Abstract
NF-κB signaling has been discovered for nearly 40 years. Initially, NF-κB signaling was identified as a pivotal pathway in mediating inflammatory responses. However, with extensive and in-depth investigations, researchers have discovered that its role can be expanded to a variety of signaling mechanisms, biological processes, human diseases, and treatment options. In this review, we first scrutinize the research process of NF-κB signaling, and summarize the composition, activation, and regulatory mechanism of NF-κB signaling. We investigate the interaction of NF-κB signaling with other important pathways, including PI3K/AKT, MAPK, JAK-STAT, TGF-β, Wnt, Notch, Hedgehog, and TLR signaling. The physiological and pathological states of NF-κB signaling, as well as its intricate involvement in inflammation, immune regulation, and tumor microenvironment, are also explicated. Additionally, we illustrate how NF-κB signaling is involved in a variety of human diseases, including cancers, inflammatory and autoimmune diseases, cardiovascular diseases, metabolic diseases, neurological diseases, and COVID-19. Further, we discuss the therapeutic approaches targeting NF-κB signaling, including IKK inhibitors, monoclonal antibodies, proteasome inhibitors, nuclear translocation inhibitors, DNA binding inhibitors, TKIs, non-coding RNAs, immunotherapy, and CAR-T. Finally, we provide an outlook for research in the field of NF-κB signaling. We hope to present a stereoscopic, comprehensive NF-κB signaling that will inform future research and clinical practice.
Similar content being viewed by others
Introduction
In 1986, Ranjan Sen and David Baltimore first identified the nuclear factor in B lymphocytes binding to the kappa enhancer of the gene encoding the κ light- chain of immunoglobulin by electrophoretic migration assays of end-labeled DNA fragments and named it nuclear factor binding near the κ light- chain gene in B cells or NF-κB.1,2 Over the next 3 years, David Baltimore’s laboratory successively uncovered the significance of kappa enhancer’s protein-binding site κB in promoting transcriptional activity and inducibility in B cells, as well as NF-κB as a molecule involved in several pathways.3,4,5 The involvement of NF-κB in inflammation and immune responses is indisputable, and this is the most important role that NF-κB signaling plays in biology. The understanding of NF-κB signaling should not be limited to its “results”. It is more meaningful to investigate the intricate biological mechanisms by which NF-κB signaling induces alterations in molecules, cells, tissues, and even organisms across diverse species and diseases. Additionally, it is crucial to comprehend the dualistic nature of NF-κB signaling, which can act as both a “foe” and a “friend”. The greater the depth and breadth of our comprehension of NF-κB signaling, the more assured we become in our ability to exploit this pathway for gene manipulation, modulation of cellular behavior, and therapeutic intervention. We will commence by presenting the biological underpinnings of NF-κB signaling and elucidate the mechanisms of its self-regulation and crosstalk with other pathways. Further, we provide a comprehensive overview of the role of NF-κB signaling in the pathogenesis of diverse organ systems and discuss therapeutic strategies targeting this pathway, which will be beneficial to better understand the research process of NF-κB signaling.
The history and development of NF-κB signaling
The mammalian NF-κB transcription factor family consists of five members, namely NF-κB1 (p105/p50), NF-κB2 (p100/p52), p65 (RELA), V-Rel reticuloendotheliosis viral oncogene homolog B (RELB), and c-REL. Due to the sharing of the conserved Rel homology domain (RHD), any two members of the NF-κB transcription factor family can form homo- or heterodimers, which bind to IκB and sequester in the cytoplasm in an inactive form, with p65/p50 being the most common dimerization form.6 Specific functions of several NF-κB complex types are involved in the development of regulatory T cells.7 RELA, RELB, and c-REL harbor transcriptional activation structural domains (TAD) with transcriptional activation activity. While p50 and p52 do not contain TAD and their homodimers are transcriptional repressors, p50 and p52 form heterodimers with TAD-containing family members to further stimulate transcription or alter the specificity of the κB site.8,9 (Fig. 1)
Overview of canonical and non-canonical NF-κB signaling. Canonical NF-κB signaling is primarily activated by BCR, TCR, TLR, IL-1R, and TNFR. BCR and TCR initiate a multistage enzymatic reaction that activates the CARMA1/BCL-10/MALT1 complex. TLR, IL-1R, and TNFR primarily promote activation of the TAK1/TAB complex. Activated CARMA1/BCL-10/MALT1 complex and TAK1/TAB complex phosphorylate the IKKα/IKKβ/NEMO (IKKγ) complex. IKKα and IKKβ phosphorylate IκBα, leading to its ubiquitination and subsequent proteasomal degradation. This results in the release of p50/RelA, which acts as a transcription factor to activate the transcription of target genes. Canonical NF-κB signaling primarily promotes cell survival and mediates inflammatory and immune responses. In non-canonical NF-κB signaling, CD40, RANK, LT-βR, and BAFF-R activate NIK, which further phosphorylates IKKα and promotes the degradation of p100 to p52. The p52 subunit then binds to RelB and undergoes nuclear translocation, promoting lymphocyte generation, survival, maturation, and adhesion. A20 TNF alpha-induced protein 3, BAFF B lymphocyte activating factor, BAFF-R B lymphocyte stimulating factor receptor, Bcl10 B cell leukemia/lymphoma 10, BCR B-cell receptor, BLNK B cell linker, BTK Bruton tyrosine kinase, CARMA1 caspase recruitment domain family member 11, CD40L CD40 ligand, CYLD cylindromatosis, IAP inhibitor-of-apoptosis protein, IKK I-kappaB kinase, IL-1 interleukin 1,IL-1R interleukin 1 receptor, IRAK interleukin 1 receptor-associated kinase, IκB IkappaB protein, LAT linker for activation of T cells, LCK lymphocyte cell-specific protein tyrosine kinase, LIGHT tumor necrosis factor ligand superfamily member 14, LPS lipopolysaccharide, LTA lymphotoxin alpha, LTB lymphotoxin beta, LT-βR lymphotoxin beta receptor, LUBAC linear ubiquitin chain assembly complexes, LYN LYN proto-oncogene, Src family tyrosine kinase, MALT1 MALT1 paracaspase, MHC major histocompatibility complex, MyD88 MYD88 innate immune signal transduction adapter, NEMO inhibitor of nuclear factor kappa-B kinase subunit gamma, NIK NF-κB-inducing kinase, PKC protein kinase C, PLC phospholipase C, RANK receptor activator of NF-KappaB, RANKL receptor activator of NF-KappaB ligand, RIP1 receptor-interacting serine/threonine-protein kinase 1, SYK spleen associated tyrosine kinase, TABTAK1-associated binding protein, TAK1 TGF-beta activated kinase 1, TCR T-cell receptor, TIRAP TIR domain containing adapter protein, TLR toll-like receptor, TNF tumor necrosis factor, TNFR TNF receptor, TRADD tumor necrosis factor receptor type 1-associated DEATH domain protein, TRAF tumor necrosis factor receptor-associated factor, TRAM TRIF-related adapter molecule, TRIF toll-like receptor adapter molecule 1, ZAP tyrosine-protein kinase ZAP-70
The I-kappaB kinase (IKK) kinase complex constitutes a key component of the NF-κB signaling cascade.10 The IKK complex consists of IKKα, IKKβ, and NEMO (IKKγ), of which IKKα and IKKβ are the kinases and IKKγ is the subunit that exerts the regulatory function. IKKα and IKKβ share 50% sequence identity, and both molecules include an amino-terminal kinase domain, a helix-loop-helix (HLH) responsible for regulating IKK kinase activity, and a leucine zipper (LZ) mediating kinase dimerization.10
Canonical NF-κB pathway
Components of canonical NF-κB pathway
NF-κB family
The precursor molecule p105 undergoes ubiquitination upon induction by the ubiquitin ligase Kip1 ubiquitination-promoting complex subunit 1 (KPC1), followed by proteasomal disassembly into the active form p50, and the nuclear localization sequence (NLS) masked by the remote structural domain of the p110 precursor is exposed, allowing cytoplasmic/nuclear signaling to proceed.11,12 When combined with RelA to form a heterodimer, p50 participates in the transmission of canonical NF-κB signaling.
IκB family
The IκB (IkappaB protein, inhibitor of NF-κB) family comprises p100, p105, IκBα, IκBβ, IκBε, IκBζ, BCL-3, and IκBNS.13 IκB binds to NF-κB through 3–8 ankyrin repeats at the C-terminus, masking the nuclear localization sequence (NLS) of NF-κB and inhibiting its activity. The N-terminus contains phosphorylation and ubiquitination sites, which are signal-responsive regions involved in the induced degradation of IκB.
IκBα, IκBβ, and IκBε are present as typical IκB proteins in the cytoplasm of resting cells, and stimulation may induce degradation and resynthesis of typical IκB proteins.8 Unlike the rapid and transient activation of IκBα-mediated NF-κB signaling, IκBβ sustainably activates NF-κB and maintains long-term expression of pro-inflammatory target genes such as tumor necrosis factor-α (TNF-α) through p65:c-Rel heterodimer.14,15 IκBγ is mainly found in lymphocytes.16 Synthesis of IκBα is specifically induced by the p65 subunit of NF-κB. IκBα binds to the p65 subunit and is present in the cytoplasm, inhibiting the transcription factor activity of NF-κB.17,18,19,20 In response to activated IKK, IκBα is phosphorylated at serine/threonine residues and degraded. The cytoplasmic complex composed of NF-κB and IκB dissociates, and NF-κB is released into the nucleus where it activates the transcription of downstream target genes.17,18,19,20
Cell activation stimulates the creation of atypical IκB proteins (IκBζ, BCL-3, and IκBNS), which then play their respective roles in the nucleus.8,21,22,23,24,25 Different from the above IκB family members, the proto-oncogene Bcl-3 can also bind tightly to p50/p52 homodimers and DNA in the nucleus to transactivate through the κB motif.26,27,28 Bcl-3 is not only an inhibitor that sequesters NF-κB to the cytoplasm and inhibits its activity, but also participates in the transcriptional process as a transcriptional coactivator.8 IκB acts on the transcription of NF-κB by affecting the production, stability, and reactivity of NF-κB complexes.8
IKK family
Amino acid regions (aa 705–743) at the carboxyl terminus of IKKα and IKKβ mediate interaction with NEMO. IKK performs a dual function of activating NF-κB and inhibiting the cell death pathway.7 In unstimulated cells, IκB inhibits the DNA-binding activity of NF-κB dimers and keeps them homeostatic localization in the cytoplasm. In stimulated cells, phosphorylation of serine residues located in IKKα proteins 176 and 180 and serine residues in IKKβ proteins 177 and 181 leads to changes in protein conformation and activation of the kinase.
Phosphorylation of IKKβ is required for canonical NF-κB signaling, and TGF-beta activated kinase 1 (TAK1) is responsible for IKKβ phosphorylation upon binding to the cofactor TAK1-associated binding protein (TAB1/2/3). IκB is phosphorylated by the active IKK complex, which causes ubiquitination and eventual destruction of IκB. NF-κB dimer is released and nuclear transposed, binding to the κB site in the promoter or enhancer and activating the transcription of specific genes. Therefore, IKK is a key regulatory event for NF-κB activation. In addition, IKKα and IKKβ are also involved in the phosphorylation of the p65 subunit.29,30,31,32
NEMO is also necessary for canonical NF-κB signaling activation. The IKK-binding domain (IBD) at the N-terminal of NEMO binds to the NEMO-binding domain (NBD) of IKK, and the C-terminal end mediates interactions with upstream signaling molecules, such as RIP, which promotes oligomerization of NEMO and phosphorylation of IKKα/β, and plays a crucial role in TNF-α, interleukin (IL)-1-activated NF-κB signaling.33,34,35,36,37 NEMO acts as a scaffold in the recruitment of IκBα by IKKβ.38 In the case of the NEMO mutation, IKKβ undergoes hyperphosphorylation upon activation by IL-1 but fails to recruit IκB.38 Yu et al. found that ubiquitin carboxy-terminal hydrolase 16 (USP16) competitively binds IKKα and IKKβ to NEMO, thereby inhibiting the interaction of IKKβ with NEMO.39 During antigen-induced activation of NF-κB signaling in T or B cells, IKK is phosphorylated in response to stimulus-dependent conformational changes or oligomerization activation, which may be related to NEMO.
Activation and regulation of canonical NF-κB pathway
NF-κB signaling may be activated by a diverse range of stimuli, including bacterial and viral products, cytokines, ultraviolet and ionizing radiation, growth factors, reactive oxygen species, and oncogenic stresses.6,40 Immune cells utilize unique, dynamic quantitative signal signatures that stimulate NF-κB signaling outside the cell or intracellular to transmit important biological information about the microenvironment.41 Dangerous stimuli such as pathogen invasion initiate innate immune responses, and dynamically encode specific information such as ligand dose, duration, and distance through wave propagation of NF-κB signaling, forming gene expression regions in response cells.41,42 The main activators of canonical NF-κB signaling include TNF-α, interleukin (IL)-1β, lipopolysaccharide (LPS), and antigen. These activators will bind to cell surface receptors and trigger the activation of NF-κB signaling in response to multiple bridging proteins. In the following section, we will describe the conduction and regulation process of canonical NF-κB signaling induced by different stimuli respectively.
TNF-α induced canonical NF-κB pathway
Hailing Hsu et al. discovered the tumor necrosis factor receptor type 1-associated DEATH domain (TRADD), which interacts with the intracellular structural domain of the TNF receptor 1 (TNFR1), in 1995, and suggested that TRADD is implicated in TNF-induced NF-κB signaling.43 Subsequently, the team found that TRADD directly interacts with the ubiquitin ligase tumor necrosis factor receptor-associated factor (TRAF2) and protein kinase receptor-interacting serine/threonine-protein kinase (RIP), activating NF-κB signaling.44,45 Further investigations have revealed that TRAF2 exhibits a higher binding affinity towards TRADD for signaling, rather than TNFR1, and impedes apoptosis by recruiting inhibitor-of-apoptosis proteins (clAPs).46 Lipid rafts, which are membrane microdomains enriched in cholesterol and sphingolipids, serve as a structural foundation for the assembly of TNFR1-RIP-TRADD-TRAF2 complexes.47 Within these complexes, where TNFR1 and RIP are ubiquitinated for NF-κB signaling.47 The TNFR1-RIP-TRADD-TRAF2 complex plays a crucial role in regulating cell survival and apoptosis48,49, and when TNFR1-mediated signaling successfully activates the complex and NF-κB signaling, the cells will survive in the presence of FLICE inhibitory proteins (FLIP, Caspase-8 inhibitor), and conversely lead to cell death.14,15
TNF alpha-induced protein 3 (A20) and CYLD lysine 63 deubiquitinase (CYLD) are key deubiquitinases in the downregulation of NF-κB signaling.50 A20 is an NF-κB signaling inhibitor comprising two structurally independent domains. The N-terminus of A20 functions as an ovarian tumor family deubiquitinating enzyme with linear linkage specificity (OTULIN), which specifically cleaves K63-linked ubiquitin chains from RIP. The C-terminus of A20 acts as a ubiquitin ligase for K48-linked polyubiquitination, leading to the proteasomal degradation of RIP.51 CYLD clears non-K48-linked polyubiquitin chains on a range of NF-κB signaling proteins and negatively regulates TRAF2- or TRAF6-mediated IKK activation through deubiquitination.50,52,53 The E3 ligase linear ubiquitin chain assembly complexes (LUBAC) consist of a catalytic HOIL-interacting protein (HOIP) and a regulated Shank-associated RH domain-interacting protein (SHARPIN) and Heme-oxidized IRP2 ubiquitin ligase 1 (HOIL-1L) composition.54,55,56 Since LUBAC promotes linear ubiquitination of NEMO and RIP1, it is considered a key mechanism for the activation in response to specific stimuli or overactivation of NF-κB signaling.57,58
IL-1β induced canonical NF-κB pathway
The investigation into interleukin 1 receptor-associated kinase (IRAK) as an essential component for IL-1 activation of NF-κB signaling originated from the discovery by Zhaodan Cao et al. that IRAK promptly binds to and phosphorylates the interleukin 1 receptor, type I (IL-1RI) in tool cells (HEK 293 and HeLa).59,60 IRAK shares similarity in the primary amino acid sequence with Pelle, a protein kinase essential for activation of the Drosophila NF-κB homolog.59,60 In the same year, the team found that TRAF6, a member of the TRAF family, is induced by IL-1 to bind with IRAK and is rapidly recruited to IL-1R, implying that TRAF6 is also involved in IL-1-NF-κB signaling.61 Marta Muzio’s team identified IRAK-2 and MYD88 innate immune signal transduction adapter (MyD88) as mediators for IL-1R-induced NF-κB signaling, and MyD88 serves as a signal transduction adapter to mediate the binding of IRAK to IL-1R, which provides possible targets for the treatment of inflammatory diseases.62,63,64 MyD88 recruits IRAK1 and IRAK4 via death structural domain, and IRAK4 triggers autophosphorylation and subsequent dissociation of IRAK1. TRAF6 functions as a signal transducer primarily involved in canonical NF-κB signaling activated by IL-1 and toll-like receptor (TLR). The activation of IKK by TRAF6 is achieved through the synthesis of lysine-63 (K63)-linked polyubiquitin chains catalyzed by the ubiquitin ligases Ubc13 and Uev1A, and the TAK1/TAB1/TAB2 protein kinase complex phosphorylates and activates IKK with the assistance of the polyubiquitin chains.65,66,67
LPS induced canonical NF-κB pathway
TLR recognizes molecules such as LPS, DNA, and RNA from viruses, bacteria, and fungi as sensors for detecting possible infections and initiating an immune cascade response for host defense.68 The toll-IL-1 receptor (TIR) structural domain of TLR4 recruits the TLR adapter molecule MyD88 and the TIR domain containing adapter protein (TIRAP), which subsequently activate IRAK1/4 and TRAF6, and involved in TLR-stimulated NF-κB signaling are also toll-like receptor adapter molecule 1 (TRIF) and TRIF-related adapter molecule (TRAM), both signaling modes are dependent on interaction with TLR4.69,70 The ubiquitin-conjugating enzyme complex composed of TRAF6/ Ubc13/ Uev1A catalyzes the formation of K63-linked polyubiquitin chain, which activates TAK1.65,71
Antigen induced canonical NF-κB pathway
T cells and B cells are the main cell types responsible for adaptive immune. T-cell receptor (TCR) is activated upon binding to the major histocompatibility complex (MHC)-antigen peptide complex. TCR is first recruited through the intracellular structural domains of CD4 and CD8 by lymphocyte cell-specific protein tyrosine kinase (LCK) to phosphorylate immunoreceptor tyrosine activation motifs (ITAM) and activate the tyrosine-protein kinase ZAP-70.72,73 ZAP-70 phosphorylates the activating linker for the activation of T cells (LAT), which promotes the recruitment of multiple junction proteins and effector molecules including phospholipase C γ1 (PLCγ1) and the formation of the LAT signalosome complex.74 PLCγ1 catalyzes the synthesis of diester glycerol and inositol (1,4,5)-trisphosphate as second messengers that trigger mitogen-activated protein kinase, protein kinase Cθ (PKCθ) and calmodulin phosphatase.73,75 The B-cell receptor (BCR) upon binding to antigen, first recruits spleen associated tyrosine kinase (SYK) and SRC proto-oncogene, non-receptor tyrosine kinase (SRC), which determines the initiation of BCR signaling and subsequent conductance efficiency.76 As a member of the SRC kinase family, LYN proto-oncogene, Src family tyrosine kinase (LYN) phosphorylates tyrosine residues of ITAM and SYK in CD79A and CD79B.76,77 B cell linker (BLNK), a substrate for SYK, promotes the recruitment of Bruton tyrosine kinase (BTK) and PLCγ2, and BTK phosphorylation activates PLCγ2 and PKCβ, which leads to intracellular calcium mobilization and activation of NF-κB signaling.76 Activated PKCθ and PKCβ recruit caspase recruitment domain family member 11 (CARMA1), B cell leukemia/lymphoma 10 (Bcl-10), and MALT1 paracaspase (MALT1), and the complex composed of CARMA1/BCL-10/MALT1 was found to be active in NF-κB and c -Jun N-terminal kinase (JNK) signaling, which mediates immune cell activation, proliferation, and differentiation, and its aberrant expression has been associated with autoimmune diseases and lymphoma formation.78,79 PKCθ and PKCβ mediate the interaction between TAK1 and CARMA1 and recruit IKK, which activates downstream NF-κB signaling.76,80
Termination of NF-κB is associated with nuclear degradation and re-localization of NF-κB subunits, and dissociation of coactivators.8 NF-κB signaling promotes the expression of IκBα, which is newly synthesized to enclose it in the cytoplasm by conjugation with the NF-κB dimer, thereby promoting the termination of transcriptional responses, and plays a significant role in the negative feedback loop of NF-κB signaling.81
Non-canonical NF-κB pathway
Components of non-canonical NF-κB pathway
Non-canonical NF-κB signaling activated by stimuli such as B lymphocyte activating factor (BAFF), CD40 ligand (CD40L), and lymphotoxin β (LTβ) does not require IKKβ or NEMO but instead relies on NF-κB-inducing kinase (NIK) and IKKα.82 NIK is a central component of non-canonical NF-κB signaling. The hallmark of non-canonical NF-κB signaling is the stabilization of NIK via ubiquitination and proteasomal degradation.83,84 NIK not only can activate IKKα but also facilitates binding between IKKα and p100, a process that is dependent on two amino acid residues of p100 (aa 866, 870).85 IKKα binds to p100 and phosphorylates serines 99, 108, 115, 123, and 872 on p100.85 p100 is subsequently ubiquitylated and partially degraded to active p52 by β-transducin repeats-containing proteins (β-TrCP) ubiquitin ligase and the 26 S proteasome.8 p100 also functions to inhibit RelB nuclear translocation.83,86
Non-canonical NF-κB signaling activated by receptors such as CD40, B lymphocyte stimulating factor receptor (BAFF-R), and lymphotoxin beta receptor (LTβR) involves the degradation of TRAF3, which is dependent on cIAP1/2 and TRAF2.87 IAP promotes the proteasomal degradation of NIK via the E3 ubiquitin ligase activity promotes proteasomal degradation of NIK, which can act as a regulator of NF-κB signaling.88 Activation of NF-κB signaling and TNF-α production by IAP antagonist compounds (IACs) was observed in tumor cell lines.89 The binding of TRAF2 to cIAP1/2 promotes TRAF2 and TRAF3 dimerization and recruitment of NIK.90 TRAF3 binds to the sequence motif ISIIAQA at the N-terminal end of NIK and promotes proteasomal degradation of NIK, thus acting as a negative regulator of NIK.84 NIK dissociates from the cIAP1/2-TRAF2 ubiquitin ligase complex and activates downstream IKKα.87
Activation and regulation of non-canonical NF-κB pathway
Most of the non-canonical NF-κB receptors belong to the TNFR superfamily, including BAFF-R, CD40, LTβR, and receptor Activator of NF-KappaB (RANK), which are associated with the recruitment of different TRAF members and bind to the corresponding ligands as complexes.83 TRAF members trigger the disassembly of the receptor-ligand complexes and further trigger the activation of NIK.
LT and tumor necrosis factor ligand superfamily member 14 (LIGHT) expressed in lymphocytes can act as ligands that bind to LTβR on the surface of lymphoid stromal cells and epithelial cells to activate NIK and mediate canonical and non-canonical NF-κB signaling by recruiting TRAF2/3/5.83 In canonical NF-κB signaling, LTβR promotes the expression of inflammatory genes such as macrophage inflammatory protein-1β (MIP-1β), MIP-2, and vascular cell adhesion molecule-1 (VCAM-1).91 In non-canonical NF-κB signaling, LTβ R mainly mediates B lymphocyte chemoattractant (BLC), EBI-1-ligand chemokine (ELC), secondary lymphoid tissue chemokine (SLC), stromal cell-derived factor-1 α (SLC), secondary lymphoid tissue chemokine (SDF-1α), and BAFF, and other genes related to secondary lymphoid organogenesis and homeostasis.91 BAFFR expressed in B cells preferentially induces the non-canonical NF-κB signaling pathway, which mediates B cell survival, development, and maturation.83,92,93,94 73-75 BAFF-R binds more strongly and rapidly to TRAF3, a property that is primarily associated with the BAFF-R signaling motif PVPAT.93 Degradation of TRAF3 activates non-canonical NF-κB signaling, and induction of the canonical NF-κB pathway requires TRAF2.83 CD40 is primarily expressed in B cells, and upon binding to CD40L on the surface of activated T cells, one pathway activates non-canonical NF-κB signaling through the recruitment of TRAF2 and TRAF3, and the other pathway participates in canonical NF-κB signaling through the recruitment of TRAF6.83 CD40-activated non-canonical NF-κB signaling is mainly involved in the regulation of T-B cell interactions, B cell proliferation, survival, and antibody isotype switching.95 Receptor activator of NF-KappaB ligand (RANKL)/RANK interaction is not only involved in the regulation of osteoclast development and activation, but also mediates immune cell survival, communication, and lymphoid organ formation.96 RANK-activated non-canonical NF-κB signaling promotes osteoclastogenesis and differentiation.97,98
Crosstalk of NF-κB signaling
Signaling molecules transmit regulatory signals intracellularly or extracellularly and act as receptors, ligands, protein kinases, or transcription factors in signaling pathways. The different signaling pathways constitute a signal transduction network with a fine-grained regulatory system through mutual interactions. NF-κB signaling is not isolated in the regulation of numerous physiological and pathological processes in which it is involved, and there may be direct or indirect regulation with other molecules, which in consequence, triggers interactions with other signaling pathways. Classical signaling pathways include NF-κB, PI3K/AKT, MAPK, JAK-STAT, TGF-β, Wnt, Notch, and Hedgehog signaling. These signaling pathways may interact with NF-κB signaling in the involvement of biological processes such as cell proliferation, differentiation, survival, death, development, immunity, inflammation, and tumorigenesis. In addition, members of the TLR receptor family are also engaged in NF-κB signaling by recognizing antigenic components of microorganisms. When placing vision in the sophisticated molecular regulatory network, it contributes to our better comprehension of NF-κB signaling by shedding light on its interactions with the abovementioned pathways (Fig. 2).
