Abstract
Study design
A questionnaire validity study.
Objectives
To perform the translation, cross-cultural adaptation, and analysis of the measurement properties of the Brazilian Portuguese version of the Spinal Cord Injury Pain Instrument (SCIPI) for the screening of neuropathic pain in spinal cord injury.
Setting
Neurorehabilitation hospital in north-eastern Brazil.
Methods
We performed the translation and cross-cultural adaptation of the SCIPI. The pre-final version was applied in 10 patients with spinal cord injury sequelae and pain report. The final version of the SCIPI was applied to 100 patients. The measurement properties evaluated were structural validity, test-retest reliability, internal consistency, construct validity, and diagnostic accuracy.
Results
None of the items in the pre-final version of the SCIPI had any comprehension problems. The one-dimensional structure of the final version of the SCIPI was adequate. There were significant correlations between the SCIPI and the Douleur Neuropathique 4 (rho = 0.546), as well as adequate test-retest reliability (intraclass correlation coefficient = 0.89, kappa ≥ 0.79), internal consistency (Cronbach’s alpha ≥ 0.76), and diagnostic accuracy (area under the curve = 0.860).
Conclusion
The Brazilian version of the SCIPI presents measurement properties that are suitable for measuring neuropathic pain related to spinal cord injury.
Similar content being viewed by others
Introduction
Spinal cord injury generates important impacts in terms of healthcare, disabilities, economic level, and quality of life [1]. Pain is a frequent problem in this population, with 70% presenting chronic pain symptoms [2] and approximately 57.6% of the pains having neuropathic characteristics [1].
The association between nociceptive pain and neuropathic pain is common in people with spinal cord injury sequelae [3]. The correct and accurate diagnosis of pain, taking into account the different pathophysiological mechanisms (nociceptive, neuropathic, and nociplastic), is of great importance for successful treatment, considering that conduction of the treatment of neuropathic pain is different from that of a nociceptive pain [4, 5].
Considering the current lack of a gold standard method to diagnose neuropathic pain, there are several instruments to perform this screening [6]. In Brazil, adapted and validated versions of the Douleur Neuropathique 4 (DN4), Leeds Assessment of Neuropathic Pain Questionnaire (LANSS), Neuropathic Pain Symptom Inventory (NPSI), and Pain Quality Assessment Scale (PQAS) are available [7].
These more generic neuropathic pain tracking instruments, which are widely used for peripheral causes of pain, have lower measurement properties when applied to homogeneous samples and from central causes of pain (as in the case of pain related to spinal cord injury), reaching indices of diagnostic accuracy from 55 to 88% depending on the instrument applied [8]. Another aspect to be considered is that these instruments are based on physical examination, in addition to the verbal descriptors of pain, as spinal cord injury has peculiarities inherent to the injury, such as changes in skin sensitivity, in which the items of these generic instruments cannot be sensitive, thus interfering with accuracy [8, 9].
The Spinal Cord Injury Pain Instrument (SCIPI) is a rapid, standardised and valid (for English and German) screening instrument to detect neuropathic pain manifestations in a population with spinal cord injury sequelae [8, 9]. Its differential is based on an updated review of the definition of neuropathic pain and updated guidelines for detecting this condition. It is administered in the form of an interview or self-report, does not require clinical physical examination, and can be applied when the patient is unavailable in person. In addition, the SCIPI has an item aimed at the changes in skin sensitivity caused by spinal cord injury [8, 9].
Thus, the aim of this study was to perform the translation, cross-cultural adaptation, and analysis of the measurement properties of the Brazilian Portuguese version of the SCIPI for the screening of neuropathic pain in spinal cord injury.
Methods
Study design
This is a questionnaire validation study carried out according to the Guideline for the Process of Cross-cultural Adaptation of Self-Report Measures [10] and the Consensus-based Standards for the Selection of Health Measurement Instruments [11]. The authorisation to carry out the translation and adaptation of the SCIPI into Brazilian Portuguese was granted via email by one of the authors of the questionnaire (Dr. Thomas N. Bryce).
We carried out this research at the Sarah Network of Rehabilitation Hospitals (São Luís, MA, Brazil); all study procedures were previously approved by the institution’s research ethics committee (opinion number 3.714.777).
