Abstract
Introduction
Intramedullary metastasis of Ewing sarcoma is extremely rare. Here, we report an adult case of cervical intramedullary recurrent Ewing sarcoma after a 10-year disease-free survival after the initial surgery for a thoracic lesion.
Case presentation
A 39-year-old man with a history of surgery and chemoradiotherapy for thoracic Ewing sarcoma ten years ago presented with neck pain and incomplete motor paralysis in the right upper extremity, which had suddenly appeared three months before. Cervical magnetic resonance imaging revealed a tear-drop-shaped intramedullary lesion at the C3 level accompanied by diffuse edematous change. Because of the rapid progression of his myelopathy, he underwent surgery for this intramedullary lesion. Intraoperatively, the tumor exhibited an orangish exophytic appearance. The unclearness of the tumor boundary compelled us to perform a partial resection. The histopathology showed the tumor comprised small round atypical cells with immunoreactivity for Nkx2.2 and CD99, diagnosing a metastatic Ewing sarcoma. Postoperatively, although his myelopathy improved transiently and adjuvant chemotherapy radiation was undergone, he died of cranial dissemination of the tumor two months and a half later.
Discussion
To our knowledge, 31 cases of primary and only 4 cases of recurrent intramedullary spinal Ewing sarcoma have been reported to date; however, this is the first case of recurrent intramedullary Ewing sarcoma with a 10-year disease-free survival. Sadly, the prognosis of the current case was extremely poor. There is no clear treatment guideline for recurrent intramedullary Ewing sarcoma because of its rarity, and further collection of similar cases would be required.
Similar content being viewed by others
Introduction
Ewing sarcoma is a highly aggressive round cell mesenchymal neoplasm affecting children and adolescents, commonly metastasizing to the bones, bone marrow, and lungs [1, 2]. Ewing sarcoma can also involve or metastasize into the structures protecting the central nervous system (spinal vertebrae and meninges), wherefrom it can compress or invade the central nervous system. However, Ewing sarcoma occurring within the spinal cord is extremely rare. To the best of our knowledge, there have been 35 previous reports of spinal intramedullary Ewing sarcoma including both primary (n = 31) and recurrent (n = 4) cases (Tables 1, 2) [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Herein we report a recurrent case of cervical intramedullary Ewing sarcoma with 10-year disease-free survival after the primary surgery for a thoracic lesion, followed by an extremely poor postoperative prognosis.
Case presentation
A 39-year-old man with a three-month history of neck pain was referred to our department. The patient also exhibited a right-hand clumsiness gradually deteriorating, whose onset was concomitant with the neck pain. He was recently diagnosed with an intramedullary tumor at the cervical C3 level based on magnetic resonance imaging (MRI) in a previous hospital. He had a history of surgery and chemoradiotherapy for a thoracic paravertebral primary Ewing sarcoma located at Th4-5 level. During the primary surgery performed 10 years ago in another hospital, he underwent a gross total resection of the thoracic tumor and had been subsequently asymptomatic.
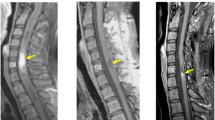
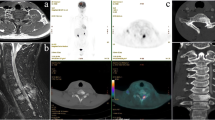
Physical examination revealed a numbness in the upper extremity and hypoesthesia below C5 level. The muscle strength of his right finger extensor and flexor measured by manual muscle testing was scored 3, and no muscle weakness of lower extremities was noted, showing a normal ambulatory ability. His deep tendon reflexes of lower extremities were upregulated with positive Babinski reflexes bilaterally. He exhibited no bladder or rectal disorders. His right hand showed myelopathy signs with 14 times of grip and release during the so-called 10-s test, while his left hand scored 22 times. The hand grip strength was 6.7 kg on the right and 35 kg on the left, respectively. While serial radiographs of the cervical spine showed no structural abnormality, cervical MRI revealed a well-demarcated intramedullary lesion at the C3 level, which appeared isointense on both T1- and T2-weighted images with marked heterogeneous gadolinium-enhancement, accompanied by extensive edematous changes on T2 sagittal MRI image within the cord extending from the medulla oblongata to Th1 (Fig. 1). The appearance of the tumor on T1-weighted gadolinium (Gd)-enhanced sagittal MRI image was spherical on its rostral edge while adopting a tear-drop shape at the caudal edge (Fig. 1c). On positron emission tomography (PET)/computed tomography (CT) using fluorodeoxyglucose (18F) (FDG), a hot spot with FDG-uptake was detected only at C3 level of the spinal cord (Fig. 2). No other lesion was detectable on PET/CT in his whole body (Fig. 2b). The differential diagnosis of this intramedullary lesion was presumed to be ependymoma, astrocytoma, or metastasis of Ewing sarcoma based on MRI findings. Given his rapidly progressive cervical myelopathy manifested by right-hand motor weakness, he underwent surgery for this intramedullary lesion in our hospital.
