Abstract
Introduction
Aggressive osteoblastoma (AO) represents a rare tumor with borderline features between benign osteoblastoma and osteosarcoma. Having a local aggressive behavior without metastasizing attitude, radical excision is a mainstay treatment. Conversely, spine fusion technique is still debated. We report a rare case of cervicothoracic junction (CTJ) AO and the tailored decision-making process to choose the best treatment.
Case presentation
A 34-year-old man complaining of neck pain was admitted to our department. Cervicothoracic MRI revealed a well-circumscribed lesion involving C7 left lamina with cortical erosion and mild spinal canal invasion. Additionally, STIR sequences exhibited a bright signal spreading through the posterior third of the C7 and T1 vertebrae which on T1-weighted and T2-weighted sequences appeared isointense and hyperintense, respectively. Therefore, the patient underwent a C7 laminotomy. Histology revealed an aggressive variant of osteoblastoma. Therefore, tumor was classified as AO and surgical management was reconsidered. A combined anterior and posterior approach was recommended to reach oncological radicality and spinal stability. At 6-years follow-up, patient remained neurologically intact without signs of recurrence and/or of instability.
Discussion
Due to its rarity and mimicking features, diagnosis of AO results challenging. Due to its aggressive behavior, radical surgery is the mainstay treatment. Conversely, the most suitable fusion technique is still debated. A proper surgical management should be focused on oncological radicality to guarantee the total tumoral removal avoiding progression or recurrences. Similarly, a proper evaluation of the long-term spinal balance should be assessed to avoid developing of spinal deformities or instrumentation failures.
Similar content being viewed by others
Introduction
Aggressive osteoblastoma (AO) was firstly described by Dorfman in 1984 [1]. AO represents a tumor with borderline features between benign osteoblastoma and osteosarcoma. The involvement of spine has been well documented for osteoblastoma and osteosarcoma, accounting about 30% of cases with a predilection for the posterior elements as lamina and pedicles [2,3,4]. Conversely, due to its rarity, the incidence and distribution of its aggressive variant are currently unknown. Having a local invasive behavior without metastasizing attitude, radical resection represents the most recommended treatment for AO [2, 3]. Nevertheless, surgical technique is still debated. Indeed, surgical management for spinal AO requires a careful evaluation of both oncological radicality and spinal stability. To our knowledge, we report the second case of a cervicothoracic junction (CTJ) AO, which requires a peculiar surgical decision-making process.
Case report
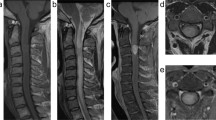
A 34-year-old man with a 6-month history of neck pain, that occasionally radiating towards the proximal left arm, was admitted to our department. His physical exam was normal, without detectable radicular deficits. Cervicothoracic Magnetic Resonance Imaging (MRI) revealed a well-circumscribed T1-isointense and T2-hyperintense lesion, involving the C7 left lamina with cortical erosion and mild spinal canal invasion. After injection of gadolinium, the lesion appeared hyperintense due to the calcification of the matrix and the highly vascular nature of this lesion. Additionally, STIR sequences exhibited a bright signal spreading through the posterior third of the C7 and T1 vertebrae. On T1-weighted and T2-weighted sequences, lesion appeared isointense and hyperintense, respectively. A T2-hyperintense signal around paraspinous soft tissues, known as “flare phenomenon”, was observed. Using 18F- FDG-PET scan, a high uptake limited at C7 half lamina was confirmed (Fig. 1).
a Axial cervical MRI at C7 showing a well-circumscribed T1-isointense lesion involving C7 left lamina with cortical erosion and mild spinal canal invasion; b Sagittal cervical MRI on STIR sequences revealed a high signal involving the posterior third of C7 and T1 vertebrae and spreading through pedicles, laminae, and spinous processes. Additionally, a T2-hyperintese signal around paraspinous soft tissues al C7-T1 levels, namely the “flare phenomenon”, was observed too; c 18F- FDG-PET scan demonstrating a high uptake al the C7 left-half lamina; d Axial CT scan following a left C7 laminotomy confirmed the excision of the growing lytic nidus and the presence of a peripheric sclerotic rim and matrix calcification; e Coronal pre-operative CT angiography demonstrated a normal vertebral artery (VA) course. To note the wide sclerotic involvement and matrix calcification of the C7 and T1 vertebrae.
