Abstract
Study design
Observational study
Objective
To describe body mass index (BMI) during rehabilitation in people with a newly sustained spinal cord injury (SCI).
Setting
Inpatient SCI rehabilitation in Denmark.
Participants
Inpatients, >18 years, having sustained a SCI within the last 12 months at admission to primary rehabilitation, inclusive of various SCI etiology, neurological level, completeness of the lesion or mobility status.
Methods
Measures of BMI were obtained at admission and discharge as part of standard care. At one SCI center measures of BMI were sampled at follow up 9.5 months after discharge as well. BMI was described by mean and standard deviation (SD). Paired t-test was used to test difference in BMI between admission and discharge. Repeated measures Analysis of Variance (ANOVA) was used for analyzing BMI deriving from three time points.
Results
Overall BMI was stable with no change (25.4 kg/m2 at admission and 25.6 kg/m2 at discharge) during rehabilitation at the two national centers. In participants with an American Spinal Injury Association (ASIA) Impairment Scale (AIS) D classification, BMI was higher during rehabilitation compared to the other groups and increased significantly (p = 0.008) from discharge to follow up.
Conclusions
Overall BMI was stable but higher than recommended in people with SCI undergoing rehabilitation at the two national centers in Denmark. Participants with an AIS D SCI were obese according to SCI adjusted BMI and the World Health Organization (WHO) recommendations during rehabilitation and at follow up.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) predisposes to overweight and a conservative estimate of the overweight prevalence in people with SCI is of 66% [1]. Obesity was found to reduce functional outcomes in patients with SCI undergoing rehabilitation [2] just as overweight was found to be one of the most common cardio-metabolic risk factors among people with SCI [3, 4]. In people with paraplegia who were wheelchair users overweight increased the cardiovascular risk profile [5].
When sustaining a SCI people experienced a loss of muscle mass. The severity of the loss increased in accordance with a higher neurological level of injury and with the completeness of the SCI [6, 7]. Energy expenditure decreased significantly and remained low due to loss of muscle mass and inactivity [6, 8]. During the first year after injury, a loss of lean body mass and increase in body fat occurred [8, 9]. Improvement in severity during the first year after SCI with a potential to gain in lean body mass and thus impact on body weight has been reported [10].
Due to these changes and fluctuations in body weight, body composition and SCI severity over time, it is important to monitor body weight in order to identify and manage obesity in daily clinical practice due to the cardio-metabolic risk factor [11]. Although body mass index (BMI) lacks the sensitivity to distinguish between fat mass and lean body mass, and despite challenges with accurate assessment of body weight and height, needed for the calculation of BMI, it is the most widely used outcome measure for body mass in people with SCI in clinical practice [12]. The BMI cut off for overweight in the able bodied population seemed to underestimate obesity in people with SCI [6, 11,12,13]. Therefore, the clinical practice guideline for identification and management of cardio-metabolic risk after SCI recommended the use of the SCI adjusted BMI cut off ≥22 kg/m2 for overweight in people with chronic SCI [1, 12].
In Denmark 65–80% of all newly injured people with SCI are classified as American Spinal Injury Association (ASIA) Impairment Scale (AIS) D at discharge from rehabilitation, and 60% are non-traumatic. The number of patients undergoing rehabilitation in Denmark is highest in individuals who are 60–74 years old [14]. However, previous studies on the course of BMI during rehabilitation and after discharge were focusing on participants who were 18–65 years of age, being a wheelchair user or expected to be a wheelchair user on a permanent basis, even in individuals classified as AIS D [15,16,17]. One study included participants with a traumatic SCI only [18]. In these studies, the course of BMI over time showed different patterns for different subgroups according to e.g., level and severity of injury, age and gender. However, these studies did not include people who were not using a wheelchair or older than 65 years of age which is the most common group of people with SCI in Denmark and the Nordic countries. Therefore, this study will provide useful information to clinicians about the course of BMI in a sample reflecting clinical practice in their daily work trying to prevent overweight in people with a new SCI.