Sagittal images of T1-weighted (a), T2-weighted (b), and T1-weighted post-Gd (c) contrast administration and axial images of T2-weighted (d), and T1-weighted post-Gd (e) administration are presented. The tear-drop shaped intramedullary tumor at the C3 level was iso-intense on T1- and T2-weighted images and demonstrated strong and diffuse enhancement with Gd. On T2-weighted sagittal image, the tumor was accompanied with diffuse intramedullary high intensity edematous change.
After a C2 double-door laminoplasty and C3–C4 wide laminectomy with the muscle-preserving technique of attaching to C2–C4 spinous processes [33], we confirmed the rostro-caudal limits of the tumor using ultrasonography. Then we incised the dura and arachnoid matter under surgical microscope. Macroscopically, the tumor appeared orangish with dorsally exophytic growth over the pia mater (Fig. 3a), containing cloudy serous fluids. We performed a myelotomy via the posterior median sulcus of the cord and tried to dissect out the tumor. However, the boundary between the tumor and the normal cord was unclear, and the tumor tissues were easily hemorrhagic. Furthermore, his heart rate became bradycardic while dissecting the tumor’s ventral boundary, resulting in subtotal resection of the tumor (Fig. 3b). A piece of tumor specimen was examined by a pathologist intraoperatively, resulting in the diagnosis of malignant small round cell tumor.
Histopathological analysis using paraffin sections of the resected specimen showed a solid tumor composed by monotonous small round cells with high N/C ratio, hyperchromatic nuclei, and high mitotic rate (Fig. 4a, b). Immunohistochemistry revealed that the resected tumor cells were positive for CD99 (Fig. 4c), Nkx2.2 (Fig. 4d), INI1 (retained, not shown), S-100 (not shown), and synaptophysin (not shown), but negative for cytokeratin AE1/AE3 (Fig. 4e), CD79a, TdT (terminal deoxynucleotidyl transferase), desmin, and myogenin. Taken together with the history of thoracic paravertebral Ewing sarcoma, although we did not confirm the presence of chimeric genes such as EWS-FLI1, the pathological findings led to the final diagnosis of intramedullary metastasis of Ewing sarcoma.
Low (a) and high (b) magnification views of hematoxylin and eosin (H-E) stain revealed the solid tumor composed by small round cells with high N/C ratio and hyperchromatic nuclei. Immunohistochemistry showed that the resected tumor cells were positive for CD99 (c) and Nkx2.2 (d), but negative for cytokeratin AE1/AE3 (e). (Scale bars: 4 mm in a, and 100 µm in b–e).
Postoperatively, the right-hand clumsiness was improved, and the patient could ambulate independently. He was transferred to another hospital for an adjuvant chemotherapy on the 15th day after surgery. However, two and a half months after the surgery for intramedullary lesion, he died of cranial dissemination of the tumor.
Discussion
Ewing sarcoma comprises 10–15% malignant bone tumors and 40–45% pediatric malignant bone tumors. The overall 5-year survival rate is of 70% in localized cases and 30% in metastatic cases [34]. To the best of our knowledge, only 35 cases of intramedullary spinal Ewing sarcoma were identified in the literature, including 31 primary (Table 1) and four recurrent/metastatic intramedullary cases (Table 2) [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Intramedullary Ewing sarcomas are commonly located at thoracic or lumbar spinal cord [25]. Initial intramedullary Ewing sarcoma symptoms are similar to other intramedullary spinal cord tumors such as ependymoma or astrocytoma, which include sensory disturbance first followed by motor paralysis and bladder and bowel dysfunctions [31]. Unlike usual spinal intramedullary tumors, intramedullary Ewing sarcomas are presumed to show an acute onset with rapid progressive aggravation of myelopathy, consequently followed by severe myeloplegia [31].
