Abstract
Study design
Retrospective case series
Setting
Three hospitals in China.
Objective
Previous research indicates that only neurological status on admission determines prognosis of acute hyperextension myelopathy (AHM). The object of this study is to analyze other unfavorable predictors of AHM in children.
Methods
The clinical data of children with AHM were retrospectively analyzed. The ASIA impairment scale (AIS) grade was recorded upon admission and at last follow-up. Intramedullary lesion length (IMLL) was measured in the sagittal T2-weighted imaging (T2WI) within two weeks after onset; gadolinium enhancement in the cord was recorded for each patient. Relationships among AIS grade, IMLL, gadolinium enhancement in the cord, and clinical improvement were assessed.
Results
A total of 33 patients were included in this retrospective study. IMLL between complete and incomplete injury was significantly different (p < 0.01) in the subacute stage, and no difference was observed in the acute stage. Correlation analysis revealed that AIS grade on admission (r = 0.906, p < 0.001) was significantly positively correlated with clinical improvement. IMLL (r = −0.608, p < 0.001) and abnormal gadolinium enhancement (r = −0.816, p < 0.001) in the cord in the subacute stage were significantly negatively correlated with clinical improvement. There were no associations between IMLL in the acute stage and clinical improvement (r = −0.248, p = 0.242). The statistically significant predictors of clinical improvement were AIS grade on admission, IMLL in the subacute stage, and abnormal gadolinium enhancement.
Conclusion
IMLL in the subacute stage and abnormal gadolinium enhancement in the cord are two other prognostic predictors of AHM in children.
Similar content being viewed by others
Introduction
The term acute hyperextension myelopathy (AHM) was first recommended to replace the term surfer’s myelopathy (SM) [1]. SM or AHM is a rare, acute, non-traumatic thoracic spinal cord injury (SCI) [2,3,4]. The initiating factor of SM among novice surfers is excessive and/or repetitive spine hyperextension when paddling [3,4,5,6]. Recently, SM has been reported among adults, adolescents, and children with no history of surfing [1, 5, 7,8,9,10,11,12]. Additionally, the characteristic of these patients before onset was that all had been involved in some sustained and/or repeated spine hyperextension activities [1, 5, 7,8,9,10,11,12,13,14,15,16], such as yoga, gymnastics, ballet, cheerleading, acrobatics, pilates, backbends, etc., which were not associated with surfing. A novel broader term acute hyperextension myelopathy (AHM) was introduced [1, 7, 8], allowing the inclusion of all patients with non-traumatic thoracic SCI due to repeated spinal hyperextension.
The exact etiology of AHM or SM remains unknown [2,3,4,5, 14, 15], but spinal cord ischemic injury due to spinal venous hypertension resulting from venous drainage disorders of the cord after repeated hyperextension of the spine plays a critical role in the pathological process [6, 7, 17,18,19]. Due to its rarity and scarce serial follow-up, the long-term follow-up evolution of imaging findings and clinical data is not well-documented [2,3,4,5,6,7, 13, 15]. As scattered reports on AHM lack systematic research, this disease is not well known.
Many children become involved in repeated spine hyperextension activities, and some have experienced AHM [1, 3, 7, 9, 12, 14,15,16]. Serious complications will occur in children with complete SCI [7]. Early and accurate prediction of prognosis can benefit patients’ rehabilitation and prevention of related complications. Previous studies indicated that only neurological status (American Spinal Injury Association Impairment Scale [AIS]) on admission determined the prognosis of AHM [2,3,4,5,6, 15, 20], and magnetic resonance imaging (MRI) characteristics were not associated with clinical improvement [13], which contradicts other findings [7, 21,22,23,24]. Recent findings indicate that there are limitations in using AIS in young children, and exact assessment of the severity and AIS grade of the SCI is difficult with children [16, 25]. Other predictors are needed to assess the severity of neurological defects. Here, we performed a retrospective study that aimed to analyze other predictors of AHM, which can facilitate future research and treatment.