Participants
All participants included in the research were treated under the Hospital’s Spinal Cord Injury Program. Patients of both sex, aged over 18 years, and with painful complaints arising after spinal cord injury were included. We consider the following exclusion criteria: Patients diagnosed with severe cognitive and/or psychiatric disorders (by the team of doctors and psychologists of the hospital during routine assessments); pain above the level of spinal cord injury (since pain above the level of spinal cord injury may be related to another cause, e.g., musculoskeletal disorders); and patients who did not speak Brazilian Portuguese as their native language.
A minimum sample size of 100 patients was used for validity analyses [11]. For reliability analyses, we used a sub-sample composed of 50 patients; the questionnaire was applied at two timepoints by one of the physiotherapists from the spinal cord injury program team, with an interval of 4 to 7 days between assessments [11].
Spinal Cord Injury Pain Instrument (SCIPI)
The SCIPI is a 4-item instrument that investigates the pain characteristics of patients with spinal cord injury. For each item, a dichotomous answer is possible (yes = 1, no = 0). The total score ranges from 0 to 4; a score ≥ 2 indicates the presence of neuropathic pain.
Translation and cross-cultural adaptation
The SCIPI translation and cross-cultural adaptation process into Brazilian Portuguese followed the criteria of Beaton et al.[10]; however, the pre-final version test was applied to 10 patients [9, 12]. The stages for the translation and cross-cultural adaptation of the SCIPI are described below.
-
1.
Translation: Two independent translators (both with Brazilian Portuguese as their mother tongue and who were fluent in English) translated the original version of the SCIPI into Brazilian Portuguese.
-
2.
Synthesis of translations: After discussions and revisions, the two translators (under observation by one of the researchers) synthesized the two versions of the questionnaire translated independently and produced a single version of the SCIPI in a consensual manner.
-
3.
Back-translation: Two independent translators (without technical knowledge of subjects in the health area), both with English as their mother tongue and who was fluent in Portuguese, translated the Brazilian Portuguese version of the SCIPI back into English, with no prior knowledge of the original version of the questionnaire.
-
4.
Analysis by the expert committee: Two specialists in the field of pain and rehabilitation, together with the four translators involved in the adaptation process, defined the pre-final version of SCIPI in a manner agreed upon by all members of the committee.
-
5.
Pre-final version test: Considering the characteristics of the population with spinal cord injury sequelae, we applied the pre-final version of the SCIPI in a sample size of 10 individuals [9, 12], according to the same inclusion criteria established here. Participants answered the SCIPI questions in the form of an interview (carried out by the interviewer without receiving any kind of influence); at the end of the interview, answered “yes” or “no” about the understanding of each question in the questionnaire. Questions that were not understood by more than 20% of the participants would be reformulated and tested again in a new sample of 10 participants until the desired level of understanding was reached [13], thus establishing the final version of the SCIPI in Brazilian Portuguese.
-
6.
Test of the final version: In order to verify the measurement properties of the instrument, the cross-culturally adapted final version of the SCIPI was applied to 100 patients.
Other clinical measurements
In addition to the final version of the SCIPI, we applied the instruments DN4, LANSS, Numerical Pain Scale (NPS), and the anamnesis form containing information on sociodemographic, clinical, and spinal cord injury characteristics. The DN4 was used to validate the construct, and the LANSS, and NPS were used to characterize the pain of the sample; the assessments were performed by a physiotherapist from the neurorehabilitation team in spinal cord injury.
Statistical analysis
In descriptive analysis, we used mean, standard deviation (SD), frequency and percentage. These data were processed using the Excel program (Microsoft, Redmond, WA, USA).
Internal consistency was obtained using Cronbach’s alpha, with an acceptable value above 0.70 [14]. Test-retest reliability was assessed via intraclass correlation coefficient (ICC) with a 2-way random-effects model and acceptable values above 0.75 [15]. Also, the standard error of measurement (SEM) was calculated [16]. For each item of the SCIPI, the kappa value was calculated, with acceptable values above 0.60 [17].
For the correlations between the questionnaires, the normality of the data was initially verified using the Kolmogorov-Smirnov test. For the construct validity, Spearman’s correlation coefficient (rho) was used to determine the magnitude of correlation between the SCIPI and the DN4. Our hypothesis is that the SCIPI demonstrates a good correlation, with a correlation magnitude greater than 0.50 with DN4, as it presents similar constructs [11, 18].