The crosstalk between NF-κB signaling and other signaling pathways. (1) The activation of PI3K by BCR and IL-7R via the cIAP-IKK pathway results in the stimulation of NF-κB. Hepatitis B virus X protein induces aerobic glycolysis and produces lactate through the NF-κB/hexokinase 2 pathway, activating the PI3K/AKT signal; (2) NF-κB inhibits TNF-α-mediated JNK signaling; (3) The product of NF-κB signaling, IL-6, can activate STAT3. JAK-STAT3 can act as an upstream regulator of NF-κB, promoting NF-κB signal transduction, while STAT1 inhibits NF-κB-mediated tumor cell survival; (4) TAK1 promotes NF-κB transcriptional activity; (5) Wnt/β-catenin signaling activates NF-κB in the cytoplasm. Dvl inhibits NF-κB signaling in the nucleus; (6) Notch1 binds to NF-κB, promoting NF-κB transcriptional activity. APC adenomatosis polyposis coli protein, CK1α casein kinase 1 alpha, DAP12 DNAX-activating protein of 12 kDa, Dvl disheveled, EMT epithelial-mesenchymal transition, ERK extracellular regulated protein kinase, GSK3β glycogen synthase kinase 3 beta, IL interleukin, IL-7R interleukin 7 receptor, JAK Janus kinase 2, JNK c-Jun N-terminal kinase, LPS lipopolysaccharide, LYN LYN proto-oncogene, Src family tyrosine kinase, MEK mitogen-activated protein kinase, MyD88 MYD88 innate immune signal transduction adapter, NF-κB nuclear factor kappa B, NICD Notch intracellular domain, PI3K phosphatidylinositol 3-kinase, STAT signal transducer and activator of transcription, SYK spleen associated tyrosine kinase, TAK1 TGF-beta activated kinase 1, TBK TANK-binding kinase, TGF transforming growth factor, TLR toll-like receptor, TNF tumor necrosis factorα, TRIF toll-like receptor adapter molecule 1, TβR TGF-beta receptor, Wnt wingless-type MMTV integration site family
Crosstalk of NF-κB signaling with PI3K/AKT signaling
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, as a crucial cellular signaling pathway, is involved in the regulation of several biological activities, which include, but are not limited to, cellular metabolism, proliferation, survival, and angiogenesis, and is in turn involved in the regulation of oncology, metabolism, immunity, angiogenesis, and cardiovascular homeostasis.99,100,101 Signals from growth factors, cytokines, and cytokines bind to the cell surface receptor tyrosine kinase (RTK) or G protein-coupled receptor (GPCR) and promote PI3K-catalyzed production of phosphatidylinositol trisphosphate (PIP3), and PIP3 is the second messenger that activates AKT.100,102 Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) encodes the p110α catalytic subunit of PI3K, and its mutation is one of the most common somatic alterations in solid tumors.103
The interplay between NF-κB signaling and PI3K/AKT signaling in diffuse large B cell lymphoma (DLBCL) is a significant phenomenon. The proliferation and survival of activated B-cell type diffuse large B cell lymphoma (ABC-DLBCL) cells require active BCR signaling, and activation of NF-κB signaling is detected in ~10% of ABC-DLBCL, and the BCR-PI3K-NF-κB signaling cascade has been suggested as a potential target for the treatment of DLBCL as a potential target.104,105 Recent findings have revealed that PI3K activates NF-κB signaling through the cIAP-IKK pathway, and copanlisib, a dual inhibitor of PI3Kα/δ, may effectively block PI3K/AKT signaling and NF-κB signaling in ABC-DLBCL, leading to tumor regression.106 Inhibition of PI3Kβ/δ in DLBCL was also found to decrease NF-κB activity.107 The dual inhibitor of PI3K and HDAC, CUDC-907, also reduced the activity of AKT, p65, and BCL-XL in multiple myeloma (MM) in a dose-dependent manner.108 Another exemplary case is atherosclerosis. IL-7, which is essential for T cell development and balance, activates NF-κB signaling via the PI3K/AKT pathway, upregulates the expression of monocyte chemotactic protein 1 (MCP-1) and cell adhesion molecule (CAM) in macrophages and human aortic endothelial cells, and plays an active role in atherosclerosis.109 Increased secretion of the pro-inflammatory factor galectin-3 (Gal-3) in atherosclerosis activates the PI3K/AKT pathway and inhibits autophagy upon binding to CD98, whereas inhibition of Gal-3 reduces the activity of the NF-κB pathway, suppresses inflammation, and enhances autophagy.110
NF-κB signaling may also interact with PI3K/AKT signaling through metabolic pathways. In hepatitis B Virus (HBV)-related hepatocellular carcinoma (HCC), hepatitis B protein X (HBx) induces aerobic glycolysis and produces a large amount of lactic acid through NF-κB/hexokinase 2 (HK2) signaling, which further activates PI3K/AKT signaling and improves the malignant proliferation ability of HCC cells.111 Somatostatin receptor subtype 2 (sst2) inhibits KRAS-activated PI3K signaling. Studies in KRASG12D, sst2± hybrid mice demonstrated that PI3K/AKT signaling activates NF-κB signaling and activates KRAS, promoting the release of CXC chemokine ligand 16 (CXCL16) and IL-6, ultimately leading to the progression of pancreatic ductal adenocarcinoma (PDAC).112 The PI3K/AKT/NF-ΚB signaling system also facilitates the epithelial-mesenchymal transition (EMT).113
Crosstalk of NF-κB signaling with MAPK signaling
The mitogen-activated protein kinase (MAPK) belongs to the serine/threonine kinase family and plays an important role in diverse cellular programs such as proliferation, differentiation, development, transformation, inflammatory responses, and apoptosis by transmitting, amplifying, and integrating signals from a broad spectrum of stimuli. MAPK signaling is a conserved enzymatic cascade that mediate signal transduction from the cell surface to the nucleus through phosphorylation events. This pathway involves three key enzymes: mitogen-activated protein kinase kinase kinase (MAPKKK), mitogen-activated protein kinase kinase (MAPKK), and mitogen-activated protein kinase (MAPK). MAPK is responsible for phosphorylating target proteins in the cytoplasm or nucleus. MAPKs in mammalian cells mainly include extracellular regulated protein kinase (ERK), p38 MAPK, c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinase 5 (ERK5). The transcriptional specificity of NF-κB can be achieved through interaction with the MAPK pathway.8 Evidence of NF-κB signaling’s interaction with MAPK signaling has primarily centered on JNK signaling. TAK1 serves as an upstream kinase for both NF-κB signaling and JNK signaling.10 The JNK pathway regulates cell cycle progression through multiple mechanisms. JNK activates c-Jun and activator protein-1 (AP-1) to exert pro-oncogenic effects, while simultaneously inducing apoptosis.114 Cellular responses exhibit variability based on the nature of the stimulus, the extent of JNK activation, and the duration of the response.114 Studies investigating the interaction of NF-κB signaling with JNK signaling have revealed that although JNK signaling regulates cell death or survival, the ultimate fate of the cell is determined by NF-κB, and activation of NF-κB signaling is capable of inhibiting pro-apoptosis induced by caspases, JNK, and reactive oxygen species (ROS).115 Negative regulation of TNF-α-mediated JNK signaling by NF-κB has been identified in murine embryonic fibroblasts, and it is important to note that this negative crosstalk is specific to TNF-α signaling.116,117 NF-κB was also observed to block TNF-induced apoptosis through the downregulation of JNK and c-Jun/AP-1 in rat hepatocytes.118 Sst2 also activates NF-κB signaling through Src homology region 2domain-containing phosphatase 1 (SHP-1), leading to the inhibition of JNK phosphorylation and apoptosis.119 During acute liver failure, interleukin 1 receptor type 1 (IL-1R1) is stimulated by IL-1 and activates the NF-κB signaling, which promotes transcriptional upregulation of inflammation-related genes and recruitment of immune cells, while NF-κB inhibits TNF-activated JNK/ERK signaling and prevents caspase 3-mediated apoptosis, which further amplifies inflammatory responses and exacerbates hepatic injury.120
Crosstalk of NF-κB signaling with JAK-STAT signaling
Janus kinase 2 (JAK) binds non-covalently to cytokine receptors, mediates the tyrosine phosphorylation of the receptor, and recruits one or more signal transducer and activator of transcription (STAT) proteins. Upon phosphorylation, STAT proteins translocate across the nuclear membrane to modulate the activity of specific genes. The JAK family comprises JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2).121 Erythropoietin mediates the activation of JAK2 in neurons, which further activates NF-κB signaling and initiates the transcription of genes with neuroprotective effects.122 The STAT family consists of STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6.123 Each STAT protein exerts unique biological effects and plays a regulatory function in cell survival, differentiation, metabolism, and immune response, and plays a key role in malignant tumors and autoimmune diseases.124 STAT1 helps boost immunity against tumors, yet STAT3 and other types of proteins may trigger pro-cancer inflammation.125 A close interaction between STAT3 and NF-κB signaling has been observed. IL-6, a gene product regulated by NF-κB signaling, is an important STAT3 activator.126 IL-10 and CpG synergistically activate STAT3 and NF-κB in a human B cell line induced by MYC.127 STAT3 also inhibited the expression of molecules essential for NF-κB and STAT1-mediated antitumor immunity, including IL-12 and interferon (IFN)-γ.128,129 STAT3-mediated acetylation of RelA promotes NF-κB to exert pro-transcriptional activity in the nucleus, a phenomenon observed in both tumor cells and tumor-associated hematopoietic cells.130 Deletion of Abelson interactor 1 (Abi-1) may lead to increased activity of STAT3 and NF-κB, which may be a potential mechanism leading to primary myelofibrosis.131 In colorectal cancer, IKKα induces the cytokine leukemia inhibitory factor (LIF) by inducing NF-κB dependent transcriptional activity, thereby activating STAT3.132 In NIK-positive anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma cells, STAT3 promotes the expression of p52 and CD30, thereby inducing sustained activation of non-canonical NF-κB signaling.133 STAT3 promotes the degradation of p100 to p52 through the activation of IKKα. This process necessitates the activation of STAT3 by cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB)-binding protein (CBP)/p300.134 STAT3 not only promotes tumor cell proliferation, survival, neovascularization, and metastasis but also exerts an inhibitory effect on anticancer immunity.125 IFN-γ and TNFα promote the inducible nitric oxide synthase (iNos) gene promoter’s response to NF-κB through activation of JAK-STAT signaling in muscle fibroblasts recruitment, thereby activating the iNOS/nitric oxide (NO) pathway and inducing muscle atrophy.135
Crosstalk of NF-κB signaling with TGF-β signaling
Members of the transforming growth factor (TGF)-β family include TGF-β, activating factor, and bone morphogenetic protein (BMP). These cytokines play crucial roles in diverse cellular processes, including cell proliferation, migration, metabolism, immune regulation, and inflammatory response.136 The TGF-β family of receptors comprises the type I receptor TGF-beta receptor (TβR) I, the type II receptor (TβRII), and the type III receptor (TβRIII), among which TβRI and TβRII possess intrinsic kinase activity, which is essential for TGF-β signaling. Upon binding to the ligand, TβRII phosphorylates the serine and threonine residues of TβRI. Activated TβRI subsequently phosphorylates the downstream signaling molecule Smad, leading to its nuclear accumulation and transcriptional regulation as a transcription factor. In the early stage of tumorigenesis, the TGF-β family exerts an oncogenic effect by inhibiting cell proliferation. However, as the tumor continues to progress, tumor cells develop resistance to TGF-β-mediated growth inhibition, which is attributed to mutations in genes encoding signaling intermediates.137,138 TCR inhibits TβRI expression and TGF-β signaling through activation of NF-κB signaling and CARMA1, resulting in the quiescence of T cells.139 Smad7 inhibits TNF signaling by forming a complex with TAB2 and TAB3, thereby suppressing NF-κB activation and inflammatory responses.140 However, NF-κB in glioblastoma activates TGF-β by inducing miR-148a or miR-182, leading to hyperactivation of both NF-κB and TGF-β signaling.141,142 TAK1 promotes the phosphorylation and transcriptional activity of NF-κB, which mediates inflammatory response, EMT, tumor metastasis, chemoresistance, etc.143,144,145 Whereas TAK1 exerts a negative regulatory effect on IKK in neutrophils after stimulation by LPS, which is in contrast to the previous perceptions.146 TGF-β induces ubiquitination degradation of MyD88 to negatively regulate pro-inflammatory signaling, specifically through the recruitment of Smad ubiquitination regulatory factor (Smurf) 1 and Smurf2 with E3 ubiquitin ligase activity by Smad6.147 TGF-β also stimulates cardiac inflammation and fibrosis through activation of NF-κB signaling.148
Crosstalk of NF-κB signaling with Wnt signaling
The wingless-type MMTV integration site family (Wnt) signaling pathways encompass Wnt/β-catenin, Wnt/planner cell polarity (PCP), and Wnt/Ca2+ pathways. The Wnt/β-catenin signaling pathway is a β-catenin-dependent class of Wnt signaling, also known as the canonical pathway, which mainly controls cell proliferation. The Wnt/PCP and Wnt/Ca2+ pathways are not dependent on β-catenin and are known as non-canonical pathways that regulate cell polarity, adhesion, and migration. In the Wnt/β-catenin pathway, lipoprotein receptor-related protein (LRP) and frizzled (FZD) act as Wnt receptors and form a complex with Wnt proteins to activate downstream signaling. During the development of acute myocardial infarction, elevated Wnt2 promoted β-catenin/NF-κB signaling by binding to Fzd4 and LRP6, and elevated Wnt4 activated the same signaling by binding to Fzd2 and LRP6, resulting in a pro-fibrotic effect.149 Axis inhibition protein (Axin)/ adenomatosis polyposis coli protein (APC)/ glycogen synthase kinase 3 beta (GSK3β)/ casein kinase 1 alpha (CK1alpha) complex phosphorylates and inactivates β-catenin. NF-κB transcriptional activation is decreased in GSK3-deficient embryonic fibroblasts without affecting IκB degradation and nuclear translocation of NF-κB.150 Disheveled (Dvl) impedes the Axin/APC/GSK3β/CK1α complex in the cytoplasm, which inhibits the degradation of β-catenin and promotes its translocation to the nucleus, and activates proliferation- and differentiation-related genes by interacting with the T-Cell factor (TCF) family of transcription factors and activating coactivators.151,152 In contrast, it has been revealed that Dvl interacts with p65 in the nucleus and inhibits NF-κB-mediated transcriptional activation, and promotes apoptosis, independently of Wnt or β-catenin.153 β-TrCP, a ubiquitin E3 ligase, promotes ubiquitylated degradation of β-catenin in response to resting Wnt signaling. During endotoxemia, NF-κB and Wnt/β-catenin signaling are mutually activated, and β-TrCP mediates the degradation of IκB to upregulate NF-κB signaling. Activated NF-κB, in turn, promotes the production of Wnt, β-catenin, and β-TrCP, which leads to cytokine storms, liver injury, and even death.154 Wnt signaling may also interact with non-canonical NF-κB signaling. LTβR was found to inhibit WNT/β-catenin signaling in alveolar epithelial progenitor cells by activating non-canonical NF-κB signaling, thereby promoting lymphocyte apoptosis and inhibiting regeneration.155
Crosstalk of NF-κB signaling with Notch signaling
The Notch signaling consists of Notch receptors, Notch ligands, CBF-1/Suppressor of hairless/Lag (CSL)-DNA-binding proteins, intracellular effector molecules, and regulators of Notch, which regulate diverse cellular processes including proliferation, stem cell maintenance, differentiation, and death.156 The classical NOTCH signaling does not necessitate amplification by a cascade of second messengers and protein kinases, and the receptor is directly transported to the nucleus after three cleavage events.157 The Notch receptor consists of an extracellular domain (NEC), a transmembrane fragment (NTC), and an intracellular domain (NTC). Notch intracellular domain (NICD).
When Notch signaling is transmitted in two neighboring cells, the Notch receptor interacts with the ligand and undergoes triple shearing, releasing the activated form of Notch, NCID, into the nucleus and binding to the transcription factor CSL to regulate downstream gene expression. The network of interactions between Notch and NF-κB may contribute to the pathogenesis of T-cell acute lymphoblastic leukemia.158,159 One possible mechanism is that the intracellular structural domain of Notch1 may compete with IκBα for binding to NF-κB and promote the transcriptional activity of NF-κB.160 It has also been found that Notch inhibits the deubiquitinase CYLD (a negative regulator of IKK) via HES1 to maintain NF-κB activity.161 In Barrett’s esophagus mouse model, Notch signaling activates NF-κB and regulates the differentiation of gastric cardia progenitor cells.162 Apurinic/apyrimidinic endonuclease (APE1) is activated in a variety of cancers and induces transcription of target genes by interacting with several redox-dependent transcription factors.163,164 APE1 promotes the activation of Notch signaling in esophageal adenocarcinoma through redox-dependent NF-κB activation and upregulation of delta-like protein 1 (DLL1) (Delta-type Notch ligand), which is critical for cancer cell stemness, inflammation, and embryonic development.164 In myeloproliferative disorders, the transcription of miR-155 is inhibited by Notch/RBPJ, leading to attenuated miR-155 inhibition of κB-Ras1 (an inhibitor of NF-κB), thereby promoting NF-κB signaling as well as the production of pro-inflammatory cytokines.165 The target gene of Notch signaling, HES1, represses Deltex1 transcription by binding directly to a site located 400 bp upstream of the Deltex1 transcriptional start site, thereby leading to the restoration of Notch1 expression.166 In medullary thyroid carcinoma with RET mutation, nuclear translocation of NF-κB binds to and enhances the expression of the miR-182 promoter, inhibits HES1 and upregulates Deltex1, ultimately promoting tumor invasion and migration.167 Overexpression of p52 and RELB in a mouse pluripotent stem cell line resulted in elevated levels of RBP and HES1, which were dependent on NICD.168 Notch is also an important upstream regulator of non-canonical NF-κB signaling, and it was found that γ-secretase inhibitor (GSI) XII inhibited Notch signaling in Hodgkin’s and Reed-Sternberg’s cells, further down-regulated the expression of p52 and RelB, and inhibited the conversion of p100 to its active form, p52.169
Crosstalk of NF-κB signaling with Hedgehog signaling
Hedgehog signaling is a highly conserved pathway with important roles in the control of cell proliferation, tissue homeostasis, tumorigenesis, and embryonic development.170,171 Members of the Hedgehog gene family include Sonic Hedgehog (SHh), Indian Hedgehog (IHh), and Desert Hedgehog (DHh), of which SHh has been the most widely and intensively studied. In the resting state, Smoothened (Smo) is inhibited by Hedgehog’s receptor PTCH. When Hedgehog binds to PTCH, activated Smo transmits signals through Gli, Sufu, and Kif7, resulting in the generation of the Gli-activated form (GliA), which translocates to the nucleus and leads to transcriptional activation of Hh target genes.170,171 NF-κB signaling plays a pivotal role in the generation of apical ectodermal ridges of limb buds during development, and inhibition of NF-κB in vertebrate limb mesenchyme downregulates the expression of SHh and Twist.172 In chronically damaged fibrotic livers, Smo suppresses transcriptional expression of miR-378a-3p via p65 activation, which subsequently upregulates the expression level of Gli3.173 High expression of p65, SHh, and Gli1 was observed to be associated with poorer prognosis in patients with advanced prostate cancer. Experimental verification in cell lines observed inconsistent results, although NF-κB signaling and SHh-Gli1 signaling activation were observed in both PC3 and DU145 cell lines, whereas in PC3 cell lines, Gli1 activation was only dependent on SHh, while in DU145 cells, Gli1 expression was neither dependent on SHh nor NF-κB.174 Therefore, further investigation is required to elucidate the crosstalk mechanism between NF-κB and Hedgehog signaling.
Crosstalk of NF-κB signaling with TLR signaling
Toll-like receptor (TLsR) is a single-channel transmembrane protein consisting of an extracellular region, a transmembrane region, and an intracellular region, and it belongs to pattern recognition receptors (PPRs). Mammalian TLRs are expressed in a number of cell types, including macrophages, dendritic cells, B cells, stromal cells, and epithelial cells. TLR recognizes and interacts with surface and intracellular components of microorganisms, activates innate immunity and mediates the development of acquired immunity.175 The TLR signaling pathway originates from a conserved intracellular structural domain of the receptor consisting of ~200 amino acids, the Toll/IL-1R (TIR) domain. TLR binding to ligands induces the formation of dimers or conformational changes that activate TLR signaling, recruit downstream signaling molecules, and ultimately lead to the activation of NF-κB and MAPK signaling, among others.176
However, TLR does not necessarily always mediate the activation of NF-κB signaling. There may be a negative regulatory relationship between the two under the influence of other molecules or pathways. A typical case is found in macrophages, where LPS inhibits NF-κB signaling by inducing Inducible cAMP early repressor (ICER) expression via p38-mediated cAMP response element-binding protein (CREB), a negative feedback loop that is an important mechanism for preventing TLR-driven excessive inflammation.177 The downstream kinase mitogen-and stress-activated protein kinase (MSK)1/2 of p38 phosphorylates and activates the transcription factor CREB, which promotes the transcription of related genes.178 Phosphorylated CREB inhibits NF-κB activation by competing with p65 for binding to CREB-binding protein (CBP).179 ICER is induced by CREB and constitutes a negative regulatory loop by binding and inhibiting the cAMP response element.180 Another prominent example pertains to CD300b, which functions biologically as a binding receptor for LPS. CD300b and its adapter, DAP12, activated splenic tyrosine kinase (Syk) and PI3K upon binding to LPS and TLR4, promoting the dissociation of MyD88-TIRAP, which further inhibited the activation of the MEK1/2-ERK1/2 and NF-κB pathways via AKT, thereby inhibiting the production of the anti-inflammatory factor IL-10 and driving the cytokine response and aggravating septic shock.181
Physiology and pathology of NF-κB signaling
Physiological roles of NF-κB signaling
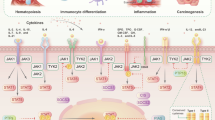
NF-κB plays a key role in cellular responses to external stimuli such as cytokines, stress, UV light, antigens, and heavy metals. Existing studies have demonstrated that the NF-κB signaling is involved in a diverse array of physiological and pathological processes, including immune and inflammatory responses, cell survival and proliferation, metabolism, as well as synaptic plasticity and memory-related activities81,182,183 (Fig. 3a).
The biological functions of NF-κB signaling. a The NF-κB signaling supports cell survival under physiological settings, modulates inflammation and immunological responses to external stimuli, and also helps to regulate metabolism and homeostasis. Overactivation of the NF-Κb signaling increases tumor malignancy in pathological settings, including angiogenesis, EMT, invasion, metastasis, and treatment resistance. Furthermore, NF-κB signaling dysregulation can result in inflammatory storms and metabolic problems. BAFF TNF superfamily member 13b, BAFF-R TNF receptor superfamily member 13 C, BCR B-cell receptor, IKK I-kappaB kinase, IKK I-kappaB kinase, IκB IkappaB protein, LIGHT tumor necrosis factor ligand superfamily member 14, LPS lipopolysaccharide, LTA lymphotoxin alpha, LTB lymphotoxin beta, LT-βR lymphotoxin beta receptor, MHC major histocompatibility complex, NEMO inhibitor of nuclear factor kappa-B kinase subunit gamma, NIK mitogen-activated protein kinase kinase kinase 14, RANK TNF receptor superfamily member 11a, RANKL TNF superfamily member 11, TCR T-cell receptor, TLR toll-like receptor, TNF tumor necrosis factor, TNFR TNF receptor. b NF-κB plays a pivotal role in both innate and adaptive immunity. In the context of innate immunity, NF-κB promotes the differentiation of macrophages into M1 phenotype. Additionally, NF-κB facilitates dendritic cell maturation and neutrophil recruitment. Concerning adaptive immunity, NF-κB enhances the activation, proliferation, maturation, and selection of B cells. Moreover, under the stimulation of different cytokines, NF-κB can drive the differentiation of CD4 T cells into various subtypes. TH helper T cell, Treg regulatory T cell, IL-12 interleukin-12, TNF-α tumor necrosis factor-alpha, Foxp3 forkhead box protein 3. c Tumor occurrence and progression are closely linked to TME. Overactivation of NF-κB signaling not only promotes tumor cell survival, invasion, metastasis, genomic instability, and metabolic abnormalities, but also reshapes the immune-suppressive microenvironment, promoting immune escape and resistance to immunotherapy. CAF cancer-associated fibroblasts, EMT epithelial-mesenchymal transition, MDSC myeloid-derived suppressor cell, PD-1 programmed death 1, TAM tumor-associated macrophage, TME tumor microenvironment
NF-κB signaling is particularly important in regulating cellular adaptation to environmental changes. In response to inflammatory stimuli, immune cells reconfigure metabolism through cellular responses mediated by NF-κB signaling. Drosophila studies revealed that NF-κB maintains the coordination of innate immune-metabolic responses by inhibiting Foxo-mediated lipolysis.184 Muscle contraction involves activation of NF-κB signaling by Ca2+, peroxides, and nitrogen oxides.185,186,187 It has been demonstrated that NF-κB signaling is activated during the strenuous exercise of the organism, either in normoxia or acute hypoxia, which includes the increase of p105, p50, IKKα, IκBβ, and glutathione reductase protein levels as well as CaMKII δD phosphorylation. When exercise ends and the muscle resumes open circulation, these changes return. The design of the new study needs to take into account the rapid changes in NF-κB signaling during exercise cessation.188
TLR-induced NF-κB activation upregulates the transcription of genes encoding inflammatory vesicles and initiates immune responses.189 Inflammation serves as a pivotal defense mechanism against bacterial and viral infections. Serine/threonine kinase 4 (Stk4) and NF-κB are involved in the activation and homeostasis of regulatory T (Treg) cells and promote Treg cell-mediated immune tolerance. Deletion of Stk4 in mouse Treg cells inhibits p65 expression, p65-Foxp3 complex formation, and Treg cell activation, ultimately leading to autoimmune lymphoproliferative disorders.190 The IKK complex protects mature T cells from TNF-induced cell death and is important for their normal homeostasis and function.7 The integrity of cellular function requires rapid activation and termination of NF-κB signaling, and this tight regulation is essential for normal cellular and organismal homeostasis.191 N6-methyladenosine (m6A) mRNA modification is involved in the maintenance of colonic epithelial cells and stem cell homeostasis. Studies in mouse colon epithelial cells have revealed that methyltransferase 14 (Mettl14) inhibits colonic epithelial cell apoptosis by modulating the NF-κB pathway.192
Pathological roles of NF-κB signaling
The pathological effects of NF-κB signaling include immune disorders, malignant behavior of tumor cells, metabolic dysregulation, and skeletal disorders. These effects are further described below.
Due to the key regulatory role of NF-κB signaling in immune and inflammatory responses, its dysregulation has been strongly associated with a variety of human diseases, including cancer, inflammatory diseases, autoimmune disorders, viral infections, and infectious shock.81,189,193 During inflammation, the NF-κB signaling is hyperactivated, leading to the abundant expression of inflammation-associated genes. Initially, researchers discovered that NF-κB potentially contributes to the pathogenesis of acquired immune deficiency syndrome (AIDS) by synergizing with and stimulating the transcription of human immunodeficiency virus (HIV).194 Research on p50-deficient mice has demonstrated the crucial involvement of NF-κB in both specific and non-specific immune responses, and although there is no evidence for the involvement of NF-κB in the developmental process.195
As a chronic ailment, the prevalence and fatality of neoplasms persistently escalate, posing a significant peril to human existence and well-being. NF-κB signaling is involved in tumorigenesis, progression, EMT, tumor metastasis, and drug resistance.6,191 NF-κB signaling is a major pathway mediating the interaction between inflammation and cancer. As a result of alterations in the inflammatory microenvironment and oncogenic mutations, sustained NF-κB activation and dysregulation of cellular functions are observed in cancer, leading to genomic instability and gene mutations, creating a microenvironment that promotes tumor progression and promotes proliferation and angiogenesis of tumor cells while inhibiting their apoptosis.6,191,196
Recent research has unveiled the pivotal role of NF-κB in the cellular response of tumors to nutrient-deprived microenvironments, and the main mechanism is to remodel the local metabolism by coordinating the actions of glycolysis, glutaminolysis, and oxidative phosphorylation pathways.184,197,198,199,200,201 Impairment of canonical and non-canonical NF-κB signaling may lead to specific developmental and immune deficiencies.202,203 For instance, germline mutations in NFKB2 in non-canonical NF-κB signaling affect the nuclear translocation of p52, which is thought to be the genetic cause of primary immunodeficiency syndromes.204
The non-canonical NF-κB pathway is crucial in lymphoid organ development, lymphocyte survival and homeostasis, dendritic cell activation, osteoclastogenesis, etc., and its aberrant activation may lead to rheumatoid arthritis, ulcerative colitis, osteoporosis, and lymphoid malignancies.83,205,206,207,208 Expression of RelB subunits is associated with the differentiation of dendritic cells and thymic UEA-1+ medullary epithelial cells, which provides the basis for its involvement in immune responses.209 NF-κB receptor activator ligand (RANKL), an osteoclast differentiation factor, has an influential role in osteoclastogenesis, linking the activated immune system to bone loss.210,211,212,213
NF-κB signaling, immune system, and inflammation
The NF-κB family is a crucial component of both innate and adaptive immunity, and plays a vital role in immune response regulation. Upon stimulation by various inducers, NF-κB undergoes translocation to the nucleus, where it binds to specific DNA sites and orchestrates the transcriptional control of numerous genes. These genes encompass antimicrobial peptides, cytokines, chemokines, stress response proteins, and anti-apoptotic proteins, among others.79 Persistent activation of the NF-κB pathway is frequently implicated in inflammatory conditions like rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis (MS), and asthma.214 Gaining deeper insights into the modulation of the NF-κB pathway holds the potential to establish targeted therapies for inflammatory diseases. In this section, we will delve into the interplay between the NF-κB signaling pathway and the immune system, spanning both innate and adaptive immunity (Fig. 3b).
Innate immunity
Innate immune cells, such as macrophages, dendritic cells, and neutrophils, play a critical role in innate immunity and the inflammatory response. These cells express pattern recognition receptors (PRRs) that are capable of detecting a wide range of microbial components known as pathogen-associated molecular patterns (PAMPs).215,216 Additionally, PRRs are also involved in recognizing molecules called damage-associated molecular patterns (DAMPs), which are released by necrotic cells and damaged tissues.
One crucial signaling pathway activated by PRRs is the canonical NF-κB pathway, which plays a significant role in the induction of pro-inflammatory cytokines, chemokines, and other inflammatory mediators in various innate immune cell types. These inflammatory mediators can directly contribute to inflammation or indirectly promote the differentiation of inflammatory T cells.