Methods
This study was a retrospective review of the data of pediatric patients diagnosed with AHM from July 2015 to August 2020. The patients were selected using the following inclusion criteria: (1) acute onset of atraumatic thoracic SCI after repeated hyperextension of the spine; (2) healthy and without any other neurological defects before onset; and (3) follow-up time of more than 6 months. Patients with the following exclusion criteria were excluded: (1) traumatic SCI; (2) patients with fracture or dislocation of the spine; (3) children with severe neurological diseases or infection, neoplasm, vascular malformation, etc. (4) cervical spinal cord injury. This study was approved by the Ethics Committee of the Hospital and Medical College.
Neurological function was assessed using the AIS grade on admission and at follow-up. The patients were divided into two groups according to their AIS grade. Group A was complete SCI, that is AIS A, and group B was incomplete SCI, that is AIS B, C, or better. Clinical improvement was defined as a shift toward improved AIS grade from admission to follow-up. Improvement in AIS grade was recorded for each patient. Initial and follow-up MRI data were analyzed and compared. We substituted the number of vertebral segments involved in intramedullary high-intensity on MRI for intramedullary lesion length (IMLL) [7, 13, 15, 21, 22]. IMLL within two weeks after onset was recorded by the number of vertebral segments each time. The contrast-enhanced MRI outcomes were also analyzed.
Statistical analyses
Descriptive statistics were used to describe the patient characteristics. Continuous variables are presented as median and standard deviation (SD). The proportions are presented as numbers and percentages. Comparisons between groups of continuous variables were performed using unpaired t-tests. The chi-square test or Fisher’s exact test, when appropriate, was used to estimate all associations between categorical variables. Correlations among clinical improvement, AIS grade on admission, IMLL in acute or subacute stage, and gadolinium enhancement in the spinal cord were evaluated using the Spearman non-parametric test. Statistical significance was set at P < 0.05.
Results
Study population
We included 33 patients (30 females and 3 males), aged 3.5–10 years (mean 6.0 years). All patients were healthy without fever, diarrhea, or vaccination before onset. None of the patients had acute trauma, pre-existing neurological dysfunction, or other symptoms. The common characteristic of movements before onset was sustained and/or repeated hyperextension of the spine. Group A included 20 patients with complete SCI, group B included 13 patients with incomplete SCI. The follow-up time was 6–61 months (mean, 26 months). There were no significant differences in age, sex, symptoms, and follow-up time between groups A and B (Table 1).
Clinical characteristics
Patients presented with acute back pain, progressive bilateral lower-extremity weakness, paresthesia, urinary retention, bowel and bladder dysfunction, and paralysis. The back pain was mild or severe. Soon after (incubation period ranged from 10 min to 8 h), the patients developed progressive paresthesia, weakness, or paraplegia. At the post-admission assessment, the patients’ neurological dysfunction seldom deteriorated. The neurological level of SCI ranged from T6 to L3, and the highest (84.8%) was at T9 to T12. No abnormalities were observed in the nerve functions of the upper limbs or brain. Blood tests and diagnostic analysis of cerebrospinal fluid (CSF) were negative or unremarkable, without any diagnostic significance.
MRI findings
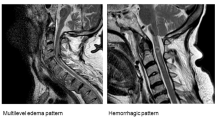
In group A, 16, and 21 MRIs were performed within the acute stage (the time to perform MRI was 2–46 h after onset, mean 16.1 h) and subacute stage (the time was 3–14 days, mean 7.7 days), respectively. Similarly, 8 and 12 MRIs in group B were performed within the acute stage (the time to perform MRI was 6–44 h after onset, mean 18.5 h) and subacute stage (the time was 5–12 days, mean 7.4 days), respectively. The time to perform MRI in the acute stage and subacute stage between Group A and Group B was not significantly different (p > 0.05). There was no ligament or disc injury, spinal stenosis, neoplasm, vascular malformation, or hematoma in any of the patients. All patients had a longitudinally diffuse intramedullary high-intensity signal (extending > 5 vertebral segments) in the acute stage, from the mid-thoracic down to the lower thoracic level (most ranged from T5 to T9) to the conus on the sagittal T2WI (Figs. 1 and 2). In the subacute stage, the MRI showed cranial progression of T2-hyperintensity (mean: 4.5 ± 2.5 vertebral segments) in group A (Fig. 2), and no neurological deterioration was detected. However, in group B, the T2-hyperintensity resolved at different levels in most patients in the subacute stage (Fig. 1).