The internal structure of the SCIPI was evaluated through confirmatory factor analysis (CFA), with tetrachoric correlations and the robust diagonally weighted least squares (RDWLS) extraction method [19]. CFA processing was performed in R Studio (version 1.1.453, Boston, MA, USA), using the lavaan and semPlot packages. The Model fit had the following classification: values greater than 0.90 were considered adequate for comparative fit index (CFI) and Tucker-Lewis index (TLI), while values less than 0.08 were considered adequate for root mean square error of approximation (RMSEA) and standardised root mean square residual (SRMR). Values below 3.00 were considered adequate in the interpretation of the chi-square/degree of freedom (DF). In the CFA, factor loadings equal to or greater than 0.40 were considered adequate for the domain [14, 20].
We used the ROC curve to determine the diagnostic accuracy, with the following interpretation of the area under the curve (AUC):[21, 22] 0.5 (due to chance); > 0.5 to 0.7 (low degree of precision), > 0.7 to 0.9 (moderate degree of precision); > 0.9 and < 1.0 (high degree of accuracy); and 1.0 (perfect test). Determination of the best cut-off point was based on the lowest value obtained from the equation (1 – sensitivity)2 + (1 – specificity)2 [22]. In addition, positive and negative predictive values and positive and negative likelihood ratios were calculated [23]. Data were processed by SPSS (version 17, Chicago, IL, USA).
Results
Sample characteristics
The sample consisted of 100 patients, 69% male, with a mean age of 41 years (SD = 12.16); in addition, 53% were married, 45% had completed/incomplete secondary education, and 81% had no occupational activity. Details of the characteristics of spinal cord injury and pain are described in Table 1.
Regarding the characteristics of spinal cord injury, 67% had a cause of injury of traumatic origin. In total, 58% of patients presented a neurological level of thoracic spinal cord injury and 58% of spinal cord injuries were incomplete (ASIA-AIS Deficiency Scale classified as B, C, or D). The mean duration of spinal cord injury was 7 years (SD = 8.55); pain complaints at the neurological level of spinal cord injury were reported in 60% of patients, with mean pain duration of 60 months (SD = 74.32). The number of medications used on average was 4.24 (SD = 2.25), including several categories of medications for the management of secondary dysfunctions (caused by spinal cord injury or previous comorbidities).
Regarding to the questionnaires used in the study, we observed the following mean scores: 2.47 (SD = 1.11) for SCIPI, 5.48 (SD = 2.34) for DN4, 11.28 (SD = 5.95) for LANSS and 6.16 (SD = 2.40) for the NPS.
Cross-cultural adaptation
In the stage of translation and cross-cultural adaptation, there was no need to adapt any specific term. The pre-final version of the SCIPI was applied to 10 patients with spinal cord injury sequelae and who had pain complaints; no item in the questionnaire reached a misunderstanding rate greater than 20%. Thus, the final version of the SCIPI was established in the Brazilian Portuguese language (Supplement 1).
Structural validity
Based on the structure presented in the study by Franz et al. [9], we performed CFA considering the 4 items and one-dimensional structure. Thus, the fit indices with adequate values were found: Chi-square/DF = 0.808, CFI = 1.000, TLI = 1.000, RMSEA (90% CI) = 0.000 (0.000–0.187), and SRMR = 0.061. Regarding the factor loading, as shown in Fig. 1, only item 4 presented a value lower than 0.40; however, considering the good fit indices, we chose to keep the original SCIPI structure.
Construct validity
The SCIPI score was correlated with the DN4 score. Significant correlation was found with correlation magnitude greater than 0.50 (rho = 0.546, p-value < 0.001).
Reliability and internal consistency
Table 2 shows the appropriate values for test-retest reliability and internal consistency, with ICC = 0.89 (p-value < 0.001), kappa ≥ 0.79 (p-value < 0.001), and Cronbach’s alpha ≥ 0.76.
Accuracy
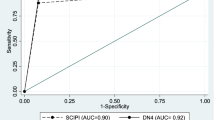
For this analysis, we considered DN4 to be a reference instrument to classify patients into two categories (with or without neuropathic pain) due to its higher correlation magnitude. The distribution of volunteers with and without neuropathic pain according to the DN4 and SCPI instruments is shown in Table 3. The SCIPI had moderate diagnostic accuracy, as described in Fig. 2 and Table 4, with an AUC value of 0.86, sensitivity of 93.8%, and specificity of 65%.