To address the previous divergent findings, we formed a longitudinal observational study including inpatients who had recently sustained a SCI and were admitted to one of the two national centers in Denmark. The annual incidence of traumatic and non-traumatic SCI admitted to the two SCI centers in Denmark is of 25–30 per million [14]. Based on clinical experience and previous studies, we hypothesized that BMI would increase during rehabilitation and continue increasing from discharge to follow up [15].
Methods
Participants and eligibility criteria
This study was an observational study on BMI at admission and discharge with consecutive enrollment during a 10 months period of all patients with a new SCI hospitalized at the Department for Spinal Cord Injuries in Eastern Denmark (DSCIED) and the Spinal Cord Injury Center of Western Denmark (SCICWD). At DSCIED, data on BMI at follow up 9.5 months after discharge was collected as well.
Inclusion criteria were: Inpatients; 18 years of age or older; having sustained a SCI within the last 12 months at admission to rehabilitation regardless of SCI etiology, neurological level, completeness of the lesion or mobility status.
Eligible patients gave informed written and verbal consent. Data on BMI at admission and discharge as well as core characteristics of the participants including age, gender, time since injury, and neurological status according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [19] were obtained from the electronic medical record at both centers.
BMI data
All data on measured body weight (kg) and self-reported height (cm) for calculating BMI (kg/m2), as well as ISNCSCI were collected and registered as part of standard care. Therefore, no specific instructions on how to collect data in relation to the study were given. BMI was calculated by the corresponding author if only data on body weight and height were registered in the medical record, and a telephone call was made to the participants involved if body height was not registered, resulting in their self-reported height, whereby calculation of BMI was possible. At follow up the participants were dressed during measurement of body weight and therefore one kilogram was subtracted [20].
For participants having data at both admission and discharge, data analysis was performed for overall BMI nationwide and for each center. Also, analysis of BMI according to SCI severity was performed nationwide and for each center. SCI severity was described according to the AIS classification and the participants were divided into three subgroups according to the standardization of reporting in the International SCI Core Data Set [21]. The three groups established were: C1-C8 AIS A,B,C; T1-S5 AIS A,B,C and AIS D regardless of injury level [21]. Likewise, age was divided into three subgroups for analysis in the order 18–29; 30–59 and 60+ years [21]. Ideally, BMI at admission, discharge and follow up was sub-grouped according to the neurological level of injury and AIS classification at the respective time points. If the neurological level and AIS classification for a specific time point was not present, the most recently registered was used when sub-grouping. Likewise, if a conversion of the severity subgroup occurred, e.g., due to neurological recovery, BMI was categorized and analyzed according to the newest severity sub-group. Because a high proportion of participants with an AIS D SCI was expected, BMI was analyzed according to cut-off points used for the general population (underweight: BMI < 18.5 kg/m2; normal: 18.5 ≤BMI < 25 kg/m2; overweight: 25 ≤ BMI < 30 kg/m2; obese: BMI ≥ 30 kg/m2) [11], as well as the adjusted cut-off points for people with SCI (recommended: BMI < 22 kg/m2; overweight: 22 ≤ BMI < 25 kg/m2; obese: BMI ≥ 25 kg/m2) [22].
Statistical analysis
Median and interquartile range (IQR) was used to report personal descriptive data at admission to the rehabilitation. BMI data were described by mean and standard deviation (SD). Paired t-test was used to test any differences in BMI between admission and discharge and independent T-test for differences between centers. Repeated measures Analysis of Variance (ANOVA) was used for analyzing BMI at three or more time points. The relationship between BMI and personal characteristics (severity, age and gender) was analyzed using univariate analysis. A significance level of α < 0.05 was considered significant. All statistical analyses were carried out using IBM SPSS statistics version 22.
Results
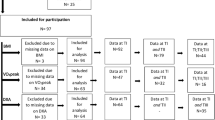
Of 137 eligible patients, informed consent was not retrieved from 30 patients at SCICWD and 9 patients at DSCIED. Therefore, 39 patients (28%) were not included in the study (non-participants) (Table 1) (Fig. 1).