A MRI in acute stage shows intramedullary high-intensity from mid-thoracic cord to the conus in a 7-year-old girl with incomplete SCI. B Contrast-enhanced MRI at day 5 shows peripheral enhancement of distal spinal cord. C The MRI at day 5 shows intramedullary high-intensity resolved with neurological recovery.
A MRI in acute stage shows intramedullary high-intensity from mid-thoracic cord to conus in a complete SCI patient. B The MRI at day 3 shows a cranial progression of the T2-hyperintensity, and hematoma in cord. C Contrast-enhanced MRI shows abnormal gadolinium enhancement in low thoracic cord. D Atrophic spinal cord is found.
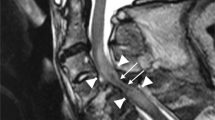
In the acute stage, IMLL was 6.9 ± 1.7 vertebral segments in group A and 6.7 ± 1.3 in group B. In the subacute stage, IMLL was 11.6 ± 2.3 vertebral segments in group A and 4.9 ± 3.8 in group B. IMLL did not differ significantly between groups A and B (p = 0.710) in the acute stage, but was significantly different in subacute stage (p < 0.0001). IMLL in group A was significantly different between the acute and subacute stages (p < 0.0001), but no significant difference was observed in group B (p = 0.242) (Table 1). IMLL in group B decreased rapidly in the subacute stage and recovered significantly faster among patients with milder neurological dysfunction. There were no associations between IMLL in the acute stage and AIS grade on admission (r = −0.161, p = 0.453), but there was a significantly negative association between IMLL in the subacute stage and AIS grade on admission (r = −0.696, p < 0.01). In the subacute stage, hemorrhage at the low spinal cord was observed in two patients in group A (Fig. 2), and no hemorrhage was observed in group B (Fig. 1).
Five and 2 contrast-enhanced MRIs were performed within the acute stage in Group A and Group B, respectively, and no abnormal gadolinium enhancement in the cord was noted. Contrast-enhanced MRI was performed in the subacute stage for 15 patients in group A, and peripheral contrast and abnormal gadolinium enhancement in the distal cord was noted in 14 patients (Fig. 2). In group B, contrast-enhanced MRI was performed for 6 patients in the subacute stage, only peripheral pial enhancement of the low thoracic cord was detected, and no abnormal gadolinium enhancement in the cord was noted (Table 1, Fig. 1). There was a significant negative association between abnormal enhancement in the cord and AIS grade on admission (r = −0.720, p < 0.001). Fourteen patients from group A displayed an atrophic spinal cord from the mid-thoracic cord (ranging from T6–T10) to the conus at 0.5–4 months later (Fig. 2). The atrophic segments of the spinal cord were consistent with peripheral contrast and abnormal gadolinium enhancement segments in the low thoracic cord. In group B, no atrophic cords were observed.
Prognosis correlation
Systematic comprehensive treatments were applied [3,4,5, 7, 13, 16]. However, during follow-up, conversion from a complete to incomplete injury was not detected in group A, and 14 patients in this group had marked spinal cord atrophy from the mid- or low-thoracic cord to the conus. Correlation analysis revealed that AIS grade on admission was significantly positively correlated with clinical improvement (r = 0.906, p < 0.001). The milder the neurological dysfunction, the better the neurological recovery. A good prognosis was expected for patients presenting with AIS C or better (Table 2).
The statistically significant predictors of clinical improvement were AIS grade on admission, IMLL in the subacute stage, and abnormal gadolinium enhancement (Table 3). Correlation analysis also found that there were no associations between IMLL in the acute stage and clinical improvement (r = −0.248, p = 0.242), and IMLL in group A was not different from that in group B in the acute stage (Table 1). However, there was a significant negative association between IMLL in the subacute stage and clinical improvement (r = −0.608, p < 0.001). Furthermore, clinical improvement was significantly negatively associated with abnormal gadolinium enhancement in the low thoracic cord (r = −0.816, p < 0.001).