As shown in the Table 2, there were only four cases of recurrent intramedullary metastasis of Ewing sarcoma in the literature. The mechanism of metastasis from the brain lesion was presumed to be drop metastasis via cerebrospinal fluid. Although there was no consistent trend of these recurrent cases due to the small number of cases, notably, the current case was the first case of intramedullary metastasis of Ewing sarcoma with 10-year disease-free survival after treatment for a primary lesion of Ewing sarcoma (Table 2). Despite very late (16 and 19 years) local recurrence of Ewing sarcoma in extremities and pelvis has been reported [35], the current case was the first case of intramedullary metastatic case after a long latent phase. The recent advances in the treatment strategy for Ewing sarcoma, including neoadjuvant and adjuvant chemotherapies with surgery and/or radiotherapy, could succeed in improving the prognosis of primary localized Ewing sarcoma lesion, and could increase the number of cancer survivors in the adolescent and young adult (AYA) generation like this case [34, 36]. Survivors of AYAs sarcoma face significantly higher risks for cardiovascular disease, infertility, and secondary malignancies due to the adjuvant therapy [36]. Therefore, careful long-term follow-up is essential for AYAs sarcoma. On the other hand, the prognosis of metastatic forms of Ewing sarcoma is poorer than that of localized ones [37]. The current case of intramedullary metastatic Ewing sarcoma showed indeed an extremely poor prognosis with less than 6 months of survival from the onset. It is possible that the surgical manipulation of the metastatic lesion might have facilitated the dissemination into the cerebrospinal fluid.
A well-known fusion protein, EWS-FLI1, plays a crucial role in Ewing sarcoma’s development, maintenance, and progression [38, 39]. In addition to EWS-FLI1, the expression of CD99, or MIC-2 gene product, on the cell surface is a sensitive diagnostic maker, although its specificity is relatively lower than EWS-FLI1 [40]. The resected specimen of the present case was indeed immunoreactive for both CD99 and Nkx2.2 (Fig. 4c, d). The combined use of these two markers is advocated as a powerful diagnostic tool that can differentiate Ewing sarcoma from other small round cell tumors with high sensitivity and specificity [41]. Although we have not confirmed the expression of EWS-FLI1 fusion protein in this case, together with the histologic features, the immunoreactivity for both CD99 and Nkx2.2 led to the final diagnosis of intramedullary Ewing sarcoma.
In conclusion, a rare case of cervical intramedullary recurrence of Ewing sarcoma with 10-year disease-free survival is reported. We performed tumor resection that resulted in partial resection of the tumor, followed by an extremely poor evolution with the patient’s death two and a half months after the surgery, most likely due to the cranial dissemination of tumor cells. Although the rapid aggravation of the myelopathy due to cervical intramedullary metastatic lesion growth compelled us to perform surgical treatment, there is no established treatment guideline due to the rarity of spinal intramedullary metastasis of Ewing sarcoma. Further collection of similar cases would be required.
References
Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, et al. The Ewing family of tumors-a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–9.
Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, et al. Ewing Sarcoma: current management and future approaches through collaboration. J Clin Oncol. 2015;33:3036–46.
Kosnik EJ, Boesel CP, Bay J, Sayers MP. Primitive neuroectodermal tumors of the central nervous system in children. J Neurosurg. 1978;48:741–6.
Jaksche H, Wockel W, Wernert N. Primary spinal medulloblastomas? Neurosurg Rev. 1988;11:259–65.
Freyer DR, Hutchinson RJ, McKeever PE. Primary primitive neuroectodermal tumor of the spinal cord associated with neural tube defect. Pediatr Neurosci. 1989;15:181–7.
Ogasawara H, Kiya K, Kurisu K, Muttaqin Z, Uozumi T, Sugiyama K, et al. Intracranial metastasis from a spinal cord primitive neuroectodermal tumor: case report. Surg Neurol. 1992;37:307–12.
