Abstract
Cancer is one of the major diseases threatening human life and health worldwide. Epigenetic modification refers to heritable changes in the genetic material without any changes in the nucleic acid sequence and results in heritable phenotypic changes. Epigenetic modifications regulate many biological processes, such as growth, aging, and various diseases, including cancer. With the advancement of next-generation sequencing technology, the role of RNA modifications in cancer progression has become increasingly prominent and is a hot spot in scientific research. This review studied several common RNA modifications, such as N6-methyladenosine, 5-methylcytosine, and pseudouridine. The deposition and roles of these modifications in coding and noncoding RNAs are summarized in detail. Based on the RNA modification background, this review summarized the expression, function, and underlying molecular mechanism of these modifications and their regulators in cancer and further discussed the role of some existing small-molecule inhibitors. More in-depth studies on RNA modification and cancer are needed to broaden the understanding of epigenetics and cancer diagnosis, treatment, and prognosis.
Similar content being viewed by others
Introduction
Cancer is one of the main threats to human health worldwide.1,2,3 Over the past two decades, cancer incidence and mortality have been growing rapidly.4,5 In 2020, there was an estimated 19.3 million new cancer cases worldwide and nearly 10 million cancer deaths.6 Lung cancer has always been cancer with the highest worldwide incidence.7,8,9,10 However, the latest data suggest that new breast cancer (BRC) cases reached 2 million in 1 year.11,12 BRC incidence has surpassed lung cancer incidence and has become the first cause of global cancer.13,14 A series of reasons, such as environmental pollution,15 bad living habits,16 dietary structure,17 and population aging,18 lead to the emergence of this phenomenon. Although significant progress has been made in cancer treatment, such as surgery, radiotherapy, chemotherapy, immunotherapy, and biological therapy, the prognosis for many patients with cancer remains poor.19,20 Therefore, exploring the underlying molecular mechanisms of cancer occurrence and development is of great significance for the early identification of cancer and the establishment of new treatment options and is also crucial for improving the prognosis of cancer patients.
Epigenetic modification refers to a heritable change in the genetic material without any change in the nucleic acid sequence and results in a heritable phenotypic change.21,22,23 These changes included DNA methylation, histone modifications, chromatin remodeling, and RNA interference (RNAi).24,25,26 Epigenetic modification regulates many biological processes in the human body, such as growth,27 aging,28 and various diseases.29,30,31 DNA methylation, a form of DNA chemical modification, is the selective addition of methyl groups to DNA molecules under the action of DNA methyltransferase. DNA methylation can occur at the C-5 position of cytosine, the N-4 position of adenine, and the N-6 position of guanine.32,33 5-Methylcytosine (m5C), the addition of a methyl group to cytosine, is the most common way of DNA modification in higher organisms in mammalian cells.34,35,36 m5C could effectively upregulate gene expression levels and inhibit some tumor suppressor genes through hypermethylation of the promoter region. In addition to m5C, a more complex and dynamic DNA epigenetic regulatory network, including 5-hydroxymethylcytosine (5hmc), 5-formylcytosine (5fC), and 5-carboxycytosine (5caC), has also been identified.37,38,39 Methylation is ubiquitous throughout the genome, and hypomethylation occurs mainly in DNA repeats.40 The role of DNA methylation in cancer has been extensively studied. This extensive change in methylation levels can cause gene instability, leading to various tumors, such as hepatocellular carcinoma (HCC),41,42 urothelial carcinoma,43 and cervical cancer (CC).44
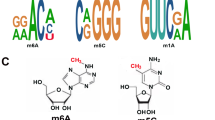
With the advancement of technologies, such as RNA sequencing and fluorescence quantification,45 the role of RNA methylation in cancer progression has gradually become prominent and an international scientific research hotspot. In addition, research on RNA modification has also made great progress. These modifications were originally thought to be fine-tuned chemical structural features of non-protein-coding RNAs. However, they were now considered dynamically regulated with the identification of a growing number of posttranscriptional regulators. More than 60% of RNA modifications were methylation modifications. Mammalian RNA methylation modifications mainly included N6-methyladenosine (m6A), N1-methyladenosine (m1A), N6,2′-O-dimethyladenosine (m6Am), 7-methylguanine (m7G), pseudouridine (Ψ), and m5C.46,47,48,49 As the most prevalent RNA methylation modification, m6A accounts for 60% of RNA methylation modifications.50,51 m6A modification has been found in eukaryotic mRNA and long noncoding RNA (lncRNA). It can occur on the adenine of RNA, mRNA, and lncRNA. m6A modification plays a significant role in RNA stabilization, localization, transport, splicing, and translation.52 m6A modification is reversible via the regulation of methyltransferases (“writers”), demethylases (“erasers”), and methylation reading proteins (“readers”).53,54 Methyltransferases, such as METTL3/14,55 WTAP, and KIAA1429, could catalyze the m6A modification of adenosine on mRNA.53,56 Demethylases, including FTO and ALKHB5, are used to demethylate bases that have undergone m6A modification.57,58,59 The main function of reader proteins is recognized, which is binding to bases with m6A modification, thereby activating downstream regulatory pathways, such as RNA degradation and microRNA (miRNA) processing.60,61,62 The abnormality of enzymes involved in m6A modification will cause a series of diseases, including tumors, musculoskeletal diseases, and rheumatoid arthritis.63,64
RNA m5C modification refers to the methylation of the fifth C atom of RNA cytosine.65,66 It is widespread in various RNA molecules, including tRNA, rRNA, mRNA, and ncRNA.67,68 m5C RNA functions by maintaining RNA stability and regulating protein synthesis and translation.66,67,68 m5C of tRNA could regulate translation. m5C of rRNA could control the quality of ribosome biosynthesis.68 m5C of mRNA could affect mRNA structure, stability, and translation process.68 RNA m5C was also regulated by “writers”, “erasers”, and “readers”. m5C could be regulated by a series of m5C methyltransferases (“writers”), such as NOP2, NSUN2, NSUN3, NSUN4, NSUN5, NSUN6, NSUN7, DNMT1, TRDMT1, DNMT3A, and DNMT3B.69 The removal process is catalyzed by TETs. The Aly/REF nuclear export factor (ALYREF) could recognize and bind to m5C sites for biological function.70,71 The level of m5C is closely related to tumorigenesis. NSUN2 promoted gastric cancer (GC) cell proliferation, migration, and invasion by upregulating the m5C level.72 The m5C alteration of PKM2 mRNA improves glucose metabolism in bladder cancer (BLC).73
Ψ, one of the trace bases of nucleic acid, is formed by linking the fifth position of uracil and ribose to form pyrimidine nucleoside. The base of Ψ and ribose is not connected by the N–C bond but the C–C bond, which is different from uridine.74,75 Ψ, the most abundant modified nucleotide in RNA,76,77 is ubiquitous and mainly presented in ncRNA.78 Ψ enhances tRNA and rRNA function by stabilizing the RNA structure.79 It is directly excreted in the urine, making Ψ a promising biomarker in cancer diagnosis and therapy. In addition, it plays an important role in the regulation of cellular biological functions in tumors.