Discussion
We observed that the Brazilian version of the SCIPI has a reliable, one-dimensional structure, and a valid construct. We used the CFA to verify the internal structure of SCIPI, which allowed the theoretical factor structure of the observed data to be tested [24] and demonstrated that the instrument has a one-dimensional structure with observed variables is related to the latent variable. Only item 4 of the SCIPI in the Brazilian version had a factor loading of less than 0.40 (this item investigates whether the pain occurs in a region of the skin that is not sensitive). Our hypothesis for this aspect is that regions with partial changes in sensitivity were not considered, such as hypoesthesia, a frequent condition in incomplete spinal cord injury or spinal cord injuries from non-traumatic causes.
Our study demonstrated that the SCIPI has a good correlation with the DN4 (rho = 0.546). As there is no gold standard tool/method for neuropathic pain assessment, construct validity was verified by correlating the SCIPI score with the DN4 score. We chose to use DN4 for two reasons: it is an instrument recommended by the Neuropathic Pain Special Interest Group for neuropathic pain screening [6], and it has suitable measurement properties into the Brazilian Portuguese language [25, 26].
The DN4 was translated, adapted, and validated for 11 languages, including Brazilian Portuguese. All adaptations demonstrated low levels of cross-cultural validity, as shown in the systematic review conducted by Mathieson et al. [6], although this review emphasises that the Brazilian Portuguese version has more satisfactory evidence among all other versions. In the validation of the Brazilian Portuguese version carried out by Santos et al. [24], the DN4 obtained a Cronbach’s alpha of 0.713 (considered reasonable), ICC of 0.62 (considered moderate), and factor loadings between 0.502 and 0.817 in the factor analysis.
Regarding diagnostic accuracy, the Brazilian version of the SCIPI had a sensitivity value of 84%, similar to the German version (86%), and higher than the original version (72%). The Brazilian version of the SCIPI proved to be less specific (65%) when compared to the original (78%) and the German (84%) versions [8, 9]. The assessment of the diagnostic accuracy of the original and German version SCIPI used the diagnosis issued by a committee of medical experts as the gold standard, while our study used the DN4 as a reference. We also verified other forms of accuracy, such as predictive values and likelihood ratio, but these data were not presented in other SCIPI validation studies.
Although the DN4 has adequate measurement properties, SCIPI was developed based on the clinical peculiarities of a person with spinal cord injury, validated in its original version according to revised definitions and guidelines for the clinical detection of neuropathic pain.
This study has limitations that must be described. The diagnostic evaluation during the performance of the accuracy was not performed by physicians’ team specializing in neuropathic pain. Inter-examiner reliability was not tested, as we considered that assessments performed by two examiners in sequence using a self-report instrument with only 4 items would likely generate equal responses due to the patient’s memory. Finally, considering the COVID-19 pandemic period, we performed the pre-final test phase in only 10 patients, although there was 100% understanding for all items.
Conclusion
The Brazilian version of the SCIPI presents measurement properties suitable for measuring neuropathic pain related to spinal cord injury.
Data availability
The database of the present study is available in Supplement 2.
References
Teixeira MJ, Paiva WS, Assis MS, Fonoff ET, Bor-Seng-Shu E, Cecon AD, et al. Neuropathic pain in patients with spinal cord injury: Report of 213 patients. Arq Neuropsiquiatr. 2013;71:600–3. https://doi.org/10.1590/0004-282X20130103.
Aquarone RL, Faro ACMe, Nogueira PC. Central neuropathic pain: Implications on quality of life of spinal cord injury patients. Rev Dor. 2015;16:280–4. https://doi.org/10.5935/1806-0013.20150057.
Burke D, Lennon O, Fullen BM. Quality of life after spinal cord injury: The impact of pain. Eur J Pain (UK). 2018;22:1662–72. https://doi.org/10.1002/ejp.1248.
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976–82. https://doi.org/10.1097/j.pain.0000000000001939.
de Miguel M, Kraychete DC. Pain in Patients with Spinal Cord Injury: A Review. Braz J Anesthesiol. 2009;59:350–7. https://doi.org/10.1590/s0034-70942009000300011.
Mathieson S, Maher CG, Terwee CB, Folly De Campos T, Lin CWC, et al. Neuropathic pain screening questionnaires have limited measurement properties. A systematic review. J Clin Epidemiol. 2015;68:957–66. https://doi.org/10.1016/j.jclinepi.2015.03.010.
Eckeli FD, Teixeira RA, Gouvêa ÁL. Neuropathic pain evaluation tools. Rev Dor. 2016;17:20–22. https://doi.org/10.5935/1806-0013.20160041.