A computed tomography (CT)-guided cervical bone biopsy was performed, but the sample was inadequate for diagnostic purposes. Therefore, the patient underwent to a C7 laminotomy to obtain a more adequate sample. Histology revealed epithelioid-shaped osteoblasts arranged both in a single layer and aggregated, mixed in a loose fibrovascular stroma with anastomosed bone trabeculae. Basing upon the local aggressive behavior the tumor was classified as a S3 according to Enneking classification and a surgical treatment was recommended.
As pre-operative planning, CT-angiography (CTA) was performed, aiming to evaluate the eventual vertebral artery (VA) pathologies as anomalous course, duplication, fenestration, tortuosity, or kinking. CTA was negative for aberrant course or VA pathologies; further data about degree of sclerosis, and the extent of bony involvement were obtained.
Surgical technique
To obtain a wide excision and to guarantee spinal stability, a combined anterior-posterior approach was recommended. In details, firstly an anterior two-level corpectomy, followed by intersomatic fixation with titanium mesh and cervical anterior plating, was performed; second stage consisted in a complete removal of vertebral arches and pedicles at C7 and T1 levels, followed by posterior C5-T3 screw fixation.
At first, patient was supine, and fiber-optic intubation was performed. Continuous MEP/SSEPs and EMG monitoring was performed. A left-sided approach was chosen. An oblique skin incision, along medial margin of sternocleidomastoid muscle (SCM), was performed. Anterior vertebral bodies of C7 and T1 vertebrae were reached by blunt dissection. Total somatectomy by high-speed drilling combined with ultrasonic aspirator was completed. Finally, a tailored titanium mesh and a cervical anterior plate were positioned. Subsequently, patient was positioned prone and a midline cervical skin incision from C5 to T3 was performed. Paravertebral muscles were detached from transverse processes. The laminae, pedicles, and spinous processes of C7 and T1 vertebrae were removed. Ultimately, we performed a lateral mass screw fixation on C5-C6 and a pedicle screw fixation on T2-T3.
Follow-up
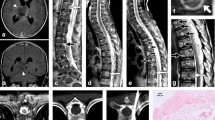
Post-operative CT scan revealed a wide tumor resection and optimal instrumentation placement. On post-operative evaluation, patients complained of mild dysphonia and dysphagia, that gradually recovered within following days. On 10th post-operative day patient was discharged home. At 3 months follow-up, a further cervical MRI confirmed previous data. Adjuvant therapy was not required. An annual clinical and radiological follow-up was recommended. Signs of recurrence and/or instability were not observed on 6-years follow-up, and patient remained neurologically. Therefore, no further follow-up was recommended (Fig. 2).
a Post-operative sagittal CT scan confirmed the extent of resection and the correct placement of the spinal implants; b Sagittal T2-weigheted MRI at 3 months follow-up confirmed oncological radicality and the optimal instrumentation placement; c At 1-year follow-up a conventional lateral cervical radiography confirmed the absence of surgical complication and/or instability; d At 6-year follow-up sagittal cervical MRI showed none sign of recurrence and/or of instability on STIR sequences. AO aggressive osteoblastoma, ASIA American Spinal Injury Association, CT computed tomography, CTJ cervicothoracic junction, ISNCSCI International Standards for Neurological Classification of Spinal Cord Injury, MRI magnetic resonance imaging, SCM sternocleidomastoid muscle, STIR short-TI inversion recovery, VA vertebral artery.
Discussion
Osteoblastoma is a rare tumor, representing about 1% of all bone tumors [2, 3]; and its aggressive variant is even rarer. Being its differential features controversial, the diagnosis results challenging [1, 5, 6]. Classically, AO has a more aggressive local growth and a higher risk of local recurrence than osteoblastoma, but no tendency to metastasize, contrary to osteosarcoma [1, 4]. AO potentially can be found in any bone, however a slight predilection for posterior vertebral element of spine has been reported [4, 7]. Typically, pain is the main symptom [8,9,10,11,12], but also neurological deficits and spine deformity may occur [2]. On imaging, different osteogenic/lytic patterns have been related to AO, mimicking tumors as the aneurysmal bone cyst or the giant cell tumor. CT scan has been suggested as the best tool to analyze the aggressive behavior that distinguishes this tumor. Indeed, CT scan allows to better define bone changes than MRI, that conversely could overestimate tumoral extension. AO tend to display an expansive growth with a lytic nidus surrounded by a sclerotic rim, matrix calcification and cortical bone invasion on CT scans [3, 13]. In the management of this rare tumor, MRI play a significant role in the evaluation of the relationship of the tumor with neighboring vascular and nervous structures. On T1-weighted sequences, AO showed a iso-/low signal whit a high contrast-enhancement due to its high vascularization [3, 13, 14]. A T2-hyperintense signal involving surrounding soft tissue and related to osteoblastoma spreading was firstly described by Crim et al. They called this finding “flare phenomenon” and it represents an intense inflammatory response to tumoral growth [15]. Interestingly, these radiological data are not specific for AO, being exhibited by other spinal tumors as aneurysmal bone cysts, osteosarcomas, Ewing’s sarcomas, lymphomas or bone metastases [2, 3, 13,14,15]. From a histological standpoint, epithelioid osteoblasts, that are polygonal shaped cells with regular oval nuclei and prominent nucleoli, are a typical finding. These cells are usually arranged in clusters or in bundle and mixed to an osteoid matrix. Widespread vascular spaces and a variable number of typical mitotic figures are observed, too [4, 16]. Furthermore, as reported by Gahlot et al., AO can be associated to aneurysmal bone cyst, making its histological differentiation more complex [8].