Participants without data needed for calculating BMI at admission and/or discharge were excluded from the data analysis (N = 18) (Table 1). However, there was no significant difference in age between this subgroup (58.0, (SD 26.2)), (95% CI, 55.1–62.6) and participants with BMI at both time points (60.0 (SD 26.5)), (95% CI, 57.3–67.1) (p = 0.360) but slightly more women represented (Table 1). In addition, there was 58% (N = 11) participants classified as AIS D compared to 52.5% (N = 42) in study participants with BMI at both time points. Data from DSCIED showed a similar distribution of SCI etiology with 66% (N = 8) being non-traumatic compared to 63% (N = 25) in participants with data at both time points.
Therefore, data analysis on BMI at both admission and discharge was performed in 80 participants. Age and gender distribution were similar to age and gender distribution in all the participants initially included (Table 1). Participants classified as AIS D was 52.5% (N = 42) at admission and 65% (N = 52) at discharge.
Overall BMI
Overall BMI was stable with no change during rehabilitation at the two national centers while BMI was 25.4 kg/m2 (5.1) at admission and 25.6 kg/m2 (4.8) at discharge (N = 80) (Table 2). Participants with a BMI above the recommended 22 kg/m2 for individuals with SCI were 75% (N = 60) at admission and 82.5% (N = 66) at discharge. According to World Health Organization (WHO) BMI cut-off points used for the general population 43.7% (N = 35) and 42.5% (N = 34) respectively were overweight.
Neurological level and severity of SCI and BMI
Compared to the recommendations for people with SCI ( < 22 kg/m2) a high but stable BMI at admission and discharge was present in all three sub-groups (C1-C8 AIS A,B,C; T1-S5 AIS A, B, C and AIS D), with no significant changes (Table 2). The highest mean BMI was observed in participants classified as AIS D with high BMI values corresponding to overweight according to WHO BMI cut-off points used for the general population (Table 2). In participants ≥65 years of age classified as AIS D BMI was 27.7 kg/m2 at both admission (95% CI, 25.5–29.9) and discharge (95% CI, 24.2–28.1).
Neurological recovery to AIS D was observed in three participants at DSCIED and seven at SCICWD. In total four improved from C1-C8 AIS A, B, C to AIS D and six from T1-S5 AIS A, B, C to AIS D. However, no significant change in BMI between admission and discharge was present among participants converting from AIS A, B, C (C1-S5) to AIS D with a mean increase of 0.3 kg/m2.
BMI at different time points during rehabilitation
Data on BMI at admission, discharge and follow up on average 9.5 months after discharge was present in 18 participants (median age 56 (IQR 26.0), 14 men and 4 women) (Fig. 2). In these participants a non-significant increase in overall BMI from 25.3 kg/m2 (5.2) (95% CI, 22.7–27.2) at discharge to 26.5 kg/m2(5.5) (95% CI, 24.0–29.4) at follow up was present. However, in participants with an AIS D classification (n = 12) a significant increase occurred by 2.0 kg/m2 from 26.2 kg/m2 (5.3) (95% CI, 23.2–29.1) at discharge to 28.2 kg/m2 (5.8) at follow up (95% CI, 24.8–31.4) (p = 0.008).
Discussion
This study was an observational study on BMI at admission and discharge from rehabilitation collected and registered as part of standard care at the two national centers in Denmark. We hypothesized that BMI would increase during rehabilitation and continue increasing from discharge to follow up. Although high, BMI was stable during rehabilitation and only increased from discharge to follow up in people with AIS D SCI.
The present study differed from other studies conducted on BMI, because participants included were 18 years and older regardless of SCI etiology, neurological level, completeness of the lesion or mobility status and resulted in a participant sample where 52.5% (N = 42) were classified as AIS D [15,16,17,18]. Median age was 61 years with an age range of 67 (21–88 years). Also, participants with improvements in neurological classification and severity of SCI during rehabilitation were included when calculating BMI at different time points. Therefore, our sample resembled individuals with SCI in the Nordic countries where 61% are classified as AIS D at discharge from rehabilitation, and the number of patients undergoing rehabilitation is highest in individuals who are 60–74 years old [14].