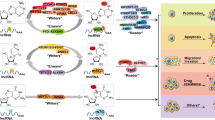
This review detailed and summarized some of the major RNA modifications, including m6A, m5C, and Ψ. The writers, erasers, and readers related to m6A and m5C were introduced, and the writers related to Ψ were introduced. Given that the pseudouridylation process is probably irreversible, there were no reports of demethylases. At the same time, no Ψ-related binding protein was found. Second, this review discussed the deposition and function of these major modifications in coding RNAs and ncRNAs. Furthermore, based on the background of RNA modifications, this review summarized and discussed the research status, expression levels, and functions of m6A, m5C, and Ψ in cancer (Fig. 1). Finally, this review discussed the impact of RNA modifications in cancer treatment and summarized some of the existing small-molecule inhibitors.

Image created with BioRender (https://biorender.com/)
Deregulation of m6A, m5C, and Ψ regulators in human cancers. Red mains an oncogenic role, while green mains a tumor suppressive role. BLC bladder cancer; HCC hepatocellular carcinoma; CC cervical cancer; GBC gallbladder carcinoma; AML acute myeloid leukemia; CRC colorectal cancer; PC pancreatic cancer; GC gastric cancer; GBM glioblastoma; PCa prostate cancer; BRC breast cancer; OC ovarian cancer; ESCC esophageal squamous cell carcinoma; NSCLC non-small cell lung cancer; LUAD Lung adenocarcinoma; LUSC lung squamous cell carcinoma; OS osteosarcoma; RCC renal cell carcinoma; EC esophageal cancer.
m6A writers, erasers, and readers
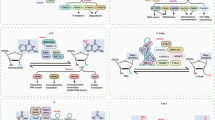
In 1974, Desrosiers et al.80 first discovered m6A on the poly(A) tract of mRNA, but due to the lack of technology to detect m6A sites in mRNA and the possibility of contamination by rRNA and snRNA, interest in m6A was greatly reduced in the late 1970s.81,82 However, interest in m6A was rekindled in 2012,83,84 with the emergence of the next-generation sequencing method MeRIP-Seq as well as genetics and biochemical research.83,84 m6A mainly appears in the RRACH sequence (where R = A or G, H = A, C, or U), and most sites are located around a stop codon and in long internal exons.85,86,87 m6A RNA modification is a dynamic and reversible posttranscriptional process.88,89,90,91 The level of m6A modification of RNA is mainly controlled by so-called “writers”, “erasers”, and “readers”. A writer, mainly including methyltransferase-like 3 (METTL3), METTL14, METTLL16, METTLL5, Wilms tumor 1-associated protein (WTAP), RNA-binding motif protein 15/15B (RBM15/15B), zinc finger CCCH-type containing 13 (ZC3H13), zinc finger CCHC-type-containing 4 (ZCCHC4), and Vir-like m6A methyltransferase associated (VIRMA, also known KIAA1429), catalyzes the formation of m6A. FTO and alkB homolog 5 (ALKBH5) are two major m6A eraser proteins that reversibly remove m6A. Readers recognize and bind to the m6A site, decode m6A methylation and generate a functional signal. These factors mainly include YT521-B homology (YTH) domain-containing protein (including YTHDFs such as YTHDF1/2/3 and YTHDCs such as YTHDC1/2), eukaryotic initiation factor (eIF) 3, IGF2 mRNA-binding protein (IGF2BP) family members (including IGF2BP1/2/3), and heterogeneous nuclear ribonucleoprotein (HNRNP) protein family members (including HNRNPA2B1, HNRNPC, and HNRNPG).
METTL3 (formerly known as MT-A70) is the most vital component of the m6A methyltransferase complex (MTC) and is highly conserved in eukaryotes.92 METTL3 was the first identified and most widely known catalytic subunit;53 it is an S-adenosylmethionine (SAM)-binding protein that catalyzes the transfer of methyl groups in SAM to adenine bases in RNA.93,94,95 The main function of METTL14 is to stabilize the MTC structure and assist METTL3 in recognizing catalytic substrates.94,96,97 WTAP is mainly responsible for recruiting METTL3-METTL14 heterodimers to nuclear speckles and for promoting m6A.98,99,100 RBM15/15B, which has no catalytic function, mainly binds to METTL3 and WTAP, directing these two proteins to target RNA sites.101,102 Schwartz et al.97 and Yue et al.103 found that VIRMA preferentially places mRNA methylation modifications near stop codon regions and the 3′-UTR. At the same time, VIRMA recruits the m6A complex to the special RNA site.97,103,104 ZC3H13 is required for nuclear localization of MTC.102,103,105 Moreover, METTL16, METTL5, and ZCCHC4 function alone to add m6A to some structural RNAs, such as U6 snRNA, 18S rRNA, and 28S rRNA.106,107,108,109,110,111 FTO and ALKBH5 were the first two proteins discovered to catalyze demethylation of m6A, which helps to maintain the dynamic balance of m6A modification.88,112,113,114 Imai et al.115 first isolated the RNA splicing-related protein YT521 in 1998. Hartmann et al.116 then identified the homologous protein YT521-B, and the YT521-B homology (YTH) domain defines a protein family.117 YTHDF1 interacts with initiation factors to initiate RNA translation.118,119 YTHDF2 mainly regulates the degradation of m6A methylated RNA.120,121 YTHDF3 acts synergistically with YTHDF1 and YTHDF2 to promote RNA translation and degradation.122,123,124 YTHDC1 facilitates RNA splicing and export,125,126 and YTHDC2 enhances translation of a target RNA and reduces target RNA abundance.127 By recognizing m6A modifications under normal and stress conditions, IGF2BP increases the stability and translation ability of mRNA.128,129 Meyer et al.130 found that a single 5′-UTR m6A results in bypassing of the 5′cap-binding protein and direct binding to eIF3 to promote translation. HNRNPC/G selectively recognizes m6A-induced splicing and regulates mRNA abundance.131,132 HNRNPA2B1 mediates the processing of primary miRNA (Fig. 2).133,134

Image created with BioRender (https://biorender.com/)
The most common types of RNA modifications and the mechanism of m6A regulation.
Functional consequences of m6A in coding RNAs and ncRNAs
m6A modification has been a hot research topic in recent years. m6A is a common modification that regulates gene expression in eukaryotes,135,136 and the discovery of FTO reveals the reversibility and dynamic balance of m6A.88 Constantly emerging studies showed that m6A is present on both mRNA and ncRNAs.137,138 This review summarized the functional consequences of m6A in mRNA and ncRNAs (mainly including miRNA, lncRNA, and circRNA).