Bryce TN, Richards JS, Bombardier CH, Dijkers MP, Fann JR, Brooks L, et al. Screening for neuropathic pain after spinal cord injury with the Spinal Cord Injury Pain Instrument (SCIPI): A preliminary validation study. Spinal Cord. 2014;52:407–12. https://doi.org/10.1038/sc.2014.21.
Franz S, Schuld C, Wilder-Smith EP, Heutehaus L, Lang S, Gantz S, et al. Spinal Cord Injury Pain Instrument and painDETECT questionnaire: Convergent construct validity in individuals with Spinal Cord Injury. Eur J Pain (UK). 2017;21:1642–56. https://doi.org/10.1002/ejp.1069.
Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Philos Pa 1976). 2000;25:3186–91. https://doi.org/10.1080/000163599428823.
Mokkink LB, Prinsen CA, Patrick DL, Alonso J, Bouter LM, de Vet HC, et al. COSMIN study design checklist for patient-reported outcome measurement instruments. Amsterdam, The Netherlands. 2019; 1–32. https://doi.org/10.1136/gutjnl-2020-320729.
Ferreira L, Neves AN, Campana MB, Tavares M da CGCF. Guia da AAOS/IWH: sugestões para adaptação transcultural de escalas. Avaliação Psicológica. 2014;13:457–61.
Da Silva Rodrigues EK, De Cássia Registro Fonseca M, Macdermid JC. Brazilian version of the Patient Rated Wrist Evaluation (PRWE-BR): Cross-cultural adaptation, internal consistency, test-retest reliability and construct validity. J Hand Ther. 2015;28:69–76. https://doi.org/10.1016/j.jht.2014.09.008.
Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. MPR-online. 2003;8:23–74.
Fleiss JL. The Design and Analysis of Clinical Experiments. John Wiley & Sons, New York. 1986.
Bassi D, Santos-de-Araújo AD, Camargo PF, Dibai-Filho AV, da Fonseca MA, Mendes RG, et al. Inter and intra-rater reliability of short-term measurement of heart rate variability on rest in diabetic type 2 patients. J Med Syst. 2018; 42:. https://doi.org/10.1007/s10916-018-1101-8.
Sim J, Wright CC. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–68. https://doi.org/10.1093/ptj/85.3.257.
Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1147–57. https://doi.org/10.1007/s11136-018-1798-3.
Asparouhov T, Muth B, Muthén B. Simple second order chi-square correction. 2010; 1–8.
BROWN TA. Confirmatory factor analysis for applied research. New York, NY US. 2006.
Greiner M, Pfeiffer D, Smith RD, Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. https://doi.org/10.1016/0009-2797(70)90001-3.
Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr Int J Paediatr. 2007;96:644–7. https://doi.org/10.1111/j.1651-2227.2006.00178.x.
Eusebi P. Diagnostic accuracy measures. Cerebrovasc Dis. 2013;36:267–72. https://doi.org/10.1159/000353863.
Jacob ALO. Uso da Análise Fatorial: Algumas Diretrizes para Pesquisadores. In: Análise fatorial para pesquisadores. LabPAM Saber e Tecnologia, Brasília, 2012
Santos JG, Brito JO, de Andrade DC, Kaziyama VM, Ferreira KA, Souza I, et al. Translation to portuguese and validation of the douleur Neuropathique 4 questionnaire. J Pain. 2010;11:484–90. https://doi.org/10.1016/j.jpain.2009.09.014.
Barbosa M, Bennett MI, Verissimo R, Carvalho D. Cross-cultural psychometric assessment of the leeds assessment of neuropathic symptoms and signs (LANSS) pain scale in the Portuguese population. Pain Pr. 2014;14:620–4. https://doi.org/10.1111/papr.12118.
Acknowledgements
We thank all patients.
Funding
This work was partially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), finance code 001.
Author information
Authors and Affiliations
Contributions
AVDF designed the study; MC and collected the data; MC, AVDF and CAFPG analyzed and interpreted the data; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (opinion number 3.714.777).
Consent for publication
The consent for publication was obtained from all individual participants included in the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cacere, M., Pontes-Silva, A., Fidelis-de-Paula-Gomes, C.A. et al. Translation, cross-cultural adaptation, and analysis of the measurement properties of the Brazilian Portuguese version of the spinal cord injury pain instrument. Spinal Cord 60, 820–825 (2022). https://doi.org/10.1038/s41393-022-00800-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00800-2