These differences among participants in the respective studies may be important. Age may have an impact due to e.g., sarcopenia or frailty prior to SCI and differences in mobility status between wheelchair users and people who ambulate may induce potential differences in muscle activity, energy expenditure and thus BMI due to differences in lean mass and fat mass [23, 24].
In this study BMI was described using the SCI adjusted cut-off points as well as the cut-off points used for the general population. The SCI adjusted cut off points are based on people with chronic SCI and therefore applying these to people with acute or subacute SCI may seem inappropriate because changes in body composition may occur within the first year after injury due to the hypercatabolic state immediately after SCI and changes in the musculoskeletal system or SCI severity [8, 10]. However, although speculative, using the SCI adjusted cut-off points during rehabilitation may be appropriate. A previous study found that 72% of the participants classified with an AIS A SCI at admission were still classified as AIS A 6 months post injury and of the 16% converting to AIS B only few became motor incomplete [10]. Because energy expenditure has shown to decrease significantly and remain low there is a risk of exceeding the SCI adjusted cut off while awaiting chronic SCI with neurological and physiological steady state. The SCI adjusted cut off points may be too extreme however when applied to people with a motor incomplete SCI and therefore the WHO BMI cut-off could be more appropriate due to a better preservation of muscle mass. Therefore, although the recommended SCI adjusted cut-off for overweight (22 kg/m2) does not take the severity of SCI into account at present, this may be important. However, this perspective needs further investigation as the causality for overweight in this study is unknown.
Overall BMI was stable with no change during rehabilitation at the two national centers, but high according to the recommended cut-off for people with SCI as well as the WHO cut-off. Our findings across SCI centers were similar compared to previous longitudinal studies by de Groot et al. and Nooijen et al. [17, 25]. However, in our study 75% (N = 60) and 82.5% (N = 66) were overweight according to the recommended 22 kg/m2 at admission to rehabilitation and discharge respectively. Likewise, 43.7% and 42.5% were overweight according to the WHO BMI cut-off points for the general population. This is much higher than previously reported. Accordingly, de Groot et al. described overweight in 56% and 63% of the participants at admission and discharge from rehabilitation using the SCI adjusted cut off and 28% and 36% when using the WHO BMI cut-off points. Although speculative, the reason for the higher percentages in our study could be that 52.5% (N = 42) of our participants were classified as AIS D which was the severity group with the highest BMI at both time points in contrast to the study by de Groot et al. where 70% (N = 124) were classified as AIS A or B. However, the increase in BMI from admission to discharge was similar in the two studies, which is worrying, due to the increased risk of cardio-metabolic complications [15].
At DSCIED, the lowest BMI observed at admission in participants with a C1-C8 AIS A, B, C classification was of 15.5 kg/m2. At present there is no definition of underweight in the clinical practice guideline for identification and management of cardio-metabolic risk after SCI [1]. However, only two out of 18 participants at admission and one out of 14 at discharge, were underweight according to the BMI classification index for able bodied persons [26]. This is in line with the findings of de Groot et al. at baseline (15%) and higher than reported by Powell et al. at baseline (4.1%). In participants classified as AIS D, BMI was >25 kg/m2 with a significant increase from discharge to follow up and higher compared to the other AIS groups which was similar to the findings of Powell et al. They suggested that the risk of weight gain was higher when neurological impairment was modest, probably due to independence in feeding and/ or higher amount of lean body mass [18]. In addition, we found that people ≥65 years of age were overweight according to the cut-off points used for the general population. In able bodied people older than 65 years of age a BMI of ≥25–29.9 kg/m2 has been associated with lower risk of all-cause mortality [27, 28]. To our knowledge this perspective has not been discussed in relation to people with SCI. However, it is a relevant area for future research because the majority sustaining a SCI in Scandinavia are 60–74 years old and classified as AIS D. If the risk of all-cause mortality is reduced in people with SCI as well, this may impact on future BMI recommendations [14].