Role of m6A in mRNA
mRNA is the template for protein biosynthesis.139 The mature mRNA of eukaryotic cells has a cap structure of trans-7-methylguanine triphosphate nucleoside at the 5′-end and a poly(A)tail structure at the 3′-end. The primary product of mRNA is known as heterologous RNA (hnRNA). After a series of posttranscriptional modifications, hnRNA is spliced into mature mRNA that is finally transported to the cytoplasm. During translocation of hnRNA into the cytoplasm, introns are spliced out and exons are joined together. After further capping and polyadenylation modifications, hnRNA becomes mature mRNA.140,141
m6A is the most abundant chemical modification in mRNA and plays an important role in multiple processes, such as cell differentiation and tissue development.122 m6A affects virtually every stage of mRNA metabolism, from processing in the nucleus to transport to translation and degradation in the cytoplasm.142 As described previously, the “readers” recognize and bind to m6A sites, decode m6A methylation, and generate functional signals. The role of m6A in mRNA is mainly recognized and regulated by “readers.” m6A “readers” have two different functional modes: direct and indirect reading. Direct reading refers to the selective binding of m6A to RNA-binding proteins with diverse cellular functions.142 If m6A modifications slightly reduce the A: U base pairing energy, this difference may alter the RNA secondary structure and thus alter RNA and protein interactions. It can cause indirect reading.143,144 The specific role of “readers” has already been elaborated on in the previous section. In general, m6A can enhance the nuclear processing, splicing, and export of mRNA. It can promote mRNA translation and affect mRNA degradation. There is a synergy among the YTHDF family. YTHDF3 affects the translation and decay of methylated mRNAs by cooperating with YTHDF1 and YTHDF2. Although both YTHDF1 and YTHDF3 contribute to the translation of their target mRNAs, they also collectively affect the segmentation of YTHDF2 by methylated transcripts to accelerate mRNA degradation.122 In addition, studies also reported that the demethylation activity of ALKBH5 significantly affects mRNA export and RNA metabolism and the assembly of mRNA processing factors in nuclear speckles.145
Role of m6A in miRNA
miRNAs are ncRNAs with ~22 nucleotides.146,147,148 Under the guidance of the RNA-induced silencing complex, miRNA and the 3′-untranslated region (3′-UTR) of the target gene pair complementarily regulate gene expression, resulting in mRNA degradation or translational inhibition at the posttranscriptional level.149,150,151 The binding of miRNAs and mRNA regulates ~60% of coding genes.152 The maturation process of miRNA mainly includes three steps.153,154 First, miRNA is transcribed into a long and capped precursor primary miRNA (pri-miRNA) by RNA polymerase II in the nucleus.155 Second, with the assistance of Drosha ribonuclease III and DiGeorge syndrome key region gene 8 (DGCR8), pri-miRNA is converted to pre-miRNA.156,157 Third, pre-miRNA is exported to the cytoplasm, where it is cleaved by the RNase III endonuclease Dicer to produce mature miRNA.158,159,160 Studies showed that m6A modification and its regulatory factors are widely involved in miRNA processing and maturation.161,162,163,164 Studies revealed the role of METTL3 in promoting miRNA maturation.163,165,166 For example, Liang et al.167 revealed that METTL3 promoted the binding of DGCR8 to miR-20a-5p and m6A modification, thereby increasing miR-20a-5p expression and inhibiting NFIC transcription. m6A induces the processing of pri-miR-17-92 in an m6A/DGCR8-dependent manner. The m6A modification that mediates this process occurs at the A879 site of pri-miR-17-92.168 METTL14 can also affect miRNA expression by regulating pri-miRNA processing and maturation.169,170 Lin et al.171 found that DCA reduces miR-92b-3p expression through m6A-relied posttranscriptional modification by promoting the dissolution of the METTL3-METTL14-WTAP complex. In addition to the role of writers in miRNA processing, erasers affect miRNA maturation. Chen et al.172 discussed the potential role of the ALKBH5/miR-194-2/RAI1 axis in esophageal squamous cell carcinoma (ESCC) treatment. ALKBH5 mainly demethylates pri-miR-194-2 and inhibits miR-194-2 biogenesis in an m6A/DGCR8-dependent manner.
Interestingly, miRNAs also affect the expression of m6A-modified proteins, modulating the level of downstream target genes.173,174 For example, METTL3 is a direct target of miR-186 in hepatoblastoma (HB) cells.175 miR-4429 inhibits SEC62 stabilization caused by m6A by targeting METTL3 in GC to suppress GC cell proliferation.176 Yue et al.177 found that miR-96 downregulates AMPKα2, increasing FTO expression. FTO in turn upregulates MYC expression by blocking m6A modification of MYC. This mechanism is involved in the pro-proliferation and antiapoptotic effects of miR-96 in colorectal cancer (CRC) cells. Xue et al.178 highlighted a positive feedback loop between ALKBH5 and miR-193a-3p. miR-193-3p targets ALKBH5 to inhibit its expression; ALKBH5 in turn inhibits miR-193a-3p expression. This positive feedback loop further promotes ESCC cell growth and metastasis.
Role of m6A in lncRNA
LncRNAs are non-protein-coding RNAs with >200 nucleotides,179,180 which regulate gene expression at the transcriptional or posttranscriptional level and are involved in physiological and pathological processes.181,182 m6A is the most abundant RNA modification in mammalian mRNA and lncRNA, with an average of three to five sites in each transcript.183 lncRNA X-inactive specific transcripts (XISTs) mediate the transcriptional silencing of genes on the X chromosome. XIST was highly methylated. METTL3 knockdown disrupted XIST-mediated gene silencing. YTHDC1 preferentially recognized m6A residues on XIST that were required for XIST to function.101,184 In addition, METTL14 inhibits the growth and metastasis of CRC by downregulating the carcinogenic lncRNA XIST.185 Hu and Ji186 found that METTL14-mediated m6A modification results in LINC01320 upregulation. Upregulated LINC01320 promotes GC cell invasion by regulating the miR-495-5p/RAB19 axis. METTL3-mediated m6A modification resulted in the downregulation of lncRNA MEG3; downregulated MEG3 regulates BTG2 expression through miR-544b.187 Erasers and readers also regulate lncRNA expression. Zhang et al.188 revealed that ALKBH5 promotes GC invasion and metastasis by reducing lncRNA NEAT1 methylation. ALKBH5 inhibits the motility of pancreatic cancer by reducing lncRNA KCNK15-AS1 methylation.189 Cui et al.190 found that FTO reduces m6A methylation of the LINC00022 transcript, resulting in the attenuation of LINC00022 inhibition by YTHDF2. Ni et al.191 pointed out that YTHDF3 is not only a new target of YAP but also has a key role in the YAP pathway by promoting the degradation of m6A-modified lncRNA GAS5.