Discussion
Certain specific movements, such as surfing, yoga, gymnastics, ballet, cheerleading, acrobatics, pilates, backbends, etc. are the inciting factors of some acute atraumatic SCIs [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. This special type of spinal cord injury is termed as SM according to activities before onset characterized by repeated hyperextension of the spine [2,3,4,5,6,7, 9,10,11,12,13, 26], AHM [1, 7, 8], or SCI without radiographic abnormality (SCIWORA) [14,15,16, 20]. Given similarities in their clinical presentation and imaging findings, AHM, which is a broader term, is defined and allows the inclusion of all patients with acute atraumatic thoracic SCI due to excessive and/or repeated spine hyperextension movement. This term would better reflect the underlying etiology [1, 7, 8], as well as allow for improved inclusion within the literature to facilitate future research and treatment.
In the present study, we detected two other predictors of poor prognosis for AHM: IMLL in the subacute stage and abnormal gadolinium enhancement in the low thoracic cord. These two predictive factors are simple and accessible in clinical practice. We observed that when IMLL was over 11 vertebral segments in the subacute stage and/or abnormal gadolinium enhancement in the low thoracic cord was present, no clinical improvement in neurological function will occur. These two predictors are often ignored in this type of disease due to their rarity and scarce serial follow-up without a long-term follow-up evolution of imaging findings and clinical data [2,3,4,5, 7, 13,14,15].
It was previously believed that neurological status on admission is the only predictor that determines the prognosis of this type of disease [2,3,4,5,6, 13, 16, 20, 27]. However, in clinical settings, exact assessment of the severity of the SCI (e.g., AIS grade, preservation of pin-prick sensation or not) is difficult with some patients, especially children [16, 25, 27]. Serious complications, such as scoliosis, subluxation of the hip, and SCI-induced osteoporotic fractures, will occur in children with complete SCI [7]. Early and accurate prediction of prognosis can benefit patients’ rehabilitation and prevention of related complications [28,29,30], and other early predictors are needed in clinical practice.
Computed tomography, MRI, and quantitative MRI can provide rich information about the cause, level, and extent of SCI [28, 30]. MRI is a simple and rapid way to evaluate SCI, and is widely used in clinical practice [29]. It has been reported that IMLL on MRI determines the long-term neurological outcome of SCI [21, 22]. However, previous studies of AHM failed to obtain the correct association between IMLL and clinical improvement.
Nakamoto et al [13] reviewed 23 cases of AHM and observed that clinical improvement was not significantly associated with any MRI characteristics, such as degree of T2-signal abnormality, length of continuous T2-signal abnormality (IMLL), and anterior-posterior diameter of the conus. Based on the neurological defects, radiographic characteristics of AHM, and statistical analyses, Nakamoto et al [13] concluded that the radiographic characteristics of AHM are not associated with clinical improvement. Thompson et al [2] and Chang et al [3] also failed to detect the correct associations between imaging characteristics and clinical improvement. Most patients were visitors to Hawaii or the Pacific Islands and transferred to hospitals in their home location for follow-up and treatment without a follow-up evolution of imaging findings [1, 4,5,6, 9,10,11,12, 14, 31, 32]. Their findings were partially consistent with our results. IMLL in the acute stage was not significantly associated with clinical improvement, and IMLL was not significantly different between groups A and B in the acute stage (p = 0.710) in our study. This may be because acute onset of AHM with complete SCI, and spinal venous hypertension is only initially limited to the mid- or lower-thoracic cord to the conus in the acute stage, which will be aggravated and progress in the subacute stage and a cranial progression of the T2-hyperintensity can be observed [6, 7]. IMLL in group A in the subacute stage was significantly different compared with that in the acute stage and group B (p < 0.001). Correlation analysis revealed that IMLL in the subacute stage was significantly negatively associated with clinical improvement (r = −0.608, p < 0.001). There is no correlation between neurologic status and early MRI findings, however, the MRI findings in subacute stage can show the severity of neurological defects. IMLL in the subacute stage is a predictor of clinical improvement.
The use of gadolinium contrast is not useful in ascertaining an AHM diagnosis, but is helpful in addressing the differential diagnosis of intramedullary lesions [2,3,4,5, 7, 13]. Gadolinium enhancement ranges from no gadolinium enhancement to peripheral contrast and abnormal gadolinium enhancement in this type of disease [13]. But most authors have failed to elucidate the correct association between gadolinium enhancement and clinical improvement. The main reason is that most studies of AHM consist of single case reports [1, 6, 8,9,10,11,12, 31,32,33]. There are only a few case series studies and these lack long-term follow-up imaging evaluation [2, 3, 13]. Contrast-enhanced MRI was not performed for all patients [2,3,4,5, 13,14,15,16], and some positive findings failed to detect accurate association with clinical improvement because no valid statistical analysis could be performed.