Systematic assessment of BMI is important in newly injured people with SCI due to reduced energy needs [8]. However, the interpretation of BMI would be improved if it was coupled with assessment of body composition as part of standard care especially in people with an AIS D SCI [1]. The use of waist circumference (WC) could be an additional and easy administered anthropometric measure because SCI specific WC cut-off point has been reported and was associated with increased cardio-metabolic risk [29].
Limitations and strengths
Compared to previous studies the number of participants was small, and because 28% (N = 39) of the patients undergoing rehabilitation in the study period were not included, the representativeness for people with SCI undergoing primary rehabilitation in Denmark may be limited. It was a strength that the survey included both national SCI centers with participants resembling individuals with SCI in Denmark regarding age and AIS classification. No specific instructions were given on how to measure body weight and height was self-reported. Therefore, caution must be taken in relation to the BMI values obtained as part of standard care with the risk of underestimating BMI due to lack of standard guidelines which is required for reliable measures of BMI. However, describing BMI in relation to the actual severity classification at different time points was a strength.
Conclusion
In people with SCI undergoing rehabilitation the overall BMI was stable but higher than recommended. Participants with an AIS D SCI were obese according to SCI adjusted BMI and the WHO recommendations during rehabilitation and at follow up. Future research should investigate the body composition to determine the reason for the higher BMI in this sub-group.
Data availability
The datasets generated and analyzed in the current study are available upon reasonable request to the corresponding author.
References
Nash MS, Groah SL, Gater DR Jr, Dyson-Hudson TA, Lieberman JA, Myers J, et al. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers. Top Spinal Cord Inj Rehabil. 2018;24:379–423.
Stenson KW, Deutsch A, Heinemann AW, Chen D. Obesity and inpatient rehabilitation outcomes for patients with a traumatic spinal cord injury. Arch Phys Med Rehabil. 2011;92:384–90.
Rajan S, McNeely MJ, Warms C, Goldstein B. Clinical assessment and management of obesity in individuals with spinal cord injury: a review. J Spinal Cord Med. 2008;31:361–72.
Libin A, Tinsley EA, Nash MS, Mendez AJ, Burns P, Elrod M, et al. Cardiometabolic risk clustering in spinal cord injury: results of exploratory factor analysis. Top Spinal Cord Inj Rehabil. 2013;19:183–94.
Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: the Stockholm spinal cord injury study. J Rehabil Med. 2011;43:237–42.
Farkas GJ, Gater DR. Neurogenic obesity and systemic inflammation following spinal cord injury: a review. J Spinal Cord Med. 2018;41:378–87.
Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol (Bethesda, Md: 1985). 2003;95:2398–407.
Felleiter P, Krebs J, Haeberli Y, Schmid W, Tesini S, Perret C. Post-traumatic changes in energy expenditure and body composition in patients with acute spinal cord injury. J Rehabil Med. 2017;49:579–84.
Singh R, Rohilla RK, Saini G, Kaur K. Longitudinal study of body composition in spinal cord injury patients. Indian J Orthop. 2014;48:168–77.
Spiess MR, Müller RM, Rupp R, Schuld C, Group E-SS, van Hedel HJA. Conversion in ASIA impairment scale during the first year after traumatic spinal cord injury. J Neurotrauma. 2009;26:2027–36.
Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C. Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med. 2011;34:416–22.
Silveira SL, Ledoux TA, Robinson-Whelen S, Stough R, and Nosek MA. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55:812–7.
Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–8.
Halvorsen, A and Pettersen AL. NordicSCIR Årsrapport 2019. 2019; https://stolav.no/Medisinskekvalitetsregistre/NorSCIR/%C3%85rsrapporter/NORSCIR%20%C3%A5rsrapport%202019.pdf.
de Groot S, Post MW, Postma K, Sluis TA, van der Woude L. Prospective analysis of body mass index during and up to 5 years after discharge from inpatient spinal cord injury rehabilitation. J Rehabil Med. 2010;42:922–8.
de Groot S, Post MW, Hoekstra T, Valent LJ, Faber WX, van der Woude LH. Trajectories in the course of body mass index after spinal cord injury. Arch Phys Med Rehabil. 2014;95:1083–92.