Role of m6A in circRNA
circRNAs comprise a special class of ncRNA molecules that form a ring structure through typical 5′-3′-phosphodiester bonds;192,193,194 no polyadenylated tail at the 3′-end and no cap structure at the 5′-end are present.195 Compared to linear ncRNA, circRNA has a high degree of stability due to its covalent closed-loop structure.196,197 According to reports, circRNAs are widely involved in the occurrence and development of cancer.198,199 More importantly, circRNAs can be detected in the blood, rendering these molecules ideal biomarkers for cancer diagnosis and prognosis. Recently, m6A modifications of circRNAs have been widespread.200,201,202 m6A can regulate circRNA translation. The initiation factor eIF4G2 and the m6A reader YTHDF drive translation initiation, and METTL3/14 further enhance translation.203,204 As mentioned above, circRNAs are more stable than other ncRNAs due to their closed-loop structure and are not easily degraded. Therefore, studies explored whether m6A modification affects circRNA degradation. The answer is yes. They found that m6A-modified circRNAs were also endoribonuclease-cleaved via the YTHDF2-HRSP12-RNase P/MRP axis.205 Guo et al.206 revealed that circ3823 degradation might be regulated by YTHDF3 and ALKBH5. In addition, m6A modification of circRNAs also suppressed innate immunity.207,208 According to Chen et al.,209 circNSUN2 is exported from the nucleus to the cytoplasm by YTHDC1 in an m6A methylation-dependent manner. Chen et al.210 also found that METTL3 induces circ1662 expression, accelerating YAP1 nuclear transport. Liu et al.211 found that circDLC1 is a new downstream effector of m6A modification mediated by KIAA1429. Xu et al.212 reported that m6A-modified circRNA ORE maintains sorafenib resistance in HCC patients by regulating the β-catenin signaling pathway. In another HCC study, the HBx protein upregulated METTL3 expression and increased the m6A modification of circARL3. YTHDC1 interacts with circ-ARL3 m6A modification to promote its reverse splicing and biogenesis.213
Role of m6A in human cancers
m6A and digestive system neoplasms
Digestive system neoplasms mainly include esophageal cancer (EC), GC, liver cancer, CRC, small bowel cancer, pancreatic cancer (PC), and gallbladder cancer. Digestive system cancer is a huge health burden worldwide, accounting for approximately 35% of global cancer-related mortality.214,215,216 Despite significant progress in digestive system cancer treatment regarding conventional surgical resection, neoadjuvant chemotherapy, radiotherapy, and immunotherapy, the overall survival rate remains very low, and morbidity and mortality rates are still rising.217,218,219 Therefore, new technologies are needed to detect early-stage digestive system cancer and to find new treatment methods and effective targets. Recently, there have been many studies on m6A expression with regard to the prognosis of digestive system cancer.220,221,222,223,224,225 Li et al.226 discussed the expression pattern and prognostic significance of m6A-related genes in ESCC, reporting that YTHDF1, HNRNPC, ZC3H13, YTHDC2, and METTL14 are dysregulated in ESCC. In addition, they found that patients with high levels of ALKBH5 have better overall survival but that patients with HNRNPC and WTAP overexpression have worse overall survival. Guan et al.227 conducted a similar study on GC: compared with normal tissues, most m6A-related genes were upregulated at both the protein and mRNA levels in GC. The expression level of m6A-related genes is also closely related to clinicopathological characteristics such as age, TNM stage, and race. High expression of WTAP and FTO indicates a poor prognosis for GC patients. Liu et al.228 analyzed RNA-seq FPKM data and matched clinical data for 331 CRC samples, finding only ALKBH5 and METTL14 to be downregulated in tumors. YTHDF1 and HNRNPC can be used as prognostic factors for CRC, with potential value in CRC treatment. Similar prediction models have been carried out in HCC and PC.129,229,230,231,232,233,234 Overall, the development of various sequencing technologies has stimulated interest in m6A and cancer research, laying a foundation for cancer markers and the development of new cancer treatment methods.
METTL3 and METTL14 are the two most common m6A methyltransferases. Research shows that METTL3 is mainly expressed as an oncogene in digestive system cancer but that METTL14 plays a role in inhibiting cancer. Indeed, METTL3 is upregulated in a variety of digestive system cancers. Highly expressed METTL3 is closely linked to lymph node involvement, distant metastasis, stage, and microvascular invasion, among other factors. It is also involved in multiple activities, such as proliferation, migration, invasion, and apoptosis.165,167,168,173,176,235,236,237,238,239 Sun et al.161 found that downregulation of METTL3 significantly inhibits the growth of CRC cells. Additionally, overexpression of miR-877 rescues the effects of METTL3 deletion on mitochondrial respiration and aerobic glycolysis. METTL3 can also promote DNA synthesis in HB. METTL3 is overexpressed in HB tissues and cell lines, and high levels are associated with the poor prognosis of HB patients.175 METTL14 is downregulated in CRC and HCC and mainly inhibits the occurrence and outcome of cancer by suppressing the proliferation and migration of cancer cells. Low expression of METTL14 is also greatly associated with clinicopathological characteristics such as TNM stage, differentiation, tumor encapsulation, tumor microsatellite, and microvascular invasion.169,170,185,240 FTO and ALKBH5 are demethylases that maintain the dynamic balance of m6A modification. According to Yue et al.,177 FTO expression is increased in CRC. FTO induces the proliferation and invasion of CRC cells by upregulating the c-Myc proto-oncogene (MYC) and inhibits apoptosis. FTO also plays a cancer-promoting role in PC.241 Zhang et al.188 studied the role of ALKBH5 in GC. Both western blot and RT–qPCR results indicated that ALKBH5 is overexpressed in GC. ALKBH5 mainly upregulates EZH2 by demethylating lncRNA NEAT1, promoting GC cell invasion and migration. The tumor-suppressor effect of ALKBH5 has been shown in ESCC and PC.172,178,189 The reason for this contradiction may be the different targets of ALKBH5. To date, most studies on m6A in cancer have been based on the m6A modification itself rather than on the underlying mechanism of reader proteins. In fact, the role of various m6A reader proteins in cancer remains largely unexplored.119,242 Table 1 summarizes the role of m6A reader in cancer reported by some current studies.243,244,245,246 For example, Chen et al.209 found that IGF2BP2 enhanced the stabilianticodon stem-loopty of HMGA2, thereby promoting the metastasis of CRC cells and exerting a cancer-promoting effect. YTHDF3 is also expressed as an oncogene in CRC,191 and Yang et al.185 described a ‘METTL14-YTHDF2-lncRNA’ regulatory axis in CRC cells (Fig. 3).