One common signaling transduction event of pattern recognition receptors (PRRs) is the activation of the canonical NF-κB pathway, which is responsible for the transcriptional induction of pro-inflammatory cytokines, chemokines, and other inflammatory mediators in different types of innate immune cells.217 The process of PRRs activating the NF-κB pathway is as follows: downstream of PRRs, LPS/TLR4 converges through myd88-dependent and TRIF-dependent signaling pathways to activate IKK via TRAFs. The dsRNA/RIG-I signal is transmitted to IKKi/TBK1 through ISP1 and then to IKK through RIP1. The signaling from NOD to NF-κB is believed to involve RIP2 oligomerization and the induction of proximity to activate IKK.218 Intestinal epithelial cells (IECs) express various PRRs, including TLRs, on their basolateral and apical cell membranes. When encountering microbial ligands, these receptors initiate cascades of signaling events leading to the activation of NF-κB and other pro-inflammatory pathways.219,220 Additionally, NF-κB serves as a central mediator for the activation initiation signal of the NLRP3 inflammasome, responding to various PRR ligands and cytokines by inducing the transcriptional expression of NLRP3 and pro-IL-1β.221
Adaptive immunity
Adaptive immunity is a specific immune response by the body against particular antigens, mainly mediated by T and B lymphocytes. NF-κB regulates the functions of multiple immune cells in adaptive immunity through gene transcription regulation. First, NF-κB participates in regulating T cell development and activation.222 Under normal conditions, most T cells are in a resting state, but when stimulated, NF-κB is activated and enters the cell nucleus, promoting the transcription of specific genes, thus initiating T cell proliferation and differentiation processes.223 Furthermore, NF-κB also regulates B cell development and function. Upon antigen stimulation, NF-κB is activated in B cells, inducing their proliferation and differentiation.224 NF-κB is also involved in regulating antibody class switching and affinity maturation in B cells.225 These processes are crucial for the formation of specific antibodies and memory responses in the body.
In addition to regulating T and B cell development and activation, NF-κB also controls the expression of pro-inflammatory cytokines in adaptive immunity.225 When immune cells are infected or damaged, NF-κB is activated and induces the synthesis of various pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α),226 interleukin-1 beta (IL-1β),227 and interleukin-6 (IL-6).228 These cytokines can trigger inflammatory reactions and attract other immune cells to eliminate pathogens or repair damaged tissues. Moreover, NF-κB also plays an important role in immune regulation in adaptive immunity. It participates in regulating immune tolerance and immune suppression. Some immune suppressive cells, such as regulatory T cells (Tregs),229 can inhibit the activity of other immune cells by activating the NF-κB pathway, maintaining immune balance and self-tolerance.
In conclusion, NF-κB plays a crucial role in adaptive immunity. It regulates T and B cell development, activation, and function, and is involved in antibody class switching and immunological memory formation. Additionally, it controls the expression of pro-inflammatory cytokines and immune regulatory processes. Further research into the mechanisms and regulatory networks of NF-κB will contribute to a better understanding of the regulatory mechanisms in adaptive immunity and may provide guidance for the development of novel immunotherapeutic strategies.
NF-κB signaling and tumor microenvironment
The tumor microenvironment comprises immune cells, fibroblasts, myeloid-derived inflammatory cells, signaling molecules, surrounding vasculature, and the extracellular matrix (ECM), which constitutes an interacting population with tumor cells and plays an integral role in tumorigenesis and malignant progression.230 Tumor-associated macrophages (TAMs) represent the predominant immune cell population within the tumor microenvironment. They engage in complex interactions with tumor cells, T cells, endothelial cells, and fibroblasts, which can either promote immune evasion, tumor growth, and invasion, or exert antitumor effects.231,232 IL-1β produced by IFN-γ-polarized TAM promotes PIM2 expression in hepatocellular carcinoma cells through MAPK signaling and NF-κB signaling, conferring the ability of tumor cells to metastasize, immune escape, and resist immunotherapy.233 Tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs) have been shown to facilitate tumor angiogenesis through the secretion of proangiogenic and pro-inflammatory factors.234 Platelets activate TGF-β/Smad and NF-κB signaling in tumor cells during intravascular transit from the primary tumor to the metastatic site, promoting tumor metastasis and EMT.235 Histamine secreted by glioblastoma stem cells triggers the activation of endothelial cells by the Ca2+-NF-κB axis, remodeling the tumor microenvironment and thereby promoting angiogenesis and tumor progression.236 CXCL2 and CXCL8 generated in tumor-infiltrating monocytes via the 6-phosphofructo-2-kinase/fructose-2,6-bis-phosphatase3 (PFKFB3)-NF-κB axis promotes neutrophil recruitment in the hepatocellular tumor microenvironment.237 The tumor microenvironment may shape gene expression and cellular phenotypes of immune cells or tumor cells, and this evolutionary change is the result of cellular adaptation under selective pressure.238,239 Activation of aryl hydrocarbon receptor (AHR) in TAMs by glioblastoma-produced kynurenine recruits CCL2 and inhibits activation of NF-κB signaling.240 Polynutrients activate NF-κB in cancer cells, leading to changes in cytokine production, neutrophil recruitment, and the immunosuppressive microenvironment to promote metastasis.241 Due to the chronic inflammatory state within the tumor microenvironment, myeloid-derived suppressor cells (MDSCs) are generated and activated to exert immunosuppressive functions.242 Cysteine-rich intestinal protein 1 (CRIP1) activates NF-κB signaling and upregulates CXCL1/5 expression to recruit MDSCs, leading to an immunosuppressive environment in pancreatic ductal adenocarcinoma.243 Tumor cells also interact metabolically with stromal cells in the tumor microenvironment.189 NIK may act as a key regulator of antitumor immunity and T-cell metabolism, and its deficiency impairs aerobic glycolysis and suppresses CD8+ effector T-cell function201 (Fig. 3c).
Tumor-induced chronic inflammatory microenvironments may lead to immunosuppression and promote immune escape.191 Overexpression of cell cycle-related kinase (CCRK) in chronic liver disease activates NF-κB signaling, promotes CXC motif chemokine ligand (CXCL)1 expression in polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSC), and remodels the immunosuppressive microenvironment to resist metastasis-associated immune surveillance.244 In addition to solid tumors, NF-κB signaling has also been implicated in the microenvironment of hematologic tumors, and it has been demonstrated that NF-κB drives pro-survival, genetic instability, and immune evasion in refractory or relapsed diffuse large B-cell lymphoma.245
Although immunotherapy may induce durable responses in cancer patients, it inevitably faces the same challenges of drug resistance as chemotherapy, targeted therapy, and other therapies.246,247 CD10 + GPR77 + CAF, defined by specific cell surface markers, promotes tumor progression and chemoresistance through sustained activation of NF-κB signaling and complement signaling and secretion of IL-8 and IL-65 to maintain stemness of tumor stem cells.248 Inactivating mutations in TRAF3, TRAF2, CYLD, and cIAP1/2 lead to persistent activation of non-canonical NF-κB signaling.206 The extracellular matrix, comprising fibronectin, glycosaminoglycans, proteoglycans, and mucus, is a dynamic collaborator of the immune system. Immune cells can directly manipulate the synthesis and catabolism of the basic components of the ECM, or they may indirectly regulate the ECM through the secretion of cytokines.249 The study on triple-negative breast cancer has revealed that the molecular and physical properties of the ECM may exert varying impacts on treatment response. The ECM of untreated tumors is thought to be a hard microenvironment, whereas a soft ECM enhances drug resistance by increasing NF-κB signaling activity and downregulating pro-apoptotic JNK signaling activity.250 The tumor microenvironment may be one of the culprits for therapeutic resistance, and researchers have found that maintaining or inhibiting the expression of certain molecules in the tumor microenvironment is a pathway for overcoming drug resistance.
NF-κB signaling in human diseases
Cancers
In normal cells, NF-κB is kept inactive in the cytoplasm by binding with IκB. Upon degradation of IκB, NF-κB translocates into the nucleus to activate target genes and carry out its biological functions. Constitutive activation of NF-κB has been implicated in various solid tumors together with hematological tumors (Fig. 4a).
NF-κB plays a crucial role in diseases affecting various organs and systems. a NF-κB is also upregulated in breast cancer cells, leading to increased downstream gene expression promoting tumor growth, metastasis, and angiogenesis. b Increased expression of NF-κB in respiratory epithelial cells exacerbates TH2 cell-related inflammatory responses and airway hyperresponsiveness, so leading to asthma. c In the kidney, activated NF-κB promotes high expression of inflammatory factors IL-1β and IL-18, leading to renal inflammation. d Activated NF-κB promotes chronic inflammation, fibroblast-like synoviocyte proliferation, and thus contributes to the development of RA in synovial tissues. e There exists a bidirectional relationship between NF-κB signaling, metabolic diseases, and inflammation. Metabolic diseases like insulin resistance, diabetes, and obesity can cause overactivation of NF-κB signaling and inflammation through the regulation of oxidative stress and macrophage function. f NF-κB also facilitates the activation of polyclonal B cells and the production of autoantibodies in patients with SLE. g In macrophages, NF-κB activation induces the secretion of pro-inflammatory cytokines, including TNF-α, IL-12, and IL-23, which directly or indirectly participate in the mucosal tissue damage typically observed in UC. h NF-κB modulate a series of inflammatory mediators and thus participates in the regulation of different cell fates in the atherosclerotic process. I Following brain injury, NF-κB is upregulated in neurons, astrocytes, and microglial cells, resulting in the secretion of more inflammatory factors such as IL-6 and iNOS, thereby triggering local brain inflammation. TBK1 serves as a protective factor by suppressing NF-κB signaling. AGE advanced glycation endproducts, AHR airway hyper reactivity, COX-2 cyclooxygenase-2, DC dendritic cell, EC endothelial cell, ER estrogen receptor, FasL factor-related apoptosis ligand, GlutR glutamyl-tRNA reductase, GM-CSF granulocyte-macrophage colony-stimulating factor, ICAM-1 intercellular cell adhesion molecule-1, iNOS inductible nitric oxide synthase, MMP9 matrix metalloproteinase-9, MN-SOD manganese superoxide dismutase, MYOCD myocardin, NETs neutrophil extracellular traps, NGF nerve growth factor, NTF neurotrophic factor, ox-LDL oxidized low-density lipoprotein, RA rheumatoid arthritis, ROS reactive oxygen species, SLE systemic lupus erythematosus, SMC smooth muscle cell, TBK1 TANK-binding kinase 1, TGF-β transforming growth factor-β, TLR4 toll-like receptor 4, UC ulcerative colitis
NF-κB can be activated by a wide range of inducers, including both extrinsic stimuli (cytokines, viral and bacterial products, carcinogens, etc.) and intrinsic stimuli (cellular stress, DNA damage, hypoxia, oncogene activation, etc.). The target genes transcriptionally regulated by NF-κB modify the gene expression pattern in cells to cope with the changes and threats faced by the organism. However, these responses can be highly pleiotropic and the outcomes of NF-κB activation largely depend on the context. Although the targets of NF-κB in tumor cells may be similar to those in normal cells, the negative feedback control is dysregulated in cancers, leading to sustained inhibition or activation of target genes.251 The effects of aberrant NF-κB include activating proto-oncogenes and genes involved in cell-cycle to promote tumor proliferation, inhibiting apoptosis to support the survival of cancer cells, regulating genes related to cell adhesion to facilitate metastasis. Additionally, NF-κB has a critical role in the metabolic reprogramming of cancer cells, promoting adaptive response to metabolic stress and thus contribute to tumor progression (Table 1).
Proliferation
NF-κB plays a pivotal role in tumor proliferation and progression. Major mechanisms include inducing the expression of cyclins and proto-oncogenes. Early in 1999, Guttridge et al revealed that NF-κB controls cell growth and differentiation through transcriptionally regulating cyclin D1 using both skeletal muscle differentiation models and normal diploid fibroblasts.252 More recent studies focusing on cancer showed that PAK upregulation enhanced cyclin D1 through NF-κB in breast cancer, consequently coordinating cell-cycle movement.253 Mutations in driver genes are also shown to have a close relationship with the NF-κB pathway in cancer cells. Gain-of-function mutations in oncogenes (such as RAS superfamily) or positive regulators (such as NF-κB inducing kinase) contribute to sustained activation of NF-κB signaling and subsequent cell proliferation. Xia et al. reported increased nuclear translocation of NF-κB was observed in K-rasG12D mutated mice, while IKKβ depletion or NF-κB signaling inhibition impairs lung adenocarcinoma development. Vreka et al. further confirmed that IKKα interacts with mutated KRAS and is necessary for the initiation and progression of KRAS-mutated lung adenocarcinoma.254 Furthermore, the dual roles of non-coding RNAs have also been reported in NF-κB-mediated tumor growth. Zhou et al., found that galectin-3 can activate TLR4 signaling and promote NF-κB translocation through the induction of lncRNA-NEAT1 (nuclear enriched abundant transcript 1) to facilitate lung adenocarcinoma cell proliferation.255 MicroRNAs, particularly miR-505, has also been reported to inhibit lung cancer proliferation through AKT/NF-κB pathway.256
Apoptosis
NF-κB has a dual role in the regulation of apoptosis, which is dependent on the balance between genes that controls cell survival and apoptosis.257 In cancer cells, NF-κB activity interacts with various apoptotic and survival proteins. NF-κB can regulate PTEN through transcriptionally activate Snail, a repressor of PTEN, and thus regulate cell survival.258 Man et al. reported a regulatory loop in the bladder cancer that overexpression of miR-130b/301b induced by NF-κB decreased USP13 expression and thus downregulate PTEN, which also facilitated the full activation of NF-κB.259 Lee et al., reported the bidirectional regulation of TRAIL, which promotes apoptosis via the ERK2/NF-κB signaling pathway in neuroepithelioma.260 YM155, a survivin inhibitor, can potentiate TRAIL-mediated apoptosis through inhibiting Mcl-1, c-FLIP, and NF-κB in breast cancer.261 Additionally, NF-κB can transcriptionally activate the Bcl-2 family, which inhibits BAX/BAK and thus prevents cytochrome C release and apoptosome formation. Furthermore, NF-κB modulates p53-mediated apoptosis via promoting the polyubiquitylation and degradation of p53.262 These findings mainly reflect its role in facilitating tumor resistance to apoptosis during tumor development.
Angiogenesis
Inducing angiogenesis is an essential hallmark of cancer. Growth factors are major regulators of angiogenesis, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF). These proangiogenic factors can be transcriptionally regulated by NF-κB in cancer, and the inhibition of NF-κB has been proven to prevent tumor angiogenesis in various cancer models, such as ovarian cancer, renal cell carcinoma, breast cancer, and colorectal cancer.263,264,265,266 Another widely investigated mechanism is its modulation of adhesion molecules in the formation of new blood vessels, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells.267,268,269 Additionally, the activation of NF-κB in immune cells and tumor-associated macrophages stimulates the release of pro-inflammatory cytokines, such as TNF-α and interleukin-6 (IL-6), which further enhance tumor angiogenesis. NF-κB also induces the expression of hypoxia-inducible factor-1 alpha (HIF-1α), which is a master regulator of genes involved in angiogenesis.270,271 Some recent studies have deepened our understanding of its role in tumor angiogenesis. For example, Herkenne et al. reported that mitochondria-shaping protein OPA1 is required in an NF-κB-dependent signaling essential for developmental and tumor angiogenesis, which revealed the role of NF-κB in mitochondrial dynamics during angiogenesis.272 It is noteworthy that matrix metalloproteinases (MMPs) are also targets of NF-κB, which promote angiogenesis and metastasis in different microenvironments.273
Metastasis
The American Cancer Society estimates that there will be 1,958,310 new cases of cancer and 609,820 cancer-related deaths in 2023.274 Metastasis is responsible for 66.7% of solid tumor-related deaths.275 Epithelial-mesenchymal transition (EMT) is an essential event in tumor metastasis. NF-κB regulates an array of EMT-associated genes, including TWIST1, SNAIL, CDH2, etc.276,277,278,279 Li et al. reported the role of TNF-α/NF-κB/TWIST1 signaling axis in EMT in breast cancer, suggesting that targeting NF-κB-mediated Twist1 upregulation may be an effective therapeutic approach for breast cancer.276 Nomura et al. reported that, in the classical lymphovascular metastatic cascade, inhibition of NF-κB by specific inhibitor decreased the expression of several EMT transcription factors (SNAI1, SNAI2, and ZEB1) and mesenchymal markers (VIM and CDH2) and prevented in vitro invasion in pancreatic cancer, which can be rescued by IKK activation.278 A more recent study used human-derived metastasis models of renal cancer to identify transcriptional enhancers responsible for metastases. Researchers functionally characterized a coregulatory enhancer cluster, which was activated by HIF2A and an NF-κB-driven lymphoid element, as a mediator of metastasis in vivo.279 Recent studies also revealed the interaction between metastasis-associated proteins and NF-κB during EMT. El-Nikhely et al. reported the bidirectional role of metastasis-associated protein 2 (MTA2) in IKK2/ NF-κB-driven lung cancer progression.280 They found that metastasis-associated protein 2 (MTA2)/nucleosome remodeling and deacetylase (NuRD) corepressor complex can downregulate NF-κB signaling and inhibit tumor growth in an IKK2-independent manner. However, when MTA2/NuRD complex dissociates from the promoter region of NF-κB target genes and IKK2-dependent positive regulation of MTA2 occurs, it leads to the activation of NF-κB signaling and further promotes EMT and tumor metastasis. Cell adhesion molecules such as selectins and integrins are also largely modulated via the NF-κB pathway,281 which facilitates cancer extravasation and colonization. It is worthy of note that NF-κB also plays a role in shaping the pre-metastatic niche. Hiratsuka et al reported that inflammation mediator serum amyloid A3 (SAA3)/TLR4 signaling stimulates NF-κB activity in both lung epithelial cells and myeloid cells to establish an inflammatory state that facilitates metastasis.282
Immune evasion
NF-κB regulates tumor microenvironment (TME) to avoid immune surveillance and promote cancer progression in various aspects. The production of cytokines and growth factors regulated via NF-κB is significant in the establishment of immunosuppressive TME. For example, TGF-β increases the expression of the transcriptional coactivator MRTF-A in non-small-cell lung cancer (NSCLC) cells. Subsequently, MRTF-A interacts with NF-κB/p65 and facilitates the binding of NF-κB/p65 to the PD-L1 promoter. The activation of PD-L1 leads to immune evasion by NSCLC cells. The downregulation of MRTF-A in vivo can effectively inhibit lung tumor progression and enhance antitumor immunity.283
The role of NF-κB was not only implicated in cancer cells, but also in surrounding immune cells, such as CAFs, TAMs, and MDSCs. Inhibition of NF-κB signaling in CAFs abolished its tumor-promoting effects, suggesting the critical role of NF-κB in CAFs-mediated protumor effects.262 A single-cell RNA sequencing analysis suggested that the pattern recognition receptor Mincle was highly expressed in TAM, largely induced in bone marrow-derived macrophages by cancer cells to promote tumor development. Researchers reported a novel Mincle/Syk/NF-κB signaling circuit in TAM, which was a requisite for maintaining TLR4-independent protumoral activities. The blockade of Mincle/Syk/NF-κB signaling can repress the TAM-driven NSCLC progression in vivo.284 A recent study demonstrated that CRIP1 facilitated MDSC trafficking and fostered an immunosuppressive TME via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma (PDAC), and inhibiting CRIP1/NF-κB/CXCL axis can sensitize PDAC to immunotherapy.243
Metabolic reprogramming
The emerging role of metabolic reprogramming has been demonstrated in the tumor progression,285 featuring high demand for nitrogen and deregulated mitochondrial oxidative phosphorylation. Remodeling of cellular metabolism via the NF-κB pathway can be addressed in various aspects.
RelA was a key component in sustaining mitochondrial oxidative phosphorylation, while its regulation of cellular metabolism depends on p53 status. In wild-type p53 colon carcinoma cells, RelA upregulates cytochrome c oxidase (synthesized by SCO2) to support oxidative phosphorylation. The inhibition of RelA leads to high vulnerability to glucose starvation and a large reduction in mitochondrial gene expression.286 However, in p53-deficient tumor, RelA interacts with heat shock protein mortalin and translocates to the mitochondria, thus repressing oxidative phosphorylation and cellular ATP levels.287
Additionally, the NF-κB pathway is critical in the metabolic adaptation to nutrient-depleted microenvironments. NF-κB transcriptionally regulates a wide array of metabolic enzymes, such as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase isoform 3 (PFKFB3), glutamate dehydrogenase 1 (GDH1), and carboxylesterase 1 (CES1), glutamine-fructose-6-phosphate transaminase 2 (GFPT2),189,197,198,199,288 facilitating metabolic adaptation to nutrient deprivation. For example, Reid et al. discovered a novel role of IKKβ in sensing glutamine-deprived environment and promoting metabolic adaptation. They found that IKKβ can directly interact with PFKFB3 at Ser269 upon glutamine deprivation to inhibit its activity, and thus downregulate aerobic glycolysis when glutamine levels are low.197
Recent studies have revealed a novel aspect of NF-κB in the cross-coordination of the immune response and metabolic systems. Researchers have reported that germinal center (GC) B cell-specific Rel loss in mice downregulate an array of metabolic genes, including genes involved in glycolysis and fatty acid oxidation, and therefore damage the formation of GCs.289 Rel also regulates an innate immune checkpoint that governs the function and metabolism of MDSCs and can promote oncogenesis via inhibiting antitumor immune responses. Rel suppression might enhance the therapeutic efficacy of current immune checkpoint immunotherapies.200,290 Infiltration of immunosuppressive macrophages is one of the defining characteristics of a pre-metastatic niche, which is a requisite for tumor metastasis. Morrissey et al. reported a novel role of NF-κB in promoting the immunosuppressive phenotype of macrophages through metabolic reprogramming. Tumor-derived exosomes (TDEs) increased glucose uptake through TLR2/NF-κB signaling and inhibits mitochondrial oxidative phosphorylation. This leads to elevated conversion to lactate, which feeds back on NF-κB and subsequently drives PD-L1 expression.291 These findings underlined the pivotal role of the NF-κB pathway in linking metabolic remodulations with inflammasome-driven cellular responses in cancer.
Drug resistance
Chemotherapy and radiotherapy largely act by inducing apoptosis in proliferating cells. However, as mentioned above, the constitutive activation of NF-κB signaling leads to evasion of apoptosis, by regulating a series of pro- and anti-apoptotic genes such as caspase-8 and c-FLIP, BCL-2, and BCL-XL.191,292 In some cases, chemotherapy and radiotherapy can activate NF-κB signaling, leading to acquired treatment resistance.293 For example, commonly used chemotherapy agents such as paclitaxel, cisplatin, gemcitabine, adriamycin, and vinblastine can activate the NF-κB cascade. Metabolic reprogramming and oncogenic signaling were significantly activated in doxorubicin-induced DLBCL-resistant cells. Further studies revealed that drug-resistant cells enhanced glycolysis through sustained activation of non-canonical NF-κB signaling, thereby promoting their survival. Targeting p52-RelB increased sensitivity to doxorubicin.294 The gut microbiota may be involved in chemotherapy resistance in malignant tumors. It has been found in a study that enrichment of Mycobacterium avium upregulated intratumoral LPS, which subsequently promoted prostate cancer progression and docetaxel resistance through activation of the NF-κB-IL6-STAT3 signaling axis.295 Tamoxifen is a commonly used endocrine medication for breast cancer, and the ensuing problem of drug resistance should not be ignored. Kotaro Azuma et al. found that tripartite motif-containing (TRIM) 47 could be a predictor of breast cancer recurrence through the analysis of 116 clinical samples of tamoxifen-treated breast cancer. Mechanistic studies found that TRIM47 promotes breast cancer proliferation and endocrine therapy resistance by forming a stable complex with protein kinase Cε (PKC-ε) and protein kinase D3 (PKD3) and activating NF-κB signaling.296 NF-κB signaling may also be implicated in the initiation and progression of castration-resistant prostate cancer (CRPCa), where it possibly relates to mutations, accumulation, and hypoactivity of the androgen receptor (AR).297 A genome-wide CRISPR-Cas9 screen performed in glioblastoma (GBM) identified E2F6 as the driver of temozolomide (TMZ) resistance, while EGFRvIII/ NF-κB is the upstream gene controlling E2F6 expression.298 Blockade of NF-κB can be a therapeutic target to reverse treatment resistance and enhance the effectiveness of anticancer treatments, which has been shown in numerous preclinical studies.299,300,301
Inflammation and autoimmune diseases
General introduction
In order to gain a better understanding of the role of NF-κB in inflammation and autoimmune diseases, it is imperative to first comprehend its function in various immune cells.
Cells of innate immunity
Macrophages have been extensively studied regarding their pro-inflammatory function mediated by NF-κB. Upon recognition of diverse PAMPs and DAMPs, macrophages rapidly activate and release substantial amounts of cytokines. Depending on specific conditions, activated macrophages can differentiate into two distinct phenotypes: classically activated (M1) and alternatively activated (M2) macrophages. NF-κB serves as a key transcription factor for M1 macrophages, regulating an array of genes involved in inflammation, including TNF-α, IL-1β, IL-6, IL-12, and cyclooxygenase-2.302 Neutrophils and dendritic cells also play essential roles in local inflammation, with NF-κB being critical for their survival and function under potentially toxic conditions. This contributes to neutrophil recruitment and dendritic cell maturation.303,304
T cells
The NF-κB signaling pathway significantly influences the activation, differentiation, proliferation, and function of T cells. Upon binding of the antigen-MHC complex and CD80 or CD86 to the TCR and CD28, respectively, NF-κB complexes containing p65 are activated, leading to delayed and sustained activation of the c-Rel complex. NF-κB activity is crucial for activated T cells as it protects against apoptosis and promotes cytokine production, particularly IL-2, which supports proliferation and differentiation.305,306
NF-κB also plays a pivotal role in the differentiation of activated T cells into effector cells including Th1, Th2, Th17, and Treg cells. This process relies on the induction of specific transcription factors, including T-bet, GATA3, RORγt, and Foxp3. Increasing evidence suggests that NF-κB family members are key regulators of these processes. For instance, studies have shown that the absence of Foxo3a results in overactivation of NF-κB and increased production of TH1 and TH2 cytokines, leading to hyperactivated immunity.307 Additionally, mice lacking the p50 subunit of NF-κB fail to develop airway eosinophilic inflammation, indicating the critical role of NF-κB in Th2 differentiation.308 The TCR/CARMA1/NF-κB axis selectively drives Th17 differentiation through mechanisms involving cell cycle progression.309 Furthermore, TCR signaling induces nuclear translocation of serine/threonine kinase 4 (Stk4), resulting in the formation of the Stk4-Foxp3-NF-κB p65 complex, which regulates Foxp3 and p65-dependent transcription programs, thereby promoting Treg cell activation.190
B cells
Regarding B cells, the development and survival of immature B cells largely depend on NF-κB signaling downstream of BCR and BAFF. Throughout B cell development, the canonical NF-κB pathway activated by BCR regulates central tolerance, survival, and differentiation. Tonic BCR and BAFF stimulation contribute to the maintenance of naive B cells through NF-κB activation. BAFF signaling coordinates the activities of RelB and cRel to ensure survival during peripheral B cell maturation.310 In the germinal center, CD40L signals cooperate with BCR signaling to induce c-Myc expression through the NF-κB pathway, promoting B cell survival and re-entry into the cell cycle.311 Subsequently, NF-κB signaling in B cells expressing specific BCR leads to class switch recombination, resulting in the differentiation of B cells into either memory B cells or plasma cells.289 In summary, NF-κB plays a crucial role in supporting B cell proliferation, differentiation, and survival.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disorder typified by inflammation of the synovial joints. Its main pathological features include inflammatory proliferation of synovial tissue, formation of pannus, and erosion and destruction of articular cartilage and surrounding tissues.312 Although the exact pathogenesis of RA remains unclear, the role of NF-κB in its development has gained increasing attention with advancements in related research. Activated NF-κB promotes chronic inflammation and excessive proliferation of fibroblast-like synoviocytes (FLS) in the synovial tissue, thereby contributing to the progression of RA.313
NF-κB activation perpetuates chronic inflammation by targeting genes involved in inflammation during the progression of RA. NF-κB is involved in signaling transduction, activation, differentiation, and production of IFN-γ and IL-17 in inflammatory T cells, which are crucial for sustaining rheumatoid synovial inflammation.314 Additionally, the proliferation of B cells and the production of autoantibodies are closely associated with activated NF-κB members.315 Regarding innate immune regulation, dysregulation of NF-κB activation in dendritic cells can induce the production of cytokines such as IL-15 and IL-18, promoting the differentiation of inflammatory T cells.316,317 NF-κB also influences non-immune cells, as evidenced by increased expression of p50 and p65 in FLS cells of RA compared to normal synovium.318 NF-κB enhances the expression of cyclin D1 and c-Myc, positively regulating the cell cycle in fibroblasts and myoblasts.319 Moreover, NF-κB activation transmits anti-apoptotic signals in FLS through the induction of c-IAP.320,321 These repetitive cycles driven by NF-κB contribute to the worsening of the disease.