Spinal venous drainage disorders resulting from sustained and/or repeated hyperextension of the spine lead to spinal venous hypertension, which plays a critical role in the development of AHM [6, 7, 17, 24]. Spinal venous hypertension can decrease the arteriovenous pressure gradient of the cord, leading to perfusion impairment and increased vascular permeability, resulting in acute ischemic spinal cord injury, which can cause blood-spinal cord barrier (BSCB) injury, further exacerbating secondary injury of the cord [6, 7, 34,35,36,37]. BSCB prevents peripheral immune cells, toxins, and other inflammatory substances from entering the spinal cord [37, 38]. We speculate that BSCB disruption plays an important role in the progression of AHM, especially incomplete injury group.
Contrast-enhanced MRI permits the non-invasive evaluation of the permeability of the BSCB [38,39,40]. Under normal physiological conditions, contrast agents such as gadopentetate dimeglumine (Gd) within the blood circulation cannot cross the BSCB into the spinal parenchyma [38]. However, in cases of SCI or other pathological conditions, damage to the BSCB permits Gd to leak into the parenchyma, resulting in increased signal intensity on T1-weighted MRI. Whether abnormal gadolinium enhancement in the cord can reflect the severity of the SCI and secondary injury [38,39,40], abnormal gadolinium enhancement is also a poor prognostic factor of SCI and some other myelopathy [41].
In this case series, we observed that clinical improvement was significantly negatively associated with abnormal gadolinium enhancement in the low thoracic cord (r = −0.816, p < 0.001). Spinal cord infarction was detected in patients who presented with abnormal gadolinium enhancement in the low thoracic cord, and some single case reports also report this outcome [6, 7, 33]. Our findings suggest that contrast enhancement of the spinal cord is also an unfavorable prognostic factor for clinical improvement of AHM.
Limitations
This study was limited by its retrospective design, which is subject to bias. The results require validation in prospective observational studies or randomized trials. Another limitation is the small sample size due to low incidence, which limits the statistical analysis that can be performed. As neurological dysfunction in mild patients usually improves within 2–5 days, these patients may not seek medical advice and are not diagnosed [5, 7]; the mild patients are fewer in this study. Thus, multicenter, prospective studies are needed to collect more cases and provide greater statistical power to detect the relationship between imaging findings and clinical improvement.
Conclusions
AHM is a rare, acute non-traumatic thoracic SCI, and inciting factors are excessive and/or repeated spine hyperextension movements. We suggest that in addition to neurological status on admission, IMLL in the subacute stage and abnormal gadolinium enhancement in the cord are two other prognostic factors for AHM in children.
Data availability
The datasets generated during this study are available from the corresponding author on reasonable request.
References
Albuja AC, Qaiser S, Lightner DD, Raslau FD, Zafar MS, Bernard PA, et al. Surfer’s myelopathy without surfing: A report of two pediatric patients. Spinal Cord Ser Cases. 2017;3:17008.
Thompson TP, Pearce J, Chang G, Madamba J. Surfer’s myelopathy. Spine (Philos Pa 1976). 2004;29:E353–6.
Chang CW, Donovan DJ, Liem LK, O’Phelan KH, Green DM, Bassin S, et al. Surfer’s myelopathy: A case series of 19 novice surfers with nontraumatic myelopathy. Neurology 2012;79:2171–6.
Freedman BA, Malone DG, Rasmussen PA, Cage JM, Benzel EC. Surfer’s myelopathy: A rare form of spinal cord infarction in novice surfers: a systematic review. Neurosurgery 2016;78:602–11.
Gandhi J, Lee MY, Joshi G, Khan SA. Surfer’s myelopathy: A review of etiology, pathogenesis, evaluation, and management. J Spinal Cord Med. 2021;44:2–7.