Image created with BioRender (https://biorender.com/)
The role of m6A in digestive system neoplasms. m6A can not only promote cancer progression, but it also plays a role in inhibiting progression. This figure mainly proposes how writers, erasers, and readers participate in the regulation of various genes and pathways in cancer progression. ESCC esophageal squamous cell carcinoma; GC gastric cancer; HB hepatoblastoma; CRC colorectal cancer; HCC hepatocellular carcinoma; PC pancreatic cancer.
m6A and respiratory system neoplasms
Among respiratory tumors, current research is mainly concerned with m6A and lung cancer. Lung cancer is a common malignant tumor, mainly divided into small cell lung cancer (SCLC) and non-SCLC (NSCLC). Among them, NSCLC accounts for about 85% of lung cancer cases.247 NSCLC can be further divided into two subtypes: lung adenocarcinoma (LUAD) and lung SCC (LUSC), accounting for 50–60% and 30% of the total cases, respectively. Studies found that the m6A modification of RNA is widely involved in the occurrence and development of lung cancer.248 It was concluded that m6A regulatory proteins, whether “writers”, “erasers”, or “readers”, play a role in lung cancer. In most cases, these regulatory proteins are mainly upregulated in lung cancer and play a cancer-promoting role.249,250,251,252,253,254 ALKBH5 increases FOXM1 expression by downregulating m6A modification on FOXM1 mRNA, ultimately promoting LUAD cell proliferation and invasion in an intermittent hypoxic environment.255 However, Jin et al.256 indicated that ALKBH5 expression is reduced in NSCLC, and high ALKBH5 expression predicts a good clinical prognosis. Molecularly, ALKBH5 reduced YAP activity by regulating the miR-107/LATS2 axis in a HuR-dependent manner, further inhibiting tumor growth and metastasis in vivo. Although there is no direct contradiction between the studies, more research is worthwhile to further verify the role and mechanism of m6A modification and its related regulators in lung cancer.
m6A and urinary system neoplasms
Bladder cancer (BLC)
BLC is one of the most common malignant tumors of the urinary tract, especially in developed countries. Despite recent advances in clinical treatment, BLC remains a major cause of cancer-related morbidity and mortality due to its high heterogeneity and recurrence rate.257,258,259 Numerous studies documented the importance of m6A modifications in biological processes and disease pathogenesis, including in BLC. Most studies revealed that METTL3, as an m6A-modified “writer,” can promote m6A levels of targeted mRNAs and translational levels and possibly plays a role in promoting mRNA stability.260,261,262 In addition, Xie et al.263 found that METTL3 and YTHDF2 synergistically degrade mRNAs of the tumor suppressors SETD7 and KLF4, thereby promoting BLC development. In another study, METTL3 may positively regulate the processing of pri-miR221/222 into mature miRNA by interacting with DGCR8, thereby playing an oncogenic role in BLC.264 Of course, other studies showed that METTL14 and FTO are under-expressed in BLC and mainly play a tumor suppressor role.58,265
Renal cell carcinoma (RCC)
RCC accounts for about 90% of renal cancers and is the most lethal of urogenital malignancies. Clear cell RCC (ccRCC) is the most common histological subtype.266,267 About 400,000 patients worldwide are diagnosed with kidney cancer each year, and the mortality rate is very high.268,269 Because RCC is resistant to chemotherapy and radiotherapy, surgery remains the most effective treatment for patients with RCC.270,271,272,273 However, patients with metastatic RCC may not have the opportunity for surgery and have a poor prognosis.274 Therefore, new targeted therapy is urgently needed to improve the survival and prognosis of RCC patients. The identification of m6A RNA methylation regulators has led to new insights into the potential therapeutic roles of m6A modifications in gene expression regulation and cancer. Genetic alterations in m6A regulators in ccRCC were identified for the first time, and a significant association between these alterations and worse clinical features was found.275 Unlike other cancers, this study showed that METTL3 was upregulated in RCC, and METTL3-negative expression was associated with larger tumor size (P = 0.010) and higher histological grade (P = 0.021). Further research revealed that METTL3 might inhibit RCC cell proliferation, migration, and invasion through the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway and play a tumor suppressor role.276 Another major m6A methyltransferase, METTL14, also inhibits RCC progression.277 Interestingly, however, WATP is upregulated in RCC and upregulates CDK2 expression by stabilizing its transcripts, promoting RCC tumorigenesis. WATP is also a methyltransferase and is mainly responsible for the recruitment of METTL3 and METTL14. However, whether this cancer-promoting effect of WATP is directly related to m6A modification was not investigated.278 More in-depth studies are needed to further explore the mechanism of action of m6A and RCC.
Prostate cancer (PCa)
PCa is also a common cancer of the urinary system and is the second leading cause of cancer-related deaths in men worldwide.267,279,280 Surgery, chemoradiotherapy, and hormone therapy are effective treatments for PCa, but the recurrence rate of patients is still high, and the mortality rate has not decreased.281 Therefore, further elucidation of the profound mechanisms involved in PCa progression is of great interest. Studies revealed the cancer-promoting role of m6A in PCa. METTL3 further affects downstream mechanisms mainly by affecting target mRNA stability and expression levels, ultimately promoting PCa cell proliferation, migration, invasion, and adhesion and inhibiting apoptosis.282,283 YTHDF2, a “reader” for m6A, is upregulated in PCa. Furthermore, miR-493-3p could target YTHDF2 and inhibit YTHDF2 expression, thereby reversing the cancer-promoting effect of YTHDF2.284
m6A and female reproductive system neoplasms
Ovarian cancer (OC)
OC is one of the most common gynecological tumors.285 Patients with advanced OC have a poor prognosis and high recurrence rates.286,287 There is an urgent need to identify and validate specific biomarkers and therapeutic targets for OC. Likewise, m6A modifications and the role of regulators in OC are constantly being reported. At present, METTL3, YTHDF1, ALKBH5, etc., function as oncogenes in OC.288,289,290,291,292 METTL3 is frequently upregulated in OC. METTL3 expression is significantly correlated with clinicopathological features, such as tumor size, tumor grade, lymph node metastasis, pT status, pN/pM status, and FIGO stage.288,289 Liu et al.290 showed that YTHDF1 promoted OC progression by enhancing EIF3C translation. EIF3C is a subunit of the protein translation initiation factor EIF3. The proposal of the novel YTHDF1-EIF3C axis helps find effective targets and mechanisms for the treatment of OC.