Osteoarthritis
Osteoarthritis (OA) is a degenerative disease characterized by the progressive deterioration of joints, with joint pain as the main clinical manifestation.322 The NF-κB pathway actively participates in and regulates various aspects of chondrocyte function, including proliferation, differentiation, apoptosis, and bone matrix metabolism.323 NF-κB plays a crucial role in the onset and progression of OA323 (Fig. 4d).
The NF-κB pathway can be activated by different ligands, such as tumor necrosis factor-alpha (TNFα).324 Upon activation, NF-κB translocates to the nucleus, leading to the transcription of downstream target genes, including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), and hypoxia-inducible factor 2-alpha (HIF2α).325,326,327 MMPs are known to degrade the extracellular matrix of articular cartilage, contributing to its breakdown.325 Furthermore, NF-κB promotes tissue inflammation and the release of catabolic factors by inducing chondrocyte apoptosis and facilitating the synthesis of prostaglandin E2 (PGE2), nitric oxide synthase (NOS), NO, and cyclooxygenase-2 (COX-2).328 These processes ultimately exacerbate joint damage in OA.
Multiple sclerosis
Multiple sclerosis (MS) is considered an immune disease primarily caused by the infiltration of peripheral immune cells into the central nervous system.329 NF-κB plays a significant role not only as a mediator of the inflammatory process in peripheral immune cells but also in microglia and astrocytes, making it important in the progression of MS.330
The differentiation of T cells into regulatory T cells (Tregs) requires the NF-κB subunit c-Rel, indicating that NF-κB could be a potential therapeutic target for MS.190,331 Furthermore, antigen-presenting cells (APCs) depend on c-Rel to produce IL-12 and IL-23, two cytokines that promote the differentiation of Th0 into Th1 or Th17 cells, respectively.309 Th17 cells secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), which plays a crucial role in promoting the progression of MS.332 The activation of the NF-κB pathway is required for astrocytes to create a local inflammatory environment by inducing the secretion of inflammatory cytokines and chemokines.333 This inflammatory response can result in local tissue damage and attract additional inflammatory cells.333 Conversely, inhibiting the NF-κB pathway in astrocytes leads to the recruitment of CD8 + CD122+ regulatory T cells, thereby alleviating MS.334,335,336 Similarly, the activation of NF-κB in microglia or infiltrating macrophages may also influence the production of inflammatory cytokines and chemokine.333,337
Ulcerative colitis
Ulcerative colitis (UC) is a persistent inflammatory disorder affecting the mucosal lining of the colon that primarily affects the rectum and extends through part or all of the colon in a continuous manner.338,339 The pathogenesis of UC involves dysregulation of cytokine production and signaling mechanisms in intestinal epithelial cells, lymphocytes, and macrophages, with the transcription factor NF-κB playing a significant regulatory role in this complex process340 (Fig. 4g).
NF-κB activation in macrophages can induce the secretion of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6. NF-κB also regulates the expression of IL-12 and IL-23,341,342 which are involved directly or indirectly in the mucosal tissue damage commonly observed in inflammatory bowel disease (IBD). The activation of NF-κB by IL-6 in colon epithelial cells may contribute to increased expression of intercellular adhesion molecule-1 (ICAM-1), an important mediator in the recruitment of neutrophils to inflammatory sites.343 Furthermore, NF-κB activation in intrinsic intestinal fibroblasts leads to increased expression of cytokines like IL-8, IL-6, and monocyte chemoattractant protein.344 This suggests that colonic fibroblasts can participate in the immunopathogenesis of IBD in an NF-κB-dependent manner.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by dysregulation of B cells and T cells, leading to the activation of polyclonal B cells and the production of autoantibodies.345 Nuclear NF-κB plays a significant role in the pathogenesis of SLE by promoting the proliferation of T cells and B cells346,347,348 (Fig. 4f).
In the context of SLE, the inflammatory environment can lead to the release of IL-6 or TNF-α, which activate the NF-κB pathway.349 This activation enhances the activity of the miR-34a promoter, resulting in the downregulation of Foxp3 expression and excessive proliferation of T cells.350 Additionally, NF-κB has been shown to be upregulated in autoreactive B cells mediated by CD40 and BAFF, promoting the secretion of autoantibodies.351,352 Furthermore, IFN-1 is a key factor in the pathogenesis of SLE, and its induction is mediated by NF-κB activation through TLR7 stimulation.353
Gluten induced enteropathy (Celiac disease)
Celiac disease (CD) is an immune-mediated intestinal disorder caused by intolerance to gluten ingestion.354 The pathogenesis of CD involves innate and adaptive immunity, primarily mediated by the infiltration of lymphocytes, particularly T cells, into the small intestinal epithelium.355 The upregulation of the NF-κB pathway and its downstream cytokines, such as IL-8, in the intestinal mucosa suggests the involvement of NF-κB in the development of celiac disease.356
In CD, various subtypes of T cells are activated, leading to the concurrent activation of different signaling cascades, including NF-κB, GATA1, JAK, and others.357 Gliadin peptides found in gluten induce changes in the oxidative balance of intestinal cells and are associated with the activation of the transcription factor NF-κB.357 NF-κB activation triggers the transcription of pro-inflammatory cytokines and enzymes like COX-2 and iNOS, leading to increased production of prostaglandins and NO metabolites, which contribute to oxidative stress. Recent studies have identified the m6A- exportin-1 (XPO1)-NF-κB axis as a potential target for CD treatment. Individuals carrying the XPO1 risk allele express higher levels of XPO1 protein, which results in increased downstream NF-κB activity and subsequent inflammation.358
Gout/Hyperuricemia
Gout is a chronic metabolic disorder characterized by the accumulation of sodium urate crystals in joints and non-articular structures.359 Sodium urate has been shown to possess pro-inflammatory activity. NF-κB is a key regulatory factor involved in controlling the production of inflammatory markers and mediators. In the context of gout, NF-κB activation induced by sodium urate contributes to systemic metabolic disturbances.360
Crystalline sodium urate acts as a damage-associated molecule that triggers innate immune pathways. For instance, sodium urate can stimulate NF-κB through TLR4 and TLR2, leading to the synthesis of pro-IL-1β and components of the inflammasome.361 Furthermore, sodium urate can induce renal inflammation in gouty nephropathy by activating tubular NF-κB signaling.362 Recent studies have also demonstrated the involvement of the pro-inflammatory NF-κB pathway in hypothalamic inflammation associated with metabolic syndrome.363,364
Periodontitis
Periodontitis is characterized by the pathological loss of periodontal ligament and alveolar bone.365 The disease involves complex interactions between specific bacterial pathogens and destructive immune responses.365,366 In a model of periodontitis, a single intra-gingival injection of LPS results in the significant expression of NF-κB p65 and pro-inflammatory cytokines in gingival tissues after 14 h. This is accompanied by increased expression of COX-2 and iNOS, as well as elevated levels of cytokines TNF-α and IL-1β. These changes are often associated with heightened nociceptive perception, supporting the involvement of NF-κB in the pathogenesis of periodontitis.367
Periodontitis is caused by the accumulation of microbial biofilms, with LPS being a major virulence factor present in the cell walls of these bacteria. LPS stimulates TLRs on microbial cells, resulting in the activation of NF-κB and subsequent upregulation of downstream inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α.368 Furthermore, periodontal fibroblasts, when exposed to both TLR activation and mechanical force stimulation, activate the p38, JNK, and NF-κB pathways. This leads to the secretion of MMPs and degradation of the bone matrix.369
Sepsis
Sepsis is a systemic inflammatory response syndrome caused by the invasion of pathogenic microorganisms, predominantly bacteria.370 In critically ill patients with sepsis, multiple organ failure often occurs in addition to the manifestations of systemic inflammatory response syndrome and the primary site of infection.371 The pathophysiology of sepsis and septic shock involves a complex network of cytokines and inflammatory mediators.372 Central to this network is the activation of NF-κB.373 Studies have revealed that increased accumulation of nuclear NF-κB in peripheral blood mononuclear cells (PBMCs) of sepsis patients is associated with higher mortality rates and poorer clinical prognosis.374
Researchers have discovered that macrophages exhibit enhanced expression of vascular endothelial growth factor receptor 3 (VEGFR-3) in response to bacterial infection or stimulation with LPS. VEGFR-3 forms a negative feedback loop that inhibits TLR4-NF-κB-mediated inflammatory responses, thereby reducing the occurrence of sepsis or endotoxic shock resulting from bacterial infections.375 Inhibition of NF-κB activation has been shown to reduce acute inflammatory processes and organ dysfunction.374 These findings highlight the potential effectiveness of targeting NF-κB for the treatment of sepsis.
Asthma
Asthma is characterized by variable airflow obstruction, typically accompanied by bronchial constriction and airway inflammation resulting from the contraction or hypertrophy of airway smooth muscle (ASM).376 Multiple pieces of evidence suggest that the NF-κB pathway is upregulated in asthmatic tissues.377 PBMCs from adult patients with uncontrolled severe and moderate asthma exhibit higher levels of NF-κB p65 protein expression compared to individuals without asthma.378 This suggests that NF-κB plays a pivotal role in the initiation and maintenance of asthmatic allergic inflammation379 (Fig. 4b).
Previous studies have shown that activation of the TLR4 pathway by ovalbumin (OVA), an allergen commonly used to induce experimental asthma, leads to the upregulation of Th2-related inflammatory responses and promotes the gene expression of inflammatory cytokines, which is mediated by NF-κB.380 NF-κB, as a multi-cellular transcription factor, also plays a major role in regulating inflammation and immune responses by modulating Th2 cell cytokine production and gene expression.381 Studies using mice lacking the NF-κB p50 subunit have demonstrated reduced eosinophilic response to inhaled allergens. This effect is attributed to the lack of secretion of Th2 cell cytokines, including IL-13, IL-4, and the eosinophil growth factor IL-5, by T cells.382
Kidney inflammation and injury
Nephritis is an immune-mediated disease characterized by the formation of immune complexes resulting from antigen-antibody binding. These immune complexes deposit in various parts of the kidney, leading to pathological damage.383 IgA nephropathy, the most common form of glomerulonephritis, involves the binding of IgA to Fcα receptors on mesangial cells, which activates NF-κB and contributes to the induction of chemokines MCP-1 and IL-8.384,385 NF-κB also plays a role in lupus nephritis. Increased expression and activation of NF-κB have been observed in glomerular endothelial cells and mesangial cells of patients with lupus nephritis, accompanied by upregulation of inflammatory cytokines.386 NF-κB is critical in regulating autoimmune nephritis involving T cells and B cells. The Th17+ T cell subset, implicated in the pathogenesis of renal inflammation, requires the involvement of the classical NF-κB pathway for the generation of Th17 cells from naive T cells387,388 (Fig. 4c).
Acute kidney injury (AKI) is commonly caused by ischemia-reperfusion, during which the kidneys experience hypoxia and reduced blood flow. Inflammation induced by AKI contributes significantly to kidney injury, and controlling inflammation has shown effectiveness in reducing kidney damage and promoting recovery.389 NF-κB is activated during ischemia-reperfusion-induced renal injury and is considered a key mediator of inflammation. Studies have demonstrated that NF-κB inhibitors can attenuate renal inflammation and reduce injury induction in animal models.390
Cardiovascular diseases
Atherosclerosis
Atherosclerosis is a chronic inflammatory disorder characterized by the aggregation of lipid particles in the arterial wall, the involvement of different cell types, and the activation of several important signaling pathways. Among them, the prominent role of NF-κB signaling in different stages of atherosclerosis is established.391 In fact, NF-κB signaling is a key element in the common pathogenesis of several atherosclerosis-associated diseases, including abdominal aortic aneurysm, peripheral artery disease, cerebrovascular disease and coronary artery disease.392,393,394,395 Endothelial cells (ECs) dysfunction plays a key role in the initiation of atherosclerosis. In response to pro-atherogenic factors including oxidized low-density lipoprotein (ox-LDL), IL-1, ROS and advanced glycation endproducts (AGEs), vascular ECs facilitate NF-κB activation and induces the expression of cytokine (such as IL-6 and TNF-α) and adhesion molecules (such as E-selectin, VCAM-1, ICAM-1).396 The process above promotes dysfunction and inflammation of ECs, and promoting the recruitment of monocytes and lymphocytes from the vascular lumen to the subendothelial layer of arterial intima. Furthermore, disturbed flow-induced ECs dysfunction through mechanotransduction pathways result in the activation of NF-κB signaling.397 Vascular smooth muscle cells (VSMCs) are the primary source of plaque cells.398 The major pathogenic mechanism underlying the formation of atherosclerotic plaques was believed to be the phenotypic switching of VSMCs from contractile to proliferative synthetic types in response to arterial inflammation.399,400 The synthetic state has also been linked to the activation of VSMCs proliferation and migration. Activated NF-κB components have been identified in VSMCs derived from human atherosclerotic lesions. Production of inflammatory cytokines such as IL-1 and TNF alter VSMCs phenotype via NF-κB mediated downregulation of contraction-relation gene expression.401 Additionally, phenotype switching, proliferation, and migration of VSMCs produced by PDGF-BB are prevented by disrupting the ROS/NF-κB signaling pathway.402 NF-κB also work in monocyte differentiation, macrophages polarization, maintenance, and transformation to foam cell. Ox-LDL engage receptor CD36 on macrophages and triggers TLR activation and excessive lipid accumulation through NF-κB activation, leading to foam cell formation in atherosclerotic plaques.403,404 Smad3/NF-κB pathways has been found to be involved in the transition between M1 and M2 subtypes in macrophages.405 Overexpressing of matrix metalloproteinase-9 (MMP-9)by macrophages promote the dissolution of plaque elastin and induces plaque disruption, which is associated with the activation of the TLR4/NF-κB pathway.406,407 In the end stage of atherosclerotic plaques, apoptosis of various cells becomes critical in controlling plaque stabilization.408 In human carotid plaques, NF-κB activation and factor-related apoptosis ligand (FasL)and active caspase-3 expression were analyzed and found to increase significantly compared to healthy controls.409 The underlying pathway regarding NF-κB induced apoptosis may be through the promotion of FasL via its receptor CD95 (Fig. 4h).
Myocardial infarction
The majority of myocardial infarction are the result of rupture of atherosclerotic plaques in blood vessels, followed by an abnormal coagulation reaction and formation of blood clot. Spontaneous or therapeutic revascularization leads to reperfusion of infarcted myocardium, which is called as reperfusion injury.410 Reperfusion injury triggers an inflammatory response, causing infiltration of inflammatory cells and excessive activation of myofibroblasts and vascular endothelial cells. The process above finally causes cardiac repair and cardiac fibrosis. In the cycle of ischemia and repair, TLR/NF-κB pathway has been widely researched and thought to be a critical role. Necrotic and damaged cells induced activation of TLR (especially TLR4), facilitate IκB kinase phosphorylation through MyD88/TAK1 pathway and finally engage NF-κB p65 and p50, leads to activation of plenty of inflammatory factors, including cytokines, chemokines, adhesion molecules and complement factor B.411 Inhibition of NF-κB has been proved to be associated with decreased reperfusion injury after myocardial infarction.412,413,414
Metabolic disorders
At the core of metabolic homeostasis is the maintenance of normal nutrient perception and regulation by the body, and the interplay of the immune and metabolic systems is an important mechanism for this.415 The metabolic challenges encountered by contemporary humans are predominantly attributed to overnutrition rather than nutritional inadequacies. Metabolic disorders frequently exhibit interplay, and individuals with obesity are predisposed to developing type 2 diabetes (Fig. 4e).
Insulin resistance and diabetes
Insulin resistance, characterized by reduced sensitivity of the body to insulin, is a prevalent feature of metabolic disorders such as type 2 diabetes mellitus, dyslipidemia, and other metabolic disorders.416 It also serves as a common pathophysiological mechanism in diseases such as cardiovascular and cerebrovascular diseases, polycystic ovary syndrome, and tumors. Insulin is a protein hormone secreted by pancreatic β-cells in the pancreas in response to endogenous or exogenous stimuli. Insulin is the sole hormone in the body that possesses hypoglycemic properties and governs the energy metabolism of the liver, skeletal muscle, and adipose tissue. When insulin resistance occurs, glucose utilization decreases while hepatic glycogen breakdown into glucose increases, resulting in elevated blood glucose levels and ultimately leading to diabetes. The insulin-insulin receptor-insulin receptor substrate-1-PI3K pathway is strongly associated with insulin resistance. The 10th edition of the Global Diabetes Map reveals that approximately 537 million adults (20–79 years old) worldwide will be afflicted with diabetes in 2021, accounting for ~10.5% of the global population within this age range, and this number is projected to escalate to 643 million in 2030.417 Insulin resistance, type 2 diabetes, and its complications are considered chronic inflammatory diseases, and NF-κB signaling is a key pathway linking inflammation and metabolism.182,418,419
TANK-binding kinase 1 (TBK1), an atypical inhibitor of the IKK family, plays an important role in the regulation of inflammatory cytokine production, innate immunity, metabolism, and autophagy.420,421,422,423 TBK1 engages in bidirectional crosstalk in energy metabolism and inflammatory signaling.424 Due to the loss of mutual inhibition with the insulin receptor, the whole-genome mutation of TBK1 confers a protective effect on the metabolism of mice receiving a high-fat diet.425,426,427 The dual small molecule inhibitor of IKKε/TBK1, Amlexanox, has entered clinical trials for the treatment of obesity and type 2 diabetes.425,426,427 In contrast, it has also been demonstrated that myeloid conditional TBK1 knockout mice develop insulin resistance, nonalcoholic steatohepatitis, and experimental colitis, which may be attributed to the absence of TBK1’s role in inhibiting the NF-κB and MAPK signaling pathways played by TBK1 in M1 macrophages.424,428
Hyperglycemia promotes ROS production, induces oxidative stress, activates pro-inflammatory NF-κB signaling, and stimulates the immune system to release excessive inflammatory mediators and cytokines, ultimately resulting in cellular damage.182,429 It has been discovered that exposure to high-efficiency particulate arrestance (HEPA)-filtered air or airborne fine particulate matter (PM2.5) with a concentration of ≤2.5 μm for 9 days can lead to downregulation of IκBα levels in the aorta of mice.430 Furthermore, after 30 days of exposure, skeletal muscle insulin-stimulated endothelial nitric oxide synthase phosphorylation is inhibited, and adipose tissue inflammation and insulin resistance are increased.430 Oxidative stress and inflammation have also been shown to be major pathophysiologic changes in diabetes-induced cognitive impairment, diabetic nephropathy, diabetes-associated colitis, and dysbiosis of the colonic microbiota, among other complications.431,432,433,434
Diabetic nephropathy is one of the most important comorbidities in patients with diabetes mellitus, and its pathogenesis involves immunoinflammatory factors, abnormal glucose metabolism, altered renal hemodynamics, and oxidative stress. The high glucose environment induces RANK expression in glomerular podocytes, which mediates the development of diabetic nephropathy by promoting the production of ROS, TNF-α, Gal-3, and IL-1β.432 Kidney risk inflammatory signature (KRIS) consisting of 17 systemic proteins enriched in TNFR superfamily members has been identified to be associated with a 10-year risk of end-stage renal disease from both three independent cohorts of patients with type 1 and type 2 diabetes mellitus.435 Metabolic insufficiency due to mitochondrial damage is also a possible mechanism of nephritis. cGAS-STING-NF-κB pathway promotes cytoplasmic translocation of mitochondrial DNA and induces expression of pro-inflammatory factors in renal tubular epithelial cells.436
Hyperglycemic state in diabetic patients leads to abnormal bone metabolism, causing peri-implantitis and alveolar bone defects in the implant area, and activation of NF-κB signaling as a mechanism to maintain a long-term inflammatory state.437 Inhibition of NF-κB signaling activation in stem cells indirectly modulates macrophage polarization toward M1, which can restore immunoregulatory capacity and reduce local inflammation.438
The evolution of diabetic cardiomyopathy to myocarditis involves strong activation of NF-κB signaling, which upregulates and secretes cytokines, chemokines, and adhesion molecules expression and secretion.439 Downregulation of cardiac-specific Jund proto-oncogene subunit (JunD) (a member of the activator protein-1 family of transcription factors) expression and upregulation of the NF-κB inflammatory pathway are observed in the myocardium of diabetic mice induced by hyperglycemia and represent defects in myocardial antioxidant and inflammatory capacity.440 The level of Salusin-β, a biologically active peptide associated with the inflammatory response of vascular endothelial cells, is elevated in patients with diabetes mellitus. Investigators found that knockdown of Salusin-β effectively inhibited oxidative stress, NF-κB activation, and upregulation of inflammatory responses via NADPH oxidase 2 (NOX2)/ROS/NF-κB signaling in a rat model of diabetic cardiomyopathy, whereas the NF-κB inhibitor Bay 11-7082 attenuated inflammatory responses only.441
Pancreatic β-cells activate NIK in response to IL-1β and IFN-γ for non-canonical NF-κB signaling.442 Interestingly, the genetic silencing of NIK, a key molecule in the non-canonical pathway, does not alter the incidence of diabetes and inflammatory response in the mouse model.443 Studies on NF-κB signaling in β-cells have mainly focused on the canonical pathway, and further studies on the role of non-canonical signaling are needed.
Obesity
Over 30% of the global population is classified as overweight or obese, with the etiology of obesity being multifactorial and capable of manifesting at any stage of human development.444 Obesity and metabolism-related diseases such as hyperglycemia, dyslipidemia, and hypertension are categorized as “metabolic syndrome”. Activation of pro-inflammatory signals such as NF-κB, JNK, and inflammasome by overnutrition explains why obesity is often accompanied by a state of chronic low-grade inflammation and insulin resistance.445 Excess body fat is initially stored in subcutaneous adipose tissue, and when the threshold of adipose tissue capacity is surpassed, the fat cells undergo a series of changes, including increased inflammation, cellular hypertrophy, and insulin resistance.446
While metabolically activated adipose tissue macrophages have been associated with deleterious effects such as insulin resistance and steatosis, they have also been shown to facilitate lysosomal extravasation for the clearance of dead adipocytes.447 The high-fat-induced metabolic disturbances and inflammatory activation are not temporary, but rather a continuous state. The activation of adipose tissue macrophages was not completely eliminated in mice that stopped receiving a high-fat diet, even after 8 weeks of weight loss.448 Single-cell sorting in mice and humans has identified triggering receptor expressed on myeloid cells-2 (TREM2) signaling as a major pathway for adipose tissue macrophage resistance to lipid imbalance and a promising therapeutic target for metabolic diseases.449,450 Downregulation of zinc finger protein 423, a transcriptional corepressor of NF-κB, activates fibroinflammatory progenitor cells around the vasculature of mouse white adipose tissue and triggers a series of inflammatory signaling cascades in response to a high-fat diet.451 High reactive oxygen species production is detected in the hair follicle stem cells of young rats fed a high-fat diet and induces the activation of lipid droplets and NF-κB signaling via IL-1R signaling, inhibiting hair follicle regeneration and accelerating hair loss.452 The increased incidence of postmenopausal estrogen receptor (ER)+ breast cancer has been found to be associated with obesity, and estrone induced by ERα through activation of NF-κB signaling not only promotes the growth of breast cancer but also possesses pro-inflammatory effects.453 Hepatic TBK1 activity decreases in the fasting state, whereas in response to the inflammatory milieu of obesity, TBK1 activity increases and promotes fatty acid re-esterification.454 High-fat diet initiates cellular inflammatory responses, and inflammation also acts as an aggravating factor for obesity. TBK1 knockdown and NOD-like receptors family pyrin domain containing 12 (NLRP12, an inhibitory immune receptor) attenuate inflammasome activation and obesity by inhibiting NF-κB signaling.424,455 It has also been found that circadian clock impairment in adipose tissue due to NF-κB signaling may be a cause of obesity and its complications.456
Nervous system diseases
Parkinson’s disease
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), leading to motor symptoms such as rigidity, bradykinesia, and resting tremor. The key mechanisms for PD include misfolded α-synuclein aggregation, neuroinflammation, and mitochondrial dysfunction in synaptic terminals.457,458 As a master regulator of various cellular functions, NF-κB has been shown to involve in the above pathophysiological process. Toll-like receptors (TLRs) are a member of the pattern recognition receptor (PRR) family, which are important in the recognition of pathogens.458 Dutta et al. reported that α-synuclein spreading depends on the TLR2/MyD88/NF-κB pathway and can be alleviated by nasal delivery of wtTIDM and wtNBD peptides.459 In addition, NF-κB activation in astrocytes and microglia modulates inflammatory responses and leads to the production of pro-inflammatory molecules such as inducible nitric oxide synthase (iNOS) and tumor necrosis factor (TNF-α).458,460 Researchers also revealed that increased translocation of NF-κB in dopaminergic neurons can activate genes encoding pro-inflammatory mediators, which initiate the generation of reactive oxygen species (ROS) through the auto-oxidation of dopamine.461,462 Furthermore, microRNAs such as miR-124 can upregulate NF-κB expression, promoting pro-inflammatory levels causing neurodegeneration in PD.463,464 Targeting the NF-κB signaling offers a new opportunity as a therapeutic approach for PD.
Brain injury
Inflammation in the central nervous system (CNS) is a well-recognized feature of various acute neurological injuries.465 Following brain injury, the release of factors from damaged cells or cell debris may trigger an inflammatory response. Injury and ischemia can activate TLRs, which subsequently activate pro-inflammatory transcription factors such as NF-κB, leading to the upregulation of numerous immune mediators466 (Fig. 4i).
Inhibiting excessive inflammation after brain injury by suppressing NF-κB activation can be beneficial in protecting neuronal cells. Research has shown that geniposide exerts neuroprotective effects against traumatic brain injury (TBI) by inhibiting phosphorylation of p38 and p65 in the NF-κB pathway.467 Other studies have also demonstrated the preventive effect of tannic acid on brain injury in rats by modulating pathways involving Nrf2, NF-κB, and apoptosis.468
Spinal cord injury
The inflammatory response plays a significant role in the pathophysiology of acute and chronic spinal cord injury (SCI), contributing to secondary injury. The NF-κB family of transcription factors is crucial for the activation of genes involved in cell inflammation, proliferation, and cell death responses.469 It has been implicated as a critical determinant of cell death and central nervous system diseases. NF-κB activates the transcription of genes encoding cytokines, COX-2, CAMs, and iNOS.470 Inhibitors of NF-κB have potential therapeutic applications in the treatment of SCI.
Valproic acid (VPA) has been shown to increase the acetylation of the STAT1/NF-κB pathway. This promotes the transition of microglia from an M1 pro-inflammatory phenotype to an M2 anti-inflammatory phenotype, inhibiting microglial activation. Consequently, VPA reduces SCI-induced inflammatory factors and alleviates the central inflammatory response mediated by microglia after spinal cord injury.471Researchers have also discovered that upregulation of Sterile alpha and Toll/interleukin 1 receptor motif-containing protein 1 (SARM1)-mediated NF-κB signaling in neurons and astrocytes promotes early neuroinflammation in SCI. Therefore, targeting SARM1 with therapeutic drugs offers a promising approach to preserving neuronal function following spinal cord injury.472
Corona virus disease 2019
Hyperactivation of the NF-κB pathway has been implicated in the pathogenesis of COVID-19, especially the severe phenotype.473 The activation of NF-κB by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to the production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).474 While these cytokines play a critical role in fighting off viral infections, cytokine storm can occur in severe cases of COVID-19. This overwhelming inflammatory response is associated with tissue damage and contributes to the development of acute respiratory distress syndrome (ARDS) and other complications.
During the initial stages of SARS-CoV-2 infection, NF-κB activation is critical in initiating the inflammatory response. Studies have highlighted that upon viral entry, SARS-CoV-2 engages with host ACE2 receptors and TMPRSS2 proteases, leading to viral entry and subsequent activation of NF-κB pathways and triggering of inflammatory cascades.475,476 The involvement of NF-κB in the outbreak stage of COVID-19 leads to an exaggerated inflammatory response characterized by the release of pro-inflammatory cytokines. This cascade of events contributes to the cytokine storm observed in severe cases, leading to tissue damage and worsening clinical outcomes.476,477 The role of NF-κB in the long-lasting stage of COVID-19 infections involves persistent inflammatory responses, potential tissue damage, and complications leading to prolonged symptoms or post-acute sequelae of SARS-CoV-2 infection (PASC). Research indicated that NF-κB activation can perpetuate the inflammatory environment even after the initial infection subsides. Carfì et al. observed persistent inflammatory markers in patients recovering from acute COVID-19, suggesting a role for NF-κB in sustaining inflammation and potentially contributing to the development of PASC.478 These studies suggested that NF-κB activation during this stage may contribute to chronic inflammation and immune dysregulation, influencing the extended duration and severity of symptoms.