Avilés-Hernández I, Garcia-Zozaya I, DeVillasante JM. Nontraumatic myelopathy associated with surfing. J Spinal Cord Med. 2007;30:288–93.
Wang Y, Zhu F, Zeng L, Wang S, Liu Y, Yang L, et al. Surfer myelopathy in children: A case series study. World Neurosurg. 2021;148:e227–41.
Cipriano K, Mansfield JT, Batal H, Gosai E. Nontraumatic acute hyperextension myelopathy associated with weightlifting. Am J Phys Med Rehabil. 2021;100:e113–5.
Wadia S, Padmanabhan P, Moeller K, Rominger A. Pediatric surfer’s myelopathy. J Emerg Med. 2015;49:e143–5.
Maharaj MM, Phan K, Hariswamy S, Rao PJ. Surfer’s myelopathy: A rare presentation in a non-surfing setting and review of the literature. J Spine Surg. 2016;2:222–6.
Teixeira S, Moser F, Kotton RH. Imaging features and differentials in surfer’s myelopathy: A case report. Emerg Radiol. 2016;23:89–92.
Dillen WL, Hendricks BK, Mannas JP, Wheeler GR. Surfer’s myelopathy: A rare presentation in a teenage gymnast and review of the literature. J Clin Neurosci. 2018;50:157–60.
Nakamoto BK, Siu AM, Hashiba KA, Sinclair BT, Baker BJ, Gerber MS, et al. Surfer’s myelopathy: A radiologic study of 23 cases. AJNR Am J Neuroradiol. 2013;34:2393–8.
Ren J, Zeng G, Ma YJ, Chen N, Chen Z, Ling F, et al. Pediatric thoracic SCIWORA after back bend during dance practice: A retrospective case series and analysis of trauma mechanisms. Child’s Nerv Syst. 2017;33:1191–8.
Tong AN, Zhang JW, Zhou HJ, Tang HH, Bai JZ, Wang FY, et al. Ischemic damage may play an important role in spinal cord injury during dancing. Spinal Cord. 2020;58:1310–6.
Zou Z, Teng A, Huang L, Luo X, Wu X, Zhang H, et al. Pediatric spinal cord injury without radiographic abnormality: The Beijing experience. Spine (Philos Pa 1976). 2021;46:E1083–8.
Griessenauer CJ, Raborn J, Foreman P, Shoja MM, Loukas M, Tubbs RS. Venous drainage of the spine and spinal cord: A comprehensive review of its history, embryology, anatomy, physiology, and pathology. Clin Anat. 2015;28:75–87.
Bickley RJ, Belyea CM, Harpstrite JK, Min KS. Surfing injuries: A review for the orthopaedic surgeon. JBJS Rev. 2021;9:e20.00152.
Xiaodong G, Yaping F, Tiansheng S, Shiqing F, Jiaguang T, Lin C, et al. Clinical guidelines for neurorestorative therapies in spinal cord injury (2021 China version). J Neurorestoratology. 2021;9:31–49.
Pang D. Spinal cord injury without radiographic abnormality in children, 2 Decades Later. Neurosurgery 2004;55:1325–43.
Aarabi B, Sansur CA, Ibrahimi DM, Simard JM, Hersh DS, Le E, et al. Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA impairment scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery 2017;80:610–20.
Aarabi B, Akhtar-Danesh N, Chryssikos T, Shanmuganathan K, Schwartzbauer GT, Simard JM, et al. Efficacy of Ultra-Early (<12 h), Early (12–24 h), and Late (>24–138.5 h) surgery with magnetic resonance imaging-confirmed decompression in american spinal injury association impairment scale grades A, B, and C cervical spinal cord injury. J Neurotrauma. 2020;37:448–57.
Flanagan EP, Krecke KN, Marsh RW, Giannini C, Keegan BM, Weinshenker BG. Specific pattern of gadolinium enhancement in spondylotic myelopathy. Ann Neurol. 2014;76:54–65.
Xie T, Pan L, Yang M, Xu Z, Wu T, Huang H, et al. Analysis of spinal angiograms that missed diagnosis of spinal vascular diseases with venous hypertensive myelopathy: the non-technical factors. Eur Spine J. 2020;29:2441–8.
