74:1275–6.
van Leeuwen JMC, Vink M, Joëls M, Kahn RS, Hermans EJ, Vinkers CH. Reward-related striatal responses following stress in healthy individuals and patients with bipolar disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:966–74.
Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258–68.
Yip SW, Worhunsky PD, Rogers RD, Goodwin GM. Hypoactivation of the ventral and dorsal striatum during reward and loss anticipation in antipsychotic and mood stabilizer-naive bipolar disorder. Neuropsychopharmacology. 2015;40:658–66.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am J Psychiatry. 2017;174:657–66.
Manelis A, Almeida JR, Stiffler R, Lockovich JC, Aslam HA, Phillips ML. Anticipation-related brain connectivity in bipolar and unipolar depression: a graph theory approach. Brain. 2016;139:2554–66.
Dutra SJ, Man V, Kober H, Cunningham WA, Gruber J. Disrupted cortico-limbic connectivity during reward processing in remitted bipolar I disorder. Bipolar Disord. 2017;19:661–75.
Ji S, Ma H, Yao M, Guo M, Li S, Chen N, et al. Aberrant temporal variability in brain regions during risk decision making in patients with bipolar I disorder: a dynamic effective connectivity study. Neuroscience. 2021;469:68–78.
Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–20.
Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81971595 and 81771812), the Key Program of the National Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC0047), and the 1•3•5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2020HXFH005).
Author information
Authors and Affiliations
Contributions
XL conceived and designed the study. XW, FT, and ZJ provided supervision. XL and XW selected the articles and extracted the data. XL contacted authors to acquire unpublished data. XL and XW analyzed and interpreted the data. XL wrote the first draft of the manuscript. XW, FT, YC, HX, and ZJ critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Long, X., Wang, X., Tian, F. et al. Altered brain activation during reward anticipation in bipolar disorder. Transl Psychiatry 12, 300 (2022). https://doi.org/10.1038/s41398-022-02075-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02075-w