Nooijen CF, Stam HJ, Sluis T, Valent L, Twisk J, van den Berg-Emons RJ. A behavioral intervention promoting physical activity in people with subacute spinal cord injury: secondary effects on health, social participation and quality of life. Clin Rehabil. 2017;31:772–80.
Powell D, Affuso O, Chen Y. Weight change after spinal cord injury. J Spinal Cord Med. 2017;40:130–7.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Whigham LD, Schoeller DA, Johnson LK, Atkinson RL. Effect of clothing weight on body weight. Int J Obes (Lond). 2013;37:160–1.
Biering-Sørensen F, DeVivo MJ, Charlifue S, Chen Y, New PW, Noonan V, et al. International Spinal Cord Injury Core Data Set (version 2.0)-including standardization of reporting. Spinal Cord. 2017;55:759–64.
Laughton GE,AC, Buchholz KA, Martin Ginis, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–62.
Dekker B, Verschuren O, Balemans ACJ, Baart N, Tubbing F, van Koppenhagen CF, et al. Energy expenditure and muscle activity during lying, sitting, standing, and walking in people with motor-incomplete spinal cord injury. Spinal Cord. 2018;56:1008–16.
Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle & Nerve. 2009;40:499–519.
de Groot S, PM, Postma K, Sluis TA, van der Woude LH. Prospective analysis of body mass index during and up to 5 years after discharge from inpatient spinal cord injury rehabilitation. J Rehabil Med. 2010;42:922–8.
Weir, CB and Jan A. BMI Classification Percentile And Cut Off Points, in StatPearls. 2019, Treasure Island (FL):StatPearls Publishing; 2019.
Kvamme JM, Holmen J, Wilsgaard T, Florholmen J, Midthjell K, Jacobsen BK. Body mass index and mortality in elderly men and women: the Tromso and HUNT studies. J Epidemiol Community Health. 2012;66:611–7.
Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99:875–90.
Gill S, Sumrell RM, Sima A, Cifu DX, Gorgey AS. Waist circumference cutoff identifying risks of obesity, metabolic syndrome, and cardiovascular disease in men with spinal cord injury. PLoS ONE. 2020;15:e0236752.
Acknowledgements
The authors would like to thank all the participants, physiotherapist Anne Christensen at SCICWD for assisting with data collection and clinical nurse specialist Line Dalsgaard and nurse Gitte Dalsborg at DSCIED for sparring about the clinical procedures related to assessment of BMI and perspectives on BMI in the elderly.
Funding
This work was supported by a research program, “Centre for Integrated Rehabilitation of Cancer Patients (CIRE) - Neuro/Psychology”, conducted collaboratively by the University Hospitals Centre for Health Care Research, University Hospital Copenhagen, Rigshospitalet, University College Copenhagen, Department of Nursing and Nutrition, and the NeuroScience Centre, Rigshospitalet.
Author information
Authors and Affiliations
Contributions
NJH was primarily responsible for the study and collecting data together with RS who was in charge of the data collection in Western Denmark. FB-S has contributed with the overall idea of the study design and data analysis. TM contributed with perspectives on the frequency of BMI measures. HK, and LS contributed with feedback on the paper. All authors contributed to the text writing and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The project is approved by the Committees on Health Research Ethics in the Capital Region of Denmark on 10.07.2018 (Journal-nr.: H-18018325). All participants received oral, as well as written information about the study, before verbal and written consent was obtained. The project is registered at Clinicaltrials.gov (NCT03369080).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holm, N.J., Steensgaard, R., Schou, L.H. et al. An observational study on body mass index during rehabilitation and follow-up in people with spinal cord injury in Denmark. Spinal Cord 60, 157–162 (2022). https://doi.org/10.1038/s41393-021-00730-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00730-5
This article is cited by
-
Obesity in wheelchair users with long-standing spinal cord injury: prevalence and associations with time since injury and physical activity
Spinal Cord (2024)
-
Monitoring outcome measures for cardiometabolic disease during rehabilitation and follow-up in people with spinal cord injury
Spinal Cord (2024)