CMulcahey MJ, Gaughan J, Betz RR, Johansen KJ. The International standards for neurological classification of spinal cord injury: Reliability of data when applied to children and youths. Spinal Cord. 2007;45:452–9.
Nakano H, Yamamoto D, Yamagami H, Sekine I, Ofuchi H. Paraplesia after surfing in a young female novice surfer: A case report on surfer’s myelopathy. Acute Med Surg. 2019;6:312–5.
Wilson JR, Jaja BNR, Kwon BK, Guest JD, Harrop JS, Aarabi B, et al. Natural history, predictors of outcome, and effects of treatment in thoracic spinal cord injury: A multi-center cohort study from the North American clinical trials network. J Neurotrauma. 2018;35:2554–60.
Zhu F, Liu Y, Zeng L, Wang Y, Kong X, Yao S, et al. Evaluating the severity and prognosis of acute traumatic cervical spinal cord injury: A novel classification using diffusion tensor imaging and diffusion tensor tractography. Spine (Philos Pa 1976). 2021;46:687–94.
David G, Mohammadi S, Martin AR, Cohen-Adad J, Weiskopf N, Thompson A, et al. Traumatic and nontraumatic spinal cord injury: Pathological insights from neuroimaging. Nat Rev Neurol. 2019;15:718–31.
Zhu F, Zeng L, Gui S, Liu Y, Wang Y, Cao X, et al. The role of diffusion tensor imaging and diffusion tensor tractography in the assessment of acute traumatic thoracolumbar spinal cord injury. World Neurosurg. 2021;150:e23–30.
Klontzas ME, Hatzidakis A, Karantanas AH. Imaging findings in a case of stand up paddle surfer’s myelopathy. BJR Case Rep. 2015;1:20150004.
Choi J, Seok HY, Kim Y, Kim BJ. Surfer’s myelopathy mimicking infectious myelitis. J Clin Neurol. 2017;13:207–8.
Takakura T, Yokoyama O, Sakuma F, Itoh R, Romero RR. Complete paraplegia resulting from surfer’s myelopathy. Am J Phys Med Rehabil. 2013;92:833–7.
Wang W, Ao Q. Research and application progress on dural substitutes. J Neurorestoratology. 2019;7:161–70.
Muralidharan R, Saladino A, Lanzino G, Atkinson JL, Rabinstein AA. The clinical and radiological presentation of spinal dural arteriovenous fistula. Spine (Philos Pa 1976). 2011;36:E1641–7.
Huang H, Chen L, Mao G, Bach J, Xue Q, Han F, et al. The 2019 yearbook of Neurorestoratology. J Neurorestoratology. 2020;8:1–11.
Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98:881–917.
Jin LY, Li J, Wang KF, Xia WW, Zhu ZQ, Wang CR, et al. Blood-spinal cord barrier in spinal cord injury: A Review. J Neurotrauma. 2021;38:1203–24.
Kataoka H, Miyamoto S, Nagata I, Ueba T, Hashimoto N. Venous congestion is a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery 2001;48:1224–30.
Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, et al. Blood-spinal cord barrier permeability in experimental spinal cord injury: Dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–41.
Cho YE, Shin JJ, Kim KS, Chin DK, Kuh SU, Lee JH, et al. The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J. 2011;20:2267–74.
Acknowledgements
We want to thank all the patients for their participation in this research.
Funding
This study was supported by National Natural Science Foundation of China (81873999, 81672158).
Author information
Authors and Affiliations
Contributions
YW: Conceptualization, Methodology, Software, Writing-Original Draft, Formal analysis, Data curation, Writing-Reviewing, and Editing. LZ: Conceptualization, Methodology, Software, Validation, Writing-Original Draft. FZ: Conceptualization, Methodology, Validation, Writing-Reviewing, and Editing. GH: Investigation, Data Curation. YW: Investigation, Software. SY: Data curation. KC: Investigation, Validation. XG: Resources, Writing-Review & Editing, Supervision, Funding acquisition. All authors revised it critically and approved the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This retrospective study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Zeng, L., Zhu, F. et al. Acute hyperextension myelopathy in children: Radiographic predictors of clinical improvement. Spinal Cord 60, 498–503 (2022). https://doi.org/10.1038/s41393-021-00739-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00739-w