Although NF-κB activation induces the expression of antiviral proteins to help control viral replication, SARS-CoV-2 has evolved mechanisms to evade the host immune response, including inhibiting some aspects of NF-κB signaling.479 The complex interaction between the virus and NF-κB signaling in COVID-19 is still being actively investigated. Targeting NF-κB signaling pathways may hold potential for therapeutic interventions, but further studies are needed to determine the efficacy and safety of such approaches.
Strategies for targeting NF-κB signaling in human diseases
IKK inhibitors
Aspirin
Aspirin, a nonsteroidal anti-inflammatory drug (NSAID), is well-known for its action in inhibiting cyclooxygenase. Elizabeth Kopp et al. first discovered in 1994 that anti-inflammatory drugs such as sodium salicylate and aspirin can inhibit the degradation of IκBα and prevent NF-κB-dependent transcription, which further confirms the important role of NF-κB signaling in inflammation and infection.480 Research suggests that the anti-inflammatory properties of aspirin are partially attributed to its specific inhibition of IKKβ, thereby preventing the activation of NF-κB and genes associated with inflammatory responses481 (Fig. 5).
Strategies for targeting NF-κB signaling in human diseases. Inhibitors of NF-κB are widely used in various clinical settings for the treatment of tumors, diabetes, and other conditions. These drugs inhibit the NF-κB pathway through different mechanisms. NSAIDs selectively inhibit IκB to suppress the activation of NF-κB. Dexamethasone inhibits NF-κB by directly coupling with the RelA subunit to block its functional activity. IMiD drugs such as thalidomide suppress the transcription function downstream of NF-κB to exert their effects. Monoclonal antibodies, including anti-PD-L1, anti IL-1, and anti TNF-α block the binding of ligands and respective receptors to inhibit their biological effects. Proteasome inhibitors include Bortezomib, Carfilzomib, Ixazomib, and Lactacystin act by halting protein degradation that ultimately results in apoptosis and cell death. Tacrolimus inhibits the protein phosphatase activity of calcineurin, preventing the nuclear translocation of NFAT and subsequently suppressing the activation of T cells. IκBα super-repressor inhibits the translocation of NF-κB into the nucleus. Tyrosine kinase inhibitors inhibits the intracellular phosphorylation of tyrosine kinase to block tumor cell growth. Natural compounds and derivatives such as resveratrol, quercetin, and isothiocyanates inhibits NF-κB through diverse mechanisms and exerts antitumor effects. Strategies targeting non-coding RNAs are also in development, such as anti-miR oligonucleotides, which can be used to inhibit miRNAs that promote the NF-κB signaling pathway. NF-κB-activated gene expression is a novel gene therapy to treat cancer by utilizing overactivation of NF-κB in cancer cells. IL-1 interleukin 1, IMiD immunomodulatory drugs, NFAT nuclear factor of activated T cells, NSAIDs nonsteroidal anti-inflammatory drugs, PD-L1 programmed death-ligand 1, TNF-α tumor necrosis factor α
Furthermore, substantial evidence indicates that aspirin and related NSAIDs possess potential antitumor activity and cancer-preventive effects, leading to increased interest in using aspirin for cancer treatment. Mechanistically, aspirin reduces the migration, invasion, and metastasis of osteosarcoma cells through the modulation of the NF-κB pathway.482 Hydrogen sulfide-releasing aspirin (HS-ASA), a novel derivative of aspirin, inhibits the growth of MDA-MB-231 breast cancer cells by downregulating the NF-κB pathway, inducing cell cycle arrest, and promoting apoptosis. HS-ASA also affects thioredoxin reductase activity and increases reactive oxygen species levels.483 Additionally, aspirin induces apoptosis in human colorectal cancer cells by inhibiting NF-κB activity, making it a potential therapeutic agent for colon cancer484 (Table 2).
Sodium salicylate
Sodium salicylate, similar to aspirin, is an NSAID. It has been discovered that sodium salicylate inhibits the activation of the transcription factor NF-κB.485 This finding opens up opportunities for exploring new applications of sodium salicylate.
In the context of anti-inflammatory therapy, both acetylsalicylic acid (aspirin) and its metabolite sodium salicylate have been found to protect against excitotoxicity caused by glutamate in neuronal cultures and hippocampal slices. This neuroprotective effect is achieved through the inhibition of NF-κB.486 Sodium salicylate is known as a COX-2 inhibitor and has been shown to improve insulin secretion defects in diabetic patients. It has been reported that this drug can prevent the dissociation of NF-κB from the NF-κB/IκB complex, thereby preventing the translocation of NF-κB from the cytoplasm to the nucleus and inhibiting the transcription of COX-2.487 Furthermore, sodium salicylate induces a shift from a proliferative to an apoptotic phenotype in human leukemia cells by inhibiting the NF-κB response and restoring TNF-induced apoptosis.488
Sulfasalazine
Sulfasalazine is primarily used as a sulfonamide antibiotic. When partially absorbed, it undergoes breakdown by intestinal microbiota into 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP). 5-ASA complexes with the connective tissue of the intestinal wall, exert antimicrobial, anti-inflammatory, and immunosuppressive effects. It inhibits the synthesis of prostaglandins and other inflammatory mediators like leukotrienes.489 Sulfasalazine (SAS) is a known NF-κB inhibitor that can inhibit the expression of TLR4, MyD88, and NF-κB p65 proteins induced by trinitro-benzene-sulfonic acid (TNBS).490
Neointimal hyperplasia (NH) induced by arterial injury is a major cause of arterial stenosis. Sulfasalazine prevents post-injury vascular stenosis by inhibiting the proliferation and migration of vascular smooth muscle cells through the NF-κB/mTOR pathway.491 Additionally, sulfasalazine plays a significant role in cancer treatment. It can promote apoptosis in U251 glioblastoma cells by inhibiting NF-κB signaling.492 The IL-1β-NFKB/CREB-Wnt signaling pathway has also been identified as a novel mechanism promoting breast cancer stem cell (CSC) colonization in the bone microenvironment. Targeting this pathway with drugs like sulfasalazine can prevent in vivo bone metastasis and colony formation of breast CSCs in vitro.493
Dexamethasone
Dexamethasone, a glucocorticoid, exhibits various pharmacological effects, including anti-inflammatory, anti-endotoxin, immunosuppressive, anti-shock, and stress response enhancement properties.494 The inhibition of NF-κB activation is one of the possible mechanisms by which dexamethasone exerts its therapeutic effects. There are two proposed mechanisms for this inhibition: Activated glucocorticoid receptors directly interact with the RelA subunit of NF-κB in the cell nucleus, leading to the inhibition of its function. Activated glucocorticoid receptors enhance the transcription of IκB and increase its levels. This, in turn, prevents the nuclear translocation of NF-κB and its binding to DNA.495 These mechanisms provide a basis for the therapeutic application of dexamethasone in diseases involving NF-κB dysregulation.
Studies have demonstrated that dexamethasone can alleviate acute pancreatitis and liver injury by inhibiting the NF-κB pathway.496 In the context of arthritis, dexamethasone can treat the condition and alleviate joint swelling symptoms by inhibiting the expression of the p65 protein in the NF-κB pathway.497 Additionally, in oral lichen planus (OLP), where the TLR4-NF-κB-p65 axis plays a crucial role, dexamethasone effectively protects against epidermal cell damage by downregulating TLR4 expression and negatively regulating the NF-κB signaling pathway in keratinocytes.498 These findings highlight the therapeutic potential of dexamethasone in NF-κB-mediated diseases and conditions. Its ability to inhibit NF-κB activation contributes to its anti-inflammatory effects and provides a rationale for its use in various inflammatory disorders.
Thalidomide
Thalidomide, initially developed as an anti-leprosy medication, has been found to have various pharmacological effects. Its mechanism of action involves immunosuppression, immune modulation, and inhibition of neutrophil chemotaxis. Multiple studies have indicated that thalidomide achieves these effects by inhibiting NF-κB activation.499,500
In terms of inflammatory therapy, thalidomide has demonstrated the ability to improve rosacea-like skin inflammation by inhibiting NF-κB activation in keratinocytes.501 Additionally, thalidomide may have therapeutic potential in tumor treatment. It can inhibit tumor necrosis factor α-induced ICAM-1 expression by suppressing the NF-κB-binding ICAM-1 promoter, leading to the inhibition of proliferation in lung cancer cells.502 Notably, the mechanism behind thalidomide-induced limb malformation is also related to NF-κB. Research has revealed that species-specific changes in the redox microenvironment, triggered by free radical generation from thalidomide, lead to the suppression of NF-κB-mediated gene expression, which is accountable for phocomelia (a congenital malformation of the limbs resembling a seal’s flippers).503
Lenalidomide
Lenalidomide, an analog of thalidomide, is an antitumor drug with various therapeutic effects, including antitumor activity, immune modulation, and anti-angiogenesis properties.504 Like thalidomide, lenalidomide also possesses the ability to inhibit NF-κB. However, it exhibits significantly higher potency in inhibiting TNF-α in vitro than thalidomide, with a 50,000-fold difference.505
Lenalidomide received FDA approval in 2003 for the treatment of relapsed or refractory multiple myeloma. It impairs the NF-κB signaling pathway in bone cells, resulting in the suppression of osteoclast-specific gene expression. This provides therapeutic effects against bone resorption and makes lenalidomide a valuable treatment option for osteolytic diseases such as multiple myeloma.506 In addition, lenalidomide has shown promise in the treatment of DLBCL. Its antitumor effect in DLBCL cells is associated with the downregulation of IRF4 and subsequent inhibition of B-cell receptor-dependent NF-κB activity.507
Pomalidomide
Pomalidomide, a next-generation immunomodulatory drug (IMiD), is primarily used in the treatment of relapsed/refractory multiple myeloma. It offers improved efficacy and toxicity characteristics compared to its sister compounds, lenalidomide and thalidomide.508 In MM cells, which heavily rely on various transcription factors, pomalidomide has been observed to inhibit NF-κB transcription and suppress COX-2 gene transcription activity, contributing to its therapeutic effects.509
Research is also being conducted to explore the potential use of pomalidomide in other diseases. For instance, it has shown the ability to enhance chemotherapy sensitivity in pancreatic cancer by inhibiting NF-κB activation induced by chemotherapy.510 Additionally, pomalidomide has demonstrated the capacity to suppress NF-κB levels and significantly reduce cortical neuron apoptosis by regulating Bax, cytochrome c, and poly (ADP-ribose) polymerase. This makes it a potential therapeutic agent for brain injury and related disorders.510,511
Pyridine derivative compound A
Pyridine and its derivatives have shown diverse biological activities and have been utilized in the synthesis of various drugs. Many of these drugs exhibit NF-κB inhibitory activity, which contributes to their pharmacological effects. Two examples of such drugs are imatinib and moxifloxacin.512
Imatinib is a well-known drug used for the treatment of diabetes and chronic myeloid leukemia (CML). During imatinib therapy, several transcription factors, including NF-κB, are activated in response to physiological and pathological changes. Studies have observed that the release of IL-6 and IL-8, as well as the activation of NF-κB and AP-1, were significantly reduced in lymphomonocytes of imatinib-treated patients. These findings suggest that the downregulation of these factors can potentially serve as favorable prognostic indicators for improved outcomes in patients receiving imatinib therapy.513 Moxifloxacin, on the other hand, is an antibiotic that exhibits anti-inflammatory effects. It achieves this by inhibiting the activation of NF-κB and mitogen-activated protein kinase in monocytes. Additionally, moxifloxacin suppresses the synthesis of pro-inflammatory cytokines, further contributing to its anti-inflammatory properties.514
New type inhibitors
From the first generation to the second generation of IKKβ inhibitors, there have been significant improvements in both the selectivity and potency of the drugs. Many IKKβ inhibitors have entered the preclinical research stage. BAY 11-7821 is an inhibitor of IκBα phosphorylation and NF-κB activation. It selectively and irreversibly inhibits TNF-α-induced IκB-α phosphorylation and reduces the expression of NF-κB and adhesion molecules. It exhibits high selectivity, primarily targeting the IKKβ subunit and does not significantly affect other related protein kinases.515 Bardoxolone methyl is a synthetic triterpenoid compound that has demonstrated inhibitory effects on NF-κB in various areas such as kidney disease and chronic obstructive pulmonary disease. Besides inhibiting NF-κB, Bardoxolone methyl also affects signaling pathways such as Nrf2 and PPAR-γ, giving it a broad therapeutic potential.516 Andrographolide is an NF-κB inhibitor derived from the Chinese medicinal herb Andrographis paniculata. It inhibits NF-κB activation by covalently modifying the cysteine residue of p50 in endothelial cells, without affecting IκBα degradation or p50/p65 nuclear translocation.517
Monoclonal antibody
Monoclonal antibodies mainly target NF-κB signaling through the inhibition of ligand-receptor interaction. Typical agents include anti-PD-1/PD-L1 and anti-IL-1. Besides, some novel monoclonal antibodies specifically targeting NF-κB signaling are under development.
Anti-PD-1/PD-L1
Programmed death-Ligand 1 (PD-L1) expression is upregulated in tumor cells or antigen-presenting cells, and evades immune surveillance after binding to programmed death 1 (PD-1) on the surface of tumor-infiltrating immune cells.518,519 RelB promotes prostate cancer immune evasion by expanding PD-L1/PD-1-mediated immune checkpoints to suppress T-cell immunity.520 TNF-α inhibits ubiquitinated degradation of PD-L1 via p65-induced COP9 signalosome 5 (CSN5), leading to immune escape.521 Immunotherapy resistance is an area of concern. TRAF2-deficient multiple myeloma cells enhance immunomodulatory drug resistance through activation of non-canonical NF-κB signaling and ERK signaling.522 Low MHC-I expression may lead to resistance to immune checkpoint inhibitors by inhibiting the IFN-γ signaling pathway, whereas guanine nucleotide-binding protein subunit gamma 4 (GNG4) maintains MHC-I expression through the NF-κB signaling.523 Inhibitors of the deubiquitinating enzyme ubiquitin-specific proteases (USP) 8 activate NF-κB signaling to trigger innate immune responses and MHC-I expression, thereby remodeling the inflammatory tumor microenvironment and enhancing the antitumor efficacy of anti-PD-1/PD-L1 therapies.524 CD11b agonists activate TAM and resist immunosuppression by degrading p65 and activating STING/STAT1 signaling.525
Anti-PD-1/PD-L1 antibodies are representative immune checkpoint inhibitors widely used in cancer therapies.526,527 These agents act by blocking the PD-1/PD-L1 interaction, thereby releasing the brakes on the immune system and combating cancer immune evasion. Currently, FDA-approved anti-PD-1/PD-L1 antibodies include pembrolizumab, atezolizumab, avelumab, durvalumab, and nivolumab.528,529,530,531 However, it is noteworthy that not all patients respond equally to these immunotherapies, and further studies to identify predictors of response and pathways strengthening the efficacy are needed to optimize their clinical use.518,532 Intriguingly, the NF-κB pathways have recently been raised as promising molecular targets to enhance the antitumor activity of checkpoint inhibitors. It has been shown that activation of the alternative NF-κB pathway combined with anti-PD-1 treatment can yield a complete and durable antitumor response in xenograft of melanoma and colorectal tumors.533 The upregulation of alternative NF-κB pathway in tumor-infiltrating DCs can be induced by anti-PD-1 treatment, leading to subsequent secretion of IL-12, which enhanced CD8 + T cell antitumor activity. Based on these findings, clinical trials investigating the combination of PD-1 inhibitors and agonistic CD40 mAbs/SMAC mimetics (which activate the alternative NF-κB signaling) for cancer treatment are currently ongoing (NCT03123783, NCT02376699, and NCT03270176).534
Anti IL-1
Anti-IL-1 inhibits the activity of interleukin-1 (IL-1), which plays a crucial role in inflammation via activation of canonical NF-κB signaling and is associated with various autoimmune and inflammatory conditions such as rheumatoid arthritis, gout, and certain skin diseases. IL-1 inhibitors, including canakinumab, rilonacept, and anakinra, have been approved for the treatment of rheumatoid arthritis,535 auto-inflammatory diseases,536 cryopyrin-associated periodic syndromes (CAPS),537,538 adult-onset Still’s disease (AOSD).539 Their applications in cancer therapy have also been under investigation. However, due to the dual role of IL-1 in tumor development, direct inhibition failed to yield satisfactory efficacy, posing challenges to the development of antitumor drugs targeting IL-1. The combination of IL-1 inhibitor with a PD-1 inhibitor in a recent clinical trial has shown clinically meaningful delays in the deterioration of symptoms for the treatment of lung cancer, although did not prolong PFS or OS (NCT03631199).540
Anti-β2-microglobulin monoclonal antibodies
Specific monoclonal antibodies that target both tumor cells and the tumor microenvironment are currently under development. One promising antibody-based novel agent in multiple myeloma is anti-β2-microglobulin monoclonal antibodies, which have shown remarkable antitumor activity on myeloma both in vitro and in xenograft with low toxicity.541,542 Studies have demonstrated that combining bortezomib with anti-β2-microglobulin monoclonal antibodies can significantly reduce NF-κB activity, induce tumor cell apoptosis, and overcome bortezomib resistance. This combination therapy also inhibits bortezomib-induced autophagy mediated by the interaction of p65 with the beclin 1 promoter.543 The enhanced effect of the combination therapy holds promise as it may allow for lower doses of either substance, reducing toxicity while enhancing efficacy. These studies highlight the potential of antibody-based therapies in multiple myeloma treatment and their ability to modulate the NF-κB signaling pathway.
Proteasome inhibitors
Proteasome inhibitors mainly target the unfolded protein response (UPR) pathway to inhibit IκBα degradation and thus suppress NF-κB. Suppression of proteasome leads to the accumulation of misfolded proteins and subsequent endoplasmic reticulum stress in cancer cells, inducing cell cycle arrest and apoptosis.544 FDA-approved proteasome inhibitors include bortezomib, carfilzomib, and ixazomib, which have been mainly used for the treatment of multiple myeloma, diffuse large B cell lymphoma (DLBCL), and other solid tumors.545
Bortezomib
Bortezomib is a first-in-class selective and reversible proteasome inhibitor, which binds to and inhibits the activity of the 26 S proteasome, a cellular complex responsible for degrading and recycling proteins.546,547 By blocking the proteasome’s function, bortezomib was initially thought to inhibit IκB degradation, which is necessary for NF-κB activation. However, it has been later confirmed that bortezomib can also induce activation of canonical NF-κB signaling in multiple myeloma in vitro.548 Further investigations indicated that this contradictory effect can be prevented by combination therapies such as the use of calpain inhibitors.549
Carfilzomib
Carfilzomib is a second-generation proteasome inhibitor targeting 20 S proteasome. Unlike bortezomib, carfilzomib irreversibly binds to the active site of the proteasome with higher selectivity, leading to a prolonged inhibition of its activity.550 This mechanism allows carfilzomib to exert a more sustained and profound effect on proteasome function. Additionally, carfilzomib has almost no off-target activity outside of proteasome and has been confirmed to inhibit NF-κB signaling in recent studies. Carfilzomib has demonstrated significant efficacy in patients with relapsed or refractory multiple myeloma in clinical trials, either as a single agent or in combination with other anticancer therapies.551,552,553
Ixazomib
Ixazomib is an oral second-generation proteasome inhibitor, acting by selectively and reversibly inhibiting the 20 S proteasome. Its oral formulation allows for easier administration and provides greater accessibility for long-term treatment. Ixazomib can effectively inhibit both activation pathways of NF-κB in MM stromal cells.554 Ixazomib is often used in combination with other anticancer drugs and has shown significant efficacy in clinical trials for the treatment of multiple myeloma. It provides an additional option for patients, expanding the range of treatment strategies available to improve outcomes and quality of life for those living with multiple myeloma.545
Marizomib
Marizomib is a novel, irreversible proteasome inhibitor developed for the treatment of relapsed or relapsed and refractory multiple myeloma. It inhibits the 3 proteolytic activities of the 20 S proteasome with specificity distinct from bortezomib and carfilzomib. Marizomib also inhibits NF-κB signaling through inhibition of IκBα degradation. Preclinical studies and early-phase clinical trials (NCT00461045, NCT00629473) have shown promising results for marizomib in terms of its anticancer activity in multiple myeloma and tolerability.555,556,557 Its use in other solid tumors such as glioblastoma and breast cancer are under preclinical investigations.558,559 Further clinical trials are still ongoing to evaluate its effectiveness as a single agent or in combination with other therapies for treating cancer.
Inhibition of nuclear translocation
Tacrolimus
Tacrolimus (FK506) is an immunosuppressive agent that is primarily used to prevent organ rejection after transplantation.560,561 It inhibits the activity of calcineurin, an enzyme that plays a crucial role in the activation of the nuclear factor of activated T cells (NFAT). By blocking NFAT nuclear translocation, tacrolimus inhibits the activation of NF-κB signaling, and thus suppress the activity of various immune cells and alleviate inflammation. Apart from preventing organ rejection, tacrolimus is also prescribed for certain dermatological conditions, such as moderate to severe atopic dermatitis (eczema) when other treatments have proven ineffective.562,563 Increasing evidence has supported tacrolimus to be used as a second-line therapeutic agent for ulcerative colitis and Crohn’s disease.560,562,564,565 Currently, tacrolimus demonstrates a relatively pronounced short-term induction of remission and is gradually being utilized in the management of traditional drug-resistant or anti-TNF-resistant IBD, while evidence regarding its long-term efficacy and safety with prolonged use remains limited.
IκBα super-repressor
The IκBα super-repressor is a genetically engineered IκB protein without IKK phosphorylation sites, leading to a sustained inhibition of NF-κB by preventing nuclear translocation. As a result, the downstream genes regulated by NF-κB is repressed. Emerging preclinical evidence has addressed the wide use of IκBα super-repressor delivered through exosome systems in various diseases, including alcohol-associated liver injury, sepsis-associated organ damage, kidney ischemia-reperfusion injury, and amyotrophic lateral sclerosis (ALS).566,567,568,569,570 By utilizing an engineered exosome technology called “exosomes for protein loading via optically reversible protein–protein interactions (EXPLOR)”, Yim et al., engineered exosomes to load super-repressor IκB (Exo-srIκB) for the efficient intracellular transfer of protein-based therapeutics.571 Although the application of IκBα super-repressor is currently limited to laboratory research settings, it demonstrates high potential as a promising intervention in clinical practice in the future.
Inhibition of DNA binding
Glucocorticoids
Glucocorticoids exert their effects by binding to specific receptors inside cells, known as glucocorticoid receptors (GRs). The precise effect of glucocorticoids on DNA binding and transcriptional regulation is complex and context-dependent. On one hand, glucocorticoid binding to the glucocorticoid response elements (GREs) can enhance gene transcription, leading to increased protein synthesis. This activation of gene expression can be seen with certain genes involved in anti-inflammatory responses or metabolic processes. On the other hand, Glucocorticoids can suppress DNA binding and gene expression by various mechanisms. GR activation can cause genome-wide blockade of NF-κB, inhibiting their ability to bind to DNA and suppressing the expression of pro-inflammatory genes.572,573,574 Additionally, the activated GRs can recruit co-repressors, which further inhibit the binding of other transcription factors or interfere with the assembly of the transcription initiation complex.
Due to their potent anti-inflammatory and immunosuppressive properties, glucocorticoids are commonly used for the treatment of a wide range of inflammatory conditions, including allergic reactions (skin rashes, bronchial constriction, etc.), autoimmune disorders (rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, etc.), asthma, IBD (Crohn’s disease, ulcerative colitis, etc.).
PPAR agonists
PPAR (peroxisome proliferator-activated receptor) agonists activate one or more isoforms of the PPAR family. There are three main isoforms of PPARs: PPAR-α, PPAR-δ/β, and PPAR-γ, each of them has distinct tissue distribution and biological functions.575,576,577,578 PPAR agonists can selectively target one or more isoforms to elicit specific therapeutic effects.579,580
PPAR-α agonists (e.g., fenofibrate and gemfibrozil) primarily target PPAR-alpha and are used to treat dyslipidemia and reduce triglyceride levels. They promote fatty acid oxidation in the liver, leading to increased clearance of triglycerides from the blood. PPAR-δ/β agonists, such as GW501516 (also known as cardarine), can enhance fatty acid metabolism, improve insulin sensitivity, and modulate skeletal muscle function. PPAR-δ/β agonists have shown potential for treating metabolic disorders such as obesity, dyslipidemia, and type 2 diabetes. PPAR-γ agonists, including pioglitazone and rosiglitazone, are widely used for managing insulin resistance and improving glycemic control in individuals with type 2 diabetes. They can enhance insulin sensitivity, promote glucose uptake by peripheral tissues, and regulate adipocyte differentiation and lipid metabolism. Additionally, investigations are ongoing to explore other potential use of PPAR agonists in various conditions, such as inflammation, neurodegenerative diseases, and cancer. It is noteworthy that studies have suggested that certain PPAR agonist (thiazolidinedione) can inhibit NF-κB DNA binding, offering a new therapeutic strategy for lymphoblastic leukemia and IBD.581,582
Tyrosine kinase inhibitors
Tyrosine kinase inhibitors (TKIs) are widely used in cancer therapy, acting by binding to the ATP-binding site of tyrosine kinases, preventing them from phosphorylating their target proteins. This disruption of kinase activity can inhibit downstream signaling pathways that promote cancer cell growth and survival. TKIs have shown efficacy in treating various types of cancer, including chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GISTs), non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and breast cancer.583
Numerous researches have suggested the interaction between TKIs and NF-κB activity. However, the relationship between TKIs and NF-κB can be complex and context-dependent. Some studies have reported that TKIs such as imatinib can suppress NF-κB signaling in certain types of cancer cells, leading to reduced cell survival and proliferation.584 On the other hand, there are also reports suggesting that some TKIs can induce NF-κB activity. The activation of NF-κB mediates resistance to TKI treatment.585,586,587 Targeting the NF-κB pathway may be a potential therapeutic approach to tackle TKI resistance.
Non-coding RNAs
Non-coding RNAs (ncRNAs) mainly include microRNA (miRNA), long non-coding RNA (IncRNA), and circular RNA (circRNA).588 These ncRNAs play important roles in various cellular processes, including gene regulation and epigenetic modulation. Increasing evidence has demonstrated that ncRNAs may engage in the aberrant regulation of NF-κB signaling.589 RNAs can act more rapidly and diversely than proteins upon stimulation, which makes them significant in the regulation of NF-κB signaling. Anti-miRNA oligonucleotides have shown effectiveness in inhibiting NF-κB in cancer.590 Targeting NF-κB signaling by ncRNAs is an emerging therapeutic strategy of cancer treatment.
Immunotherapy
Scientists have developed an inhibitor called KIC-0101, which effectively blocks the NF-κB pathway and inhibits the induction of pro-inflammatory cytokines both in vitro and in vivo. In a mouse model of rheumatoid arthritis, treatment with KIC-0101 significantly improves cartilage damage and inflammation.591 Additionally, researchers have discovered that Perillyl alcohol (POH) effectively ameliorates arthritis in rats by modulating the TLR4/NF-κB signaling pathway.592 The current research has identified several novel targets for treating osteoarthritis (OA) through the NF-κB pathway. For instance, the regulatory role of miR-214-3p in cartilage degradation in OA via the NF-κB pathway has been revealed, highlighting the potential of miR-214-3p as a therapeutic target.593
Current and future research directions mainly focus on the interplay between NF-κB and inflammation response as well as neural damage, aiming to identify therapeutic targets for improving disease progression and quality of life in multiple sclerosis (MS) patients. Sunny Malhotra et al. discovered that NF-κB is involved in the regulation of NLRP3 inflammasome, serving as a prognostic factor and potential therapeutic target in primary progressive multiple sclerosis.594 Furthermore, inhibition of poly (ADP-ribose) polymerase 1 (PARP1), an upstream regulator of NF-κB signaling, has demonstrated therapeutic potential in multiple sclerosis and its animal models.595
Mohamed El-Sherbiny et al. discovered that betulin demonstrates anti-inflammatory and anti-apoptotic effects in experimental ulcerative colitis through modulation of the TLR4/NF-κB/caspase signaling pathway.596 However, a significant limitation for its clinical application is its poor solubility in aqueous media, which calls for further research to enhance the drug’s clinical potential and expand its therapeutic applicability.