Kwon OK, Wang KC, Kim CJ, Kim IO, Chi JG, Cho BK. Primary intramedullary spinal cord primitive neuroectodermal tumor with intracranial seeding in an infant. Childs Nerv Syst. 1996;12:633–6.
Deme S, Ang LC, Skaf G, Rowed DW. Primary intramedullary primitive neuroectodermal tumor of the spinal cord: case report and review of the literature. Neurosurgery. 1997;41:1417–20.
Meltzer CC, Townsend DW, Kottapally S, Jadali F. FDG imaging of spinal cord primitive neuroectodermal tumor. J Nucl Med. 1998;39:1207–9.
Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293–5.
Mawrin C, Synowitz HJ, Kirches E, Kutz E, Dietzmann K, Weis S. Primary primitive neuroectodermal tumor of the spinal cord: case report and review of the literature. Clin Neurol Neurosurg. 2002;104:36–40.
Albrecht CF, Weiss E, Schulz-Schaeffer WJ, Albrecht T, Fauser S, Wickboldt J, et al. Primary intraspinal primitive neuroectodermal tumor: report of two cases and review of the literature. J Neurooncol. 2003;61:113–20.
Kim YW, Jin BH, Kim TS, Cho YE. Primary intraspinal primitive neuroectodermal tumor at conus medullaris. Yonsei Med J. 2004;45:533–8.
Kampman WA, Kros JM, De Jong TH, Lequin MH. Primitive neuroectodermal tumours (PNETs) located in the spinal canal; the relevance of classification as central or peripheral PNET: case report of a primary spinal PNET occurrence with a critical literature review. J Neurooncol. 2006;77:65–72.
Jain A, Jalali R, Nadkarni TD, Sharma S. Primary intramedullary primitive neuroectodermal tumor of the cervical spinal cord. Case report. J Neurosurg Spine. 2006;4:497–502.
De Tommasi A, De Tommasi C, Occhiogrosso G, Cimmino A, Parisi M, Sanguedolce F, et al. Primary intramedullary primitive neuroectodermal tumor (PNET)-case report and review of the literature. Eur J Neurol. 2006;13:240–3.
Kumar R, Reddy SJ, Wani AA, Pal L. Primary spinal primitive neuroectodermal tumor: case series and review of the literature. Pediatr Neurosurg. 2007;43:1–6.
Han IH, Kuh SU, Chin DK, Kim KS, Jin BH, Cho YE. Surgical treatment of primary spinal tumors in the conus medullaris. J Korean Neurosurg Soc. 2008;44:72–7.
Otero-Rodriguez A, Hinojosa J, Esparza J, Munoz MJ, Iglesias S, Rodriguez-Gil Y. et al. Purely intramedullary spinal cord primitive neuroectodermal tumor: case report and review of the literature. Neurocirugia. 2009;20:381–6. Discussion 386–7.
Tsutsumi S, Nonaka Y, Abe Y, Yasumoto Y, Nakazato Y, Ito M. Intramedullary primitive neuroectodermal tumor presenting with rapidly-progressive cauda equina syndrome. Neurol Med Chir. 2010;50:1031–5.
Benesch M, Sperl D, von Bueren AO, Schmid I, von Hoff K, Warmuth-Metz M, et al. Primary central nervous system primitive neuroectodermal tumors (CNS-PNETs) of the spinal cord in children: four cases from the German HIT database with a critical review of the literature. J Neurooncol. 2011;104:279–86.
Ellis JA, Rothrock RJ, Moise G, McCormick PC 2nd, Tanji K, Canoll P, et al. Primitive neuroectodermal tumors of the spine: a comprehensive review with illustrative clinical cases. Neurosurg Focus. 2011;30:E1.
Gollard RP, Rosen L, Anson J, Mason J, Khoury J. Intramedullary PNET of the spine: long-term survival after combined modality therapy and subsequent relapse. J Pediatr Hematol Oncol. 2011;33:107–12.