Different Authors have suggested radical surgery as the mainstay management, while the role of adjuvant treatments remains debated [2, 3, 14, 17]. Due to its highly vascularized nature, some Authors have suggested the pre-operative embolization to facilitate surgery. In details, Ando et al. have reported a successful total removal of a C6-C7 AO, preserving the VA and minimizing intraoperative bleeding [11]. Similarly, Schurl et al. have proved that a multidisciplinary treatment of cervical AO makes it easier to manage [10]. Regarding surgical options, Boriani et al. have proposed the employment of the Enneking staging to guide surgical treatment. Their results suggested that intralesional excision results effective for Enneking stage II tumors; while en-bloc resection with wide free margin is recommended for Enneking stage III lesions [18]. Conversely, chemotherapy and radiotherapy are suggested only for unresectable lesions and recurrences [2, 3, 14].
To choose best surgical approach, the oncological radicality as well as the long-term spinal stability have to be assessed. In literature, all Authors agree on the need of a wide resection to avoid recurrences [8,9,10,11, 19]. Conversely, the most suitable spine fusion technique is still debated. In all reported cases, only a posterior or an anterior instrumentation was preferred. To our knowledge, we present the second case of AO involving the CTJ with a peculiar decision-making process that resulted in favor of a combined anterior/posterior approach. In the reported case, 2 cardinal issues have to be faced.
From a histological standpoint, a wide excision of the C7 and T1 vertebrae guaranteed the best oncological prognosis. However, the involvement of the CTJ required further considerations. This spinal segment is a biomechanical increased stressed area due to the transition of neck, with a high flexibility, to an almost inflexible thoracic spine. Additionally, the CTJ represents the point in which the cervical lordosis curves into thoracic kyphosis. Therefore, reconstruction of the anatomical spinal integrity is mandatory to guarantee long-term function and stability.
In the decision-making process, anterior or posterior approach has been discussed. A single anterior approach would have facilitated the removal of C7 and T1 vertebral bodies involved in tumor growth, however it would have been inefficacy to guarantee a long-term spinal stability. Similarly, a single posterior approach would have led to obtain an optimal spine fusion, but it would have made challenging reaching a wide excision. Against this background, a combined anterior-posterior approach has resulted the most recommended surgical strategy in the presented case. This approach has allowed both a wide excision, with wide free-margins, and to reconstruct spinal balance. Indeed, on the long-term follow-up, no recurrences or tumoral progression were observed as well as post-operative kyphotic deformity or instrumentation failure.
AO involving CTJ has peculiar features in term of local aggressive invasion and spine balance. Consequently, a complex evaluation involving both spine stability and oncological aims is recommended.
References
Dorfman HD, Weiss SW. Borderline osteoblastic tumors: problems in the differential diagnosis of aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn Pathol. 1984;1:215–34. http://www.ncbi.nlm.nih.gov/pubmed/6600112.
Wu M, Xu K, Xie Y, Yan F, Deng Z, Lei J.et al. Diagnostic and management options of osteoblastoma in the spine. Med Sci Monit. 2019;25:1362–72. https://www.medscimonit.com/abstract/index/idArt/913666.
Galgano MA, Goulart CR, Iwenofu H, Chin LS, Lavelle W, Mendel E. Osteoblastomas of the spine: a comprehensive review. Neurosurg Focus. 2016;41:E4 http://www.ncbi.nlm.nih.gov/pubmed/27476846.