Inhibitors of NF-κB may also well improve the complications of SLE. The clinical implications of RIG-I hyperactivation caused by a novel pathogenic variant in DDX58 are explored, shedding light on its relevance to lupus nephritis and the potential involvement of the NF-κB pathway.597
CAR (chimeric antigen receptor)-T cell immunotherapy
CAR-T cell immunotherapy is an innovative and promising approach in cancer treatment. Huang et al. has recently revealed the key role of NFAT and NF-κB in the dynamic co-regulation of TCR and CAR signaling responses in human T cells.598 Despite the remarkable clinical success of CAR-T in the treatment of hematological malignancies, it also faces challenges such as T cell exhaustion. Second-generation CAR targets are thus developed, including co-stimulatory regulators 4-1BB (CD137), to increase T cell expansion and delay apoptosis.599 Recent studies have reported that human CAR with 4-1BB endodomain leads to strong NF-κB activation through the recruitment of TRAF molecules, indicating the important role of TRAF-NF-κB axis in CAR-T persistence upon antigen stimulation.600 Disruption of TRAF2 signaling inhibited IKKα and IKKβ phosphorylation and prevented tonic CAR signaling-dependent T cell toxicity. A recent study showed that cis ligation of 4-1BB relative to the TCR-CD3 complex lead to more intense canonical and non-canonical NF-κB signaling, providing a more robust induction of cell cycle and DNA damage repair gene expression.601 In addition, Jakrawadee et al has developed a composite co-stimulatory domain of a B cell signaling moiety, CD79A/CD40, to synergize with other T cell signals and enhance CAR-T cell function. In the preclinical model, CD79A/CD40 incorporating CD19CAR-T cells demonstrated higher NF-κB and p38 activity compared with the CD28 or 4-1BB incorporating CD19CAR-T cells and improved antitumor efficacy.602 Further studies are still required to optimize the design of CARs and improve CAR-T cell function and persistence.
Future directions
In summary, NF-κB signaling pathway plays a crucial role in multiple human diseases, as it operates at multiple levels of immune responses and contributes to the inflammatory lesions observed in these conditions. Therefore, developing therapeutic approaches targeting specific effectors implicated in these diseases is of utmost importance. However, the NF-κB signaling pathway encompasses multiple components and exerts its influence in various physiological responses, making it challenging to avoid potential side effects associated with NF-κB inhibitors. Hence, the future research focus lies in developing more specific NF-κB inhibitors for the treatment of inflammatory disorders.
Prospectively, strategies targeting NF-κB signaling require development in the following directions: (1) Developing NF-κB inhibitors that simultaneously target multiple key nodes; (2) Improving the bioavailability, safety, and stability of small molecule drugs; (3) Enhancing drug specificity to minimize interference with other cellular functions; (4) Exploring natural products and traditional Chinese medicine research.
Conclusion
In the field of NF-κB signaling, there have been several outstanding reviews describing the components and transmission mechanisms of canonical or non-canonical NF-κB signaling.33,83,183,189,191,217,306,603,604,605,606,607,608,609 Regarding the biological functions of NF-κB signaling, the main focus has been on inflammation, immune response, and metabolism. In conclusion, the attention of these articles centers on NF-κB signaling itself. In this review, we attempt to take a broader perspective on NF-κB signaling by describing its involvement in various human diseases and summarizing the therapeutic means for targeting NF-κB signaling, which is an important characteristic that distinguishes this review from others. We hope to be able to provide reference to scholars (beginners, scientists, or clinicians) from multiple intellectual profiles in this format.
More is known about NF-κB signaling and more is unknown. The timing of activation can affect the transcriptional responses produced by NF-κB signaling in response to stimuli. Oscillatory activation of NF-κB promotes transcription of inflammatory genes, whereas persistent activation reprograms the epigenome, involving a broader range of genes.610 Sine oculis homeobox (SIX) family transcription factors are activated through non-canonical NF-κB signaling and then bind to the promoter regions of pro-inflammatory genes to directly inhibit RELA and RELB function, a negative feedback loop that enriches the understanding of NF-κB signaling and provides a possible therapeutic target.611 Components of NF-κB signaling also act independently, a prime example being the finding that IKK controls Rel-deficient thymocytes from RIPK1-dependent cell death independently of NF-κB activation.612
The involvement of NF-κB signaling in inflammation and the immune response is re-emphasized in this review, particularly in the context of immunotherapy as a “revolution” for tumors and other diseases. Indeed, NF-κB signaling has demonstrated initial potential in a variety of diseases thought to be difficult to overcome, extending beyond those discussed in this article. Long-term latency of CD4 + T cells poses a significant challenge in the eradication of AIDS. AZD5582 has been found to effectively promote the expression of HIV and simian immunodeficiency virus (SIV) through the activation of atypical NF-κB signaling in mouse and rhesus monkey models, a finding that provides a basis for combining AZD5582 with HIV-removing drugs to eradicate AIDS.613
Promoting ongoing research from the laboratory to the clinic is a shared objective among scientists. In the 40 years of research on NF-κB signaling, an increasing number of promising targets have been identified, leading to the development of corresponding therapeutics that have either entered clinical trials or been approved for disease treatment. It is challenging to tackle the issue of how to promote the rapid and consistent application of cellular and animal studies to human applications. The diversity of the disease spectrum and inter-species variability are significant obstacles. More advanced and closer to the real human environment research methodologies are required, encompassing spatial multi-omics, single-cell sequencing, organoids, genetically engineered animal models, 3D bioprinting, and other cutting-edge techniques. A recent study utilizing time-dependent multi-omics and single-cell RNA sequencing revealed heterogeneity in Rel and RelA-mediated gene expression and specific responses, and has demonstrated that their functional antagonism arises from co-expression in the nucleus.614
While we cannot overlook the potent potential of NF-κB signaling, we also need to confront the latent apprehensions underlying it. As described in this article, therapies targeting NF-κB signaling will inevitably elicit side effects, given its involvement in a diverse array of biological processes. Therefore, precise drug design, synthesis, and delivery procedures are imperative, and the utilization of nanomaterials will be pivotal in facilitating this process. Future research needs to think about how to improve the efficiency of targeting NF-κB signaling therapy while mitigating potential adverse effects, thereby achieving a significant breakthrough in the field of immunotherapy.
References
Sen, R. & Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46, 705–716 (1986).
Singh, H., Sen, R., Baltimore, D. & Sharp, P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature 319, 154–158 (1986).
Sen, R. & Baltimore, D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47, 921–928 (1986).
Lenardo, M., Pierce, J. W. & Baltimore, D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science 236, 1573–1577 (1987).
Lenardo, M. J., Fan, C. M., Maniatis, T. & Baltimore, D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 57, 287–294 (1989).
Ahmad, S. et al. Long non-coding RNAs regulated NF-κB signaling in cancer metastasis: micromanaging by not so small non-coding RNAs. Semin. Cancer Biol. 85, 155–163 (2022).
Blanchett, S., Boal-Carvalho, I., Layzell, S. & Seddon, B. NF-κB and extrinsic cell death pathways - entwined do-or-die decisions for T cells. Trends Immunol. 42, 76–88 (2021).
Ghosh, S. & Hayden, M. S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 8, 837–848 (2008).
Gulei, D. et al. The tumor suppressor functions of ubiquitin ligase KPC1: from cell-cycle control to NF-κB regulator. Cancer Res 83, 1762–1767 (2023).
Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2, a000158 (2010).
Henkel, T. et al. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell 68, 1121–1133 (1992).
Palombella, V. J., Rando, O. J., Goldberg, A. L. & Maniatis, T. The ubiquitinproteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell 78, 773–785 (1994).
Hayden, M. S. & Ghosh, S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234 (2012).
Thompson, J. E., Phillips, R. J., Erdjument-Bromage, H., Tempst, P. & Ghosh, S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell 80, 573–582 (1995).
Rao, P. et al. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature 466, 1115–1119 (2010).
Inoue, J., Kerr, L. D., Kakizuka, A. & Verma, I. M. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell 68, 1109–1120 (1992).
Baeuerle, P. A. & Baltimore, D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242, 540–546 (1988).
Zabel, U. & Baeuerle, P. A. Purified human IκB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell 61, 255–265 (1990).
Sun, S. C., Ganchi, P. A., Ballard, D. W. & Greene, W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259, 1912–1915 (1993).
Brown, K., Gerstberger, S., Carlson, L., Franzoso, G. & Siebenlist, U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267, 1485–1488 (1995).
Dechend, R. et al. The Bcl-3 oncoprotein acts as a bridging factor between NF-κB/Rel and nuclear co-regulators. Oncogene 18, 3316–3323 (1999).
Nolan, G. P. et al. The bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that preferentially interacts with NF-kappa B p50 and p52 in a phosphorylation-dependent manner. Mol. Cell Biol. 13, 3557–3566 (1993).
Gehrke, N. et al. Hepatocyte Bcl-3 protects from death-receptor mediated apoptosis and subsequent acute liver failure. Cell Death Dis. 13, 510 (2022).
Jaiswal, H. et al. The NF-κB regulator Bcl-3 restricts terminal differentiation and promotes memory cell formation of CD8 + T cells during viral infection. PLoS Pathog. 17, e1009249 (2021).
Tang, W. et al. Bcl-3 inhibits lupus-like phenotypes in BL6/lpr mice. Eur. J. Immunol. 51, 197–205 (2021).
Bours, V. et al. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell 72, 729–739 (1993).
Westerheide, S. D., Mayo, M. W., Anest, V., Hanson, J. L. & Baldwin, A. S. The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition. Mol. Cell Biol. 21, 8428–8436 (2001).
Watanabe, N., Iwamura, T., Shinoda, T. & Fujita, T. Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generation of NF-kappaB homodimers from the cytoplasmic pool of p50-p105 and nuclear translocation. EMBO J. 16, 3609–3620 (1997).
Chen, L.-F. & Greene, W. C. Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 5, 392–401 (2004).
Ha, H., Han, D. & Choi, Y. TRAF-Mediated TNFR-Family Signaling. Curr. Protoc. Immunol. 87, 11.9D.1–11.9D.19 (2009).
Sakurai, H., Chiba, H., Miyoshi, H., Sugita, T. & Toriumi, W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274, 30353–30356 (1999).
Sakurai, H. et al. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 278, 36916–36923 (2003).
Yu, H., Lin, L., Zhang, Z., Zhang, H. & Hu, H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target Ther. 5, 209 (2020).
May, M. J., Marienfeld, R. B. & Ghosh, S. Characterization of the Ikappa B-kinase NEMO binding domain. J. Biol. Chem. 277, 45992–46000 (2002).
Marienfeld, R. B., Palkowitsch, L. & Ghosh, S. Dimerization of the I kappa B kinase-binding domain of NEMO is required for tumor necrosis factor alpha-induced NF-kappa B activity. Mol. Cell Biol. 26, 9209–9219 (2006).
Zhang, S. Q., Kovalenko, A., Cantarella, G. & Wallach, D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12, 301–311 (2000).
Poyet, J. L. et al. Activation of the Ikappa B kinases by RIP via IKKgamma /NEMO-mediated oligomerization. J. Biol. Chem. 275, 37966–37977 (2000).
Schröfelbauer, B., Polley, S., Behar, M., Ghosh, G. & Hoffmann, A. NEMO ensures signaling specificity of the pleiotropic IKKβ by directing its kinase activity toward IκBα. Mol. Cell 47, 111–121 (2012).
Yu, J.-S. et al. Substrate-specific recognition of IKKs mediated by USP16 facilitates autoimmune inflammation. Sci. Adv. 7, eabc4009 (2021).
Le Negrate, G. Subversion of innate immune responses by bacterial hindrance of NF-κB pathway. Cell Microbiol 14, 155–167 (2012).
Aqdas, M. & Sung, M.-H. NF-κB dynamics in the language of immune cells. Trends Immunol. 44, 32–43 (2023).
Son, M. et al. Spatiotemporal NF-κB dynamics encodes the position, amplitude, and duration of local immune inputs. Sci. Adv. 8, eabn6240 (2022).
Hsu, H., Xiong, J. & Goeddel, D. V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81, 495–504 (1995).
Hsu, H., Shu, H. B., Pan, M. G. & Goeddel, D. V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84, 299–308 (1996).
Hsu, H., Huang, J., Shu, H. B., Baichwal, V. & Goeddel, D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Park, Y. C. et al. A novel mechanism of TRAF signaling revealed by structural and functional analyses of the TRADD-TRAF2 interaction. Cell 101, 777–787 (2000).
Legler, D. F., Micheau, O., Doucey, M.-A., Tschopp, J. & Bron, C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 18, 655–664 (2003).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Ermolaeva, M. A. et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat. Immunol. 9, 1037–1046 (2008).
Lork, M., Verhelst, K. & Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: so similar, yet so different. Cell Death Differ. 24, 1172–1183 (2017).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430, 694–699 (2004).
Kovalenko, A. et al. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424, 801–805 (2003).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424, 793–796 (2003).
Ikeda, F. et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471, 637–641 (2011).
Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 (2011).
Courtois, G. & Fauvarque, M.-O. The many roles of ubiquitin in NF-κB signaling. Biomedicines 6, 43 (2018).
Li, M. et al. Reciprocal interplay between OTULIN-LUBAC determines genotoxic and inflammatory NF-κB signal responses. Proc. Natl Acad. Sci. USA 119, e2123097119 (2022).
Niu, J., Shi, Y., Iwai, K. & Wu, Z.-H. LUBAC regulates NF-κB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. EMBO J. 30, 3741–3753 (2011).
Cao, Z., Henzel, W. J. & Gao, X. IRAK: a kinase associated with the interleukin-1 receptor. Science 271, 1128–1131 (1996).
Croston, G. E., Cao, Z. & Goeddel, D. V. NF-kappa B activation by interleukin-1 (IL-1) requires an IL-1 receptor-associated protein kinase activity. J. Biol. Chem. 270, 16514–16517 (1995).
Cao, Z., Xiong, J., Takeuchi, M., Kurama, T. & Goeddel, D. V. TRAF6 is a signal transducer for interleukin-1. Nature 383, 443–446 (1996).
Muzio, M., Ni, J., Feng, P. & Dixit, V. M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278, 1612–1615 (1997).
Wesche, H., Henzel, W. J., Shillinglaw, W., Li, S. & Cao, Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 (1997).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Deng, L. et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 (2000).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
O’Neill, L. A. J. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Rev. 226, 10–18 (2008).
Blasius, A. L. & Beutler, B. Intracellular toll-like receptors. Immunity 32, 305–315 (2010).
Fitzgerald, K. A. et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055 (2003).
Kawagoe, T. et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat. Immunol. 9, 684–691 (2008).
Xia, Z.-P. et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 (2009).
Love, P. E. & Hayes, S. M. ITAM-mediated signaling by the T-cell antigen receptor. Cold Spring Harb. Perspect. Biol. 2, a002485 (2010).
Gaud, G., Lesourne, R. & Love, P. E. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 18, 485–497 (2018).
Hořejší, V., Zhang, W. & Schraven, B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol. 4, 603–616 (2004).
Sun, Z. et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404, 402–407 (2000).
Rickert, R. C. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat. Rev. Immunol. 13, 578–591 (2013).
Xu, Y., Harder, K. W., Huntington, N. D., Hibbs, M. L. & Tarlinton, D. M. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity 22, 9–18 (2005).
Thome, M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4, 348–359 (2004).
Li, Q. & Verma, I. M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Shinohara, H. et al. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med. 202, 1423–1431 (2005).
Hayden, M. S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008).
Park, K.-J., Krishnan, V., O’Malley, B. W., Yamamoto, Y. & Gaynor, R. B. Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol. Cell 18, 71–82 (2005).
Sun, S.-C. Non-canonical NF-κB signaling pathway. Cell Res. 21, 71–85 (2011).
Liao, G., Zhang, M., Harhaj, E. W. & Sun, S.-C. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J. Biol. Chem. 279, 26243–26250 (2004).
Xiao, G., Fong, A. & Sun, S.-C. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 279, 30099–30105 (2004).
Solan, N. J., Miyoshi, H., Carmona, E. M., Bren, G. D. & Paya, C. V. RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem. 277, 1405–1418 (2002).
Vallabhapurapu, S. et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 9, 1364–1370 (2008).
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 131, 669–681 (2007).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131, 682–693 (2007).
Zarnegar, B. J. et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9, 1371–1378 (2008).
Dejardin, E. et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17, 525–535 (2002).
Kayagaki, N. et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity 17, 515–524 (2002).
Morrison, M. D., Reiley, W., Zhang, M. & Sun, S.-C. An atypical tumor necrosis factor (TNF) receptor-associated factor-binding motif of B cell-activating factor belonging to the TNF family (BAFF) receptor mediates induction of the noncanonical NF-kappaB signaling pathway. J. Biol. Chem. 280, 10018–10024 (2005).
Claudio, E., Brown, K., Park, S., Wang, H. & Siebenlist, U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 3, 958–965 (2002).
Bishop, G. A. & Hostager, B. S. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 14, 297–309 (2003).
Theill, L. E., Boyle, W. J. & Penninger, J. M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20, 795–823 (2002).
Vaira, S. et al. RelB is the NF-kappaB subunit downstream of NIK responsible for osteoclast differentiation. Proc. Natl Acad. Sci. USA 105, 3897–3902 (2008).
Novack, D. V. et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J. Exp. Med. 198, 771–781 (2003).
He, Y. et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target Ther. 6, 425 (2021).
Vanhaesebroeck, B., Guillermet-Guibert, J., Graupera, M. & Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341 (2010).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 (2006).
Thorpe, L. M., Yuzugullu, H. & Zhao, J. J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 15, 7–24 (2015).
Hanker, A. B., Kaklamani, V. & Arteaga, C. L. Challenges for the clinical development of PI3K inhibitors: strategies to improve their impact in solid tumors. Cancer Discov. 9, 482–491 (2019).
Davis, R. E. et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463, 88–92 (2010).
Xu, W., Berning, P. & Lenz, G. Targeting B-cell receptor and PI3K signaling in diffuse large B-cell lymphoma. Blood 138, 1110–1119 (2021).
Paul, J. et al. Simultaneous inhibition of PI3Kδ and PI3Kα induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-κB and AKT. Cancer Cell 31, 64–78 (2017).
Xu, W. et al. mTOR inhibition amplifies the anti-lymphoma effect of PI3Kβ/δ blockage in diffuse large B-cell lymphoma. Leukemia 37, 178–189 (2023).
Okabe, S., Tanaka, Y. & Gotoh, A. Targeting phosphoinositide 3-kinases and histone deacetylases in multiple myeloma. Exp. Hematol. Oncol. 10, 19 (2021).
Li, R. et al. Interleukin-7 induces recruitment of monocytes/macrophages to endothelium. Eur. Heart J. 33, 3114–3123 (2012).
Wang, Z. et al. Melatonin inhibits atherosclerosis progression via galectin-3 downregulation to enhance autophagy and inhibit inflammation. J. Pineal Res. 74, e12855 (2023).
Chen, L. et al. Aerobic glycolysis enhances HBx-initiated hepatocellular carcinogenesis via NF-κBp65/HK2 signalling. J. Exp. Clin. Cancer Res. 41, 329 (2022).
Chalabi-Dchar, M. et al. Loss of somatostatin receptor subtype 2 promotes growth of KRAS-induced pancreatic tumors in mice by activating PI3K signaling and overexpression of CXCL16. Gastroenterology 148, 1452–1465 (2015).
Lin, C.-Y. et al. Role of tissue transglutaminase 2 in the acquisition of a mesenchymal-like phenotype in highly invasive A431 tumor cells. Mol. Cancer 10, 87 (2011).
Wagner, E. F. & Nebreda, Á. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 (2009).
Nakano, H. et al. Reactive oxygen species mediate crosstalk between NF-κB and JNK. Cell Death Differ. 13, 730–737 (2006).
Tang, G. et al. Inhibition of JNK activation through NF-κB target genes. Nature 414, 313–317 (2001).
De Smaele, E. et al. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414, 308–313 (2001).
Liu, H., Lo, C. R. & Czaja, M. J. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35, 772–778 (2002).
Guillermet-Guibert, J. et al. Novel synergistic mechanism for sst2 somatostatin and TNFα receptors to induce apoptosis: crosstalk between NF-κB and JNK pathways. Cell Death Differ. 14, 197–208 (2007).
Gehrke, N. et al. Hepatocyte-specific deletion of IL1-RI attenuates liver injury by blocking IL-1 driven autoinflammation. J. Hepatol. 68, 986–995 (2018).
Yamaoka, K. et al. The Janus kinases (Jaks). Genome Biol. 5, 253 (2004).
Digicaylioglu, M. & Lipton, S. A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature 412, 641–647 (2001).
Levy, D. E. & Darnell, J. E. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
Xue, C. et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct. Target Ther. 8, 1–24 (2023).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Feist, M. et al. Cooperative STAT/NF-κB signaling regulates lymphoma metabolic reprogramming and aberrant GOT2 expression. Nat. Commun. 9, 1514 (2018).
Kortylewski, M. et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 15, 114–123 (2009).
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003).
Lee, H. et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 15, 283–293 (2009).
Chorzalska, A. et al. Bone marrow–specific loss of ABI1 induces myeloproliferative neoplasm with features resembling human myelofibrosis. Blood 132, 2053–2066 (2018).
Pecharromán, I. et al. IκB kinase-α coordinates BRD4 and JAK/STAT signaling to subvert DNA damage-based anticancer therapy. EMBO J. 42, e114719 (2023).
Wang, H. et al. A novel model of alternative NF-κB pathway activation in anaplastic large cell lymphoma. Leukemia 35, 1976–1989 (2021).
Nadiminty, N. et al. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc. Natl Acad. Sci. USA 103, 7264–7269 (2006).
Ma, J. F. et al. STAT3 promotes IFNγ/TNFα-induced muscle wasting in an NF-κB-dependent and IL-6-independent manner. EMBO Mol. Med. 9, 622–637 (2017).
Massagué, J. & Sheppard, D. TGF-β signaling in health and disease. Cell 186, 4007–4037 (2023).
Markowitz, S. D. & Roberts, A. B. Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev. 7, 93–102 (1996).
Reiss, M. TGF-beta and cancer. Microbes Infect. 1, 1327–1347 (1999).
Tu, E. et al. T cell receptor-regulated TGF-β type I receptor expression determines T cell quiescence and activation. Immunity 48, 745–759.e6 (2018).
Hong, S. et al. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat. Immunol. 8, 504–513 (2007).
Wang, H. et al. NF-κB induces miR-148a to sustain TGF-β/Smad signaling activation in glioblastoma. Mol. Cancer 14, 2 (2015).
Song, L. et al. TGF-β induces miR-182 to sustain NF-κB activation in glioma subsets. J. Clin. Invest. 122, 3563–3578 (2012).
Strippoli, R. et al. p38 maintains E-cadherin expression by modulating TAK1-NF-kappa B during epithelial-to-mesenchymal transition. J. Cell Sci. 123, 4321–4331 (2010).
Santoro, R., Carbone, C., Piro, G., Chiao, P. J. & Melisi, D. TAK-ing aim at chemoresistance: the emerging role of MAP3K7 as a target for cancer therapy. Drug Resist Updat. 33–35, 36–42 (2017).
Shin, E. M. et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J. Clin. Invest. 124, 3807–3824 (2014).
Alagbala Ajibade, A. et al. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1 + CD11b+ neutrophils. Immunity 36, 43–54 (2012).
Lee, Y. S. et al. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat. Commun. 2, 460 (2011).
Szabo, P. L. et al. Tenascin-C provokes cardiac fibrosis and endothelial impairment in Duchenne muscular dystrophy. Cardiovasc. Res. 118, cvac066.152 (2022).
Yin, C. et al. Elevated Wnt2 and Wnt4 activate NF-κB signaling to promote cardiac fibrosis by cooperation of Fzd4/2 and LRP6 following myocardial infarction. eBioMedicine 74, 103745 (2021).
Hoeflich, K. P. et al. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406, 86–90 (2000).
Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 7, 3 (2022).
Nusse, R. & Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Deng, N., Ye, Y., Wang, W. & Li, L. Dishevelled interacts with p65 and acts as a repressor of NF-κB-mediated transcription. Cell Res. 20, 1117–1127 (2010).
Jang, J. et al. LGK974 suppresses lipopolysaccharide-induced endotoxemia in mice by modulating the crosstalk between the Wnt/β-catenin and NF-κB pathways. Exp. Mol. Med. 53, 407–421 (2021).
Conlon, T. M. et al. Inhibition of LTβR signalling activates WNT-induced regeneration in lung. Nature 588, 151–156 (2020).
Majumder, S. et al. Targeting Notch in oncology: the path forward. Nat. Rev. Drug Discov. 20, 125–144 (2021).
Zhou, B. et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct. Target Ther. 7, 95 (2022).
Screpanti, I., Bellavia, D., Campese, A. F., Frati, L. & Gulino, A. Notch, a unifying target in T-cell acute lymphoblastic leukemia? Trends Mol. Med. 9, 30–35 (2003).
Vilimas, T. et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat. Med. 13, 70–77 (2007).
Shin, H. M. et al. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 25, 129–138 (2006).
Espinosa, L. et al. The Notch/Hes1 pathway sustains NF-κB activation through CYLD repression in T cell leukemia. Cancer Cell 18, 268–281 (2010).
Kunze, B. et al. Notch signaling mediates differentiation in Barrett’s esophagus and promotes progression to adenocarcinoma. Gastroenterology 159, 575–590 (2020).
Shah, F. et al. Exploiting the Ref-1-APE1 node in cancer signaling and other diseases: from bench to clinic. npj Precis. Oncol. 1, 1–19 (2017).
Chen, L. et al. Activation of NOTCH signaling via DLL1 is mediated by APE1-redox-dependent NF-κB activation in oesophageal adenocarcinoma. Gut 72, 421–432 (2023).
Wang, L. et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-κB-dependent manner. Cell Stem Cell 15, 51–65 (2014).
Zhang, P., Yang, Y., Nolo, R., Zweidler-McKay, P. A. & Hughes, D. P. M. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene 29, 2916–2926 (2010).
Spitschak, A., Meier, C., Kowtharapu, B., Engelmann, D. & Pützer, B. M. MiR-182 promotes cancer invasion by linking RET oncogene activated NF-κB to loss of the HES1/Notch1 regulatory circuit. Mol. Cancer 16, 24 (2017).
Zhang, H. et al. NOTCH inhibits osteoblast formation in inflammatory arthritis via noncanonical NF-κB. J. Clin. Invest. 124, 3200–3214 (2014).
Schwarzer, R., Dörken, B. & Jundt, F. Notch is an essential upstream regulator of NF-κB and is relevant for survival of Hodgkin and Reed–Sternberg cells. Leukemia 26, 806–813 (2012).
Jing, J. et al. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target Ther. 8, 315 (2023).
Zhang, Y. & Beachy, P. A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 24, 668–687 (2023).
Bushdid, P. B. et al. Inhibition of NF-κB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature 392, 615–618 (1998).
Hyun, J. et al. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat. Commun. 7, 1–16 (2016).
Vecchiotti, D. et al. Elevated NF-κB/SHh/GLI1 signature denotes a worse prognosis and represent a novel potential therapeutic target in advanced prostate cancer. Cells 11, 2118 (2022).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001).
Barton, G. M. & Medzhitov, R. Toll-like receptor signaling pathways. Science 300, 1524–1525 (2003).
Lv, S. et al. A negative feedback loop of ICER and NF-κB regulates TLR signaling in innate immune responses. Cell Death Differ. 24, 492–499 (2017).
Ananieva, O. et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 9, 1028–1036 (2008).
Parry, G. C. & Mackman, N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J. Immunol. 159, 5450–5456 (1997).
Molina, C. A., Foulkes, N. S., Lalli, E. & Sassone-Corsi, P. Inducibility and negative autoregulation of CREM: An alternative promoter directs the expression of ICER, an early response repressor. Cell 75, 875–886 (1993).
Voss, O. H. et al. Lipopolysaccharide-induced CD300b receptor binding to Toll-like receptor 4 alters signaling to drive cytokine responses that enhance septic shock. Immunity 44, 1365–1378 (2016).
Kracht, M., Müller-Ladner, U. & Schmitz, M. L. Mutual regulation of metabolic processes and proinflammatory NF-κB signaling. J. Allergy Clin. Immunol. 146, 694–705 (2020).
Baker, R. G., Hayden, M. S. & Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 13, 11–22 (2011).
Molaei, M., Vandehoef, C. & Karpac, J. NF-κB shapes metabolic adaptation by attenuating Foxo-mediated lipolysis in Drosophila. Dev. Cell 49, 802–810.e6 (2019).
Hughes, K., Antonsson, Å. & Grundstrøm, T. Calmodulin dependence of NFκB activation. FEBS Lett. 441, 132–136 (1998).
Fan, C., Li, Q., Ross, D. & Engelhardt, J. F. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J. Biol. Chem. 278, 2072–2080 (2003).
Talbott, S. J. et al. S-nitrosylation of FLICE inhibitory protein determines its interaction with RIP1 and activation of NF-κB. Cell Cycle 13, 1948–1957 (2014).