Cervical and endometrial cancers
Cervical cancer (CC) is one of the most common gynecological malignancies, especially in developing countries.293,294 More than 90% of cervical cancers are squamous cell carcinomas.295 The early symptoms of cervical cancer are not obvious, and most patients are already in the late stage when they are found.296 Therefore, finding early cancer biomarkers is of great significance. As a way of posttranscriptional modification of RNA, m6A has stimulated the interest of researchers in recent years. Although there are few reports on m6A and CC, it is undeniable that m6A modification and its regulators play an important role in affecting CC progression.297,298,299 FTO is upregulated in CC, which not only affects the biological process of CC but also plays a role in chemotherapy resistance.298,299 In endometrial cancer, studies also found the role of m6A modification. m6A mRNA methylation promotes the proliferation of endometrial cancer by regulating Akt activity and plays a role in promoting cancer.300
m6A and nervous system neoplasms
Glioblastoma (GBM)
GBM is the most lethal primary malignant brain tumor, commonly found in adults.301 GBM is characterized by significant intratumoural and intertumoral heterogeneity. GBM contains many GBM stem cell-like cells (GSCs) that can self-renew and have greater resistance to conventional treatments, radiotherapy, and chemotherapy.301,302,303 Therefore, it is necessary to further understand the molecular mechanism of GSC and find possible therapeutic targets. An increasing number of studies revealed the role of m6A RNA methylation in regulating GSC self-renewal and GBM occurrence.304,305,306 For example, ALKBH5 demethylates FOXM1 nascent transcripts, resulting in enhanced FOXM1 expression, thereby maintaining GSC tumorigenicity. In addition, METTL3 maintains carcinogenesis in GBM by mediating nonsense-mediated degradation of SRSF mRNA.307 In another study, 2HG inhibited tumor proliferation by targeting the FTO/m6A/MYC/CEBPA signaling pathway, demonstrating an antitumor effect.308
m6A and hematological neoplasms
Acute myeloid leukemia (AML)
Normally, pluripotent hematopoietic stem cells differentiate into myeloid progenitor cells and eventually into mature myeloid cells, a process called myelopoiesis. Abnormal bone marrow production can lead to diseases, such as AML.309,310 AML, one of the most common and lethal hematopoietic malignancies, blocks its myeloid differentiation to generate self-renewing leukemia stem cells (LSCs).311,312 Abnormal cell proliferation and arrest of terminal differentiation of myeloid cells are the two hallmarks of AML.313,314 The development of specific targeted therapies for AML is a current priority. Emerging evidence suggested that RNA m6A modification is involved in various physiological and pathological processes, including hematopoiesis and leukemogenesis. At present, there are a lot of studies on m6A and AML, and m6A “writers,” “erasers,” and “readers” are involved in AML progression and play a role in promoting cancer.315,316,317,318,319 Genetic alterations in m6A regulators predicted poor survival in AML patients.320 m6A modifications and regulators mainly play important roles in the development and progression of AML and self-renewal of LSCs/leukemia-initiating cells. In terms of mechanism, METTL14 mainly regulates the expression of its mRNA targets MYB and MYC through m6A modification, thereby exerting its oncogenic effect. Furthermore, it is negatively regulated by SPI1. A novel SPI1-METTL14-MYB/MYC signaling axis plays an important role in myelopoiesis and leukemogenesis.321 In addition, Su et al.308 investigated the functional role of R-2-hydroxyglutaric acid (R-2HG), an oncogenic metabolite, in AML. Results revealed that R-2HG exerts broad antileukemic activity in vitro and in vivo by inhibiting AML cell proliferation and promoting cycle arrest and apoptosis. Mechanistically, R-2HG inhibits FTO activity and reduces the stability of MYC/CEBPA transcripts in an m6A-modified manner, resulting in the inhibition of related pathways. In conclusion, it is not difficult to see that m6A modification and its regulators mainly promote cancer in AML. Further search for molecules and sites targeting m6A to specifically manufacture related inhibitors may be expected to provide new therapies for AML diseases.
m6A and tumors of other systems
In addition to its involvement in the aforementioned systems and cancers, m6A is widely involved in breast cancer,322,323,324 melanoma,325,326,327,328 osteosarcoma,329,330 head-and-neck SCC (HNSCC),331,332,333 etc. (summarized in Table 1). In melanoma, Jia et al.328 showed that m6A levels were low in ocular melanoma samples and predicted poor prognosis. Mechanistically, YTHDF1 promoted the translation of methylated HINT2 mRNA, which inhibited ocular melanoma progression. Another study revealed that METTL3-mediated m6A RNA methylation promotes uveal melanoma cell proliferation, migration, and invasion by targeting c-Met. m6A modification acts as a key oncogenic regulator in uveal melanoma development.325 The reason for the contradiction is unclear, but more research is needed to further verify and explain this phenomenon. Based on this, the regulatory mechanism of m6A modification is very complex. First, because there are many m6A regulators, each has different functions. In addition, there are many targets for m6A modification, and the biological effects of different targets are also very different. Second, m6A modifications are located on different regulatory mechanisms in different cells. Therefore, key enzymes of m6A modification may have opposite effects on tumorigenesis in different systems, even within one specific type of tumor.
m5C writers, erasers, and readers
m5C refers to a methyl group inserted into the C atom at the fifth position of cytidine. In 1950, studies found m5C in nucleic acids. At that time, m5C was only found in the deoxypentose nucleic acids of animals and higher plants.334 In the 1970s, some research reports on m5C in RNA began to appear.80,335,336 So, far, m5C has become a research hotspot in RNA methylation modification. The technologies used to detect m5C are also constantly updated, currently mainly including bisulfite sequencing, m5C RNA immunoprecipitation sequencing (m5C-RIP-seq), 5-azacytidine-mediated RNA immunoprecipitation sequencing (Aza-IP-seq), and methyl CL-single nucleotide resolved cross-link immunoprecipitation sequencing (miCLIP-seq). Similar to m6A, m5C is also modified by three major regulators. “writers” refers to members of the DNA methyltransferase 2 (DNMT2) and NOP2/SUN RNA methyltransferase family member (NSUN) family. The latter mainly includes NSUN1, NSUN2, NSUN3, NSUN4, NSUN5, NSUN6, and NSUN7. The TET family and ALKBH1 constitute the “erasers” of m5C. As for “readers,” two main ones have been found, namely ALYREF and Y-box binding protein 1 (YBX1).