Lucas DR, Unni KK, McLeod RA, O’Connor MI, Sim FH. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. 1994;25:117–34. http://www.ncbi.nlm.nih.gov/pubmed/8119712.
Söylemez MS, Ozger H, Alpan B, Ozkan K, Salduz A, Bilgic B.et al. Clinical management of a challenging malignancy, osteoblastoma-like osteosarcoma: a report of four cases and a review of the literature. Ther Clin Risk Manag. 2016;12:1261–70. https://www.dovepress.com/clinical-management-of-a-challenging-malignancy-osteoblastoma-like-ost-peer-reviewed-article-TCRM.
Della Rocca C.Huvos AG, Osteoblastoma: varied histological presentations with a benign clinical course. Am J Surg Pathol. 1996;20:841–50. http://journals.lww.com/00000478-199607000-00007.
Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19:678–89. http://www.ncbi.nlm.nih.gov/pubmed/22052644.
Gahlot N, Jalan D, Elhence P. C4 cervical spine osteoblastoma associated with secondary aneurysmal bone cyst in an adolescent patient: 2-year follow-up. Spinal Cord Ser Cases. 2019;5:89 http://www.nature.com/articles/s41394-019-0233-5.
Nishida K, Doita M, Kawahara N, Tomita K, Kurosaka M. Total en bloc spondylectomy in the treatment of aggressive osteoblastoma of the thoracic spine. Orthopedics. 2008;31:403 http://www.ncbi.nlm.nih.gov/pubmed/19292265.
Schur S, Camlioglu E, Jung S, Powell T, Gutman G, Golan J. Preoperative embolization and complete tumoral resection of a cervical aggressive epithelioid osteoblastoma. World Neurosurg. 2017;106. http://www.ncbi.nlm.nih.gov/pubmed/28710051
Ando K, Imagama S, Kobayashi K, Nishida Y, Ishiguro N. Aggressive osteoblastoma of the cervical spine involving the canal and vertebral artery: a case report. Eur Spine J. 2017;26:111–6. http://www.ncbi.nlm.nih.gov/pubmed/27981453.
Kawahara N, Tomita K, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: surgical techniques and related basic background. Orthop Clin North Am. 2009;40:47–63. http://www.ncbi.nlm.nih.gov/pubmed/19064055.
Orguc S, Arkun R. Primary tumors of the spine. Semin Musculoskelet Radio. 2014;18:280–99. http://www.ncbi.nlm.nih.gov/pubmed/24896744.
Boriani S, Capanna R, Donati D, Levine A, Picci P, Savini R Osteoblastoma of the spine. Clin Orthop Relat Res. 1992;37–45. http://www.ncbi.nlm.nih.gov/pubmed/1563167
Crim JR, Mirra JM, Eckardt JJ, Seeger LL. Widespread inflammatory response to osteoblastoma: the flare phenomenon. Radiology. 1990;177:835–6. https://doi.org/10.1148/radiology.177.3.2243998.
Revell PA, Scholtz CL. Aggressive osteoblastoma. J Pathol. 1979;127:195–8. https://doi.org/10.1002/path.1711270406.
Versteeg AL, Dea N, Boriani S, Varga PP, Luzzati A, Fehlings MG.et al. Surgical management of spinal osteoblastomas. J Neurosurg Spine. 2017;27:321–7. https://thejns.org/view/journals/j-neurosurg-spine/27/3/article-p321.xml.
Boriani S, Amendola L, Bandiera S, Simoes CE, Alberghini M, Di Fiore M. et al. Staging and treatment of osteoblastoma in the mobile spine: a review of 51 cases. Eur Spine J. 2012;21:2003–10. https://doi.org/10.1007/s00586-012-2395-8.
López-Puerta JM, Fernández-Marín MR, Martín Benlloch JA, Lorente R. Spinal osteoid osteoma recurring as an aggressive osteoblastoma. Neurocirugia. 31:146–50. http://www.ncbi.nlm.nih.gov/pubmed/31488355
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all the following: the conception and design of the study; drafting the article or revising it critically for important intellectual content; final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
An informed consent for publication has been obtained from the patient.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Elia, A., Vitali, M., Grasso, V. et al. Tailored surgery on aggressive osteoblastoma involving the cervicothoracic junction: an oncological and spinal stability long-term follow-up. Spinal Cord Ser Cases 7, 99 (2021). https://doi.org/10.1038/s41394-021-00463-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00463-w