Gallego-Selles, A. et al. Fast regulation of the NF-κB signalling pathway in human skeletal muscle revealed by high-intensity exercise and ischaemia at exhaustion: Role of oxygenation and metabolite accumulation. Redox Biol. 55, 102398 (2022).
Capece, D. et al. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 43, 757–775 (2022).
Cui, Y. et al. A Stk4-Foxp3-NF-κB p65 transcriptional complex promotes Treg cell activation and homeostasis. Sci. Immunol. 7, eabl8357 (2022).
Xia, Y., Shen, S. & Verma, I. M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2, 823–830 (2014).
Zhang, T. et al. m6A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB-mediated antiapoptotic pathway. Sci. Adv. 8, eabl5723 (2022).
Li, D. et al. Targeting NF-κB pathway by dietary lignans in inflammation: expanding roles of gut microbiota and metabolites. Crit. Rev. Food Sci. Nutr. 63, 5967–5983 (2023).
Nabel, G. & Baltimore, D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326, 711–713 (1987).
Sha, W. C., Liou, H. C., Tuomanen, E. I. & Baltimore, D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80, 321–330 (1995).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013).
Reid, M. A. et al. IKKβ promotes metabolic adaptation to glutamine deprivation via phosphorylation and inhibition of PFKFB3. Genes Dev. 30, 1837–1851 (2016).
Wang, X. et al. α-Ketoglutarate-activated NF-κB signaling promotes compensatory glucose uptake and brain tumor development. Mol. Cell 76, 148–162.e7 (2019).
Capece, D. et al. Enhanced triacylglycerol catabolism by carboxylesterase 1 promotes aggressive colorectal carcinoma. J. Clin. Invest. 131, e137845 (2021). 137845.
Li, T. et al. c-Rel is a myeloid checkpoint for cancer immunotherapy. Nat. Cancer 1, 507–517 (2020).
Gu, M. et al. NF-κB-inducing kinase maintains T cell metabolic fitness in antitumor immunity. Nat. Immunol. 22, 193–204 (2021).
Döffinger, R. et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat. Genet. 27, 277–285 (2001).
Paciolla, M. et al. Rare mendelian primary immunodeficiency diseases associated with impaired NF-κB signaling. Genes Immun. 16, 239–246 (2015).
Chen, K. et al. Germline mutations in NFKB2 implicate the noncanonical NF-κB pathway in the pathogenesis of common variable immunodeficiency. Am. J. Hum. Genet. 93, 812–824 (2013).
Dejardin, E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharm. 72, 1161–1179 (2006).
Keats, J. J. et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12, 131–144 (2007).
Annunziata, C. M. et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12, 115–130 (2007).
Gardam, S., Sierro, F., Basten, A., Mackay, F. & Brink, R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity 28, 391–401 (2008).
Burkly, L. et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature 373, 531–536 (1995).
Yao, Z., Xing, L. & Boyce, B. F. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J. Clin. Invest. 119, 3024–3034 (2009).
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Yamashita, T. et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 282, 18245–18253 (2007).
Xu, J. et al. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 20, 7–17 (2009).
Sehnert, B. et al. NF-κB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-κB in immune-mediated diseases. Proc. Natl Acad. Sci. USA 110, 16556–16561 (2013).
Newton, K. & Dixit, V. M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4, a006049 (2012).
Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Liu, T., Zhang, L., Joo, D. & Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2, 1–9 (2017).
Kaisho, T. & Tanaka, T. Turning NF-kappaB and IRFs on and off in DC. Trends Immunol. 29, 329–336 (2008).
Wullaert, A., Bonnet, M. C. & Pasparakis, M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 21, 146–158 (2011).
Xu, C. et al. Alpha-kinase 1 (ALPK1) agonist DF-006 demonstrates potent efficacy in mouse and primary human hepatocyte (PHH) models of hepatitis B. Hepatology 77, 275–289 (2023).
Afonina, I. S., Zhong, Z., Karin, M. & Beyaert, R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 18, 861–869 (2017).
Hwang, J.-R., Byeon, Y., Kim, D. & Park, S.-G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 52, 750–761 (2020).
Mueller, K. et al. Octamer-dependent transcription in T cells is mediated by NFAT and NF-κB. Nucleic Acids Res. 41, 2138–2154 (2013).
He, Y. et al. Combination of enzastaurin and ibrutinib synergistically induces anti-tumor effects in diffuse large B cell lymphoma. J. Exp. Clin. Cancer Res. 38, 86 (2019).
Pone, E. J. et al. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway. Nat. Commun. 3, 767 (2012).
Verhelst, K., Carpentier, I. & Beyaert, R. Regulation of TNF-induced NF-κB activation by different cytoplasmic ubiquitination events. Cytokine Growth Factor Rev. 22, 277–286 (2011).
Mantsounga, C. S. et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 38, 110309 (2022).
He, G. & Karin, M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 21, 159–168 (2011).
Grinberg-Bleyer, Y. et al. NF-κB c-Rel is crucial for the regulatory T cell immune checkpoint in cancer. Cell 170, 1096–1108.e13 (2017).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Kloosterman, D. J. & Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 186, 1627–1651 (2023).
Pittet, M. J., Michielin, O. & Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 19, 402–421 (2022).
Wang, J.-C. et al. PIM2 expression induced by proinflammatory macrophages suppresses immunotherapy efficacy in hepatocellular carcinoma. Cancer Res. 82, 3307–3320 (2022).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011).
Chen, J. et al. Glioblastoma stem cell-specific histamine secretion drives pro-angiogenic tumor microenvironment remodeling. Cell Stem Cell 29, 1531–1546.e7 (2022).
Peng, Z.-P. et al. Glycolytic activation of monocytes regulates the accumulation and function of neutrophils in human hepatocellular carcinoma. J. Hepatol. 73, 906–917 (2020).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Polyak, K., Haviv, I. & Campbell, I. G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 25, 30–38 (2009).
Takenaka, M. C. et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729–740 (2019).
Ganguly, D. et al. Pleiotrophin drives a prometastatic immune niche in breast cancer. J. Exp. Med. 220, e20220610 (2023).
Hegde, S., Leader, A. M. & Merad, M. MDSC: markers, development, states, and unaddressed complexity. Immunity 54, 875–884 (2021).
Liu, X. et al. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut. 72, 2329–2343 (2023).
Zeng, X. et al. Cell cycle-related kinase reprograms the liver immune microenvironment to promote cancer metastasis. Cell Mol. Immunol. 18, 1005–1015 (2021).
Pascual, M. et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood 133, 2401–2412 (2019).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Su, S. CD10+ GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16 (2018).
Sutherland, T. E., Dyer, D. P. & Allen, J. E. The extracellular matrix and the immune system: a mutually dependent relationship. Science 379, eabp8964 (2023).
Drain, A. P. et al. Matrix compliance permits NF-κB activation to drive therapy resistance in breast cancer. J. Exp. Med. 218, e20191360 (2021).
Perkins, N. D. The diverse and complex roles of NF-κB subunits in cancer. Nat. Rev. Cancer 12, 121–132 (2012).
Guttridge, D. C., Albanese, C., Reuther, J. Y., Pestell, R. G. & Baldwin, A. S. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell Biol. 19, 5785–5799 (1999).
Zhang, Y.-C. et al. PAK5-mediated phosphorylation and nuclear translocation of NF-κB-p65 promotes breast cancer cell proliferation in vitro and in vivo. J. Exp. Clin. Cancer Res. 36, 146 (2017).
Vreka, M. et al. IκB kinase α is required for development and progression of KRAS-mutant lung adenocarcinoma. Cancer Res. 78, 2939–2951 (2018).
Zhou, W. et al. Galectin-3 activates TLR4/NF-κB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA-NEAT1 expression. BMC Cancer 18, 580 (2018).
Tang, H., Lv, W., Sun, W., Bi, Q. & Hao, Y. miR‑505 inhibits cell growth and EMT by targeting MAP3K3 through the AKT‑NFκB pathway in NSCLC cells. Int. J. Mol. Med. 43, 1203–1216 (2019).
Carrà, G., Avalle, L., Seclì, L., Brancaccio, M. & Morotti, A. Shedding light on NF-κB functions in cellular organelles. Front. Cell Dev. Biol. 10, 841646 (2022).
Escrivà, M. et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol. Cell Biol. 28, 1528–1540 (2008).
Man, X. et al. USP13 functions as a tumor suppressor by blocking the NF-kB-mediated PTEN downregulation in human bladder cancer. J. Exp. Clin. Cancer Res. 38, 259 (2019).
Lee, M. W. et al. Dual role of ERK2/NF-κB signaling in TRAIL sensitivity. Am. J. Cancer Res. 12, 3373–3389 (2022).
Pavitra, E. et al. The role of NF-κB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed. Pharmacother. 163, 114822 (2023).
Taniguchi, K. & Karin, M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Meteoglu, I., Erdogdu, I. H., Meydan, N., Erkus, M. & Barutca, S. NF-KappaB expression correlates with apoptosis and angiogenesis in clear cell renal cell carcinoma tissues. J. Exp. Clin. Cancer Res. 27, 53 (2008).
Liang, S. et al. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-κB/IL-6 signals. Cancer Lett. 386, 12–23 (2017).
Wang, R. et al. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 11, 55 (2020).
Huang, S., Robinson, J. B., Deguzman, A., Bucana, C. D. & Fidler, I. J. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 60, 5334–5339 (2000).
Jobin, C., Hellerbrand, C., Licato, L. L., Brenner, D. A. & Sartor, R. B. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 42, 779–787 (1998).
Zhu, Y. et al. Astragalus polysaccharides suppress ICAM-1 and VCAM-1 expression in TNF-α-treated human vascular endothelial cells by blocking NF-κB activation. Acta Pharm. Sin. 34, 1036–1042 (2013).
Volanti, C. et al. Downregulation of ICAM-1 and VCAM-1 expression in endothelial cells treated by photodynamic therapy. Oncogene 23, 8649–8658 (2004).
Kim, H. J., Hawke, N. & Baldwin, A. S. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 13, 738–747 (2006).
Walmsley, S. R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 201, 105–115 (2005).
Herkenne, S. et al. Developmental and tumor angiogenesis requires the mitochondria-shaping protein Opa1. Cell Metab. 31, 987–1003.e8 (2020).
John, A. & Tuszynski, G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 7, 14–23 (2001).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Dillekås, H., Rogers, M. S. & Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 8, 5574–5576 (2019).
Li, C.-W. et al. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 72, 1290–1300 (2012).
Huber, M. A. et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Nomura, A. et al. Inhibition of NF-kappa B pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab Invest. 96, 1268–1278 (2016).
Rodrigues, P. et al. NF-κB-dependent lymphoid enhancer co-option promotes renal carcinoma metastasis. Cancer Discov. 8, 850–865 (2018).
El-Nikhely, N. et al. Metastasis-associated protein 2 represses NF-κB to reduce lung tumor growth and inflammation. Cancer Res. 80, 4199–4211 (2020).
Collins, T. et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 9, 899–909 (1995).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Du, F. et al. MRTF-A-NF-κB/p65 axis-mediated PDL1 transcription and expression contributes to immune evasion of non-small-cell lung cancer via TGF-β. Exp. Mol. Med. 53, 1366–1378 (2021).
Li, C. et al. The Mincle/Syk/NF-κB signaling circuit is essential for maintaining the protumoral activities of tumor-associated macrophages. Cancer Immunol. Res. 8, 1004–1017 (2020).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Mauro, C. et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 13, 1272–1279 (2011).
Johnson, R. F., Witzel, I.-I. & Perkins, N. D. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-κB. Cancer Res. 71, 5588–5597 (2011).
Szymura, S. J. et al. NF-κB upregulates glutamine-fructose-6-phosphate transaminase 2 to promote migration in non-small cell lung cancer. Cell Commun. Signal 17, 24 (2019).
Heise, N. et al. Germinal center B cell maintenance and differentiation are controlled by distinct NF-κB transcription factor subunits. J. Exp. Med. 211, 2103–2118 (2014).
Seton-Rogers, S. Switching off MDSCs by targeting REL. Nat. Rev. Drug Discov. 19, 445 (2020).
Morrissey, S. M. et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 33, 2040–2058.e10 (2021).
Li, F. & Sethi, G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta. 1805, 167–180 (2010).
Dimitrakopoulos, F.-I. D., Kottorou, A. E., Kalofonou, M. & Kalofonos, H. P. The fire within: NF-κB involvement in non-small cell lung cancer. Cancer Res. 80, 4025–4036 (2020).
Lim, S. K. et al. Sustained activation of non-canonical NF-κB signalling drives glycolytic reprogramming in doxorubicin-resistant DLBCL. Leukemia 37, 441–452 (2023).
Zhong, W. et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome 10, 94 (2022).
Azuma, K. et al. TRIM47 activates NF-κB signaling via PKC-ε/PKD3 stabilization and contributes to endocrine therapy resistance in breast cancer. Proc. Natl Acad. Sci. USA 118, e2100784118 (2021).
Thomas-Jardin, S. E., Dahl, H., Nawas, A. F., Bautista, M. & Delk, N. A. NF-κB signaling promotes castration-resistant prostate cancer initiation and progression. Pharm. Ther. 211, 107538 (2020).
Huang, K. et al. Genome-wide CRISPR-Cas9 screening identifies NF-κB/E2F6 responsible for EGFRvIII-associated temozolomide resistance in glioblastoma. Adv. Sci. 6, 1900782 (2019).
Kong, W. et al. Hesperetin reverses P‑glycoprotein‑mediated cisplatin resistance in DDP‑resistant human lung cancer cells via modulation of the nuclear factor‑κB signaling pathway. Int. J. Mol. Med. 45, 1213–1224 (2020).
Denlinger, C. E., Rundall, B. K., Keller, M. D. & Jones, D. R. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann. Thorac. Surg. 78, 1207–1214 (2004).
Jiang, N. et al. Triptolide reverses the Taxol resistance of lung adenocarcinoma by inhibiting the NF-κB signaling pathway and the expression of NF-κB-regulated drug-resistant genes. Mol. Med. Rep. 13, 153–159 (2016).
Wang, N., Liang, H. & Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 5, 614 (2014).
Ward, C. et al. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 274, 4309–4318 (1999).
Wu, M. et al. Gene expression profiles identify both MyD88-independent and MyD88-dependent pathways involved in the maturation of dendritic cells mediated by heparan sulfate: a novel adjuvant. Hum. Vaccin. Immunother. 10, 3711–3721 (2014).
Gerondakis, S., Fulford, T. S., Messina, N. L. & Grumont, R. J. NF-κB control of T cell development. Nat. Immunol. 15, 15–25 (2014).
Hayden, M. S. & Ghosh, S. NF-κB in immunobiology. Cell Res. 21, 223–244 (2011).
Lin, L., Hron, J. D. & Peng, S. L. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21, 203–213 (2004).
Das, J. et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2, 45–50 (2001).
Molinero, L. L., Cubre, A., Mora-Solano, C., Wang, Y. & Alegre, M.-L. T cell receptor/CARMA1/NF-κB signaling controls T-helper (Th) 17 differentiation. Proc. Natl Acad. Sci. USA 109, 18529–18534 (2012).
Almaden, J. V. et al. B-cell survival and development controlled by the coordination of NF-κB family members RelB and cRel. Blood 127, 1276–1286 (2016).
Luo, W., Weisel, F. & Shlomchik, M. J. B cell receptor and CD40 signaling are rewired for synergistic induction of the c-Myc transcription factor in germinal center B cells. Immunity 48, 313–326.e5 (2018).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Ilchovska, D. D. & Barrow, D. M. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun. Rev. 20, 102741 (2021).
Tian, R. et al. 1,25(OH)2D3 promotes chondrocyte apoptosis and restores physical function in rheumatoid arthritis through the NF-κB signal pathway. Biomed. Pharmacother. 106, 149–155 (2018).
Zhang, L.-L. et al. BAFF, involved in B cell activation through the NF-κB pathway, is related to disease activity and bone destruction in rheumatoid arthritis. Acta Pharm. Sin. 42, 1665–1675 (2021).
Noort, A. R. et al. Tertiary lymphoid structures in rheumatoid arthritis: NF-κB-inducing kinase-positive endothelial cells as central players. Am. J. Pathol. 185, 1935–1943 (2015).
Raeber, M. E., Zurbuchen, Y., Impellizzieri, D. & Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 283, 176–193 (2018).
Makarov, S. S. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 3, 200–206 (2001).
Romashkova, J. A. & Makarov, S. S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401, 86–90 (1999).
Aggarwal, B. B. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann. Rheum. Dis. 59, i6–i16 (2000).
Gregersen, P. K. et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat. Genet. 41, 820–823 (2009).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Yao, Q. et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target Ther. 8, 56 (2023).
Noort, A. R., Tak, P. P. & Tas, S. W. Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res. Ther. 17, 15 (2015).
Molloy, E. S. et al. Mechanism of basic calcium phosphate crystal-stimulated matrix metalloproteinase-13 expression by osteoarthritic synovial fibroblasts: inhibition by prostaglandin E2. Ann. Rheum. Dis. 67, 1773–1779 (2008).
Shakibaei, M., John, T., Schulze-Tanzil, G., Lehmann, I. & Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharm. 73, 1434–1445 (2007).
Rius, J. et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811 (2008).
Yoon, D. S. et al. TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis. Cell Death Differ. 29, 1364–1378 (2022).
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B. & Ciccarelli, O. Multiple sclerosis. Lancet 391, 1622–1636 (2018).
Ruck, T. et al. K2P18.1 translates T cell receptor signals into thymic regulatory T cell development. Cell Res. 32, 72–88 (2022).
Danikowski, K. M., Jayaraman, S. & Prabhakar, B. S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation 14, 117 (2017).
Yang, J., Sundrud, M. S., Skepner, J. & Yamagata, T. Targeting Th17 cells in autoimmune diseases. Trends Pharm. Sci. 35, 493–500 (2014).
Mc Guire, C., Prinz, M., Beyaert, R. & van Loo, G. Nuclear factor kappa B (NF-κB) in multiple sclerosis pathology. Trends Mol. Med. 19, 604–613 (2013).
Charabati, M., Wheeler, M. A., Weiner, H. L. & Quintana, F. J. Multiple sclerosis: neuroimmune crosstalk and therapeutic targeting. Cell 186, 1309–1327 (2023).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020).
Nobili, P. et al. Therapeutic potential of astrocyte purinergic signalling in epilepsy and multiple sclerosis. Front. Pharm. 13, 900337 (2022).
Balzano, T. et al. Sustained hyperammonemia induces TNF-a IN Purkinje neurons by activating the TNFR1-NF-κB pathway. J. Neuroinflammation 17, 70 (2020).
Ordás, I., Eckmann, L., Talamini, M., Baumgart, D. C. & Sandborn, W. J. Ulcerative colitis. Lancet 380, 1606–1619 (2012).
Pang, W. et al. Serological biomarker-based machine learning models for predicting the relapse of ulcerative colitis. J. Inflamm. Res. 16, 3531–3545 (2023).
Atreya, I., Atreya, R. & Neurath, M. F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 263, 591–596 (2008).
Yan, Y.-X. et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharm. Sin. 39, 1633–1644 (2018).
Chen, X. et al. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 10, 906 (2019).
Vavricka, S. R. et al. Expression patterns of TNFα, MAdCAM1, and STAT3 in intestinal and skin manifestations of inflammatory bowel disease. J. Crohns Colitis 12, 347–354 (2018).
Xu, Q. et al. MAST3 modulates the inflammatory response and proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Int. Immunopharmacol. 77, 105900 (2019).
Kiriakidou, M. & Ching, C. L. Systemic lupus erythematosus. Ann. Intern. Med. 172, ITC81–ITC96 (2020).
Pacheco, G. V. et al. Expression of TLR-7, MyD88, NF-kB, and INF-α in B lymphocytes of Mayan women with systemic lupus erythematosus in Mexico. Front. Immunol. 7, 22 (2016).
Brightbill, H. D. et al. NF-κB inducing kinase is a therapeutic target for systemic lupus erythematosus. Nat. Commun. 9, 179 (2018).
Hung, K.-H. et al. The KDM4A/KDM4C/NF-κB and WDR5 epigenetic cascade regulates the activation of B cells. Nucleic Acids Res. 46, 5547–5560 (2018).
Huang, A.-F. & Xu, W.-D. NF-κB kinase subunit ɛ in neuropsychiatric systemic lupus erythematosus: comment on the article by Karino et al. Arthritis Rheumatol. 75, 1294 (2023).
Xie, M. et al. NF-κB-driven miR-34a impairs Treg/Th17 balance via targeting Foxp3. J. Autoimmun. 102, 96–113 (2019).
Zhang, W. et al. Aberrant CD40-induced NF-κB activation in human lupus B lymphocytes. PLoS ONE 7, e41644 (2012).
Manou-Stathopoulou, S. & Lewis, M. J. Diversity of NF-κB signalling and inflammatory heterogeneity in rheumatic autoimmune disease. Semin. Immunol. 58, 101649 (2021).
Liu, M. et al. Type I interferons promote the survival and proinflammatory properties of transitional B cells in systemic lupus erythematosus patients. Cell Mol. Immunol. 16, 367–379 (2019).
Leonard, M. M., Sapone, A., Catassi, C. & Fasano, A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA 318, 647–656 (2017).
Wang, N. et al. Effect and mechanism of peanut skin proanthocyanidins on gliadin-induced Caco-2 celiac disease model cells. Clin. Immunol. 245, 109100 (2022).
Jelínková, L., Tucková, L., Cinová, J., Flegelová, Z. & Tlaskalová-Hogenová, H. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 571, 81–85 (2004).
Capozzi, A. et al. Modulatory effect of gliadin peptide 10-mer on epithelial intestinal CACO-2 cell inflammatory response. PLoS ONE 8, e66561 (2013).
Olazagoitia-Garmendia, A. et al. Gluten-induced RNA methylation changes regulate intestinal inflammation via allele-specific XPO1 translation in epithelial cells. Gut. 71, 68–76 (2022).
Dalbeth, N., Gosling, A. L., Gaffo, A. & Abhishek, A. Gout. Lancet 397, 1843–1855 (2021).
Lu, W. et al. Uric acid produces an inflammatory response through activation of NF-κB in the hypothalamus: implications for the pathogenesis of metabolic disorders. Sci. Rep. 5, 12144 (2015).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Zhou, Y. et al. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS ONE 7, e39738 (2012).
Purkayastha, S., Zhang, G. & Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 (2011).
Zhang, X. et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Posada-López, A., Botero, J. E., Pineda-Tamayo, R. A. & Agudelo-Suárez, A. A. The effect of periodontal treatment on clinical and biological indicators, quality of life, and oral health in rheumatoid arthritis patients: a quasi-experimental study. Int. J. Environ. Res. Public Health 19, 1789 (2022).
Slots, J. Periodontitis: facts, fallacies and the future. Periodontol 2000 75, 7–23 (2017).
Gugliandolo, E. et al. Anti-Inflammatory effect of ATB-352, a H2S -releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharmacol. Res. 132, 220–231 (2018).
Venugopal, P. et al. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell Physiol. 233, 5877–5884 (2018).
Lisboa, R. A., Andrade, M. V. & Cunha-Melo, J. R. Toll-like receptor activation and mechanical force stimulation promote the secretion of matrix metalloproteinases 1, 3 and 10 of human periodontal fibroblasts via p38, JNK and NF-kB. Arch. Oral. Biol. 58, 731–739 (2013).
Fang, C. & Ma, Y. Peripheral blood genes crosstalk between COVID-19 and sepsis. Int. J. Mol. Sci. 24, 2591 (2023).
Huang, S. et al. Tim-3 regulates sepsis-induced immunosuppression by inhibiting the NF-κB signaling pathway in CD4 T cells. Mol. Ther. 30, 1227–1238 (2022).
Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet 392, 75–87 (2018).
Gotts, J. E. & Matthay, M. A. Sepsis: pathophysiology and clinical management. BMJ 353, i1585 (2016).
Abraham, E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J. Infect. Dis. 187, S364–S369 (2003).
Zhang, Y. et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-κB signaling and protects against endotoxin shock. Immunity 40, 501–514 (2014).
Sockrider, M. & Fussner, L. What is asthma? Am. J. Respir. Crit. Care Med. 202, P25–P26 (2020).
Tan, Y.-Y. et al. FGF2 is overexpressed in asthma and promotes airway inflammation through the FGFR/MAPK/NF-κB pathway in airway epithelial cells. Mil. Med. Res. 9, 7 (2022).
Adcock, I. M., Lane, S. J., Brown, C. R., Lee, T. H. & Barnes, P. J. Abnormal glucocorticoid receptor-activator protein 1 interaction in steroid-resistant asthma. J. Exp. Med. 182, 1951–1958 (1995).
Izuhara, K. et al. Periostin in inflammation and allergy. Cell Mol. Life Sci. 74, 4293–4303 (2017).
Zhang, Q. et al. Propofol inhibits NF-κB activation to ameliorate airway inflammation in ovalbumin (OVA)-induced allergic asthma mice. Int. Immunopharmacol. 51, 158–164 (2017).
Contoli, M. et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 70, 910–920 (2015).
Wu, Z., Mehrabi Nasab, E., Arora, P. & Athari, S. S. Study effect of probiotics and prebiotics on treatment of OVA-LPS-induced of allergic asthma inflammation and pneumonia by regulating the TLR4/NF-kB signaling pathway. J. Transl. Med. 20, 130 (2022).
Chadban, S. J. & Atkins, R. C. Glomerulonephritis. Lancet 365, 1797–1806 (2005).
Coppo, R. & Amore, A. Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int. 65, 1544–1547 (2004).
Suzuki, H. et al. Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int. 72, 319–327 (2007).
Chalmers, S. A., Garcia, S. J., Reynolds, J. A., Herlitz, L. & Putterman, C. NF-kB signaling in myeloid cells mediates the pathogenesis of immune-mediated nephritis. J. Autoimmun. 98, 33–43 (2019).
Ruan, Q. et al. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J. Exp. Med. 208, 2321–2333 (2011).
Zhang, H. & Sun, S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 5, 63 (2015).
Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380, 756–766 (2012).
Fujihara, C. K. et al. Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am. J. Physiol. Ren. Physiol. 292, F92–F99 (2007).
Trigueros-Motos, L. et al. Embryological-origin-dependent differences in homeobox expression in adult aorta: role in regional phenotypic variability and regulation of NF-κB activity. Arterioscler. Thromb. Vasc. Biol. 33, 1248–1256 (2013).
Saito, T. et al. Importance of endothelial NF-κB signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc. Res. 97, 106–114 (2013).
Sivandzade, F., Prasad, S., Bhalerao, A. & Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 21, 101059 (2019).
Fang, C. et al. Shengyu decoction treating vascular cognitive impairment by promoting AKT/HIF-1α/VEGF related cerebrovascular generation and ameliorating MAPK/NF-κB mediated neuroinflammation. J. Ethnopharmacol. 296, 115441 (2022).
Bai, R. et al. The role of NLRP3 inflammasome in cerebrovascular diseases pathology and possible therapeutic targets. ASN Neuro 13, 17590914211018100 (2021).
Alfaddagh, A. et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am. J. Prev. Cardiol. 4, 100130 (2020).
Tamargo, I. A., Baek, K. I., Kim, Y., Park, C. & Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 20, 738–753 (2023).
Basatemur, G. L., Jørgensen, H. F., Clarke, M. C. H., Bennett, M. R. & Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 16, 727–744 (2019).
Ross, R. & Glomset, J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 180, 1332–1339 (1973).
Francis, G. A. The greatly under-represented role of smooth muscle cells in atherosclerosis. Curr. Atheroscler. Rep. 25, 741–749 (2023).
Tang, R.-H. et al. Myocardin inhibits cellular proliferation by inhibiting NF-kappaB(p65)-dependent cell cycle progression. Proc. Natl Acad. Sci. USA 105, 3362–3367 (2008).
Lu, Q.-B. et al. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 14, 656–668 (2018).
McLaren, J. E., Michael, D. R., Ashlin, T. G. & Ramji, D. P. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog. Lipid Res. 50, 331–347 (2011).
Sukhorukov, V. N. et al. Lipid metabolism in macrophages: focus on atherosclerosis. Biomedicines 8, 262 (2020).
Yang, S. et al. MicroRNA-216a promotes M1 macrophages polarization and atherosclerosis progression by activating telomerase via the Smad3/NF-κB pathway. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1772–1781 (2019).
Gough, P. J., Gomez, I. G., Wille, P. T. & Raines, E. W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J. Clin. Invest. 116, 59–69 (2006).
Wang, N. et al. Combination of tanshinone IIA and astragaloside IV attenuate atherosclerotic plaque vulnerability in ApoE(-/-) mice by activating PI3K/AKT signaling and suppressing TRL4/NF-κB signaling. Biomed. Pharmacother. 123, 109729 (2020).