NSUN2 is the first discovered and the most thoroughly studied member of the NSUN family. For the first time, tRNA-specific methyltransferase 4 (Trm4) in yeast was involved in tRNA methylation modification.337,338 Since then, studies have identified the homologous protein of Trm4 in animals, now known as NSUN2.339,340 The process of NSUN2 catalyzing m5C methylation mainly involves the covalent binding of sulfur atoms of cysteine residues to the C6 position of the base in the target RNA, and then C5 nucleophilically attacks the methyl group of the SAM to complete the methylation.341,342,343,344 NSUN2-catalyzed methylation has been found in tRNA, mRNA, rRNA, mitochondrial tRNA (mt-tRNA), vault-derived small RNA, lncRNA, viral RNA, and other RNAs.345,346,347,348,349,350 In addition, conserved residues of the NSUN2 gene undergo missense alterations in autosomal recessive mental retardation, revealing the role of RNA methyltransferases in human neurocognitive development.351,352,353 NSUN1 (or p120, also known as Nop2 in yeast) and NSUN5 (also known as Rcm1 in yeast) are mainly related to 28S rRNA in humans.354,355,356 NSUN4 is also mainly associated with rRNA. NSUN4 is a bifunctional mitochondrial protein required for 12S rRNA methylation and the filament assembly site.357 NSUN3 initiates 5-formylcytidine biogenesis in human mt-tRNA (Met).358,359,360 Another tRNA methylation regulator is NSUN6, which mediates the specific methylation of tRNA (Cys) and tRNA (Thr) located at position C72.361 The specific role of NSUN7 is not yet fully understood. However, the continuous development of next-generation sequencing (NGS) technology will lead to a deeper understanding of the regulators of m5C in the future. DNMT2 (also known as TRMDT1) is the most widely studied methyltransferase besides NSUN2. DNMT2 does not only act on tRNA. tRNA (Asp-GTC), tRNA (Val-AAC), and tRNA (Gly-GCC) are all its substrates. It also catalyzes mRNA methylation.345,362,363,364,365
Compared to the writers of m5C, research on erasers and readers is relatively less mature. The 10–11 translocation (Tet) family of mammalian Fe(II)- and 2-oxoglutarate-dependent dioxygenases can oxidize RNA 5-methylcytidine to 5-hydroxymethylcytidine (5hmrC), 5-formylcytidine (5frC), and 5-carboxycytidine (5carC).366 Tet2 promotes myelopoiesis induced by mammalian pathogen infection by reducing m5C in mRNA.367 In addition, studies revealed a full-length isoform containing an N-terminal CXXC domain (Tet3FL). The CXXC domain binds unmethylated CpGs, but it has the highest affinity for 5caC but not m5C.368 Another currently known eraser is ALKBH1. m5C modification can be further oxidized by α-ketoglutarate and the Fe(II)-dependent dioxosome ALKBH1/ABH1 to generate 5-formylcytidine at this position.360 ALYREF is a reader protein that specifically recognizes m5C, which binds mainly to the 5′ and 3′ regions of mRNA in vivo.369 m5C RNA modification promotes retroviral replication in an ALYREF reader protein-dependent manner.370 In addition, studies revealed that, upon NSUN2 depletion, ALYREF-mediated dysregulation of mRNA output could be restored by reconstitution of wild-type but not methyltransferase-deficient NSUN2.346 In addition to ALYREF, YBX1, a newly discovered m5C reader protein, regulates mRNA stability in the cytoplasm (Fig. 4).371

Image created with BioRender (https://biorender.com/)
The mechanism of m5C regulation and the common detection methods.
Functional consequences of m5C in coding RNAs and ncRNAs
Initial studies of m5C in RNA were mainly limited to tRNA and rRNA. RNA bisulfite conversion was combined with NGS technology to whole-transcriptome detection of modified cytosine residues at single-nucleotide resolution, which found 10,275 m5C sites in mRNA and other ncRNAs in addition to tRNA and rRNA. This study provides the first map of the distribution of m5C modifications in the human transcriptome, advancing the study of posttranscriptional modifications in gene regulation.372 Next, this review mainly summarized the action sites and effects of m5C on tRNA, rRNA, mRNA, and other ncRNAs and briefly described the mechanism, hoping to provide some help for further research in the future.
Role of m5C in tRNA
tRNA acts as a carrier for amino acids, providing activated amino acids during protein synthesis.373 tRNA is generally composed of 74 to 95 nucleotides and has a stable spatial structure.374,375 The methylation sites of tRNA are mainly located at C34, C38, C40, C48, and C50 of the anticodon loop.376,377,378 m5C promotes the stability of tRNA for subsequent protein synthesis. Specifically, m5C methylation from tRNA in mice was eliminated by disrupting DNMT2 and NSUN2 tRNA methyltransferases. A dramatic reduction in the steady-state levels of unmethylated tRNA and a reduction in the overall protein synthesis rate was observed.345 Gkatza et al.379 found that loss of NSUN2 resulted in reduced methylation of tRNA-derived noncoding fragments, further resulting in impaired regulation of protein synthesis. Furthermore, NSUN2-driven RNA methylation is functionally required to adapt cell cycle progression to early stress responses. Thus, cytosine-5 RNA methylation links protein synthesis to cellular metabolism. m5C also affects tRNA cleavage, resulting in neurodevelopmental disorders.380 tRNAs with DNMT2-dependent methylation can discriminate homologous codons to accurately direct polypeptide synthesis and protect themselves from stress-induced endonucleolytic cleavage.363,364
m5C also plays an important role in mt-tRNA. NSUN3 interacts with (mt-)tRNAMet and undergoes methylation modification at its C34 wobble position, expanding codon recognition during mitochondrial translation. In addition, NSUN3 specifically recognizes the anticodon stem-loop (ASL) of tRNA, and impairing the ASL base pairing causes disease.360 NSUN3 mutation also causes insufficient formylation at the same site of mt-tRNA.358 NSUN2 also targets mt-tRNA positions 48–50 to catalyze the formation of m5C methylation. However, studies revealed that NSUN2 does not affect mt-tRNA stability and oxidative phosphorylation.347,381
Role of m5C in rRNA
rRNA and ribosomal proteins make up the ribosome, which provides a site for protein synthesis in vivo.382 rRNA accounted for about 80% of the total RNA weight.383,384 rRNA methylation plays an important role in mitochondrial ribosome biogenesis. Studies first found that yeast contains two m5C modification sites, 2278 of helix 70 and 2870 of helix 89, respectively. Further, Rcm1 and Nop2 were responsible for catalyzing the methylation of these two sites, and the cysteines in motifs IV and VI of these two proteins played an important role.355 Gigova et al.385 also revealed that methylation of the 25S rRNA IV domain is critical for ribosome stability. In addition, in humans, NSUN1 and NSUN5 catalyze m5C4447 and m5C3782 of 28S rRNA, respectively.355 NSUN5 also helps generate specialized ribosomes that fight stress, extending lifespan and stress resistance in yeast, worms, and flies.386 First, NSUN4 methylates the 12S rRNA of the small ribosomal subunit, and sequencing results showed that the methylation site is at cytosine 911. More importantly, NSUN4 can also cooperate with MTERF4 to promote ribosome assembly. Thus, NSUN4 plays a key role in controlling the final step of ribosome biogenesis.357,387 More interestingly, the YTHDF2 protein is also one of the readers of m5C in RNA, although its affinity is not as strong as that of m6A. RNA bisulfite sequencing results showed that YTHDF2 deletion significantly enhanced m5C methylation levels in rRNA. It is also involved in pre-rRNA processing.388 In conclusion, m5C modification in rRNA plays a role in ribosome assembly, synthesis, and stability, regulates cellular responses to stress, and prolongs lifespan.