Alexiou GA, Siozos G, Stefanaki K, Moschovi M, Prodromou N. Intramedullary spinal cord primitive neuroectodermal tumor presenting with hydrocephalus. J Child Neurol. 2013;28:246–50.
Coulibaly O, Gana R, Sogoba Y, Regragui A, Maaqili R, Bellakhdar F. Primary intramedullary Ewing’s sarcoma: a case report and review of the literature. Case Rep. Clin Med. 2015;4:110–3.
Wang G, Guo F. Primary intramedullary primitive neuroectodermal tumor: a case report and review of the literature. Medicines. 2017;96:e9001.
Khwaja R, Mantilla E, Fink K, Pan E. Adult primary peripheral PNET/Ewing’s sarcoma of the cervical and thoracic spine. Anticancer Res. 2019;39:4463–5.
Chen X, Zhang G. Multiple spinal intramedullary primitive neuroectodermal tumors mimicking acute myelitis. World Neurosurg. 2019;126:72–75.
Weil RJ, Zhuang Z, Pack S, Kumar S, Helman L, Fuller BG, et al. Intramedullary Ewing sarcoma of the spinal cord: consequences of molecular diagnostics. Case report. J Neurosurg. 2001;95(Suppl):270–5.
Gorgulu A, Albayrak BS, Kose T. Cervical leptomeningeal and intramedullary metastasis of a cerebral PNET in an adult. J Neurooncol. 2005;74:339–40.
Jia L, Li G, You C, He M, Ye F. Intramedullary Ewing’s sarcoma of the spinal cord associated with hydrocephalus. Neurol India. 2009;57:828–9.
Yurtsever C, Kafadar C, Sonmez G, Mutlu H. Intramedullary spinal cord metastasis from Ewing sarcoma. Spine J. 2016;16:e745–6.
Shiraishi T, Yato Y. New double-door laminoplasty procedure for the axis to preserve all muscular attachments to the spinous process: technical note. Neurosurg Focus. 2002;12:E9.
Kridis WB, Toumi N, Chaari H, Khanfir A, Ayadi K, Keskes H, et al. A review of Ewing sarcoma treatment: is it still a subject of debate? Rev Recent Clin Trials. 2017;12:19–23.
Hanna SA, David LA, Gikas PD, Tindall AJ, Cannon SR, Briggs TW. Very late local recurrence of Ewing’s sarcoma-can you ever say ‘cured’? A report of two cases and literature review. Ann R Coll Surg Engl. 2008;90:W12–5.
Reed DR, Naghavi A, Binitie O. Sarcoma as a model for adolescent and young adult care. J Oncol Pract. 2019;15:239–47.
Buckley JD, Pendergrass TW, Buckley CM, Pritchard DJ, Nesbit ME, Provisor AJ, et al. Epidemiology of osteosarcoma and Ewing’s sarcoma in childhood: a study of 305 cases by the Children’s Cancer Group. Cancer. 1998;83:1440–8.
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5.
Riggi N, Suva ML, Suva D, Cironi L, Provero P, Tercier S, et al. EWS-FLI-1 expression triggers a Ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 2008;68:2176–85.
Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67:1886–93.
Shibuya R, Matsuyama A, Nakamoto M, Shiba E, Kasai T, Hisaoka M. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465:599–605.
Acknowledgements
We thank Ms. Makiko Miyazaki, Ms. Yukari Yamanishi, and Ms. Kaoru Yasumuro for their assistance. The final draft of this manuscript has been edited by Francois Renault-Mihara (ClearBioEditing).
Author information
Authors and Affiliations
Contributions
Writing—original draft: KF and OT. Writing—review and editing: SN, SS, NN, EO, MY, RN, and KW. Data curation—KF, OT, MN, and MM. Pathological analyses—KE. OT was responsible for all work related to this submission as the corresponding author. All authors approved the final version manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The submitted manuscript does not contain information about medical device(s) or drug(s). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Rights and permissions
About this article
Cite this article
Fukushima, K., Tsuji, O., Suzuki, S. et al. Cervical intramedullary recurrent Ewing sarcoma after 10-year disease-free survival in an adult: a case report and review of literature. Spinal Cord Ser Cases 7, 45 (2021). https://doi.org/10.1038/s41394-021-00406-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00406-5