Ottonello, L., Bertolotto, M., Montecucco, F., Bianchi, G. & Dallegri, F. Delayed apoptosis of human monocytes exposed to immune complexes is reversed by oxaprozin: role of the Akt/IkappaB kinase/nuclear factor kappaB pathway. Br. J. Pharm. 157, 294–306 (2009).
Martín-Ventura, J. L. et al. NF-kappaB activation and Fas ligand overexpression in blood and plaques of patients with carotid atherosclerosis: potential implication in plaque instability. Stroke 35, 458–463 (2004).
Frangogiannis, N. G. Pathophysiology of myocardial infarction. Compr. Physiol. 5, 1841–1875 (2015).
Prabhu, S. D. & Frangogiannis, N. G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 (2016).
Tranter, M. et al. In vivo delivery of nucleic acids via glycopolymer vehicles affords therapeutic infarct size reduction in vivo. Mol. Ther. 20, 601–608 (2012).
Quan, W. et al. Cardioprotective effect of rosmarinic acid against myocardial ischaemia/reperfusion injury via suppression of the NF-κB inflammatory signalling pathway and ROS production in mice. Pharm. Biol. 59, 222–231 (2021).
Yu, P. et al. Panax quinquefolius L. saponins protect myocardial ischemia reperfusion no-reflow through inhibiting the activation of NLRP3 inflammasome via TLR4/MyD88/NF-κB signaling pathway. Front. Pharm. 11, 607813 (2020).
Hotamisligil, G. S. & Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 8, 923–934 (2008).
Huh, J. Y. & Saltiel, A. R. Roles of IκB kinases and TANK-binding kinase 1 in hepatic lipid metabolism and nonalcoholic fatty liver disease. Exp. Mol. Med. 53, 1697–1705 (2021).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Chiang, S.-H. et al. The protein kinase IKKɛ regulates energy balance in obese mice. Cell 138, 961–975 (2009).
Pilli, M. et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37, 223–234 (2012).
Yu, J. et al. Regulation of T-cell activation and migration by the kinase TBK1 during neuroinflammation. Nat. Commun. 6, 6074 (2015).
Hasan, M. et al. Chronic innate immune activation of TBK1 suppresses mTORC1 activity and dysregulates cellular metabolism. Proc. Natl Acad. Sci. USA 114, 746–751 (2017).
Runde, A. P., Mack, R., S J, P. B. & Zhang, J. The role of TBK1 in cancer pathogenesis and anticancer immunity. J. Exp. Clin. Cancer Res. 41, 135 (2022).
Zhao, P. et al. TBK1 at the crossroads of inflammation and energy homeostasis in adipose tissue. Cell 172, 731–743.e12 (2018).
Reilly, S. M. et al. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat. Med. 19, 313–321 (2013).
Oral, E. A. et al. Inhibition of IKKɛ and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab. 26, 157–170.e7 (2017).
Cruz, V. H., Arner, E. N., Wynne, K. W., Scherer, P. E. & Brekken, R. A. Loss of Tbk1 kinase activity protects mice from diet-induced metabolic dysfunction. Mol. Metab. 16, 139–149 (2018).
Gao, T. et al. Myeloid cell TBK1 restricts inflammatory responses. Proc. Natl Acad. Sci. USA 119, e2107742119 (2022).
Jin, Q. et al. Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Front. Immunol. 14, 1185317 (2023).
Haberzettl, P., O’Toole, T. E., Bhatnagar, A. & Conklin, D. J. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ. Health Perspect. 124, 1830–1839 (2016).
Yin, Q. et al. Spatiotemporal variations of vascular endothelial growth factor in the brain of diabetic cognitive impairment. Pharm. Res. 163, 105234 (2021).
Ke, G. et al. Receptor activator of NF-κB mediates podocyte injury in diabetic nephropathy. Kidney Int. 100, 377–390 (2021).
Wang, X. et al. Odd-numbered agaro-oligosaccharides alleviate type 2 diabetes mellitus and related colonic microbiota dysbiosis in mice. Carbohydr. Polym. 240, 116261 (2020).
Jha, J. C. et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1237–1254 (2014).
Niewczas, M. A. et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 25, 805–813 (2019).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e5 (2019).
Zheng, J. et al. Diabetes activates periodontal ligament fibroblasts via NF-κB in vivo. J. Dent. Res. 97, 580–588 (2018).
Tian, P. et al. Dual stimulus responsive borosilicate glass (BSG) scaffolds promote diabetic alveolar bone defectsrepair by modulating macrophage phenotype. Bioact. Mater. 26, 231–248 (2023).
Frati, G. et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc. Res. 113, 378–388 (2017).
Hussain, S. et al. Hyperglycemia induces myocardial dysfunction via epigenetic regulation of JunD. Circ. Res. 127, 1261–1273 (2020).
Zhao, M.-X. et al. Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 8, e2690 (2017).
Meyerovich, K. et al. The non-canonical NF-κB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia 59, 512–521 (2016).
Meyerovich, K., Ortis, F. & Cardozo, A. K. The non-canonical NF-κB pathway and its contribution to β-cell failure in diabetes. J. Mol. Endocrinol. 61, F1–F6 (2018).
Chooi, Y. C., Ding, C. & Magkos, F. The epidemiology of obesity. Metabolism 92, 6–10 (2019).
Catrysse, L. & van Loo, G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-κB. Trends Cell Biol. 27, 417–429 (2017).
Hammarstedt, A., Gogg, S., Hedjazifar, S., Nerstedt, A. & Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 98, 1911–1941 (2018).
Coats, B. R. et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 20, 3149–3161 (2017).
Zamarron, B. F. et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes 66, 392–406 (2017).
Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178, 686–698.e14 (2019).
Deczkowska, A., Weiner, A. & Amit, I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell 181, 1207–1217 (2020).
Shan, B. et al. Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat. Metab. 2, 1332–1349 (2020).
Morinaga, H. et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 595, 266–271 (2021).
Qureshi, R. et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab. 31, 1154–1172.e9 (2020).
Huh, J. Y. et al. TANK-binding kinase 1 regulates the localization of acyl-CoA synthetase ACSL1 to control hepatic fatty acid oxidation. Cell Metab. 32, 1012–1027.e7 (2020).
Truax, A. D. et al. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe 24, 364–378.e6 (2018).
Maury, E., Navez, B. & Brichard, S. M. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat. Commun. 12, 2388 (2021).
Surmeier, D. J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 285, 3657–3668 (2018).
Dolatshahi, M., Ranjbar Hameghavandi, M. H., Sabahi, M. & Rostamkhani, S. Nuclear factor-kappa B (NF-κB) in pathophysiology of Parkinson disease: diverse patterns and mechanisms contributing to neurodegeneration. Eur. J. Neurosci. https://doi.org/10.1111/ejn.15242 (2021).
Dutta, D. et al. Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo. Nat. Commun. 12, 5382 (2021).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Goes, A. T. R. et al. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 279, 111–120 (2018).
Hassanzadeh, K. & Rahimmi, A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: could targeting these pathways write a good ending? J. Cell Physiol. 234, 23–32 (2018).
Yao, L. et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 33, 8648–8665 (2019).
Gan, L., Li, Z., Lv, Q. & Huang, W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int. J. Pharm. 567, 118449 (2019).
Maas, A. I. R., Stocchetti, N. & Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 (2008).
O’Neill, L. A. & Kaltschmidt, C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20, 252–258 (1997).
Yuan, J., Zhang, J., Cao, J., Wang, G. & Bai, H. Geniposide alleviates traumatic brain injury in rats via anti-inflammatory effect and MAPK/NF-kB inhibition. Cell Mol. Neurobiol. 40, 511–520 (2020).
Du, S., Deng, Y., Yuan, H. & Sun, Y. Safflower yellow B protects brain against cerebral ischemia reperfusion injury through AMPK/NF-kB pathway. Evid. Based Complement Altern. Med. 2019, 7219740 (2019).
Ahuja, C. S. et al. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 3, 17018 (2017).
Brambilla, R. et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 202, 145–156 (2005).
Chen, S. et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J. Neuroinflammation 15, 150 (2018).
Liu, H. et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling. Theranostics 11, 4187–4206 (2021).
Hirano, T. & Murakami, M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity 52, 731–733 (2020).
Manik, M. & Singh, R. K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 94, 869–877 (2022).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020).
Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e9 (2020).
Zhou, Y. et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci. Rev. 7, 998–1002 (2020).
Carfì, A., Bernabei, R., Landi, F. & Gemelli Against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605 (2020).
Rashid, F. et al. Roles and functions of SARS-CoV-2 proteins in host immune evasion. Front. Immunol. 13, 940756 (2022).
Kopp, E. & Ghosh, S. Inhibition of NF-κB by sodium salicylate and aspirin. Science 265, 956–959 (1994).
Yin, M. J., Yamamoto, Y. & Gaynor, R. B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396, 77–80 (1998).
Liao, D. et al. Aspirin suppresses the growth and metastasis of osteosarcoma through the NF-κB pathway. Clin. Cancer Res. 21, 5349–5359 (2015).
Chattopadhyay, M. et al. Hydrogen sulfide-releasing aspirin suppresses NF-κB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem. Pharm. 83, 723–732 (2012).
Shao, J. et al. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene 19, 726–736 (2000).
Frantz, B. & O’Neill, E. A. The effect of sodium salicylate and aspirin on NF-kappa B. Science 270, 2017–2019 (1995).
Grilli, M., Pizzi, M., Memo, M. & Spano, P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science 274, 1383–1385 (1996).
Tran, P. O. T., Gleason, C. E. & Robertson, R. P. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes 51, 1772–1778 (2002).
Rae, C., Langa, S., Tucker, S. J. & MacEwan, D. J. Elevated NF-kappaB responses and FLIP levels in leukemic but not normal lymphocytes: reduction by salicylate allows TNF-induced apoptosis. Proc. Natl Acad. Sci. USA 104, 12790–12795 (2007).
Goldman, P. & Peppercorn, M. A. Drug therapy: sulfasalazine. N. Engl. J. Med. 293, 20–23 (1975).
Rashidian, A. et al. Atorvastatin attenuates TNBS-induced rat colitis: the involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology 24, 109–118 (2016).
Zhang, W. et al. Sulfasalazine induces autophagy inhibiting neointimal hyperplasia following carotid artery injuries in mice. Front. Bioeng. Biotechnol. 11, 1199785 (2023).
Su, J. et al. p62 participates in the inhibition of NF-κB signaling and apoptosis induced by sulfasalazine in human glioma U251 cells. Oncol. Rep. 34, 235–243 (2015).
Eyre, R. et al. Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat. Commun. 10, 5016 (2019).
Vandewalle, J., Luypaert, A., De Bosscher, K. & Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 29, 42–54 (2018).
Timmermans, S., Souffriau, J. & Libert, C. A general introduction to glucocorticoid biology. Front. Immunol. 10, 1545 (2019).
Zhang, X.-P. et al. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J. Gastroenterol. 13, 548–556 (2007).
Ge, Y. et al. The molecular mechanisms of the effect of dexamethasone and cyclosporin A on TLR4 /NF-κB signaling pathway activation in oral Lichen Planus. Gene 508, 157–164 (2012).
Shibata, Y. et al. Effects of linear polarized infrared light irradiation on the transcriptional regulation of IL-8 expression in IL-1beta-stimulated human rheumatoid synoviocytes involves phosphorylation of the NF-kappaB RelA subunit. J. Photochem. Photobio. B 94, 164–170 (2009).
Keifer, J. A., Guttridge, D. C., Ashburner, B. P. & Baldwin, A. S. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J. Biol. Chem. 276, 22382–22387 (2001).
Jin, S. H., Kim, T. I., Han, D. S., Shin, S. K. & Kim, W. H. Thalidomide suppresses the interleukin 1beta-induced NFkappaB signaling pathway in colon cancer cells. Ann. N. Y Acad. Sci. 973, 414–418 (2002).
Chen, M. et al. Thalidomide ameliorates rosacea-like skin inflammation and suppresses NF-κB activation in keratinocytes. Biomed. Pharmacother. 116, 109011 (2019).
Lin, Y.-C., Shun, C.-T., Wu, M.-S. & Chen, C.-C. A novel anticancer effect of thalidomide: inhibition of intercellular adhesion molecule-1-mediated cell invasion and metastasis through suppression of nuclear factor-kappaB. Clin. Cancer Res 12, 7165–7173 (2006).
Hansen, J. M. & Harris, C. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxid. Redox Signal 6, 1–14 (2004).
Jan, M., Sperling, A. S. & Ebert, B. L. Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide. Nat. Rev. Clin. Oncol. 18, 401–417 (2021).
Kotla, V. et al. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2, 36 (2009).
Breitkreutz, I. et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 22, 1925–1932 (2008).
Gribben, J. G., Fowler, N. & Morschhauser, F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J. Clin. Oncol. 33, 2803–2811 (2015).
Shah, U. A. & Mailankody, S. Emerging immunotherapies in multiple myeloma. BMJ 370, m3176 (2020).
Chanan-Khan, A. A. et al. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 3, e143 (2013).
Tsai, Y.-R. et al. Pomalidomide ameliorates H2O2-induced oxidative stress injury and cell death in rat primary cortical neuronal cultures by inducing anti-oxidative and anti-apoptosis effects. Int. J. Mol. Sci. 19, 3252 (2018).
Wang, J., Zheng, B., Yang, S., Zhou, D. & Wang, J. Olmesartan prevents oligomerized amyloid β (Aβ)-induced cellular senescence in neuronal cells. ACS Chem. Neurosci. 12, 1162–1169 (2021).
Marinescu, M. & Popa, C.-V. Pyridine compounds with antimicrobial and antiviral activities. Int. J. Mol. Sci. 23, 5659 (2022).
Ciarcia, R. et al. Imatinib treatment inhibit IL-6, IL-8, NF-KB and AP-1 production and modulate intracellular calcium in CML patients. J. Cell Physiol. 227, 2798–2803 (2012).
Zusso, M. et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflammation 16, 148 (2019).
Chelko, S. P. et al. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation 140, 1491–1505 (2019).
Hong, D. S. et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 18, 3396–3406 (2012).
Wang, Y.-J., Wang, J.-T., Fan, Q.-X. & Geng, J.-G. Andrographolide inhibits NF-kappaBeta activation and attenuates neointimal hyperplasia in arterial restenosis. Cell Res. 17, 933–941 (2007).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362 (2021).
Yamaguchi, H., Hsu, J.-M., Yang, W.-H. & Hung, M.-C. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat. Rev. Clin. Oncol. 19, 287–305 (2022).
Zhang, Y. et al. RelB upregulates PD-L1 and exacerbates prostate cancer immune evasion. J. Exp. Clin. Cancer Res. 41, 66 (2022).
Lim, S.-O. et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell 30, 925–939 (2016).
Liu, J. et al. ERK signaling mediates resistance to immunomodulatory drugs in the bone marrow microenvironment. Sci. Adv. 7, eabg2697 (2021).
Kawase, K. et al. High expression of MHC class I overcomes cancer immunotherapy resistance due to IFNγ signaling pathway defects. Cancer Immunol. Res. 11, 895–908 (2023).
Xiong, W. et al. USP8 inhibition reshapes an inflamed tumor microenvironment that potentiates the immunotherapy. Nat. Commun. 13, 1700 (2022).
Liu, X. et al. Context-dependent activation of STING-interferon signaling by CD11b agonists enhances anti-tumor immunity. Cancer Cell 41, 1073–1090.e12 (2023).
Topalian, S. L. et al. Neoadjuvant immune checkpoint blockade: a window of opportunity to advance cancer immunotherapy. Cancer Cell 41, 1551–1566 (2023).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20, 1239–1251 (2019).
Lee, S. M. et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet 402, 451–463 (2023).
Pal, S. K. et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet 400, 1103–1116 (2022).
Yi, M. et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 17, 129 (2018).
Garris, C. S. et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 49, 1148–1161.e7 (2018).
Eluard, B., Thieblemont, C. & Baud, V. NF-κB in the new era of cancer therapy. Trends Cancer 6, 677–687 (2020).
Abramson, S. B. & Amin, A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatol. 41, 972–980 (2002).
Spohn, G., Arenas-Ramirez, N., Bouchaud, G. & Boyman, O. Endogenous polyclonal anti-IL-1 antibody responses potentiate IL-1 activity during pathogenic inflammation. J. Allergy Clin. Immunol. 139, 1957–1965.e3 (2017).
Birk-Bachar, M. et al. Discovery of a novel missense variant in NLRP3 causing atypical CAPS with hearing loss as the primary presentation, responsive to anti-IL-1 therapy. Arthritis Rheumatol. https://doi.org/10.1002/art.42721 (2023).
Koné-Paut, I. & Galeotti, C. Anakinra for cryopyrin-associated periodic syndrome. Expert Rev. Clin. Immunol. 10, 7–18 (2014).
Junge, G., Mason, J. & Feist, E. Adult onset Still’s disease-The evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin. Arthritis Rheum. 47, 295–302 (2017).
Tan, D. S. W. et al. Canakinumab versus placebo in combination with first-line pembrolizumab plus chemotherapy for advanced non-small-cell lung cancer: results from the CANOPY-1 trial. J. Clin. Oncol. 42, 192–204 (2024).
Yang, J. et al. Targeting beta2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell 10, 295–307 (2006).
Yang, J. et al. Human-like mouse models for testing the efficacy and safety of anti-beta2-microglobulin monoclonal antibodies to treat myeloma. Clin. Cancer Res. 15, 951–959 (2009).
Vrábel, D., Pour, L. & Ševčíková, S. The impact of NF-κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 34, 56–66 (2019).
Obeng, E. A. et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 (2006).
Manasanch, E. E. & Orlowski, R. Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 14, 417–433 (2017).
Alwahsh, M., Farhat, J., Talhouni, S., Hamadneh, L. & Hergenröder, R. Bortezomib advanced mechanisms of action in multiple myeloma, solid and liquid tumors along with its novel therapeutic applications. EXCLI J. 22, 146–168 (2023).
Yadav, P., Cook, M. & Cockwell, P. Current trends of renal impairment in multiple myeloma. Kidney Dis. 1, 241–257 (2016).
Hideshima, T. et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood 114, 1046–1052 (2009).
Li, C. et al. Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IkappaB(alpha) degradation. J. Biol. Chem. 285, 16096–16104 (2010).
O’Connor, O. A. et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin. Cancer Res. 15, 7085–7091 (2009).
Kortuem, K. M. & Stewart, A. K. Carfilzomib. Blood 121, 893–897 (2013).
Dimopoulos, M. A., Sonneveld, P., Siegel, D., Palumbo, A. & San-Miguel, J. Carfilzomib and pomalidomide in patients with relapsed and/or refractory multiple myeloma with baseline risk factors. Ann. Oncol. 26, 2247–2256 (2015).
Moreau, P. et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet 397, 2361–2371 (2021).
Chauhan, D. et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin. Cancer Res. 17, 5311–5321 (2011).
Chauhan, D. et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 8, 407–419 (2005).
Richardson, P. G. et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood 127, 2693–2700 (2016).
Harrison, S. J. et al. Phase I clinical trial of marizomib (NPI-0052) in patients with advanced malignancies including multiple myeloma: study NPI-0052-102 final results. Clin. Cancer Res. 22, 4559–4566 (2016).
Raninga, P. V. et al. Marizomib suppresses triple-negative breast cancer via proteasome and oxidative phosphorylation inhibition. Theranostics 10, 5259–5275 (2020).
Di, K. et al. Marizomib activity as a single agent in malignant gliomas: ability to cross the blood-brain barrier. Neuro Oncol. 18, 840–848 (2016).
Scott, L. J., McKeage, K., Keam, S. J. & Plosker, G. L. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 63, 1247–1297 (2003).
Cross, S. A. & Perry, C. M. Tacrolimus once-daily formulation: in the prophylaxis of transplant rejection in renal or liver allograft recipients. Drugs 67, 1931–1943 (2007).
Schroer, B. & Lockey, R. Oral tacrolimus for severe recalcitrant atopic eczema. J. Allergy Clin. Immunol. 111, 1409–1410 (2003).
Fleischer, A. B. Treatment of atopic dermatitis: role of tacrolimus ointment as a topical noncorticosteroidal therapy. J. Allergy Clin. Immunol. 104, S126–S130 (1999).
Renna, S., Cottone, M. & Orlando, A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J. Gastroenterol. 20, 9675–9690 (2014).
Sandborn, W. J. et al. Tacrolimus for the treatment of fistulas in patients with Crohn’s disease: a randomized, placebo-controlled trial. Gastroenterology 125, 380–388 (2003).
Kim, H.-H. et al. Exosome-based delivery of super-repressor IκBα alleviates alcohol-associated liver injury in mice. Pharmaceutics 15, 636 (2023).
Choi, H. et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci. Adv. 6, eaaz6980 (2020).
Kim, S. et al. Exosome-based delivery of super-repressor IκBα ameliorates kidney ischemia-reperfusion injury. Kidney Int. 100, 570–584 (2021).
Dutta, K., Thammisetty, S. S., Boutej, H., Bareil, C. & Julien, J.-P. Mitigation of ALS pathology by neuron-specific inhibition of nuclear factor kappa B signaling. J. Neurosci. 40, 5137–5154 (2020).
Sheller-Miller, S. et al. Exosomal delivery of NF-κB inhibitor delays LPS-induced preterm birth and modulates fetal immune cell profile in mouse models. Sci. Adv. 7, eabd3865 (2021).
Yim, N. et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 7, 12277 (2016).
De Bosscher, K., Vanden Berghe, W. & Haegeman, G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 24, 488–522 (2003).
Uhlenhaut, N. H. et al. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol. Cell 49, 158–171 (2013).
Oh, K.-S. et al. Anti-inflammatory chromatinscape suggests alternative mechanisms of glucocorticoid receptor action. Immunity 47, 298–309.e5 (2017).
Guan, Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J. Am. Soc. Nephrol. 15, 2801–2815 (2004).
Wang, Y.-X. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 20, 124–137 (2010).
Ahmed, W. et al. PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J. Intern. Med. 262, 184–198 (2007).
Nishida, Y. et al. Influence of single-nucleotide polymorphisms in PPAR-δ, PPAR-γ, and PRKAA2 on the changes in anthropometric indices and blood measurements through exercise-centered lifestyle intervention in Japanese middle-aged men. Int. J. Mol. Sci. 19, 703 (2018).
Gross, B., Pawlak, M., Lefebvre, P. & Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 13, 36–49 (2017).
Guo, L. & Tabrizchi, R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharm. Ther. 111, 145–173 (2006).
Liu, H. et al. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARalpha/gamma ligand TZD18. Blood 107, 3683–3692 (2006).
Su, C. G. et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J. Clin. Invest. 104, 383–389 (1999).
Huang, L., Jiang, S. & Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J. Hematol. Oncol. 13, 143 (2020).
Kim, E. et al. Imatinib enhances docetaxel-induced apoptosis through inhibition of nuclear factor-κB activation in anaplastic thyroid carcinoma cells. Thyroid 22, 717–724 (2012).
Yang, T. et al. FAM167A is a key molecule to induce BCR-ABL-independent TKI resistance in CML via noncanonical NF-κB signaling activation. J. Exp. Clin. Cancer Res. 41, 82 (2022).
Challa, S. et al. IKBKE is a substrate of EGFR and a therapeutic target in non-small cell lung cancer with activating mutations of EGFR. Cancer Res. 76, 4418–4429 (2016).
Chiu, C.-F. et al. NF-κB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc. Natl Acad. Sci. USA 113, E2526–E2535 (2016).
Escárcega, R. O., Fuentes-Alexandro, S., García-Carrasco, M., Gatica, A. & Zamora, A. The transcription factor nuclear factor-kappa B and cancer. Clin. Oncol. 19, 154–161 (2007).
Liu, X., Shao, Y., Zhou, J., Qian, G. & Ma, Z. Nuclear factor κB signaling and its related non-coding RNAs in cancer therapy. Mol. Ther. Nucleic Acids 19, 208–217 (2020).
Soldevilla, M. M., Meraviglia-Crivelli de Caso, D., Menon, A. P. & Pastor, F. Aptamer-iRNAs as therapeutics for cancer treatment. Pharmaceuticals 11, 108 (2018).
Yoon, S.-B. et al. A novel IRAK4/PIM1 inhibitor ameliorates rheumatoid arthritis and lymphoid malignancy by blocking the TLR/MYD88-mediated NF-κB pathway. Acta Pharm. Sin. B 13, 1093–1109 (2023).
Puppala, E. R. et al. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-κB and Keap1/Nrf2 signaling pathways: A comprehensive study onin-vitro and in-vivo experimental models. Phytomedicine 97, 153926 (2022).
Fioravanti, A., Tenti, S. & Cheleschi, S. MiR-214-3p, a novel possible therapeutic target for the pathogenesis of osteoarthritis. EBioMedicine 66, 103300 (2021).
Malhotra, S. et al. NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain 143, 1414–1430 (2020).
Wang, Y., Pleasure, D., Deng, W. & Guo, F. Therapeutic potentials of poly (ADP-Ribose) polymerase 1 (PARP1) inhibition in multiple sclerosis and animal models: concept revisiting. Adv. Sci. 9, e2102853 (2022).
El-Sherbiny, M. et al. Anti-inflammatory/anti-apoptotic impact of betulin attenuates experimentally induced ulcerative colitis: an insight into TLR4/NF-kB/caspase signalling modulation. Environ. Toxicol. Pharm. 88, 103750 (2021).
Crow, M. K. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 82, 999–1014 (2023).
Huang, W. et al. NFAT and NF-κB dynamically co-regulate TCR and CAR signaling responses in human T cells. Cell Rep. 42, 112663 (2023).
van der Stegen, S. J. C., Hamieh, M. & Sadelain, M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015).
Li, G. et al. 4-1BB enhancement of CAR T function requires NF-κB and TRAFs. JCI Insight 3, e121322 (2018).
Otano, I. et al. CD137 (4-1BB) costimulation of CD8 + T cells is more potent when provided in cis than in trans with respect to CD3-TCR stimulation. Nat. Commun. 12, 7296 (2021).
Julamanee, J. et al. Composite CD79A/CD40 co-stimulatory endodomain enhances CD19CAR-T cell proliferation and survival. Mol. Ther. 29, 2677–2690 (2021).
Zhang, Q., Lenardo, M. J. & Baltimore, D. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell 168, 37–57 (2017).
Hoesel, B. & Schmid, J. A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 12, 86 (2013).
Oeckinghaus, A., Hayden, M. S. & Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 (2011).
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 12, 715–723 (2011).
Napetschnig, J. & Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 42, 443–468 (2013).
Sun, S.-C. The noncanonical NF-κB pathway. Immunol. Rev. 246, 125–140 (2012).
DiDonato, J. A., Mercurio, F. & Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 (2012).
Cheng, Q. J. et al. NF-κB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science 372, 1349–1353 (2021).
Liu, Z. et al. A NIK–SIX signalling axis controls inflammation by targeted silencing of non-canonical NF-κB. Nature 568, 249–253 (2019).
Webb, L. V. et al. Survival of single positive thymocytes depends upon developmental control of RIPK1 kinase signaling by the IKK complex independent of NF-κB. Immunity 50, 348–361.e4 (2019).
Nixon, C. C. et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578, 160–165 (2020).
Zhao, M. et al. NF-κB subunits direct kinetically distinct transcriptional cascades in antigen receptor-activated B cells. Nat. Immunol. 24, 1552–1564 (2023).
Xia, Y. et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat. Cell Biol. 14, 257–265 (2012).
Meylan, E. et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462, 104–107 (2009).
Lennikov, A. et al. Selective IKK2 inhibitor IMD0354 disrupts NF-κB signaling to suppress corneal inflammation and angiogenesis. Angiogenesis 21, 267–285 (2018).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 82072915 and 82373359); the Project of Shanghai Municipal Health Commission (grant no. 202140397); CSCO-ROCHE Cancer Research Fund 2019 (grant no. Y-2019Roche-171); and Chinese Young Breast Experts Research Project (grant no. CYBER-2021-001). Beijing Science and Technology Innovation Medical Development Foundation Key Project (grant no. KC2022-ZZ-0091-6).
Author information
Authors and Affiliations
Contributions
Q.G. and J.Z. designed the review. Q.G., Y.J., X.C., X.Y. and X.S. conducted literature research, graphing, and manuscript writing. Manuscripts and figures were further verified by M.L., C.Z. and T.Z. J.Z. revised the manuscript and provided general guidance. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Q., Jin, Y., Chen, X. et al. NF-κB in biology and targeted therapy: new insights and translational implications. Sig Transduct Target Ther 9, 53 (2024). https://doi.org/10.1038/s41392-024-01757-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01757-9