Role of m5C in mRNA
In early zebrafish embryos, genome-wide analysis of RNA m5C modifications revealed that m5C-modified maternal mRNAs exhibit higher stability than m5C-unmodified mRNAs. The mechanism is as follows: YBX1 acts as an m5C reader, preferentially recognizes m5C-modified mRNAs through the indole loop of the W65 cold shock domain, and recruits Pabpc1a or ELAVL1 (both mRNA stabilizers) to maintain the stability of its target mRNAs.371,389 m5C also promotes mRNA export, and ALYREF is another m5C reader. Yang et al.346 revealed the role of ALYREF and NSUN2 in regulating mRNA output. In addition, the combined modification of m5C and m6A on the same RNA will produce a synergistic effect and jointly affect the expression of subsequent proteins. Specifically, NSUN2-mediated m5C and METTL3/METTL14-mediated m6A methylation synergistically enhance p21 mRNA expression.390 Schumann et al.391 showed that cytosine methylation levels are strongly negatively correlated with mRNA translation. The contradiction between the two was explained by different m5C profiles. Huang et al.392 also pointed out that species, rather than tissue type, is the main determinant of methylation levels. In addition, downregulation of TRDMT1, a tRNA methyltransferase, affected mRNA methylation levels, further inhibiting cancer proliferation and migration.393 In conclusion, m5C mainly affects mRNA stability, export, translation level, and subsequent biological functions, and the critical role of m5C mRNA modification in early development.
Role of m5C in other ncRNAs
Cytosine methylation can also be involved in the regulation of lncRNA function. lncRNA HOTAIR and XIST are specific targets of m5C. The m5C level in the XIST A structure significantly affects the binding of XIST to the chromatin modification complex PRC2.394 In addition, NSUN2 mediates m5C-modified lncRNA nuclear magnetic resonance (NMR) by competitively inhibiting the methylation of underlying mRNAs. lncRNA NMR plays an oncogenic role in ESCC.349 In addition, m5C was also deposited in the noncoding vault RNA VTRNA1.1. NSUN2 mediates the methylation of cytosine 69 of VTRNA1.1, thereby processing VTRNA1.1 into small vault RNAs (svRNAs). svRNAs can regulate epidermal differentiation and play an important role in cell differentiation.348 Likewise, NSUN2 is the primary writer of m5C on HIV-1 RNA. NSUN2 deletion not only inhibits m5C modification of HIV-1 transcripts but also inhibits HIV viral replication.395 DNMT2 deletion in mice prevents m5C modification in sperm miRNAs and abrogates small ncRNA-mediated intergenerational transmission of metabolic disorders.396
Role of m5C in cancer
Recently, there has been a growing body of research on m5C and cancer, although the specific mechanisms of m5C in some cancers remain obscure. Current and future research will increasingly clearly describe the role and molecular mechanism of m5C in cancer. Misu is the first SUN domain-containing protein discovered in invertebrates, and its expression level in normal tissues is much lower than in tumor tissues. Misu can affect the role of Myc, a well-known proto-oncogene, in tumor cell growth and proliferation.338 In addition, NSUN2, with high sequence homology to Misu, was overexpressed in almost all cancers.397 Xiang et al.398 conducted the first comprehensive analysis of m5C regulators in gastrointestinal cancer. Also, m5C regulators were closely related to the ErbB/PI3K-Akt signaling pathway. This review summarized the current research on m5C and cancer and discussed the basic roles and possible molecular mechanisms of m5C by classifying the digestive and nondigestive system cancers, hoping to provide some help for future research (Table 2).
m5C and digestive system cancers
5 mC profiling of mRNA, circRNA, and lncRNA in human HCC and adjacent normal tissues was carried out.399,400,401 No matter which kind of RNA m5C was modified, there was a significant difference between cancer and paracancer. Despite the lack of follow-up molecular investigations, these expression profiles are important for understanding the mechanisms of m5C regulation in HCC. Through bioinformatics analysis, He et al.402 revealed the characterization and role of m5C regulators in HCC and pointed out that m5C-related gene mutations are prevalent in HCC. In addition, more m5C regulators are associated with the clinical grade and prognosis of HCC. Among them, NSUN4 and ALYREF were effective predictors of survival. Another study found that NSUN2 mediated the m5C modification of H19, promoted the specific binding of H19 to G3BP1, and further led to MYC accumulation, which subsequently promoted the occurrence, development, and poor differentiation of HCC.403
NSUN2 expression was increased in GC tissues. NSUN2 could improve GC cell proliferation in vivo and in vitro through a series of experiments, such as CCK-8 assay, colony formation assay, flow cytometry analysis, and nude mice tumorigenesis experiment. Mechanistically, NSUN2 methylated the 3′-UTR of CDKN1C (p57Kip2) mRNA, which led to p57Kip2 downregulation.404 Another study on NSUN2 and GC revealed that high NSUN2 expression was closely related to poor prognosis in GC patients. Similarly, NSUN2 also functions as an oncogene in GC cells. SUMO-2/3 interacted with NSUN2 to maintain the stability of NSUN2 and promoted its nuclear translocation, thereby enhancing the cancer-promoting effect.72 Although only the role of m5C writer in GC is currently studied, with technological progress and research accumulation, the role of m5C regulators in GC will be clearer in the future.
PC is the most dangerous type of cancer. Mortality is very high, and the prognosis is very poor.405,406,407 The role of RNA posttranscriptional modifications in PC is constantly being updated and presented. The expression profile of m5C regulators in PC has been reported.408 The differential expression of m5C-related genes in PC suggested that different regulators play different or even opposite roles. NSUN6 is a tumor suppressor gene in PC, and its expression was closely correlated with clinicopathological features, such as T stage and Ki-67+ cell rate. Moreover, NSUN6 can also predict the prognosis and recurrence of PC, and it is expected to become an excellent target for the prognosis evaluation of cancer diagnosis and treatment.409 Yuan et al.410 also constructed a prognostic risk model for 8-m5C-related lncRNAs in pancreatic ductal adenocarcinoma. Although this prognostic model is the first to be constructed, it does have a certain reference value. However, the disadvantage is that this model has not been validated by in vivo experiments. Therefore, in-depth mechanistic studies and validation in the future will better reveal the role of m5C regulators in PC.
Two studies reported the role of m5C-related regulators in ESCC. Both revealed the cancer-promoting role of m5C writer NSUN2 in ESCC. The first study showed that NSUN2 was involved in how lncRNA NMR promotes tumor progression in ESCC. NSUN2 methylation modifies NMR, which binds to the chromatin regulator BPTF to initiate downstream pathways.349 The other study found that NSUN2 exerted a procancer effect by stimulating oncogenic PI3K-Akt and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signaling by enhancing GRB2 mRNA stability.411
Similar bioinformatics studies were also conducted on colon cancer. Based on RNA sequencing data of The Cancer Genome Atlas dataset, a prediction model containing three m5C regulators, NSUN6, DNMT2, and ALKBH1, was established. The expression of these three regulators is highly correlated with colon cancer prognosis. In addition, NSUN6, DNMT2, and ALKBH1 can work together on the MAPK signaling pathway, thereby affecting the immune infiltration level in colon cancer.412
NSUN2 is one of the most well-studied and extensively studied m5C regulators. As summarized above and demonstrated in this study, NSUN2 is closely related to cell immortalization.338 Therefore, Gao et al.71 explored the role of NSUN2 in gallbladder carcinoma (GBC). The result was that NSUN2 cooperated with RPL6 to promote GBC cell proliferation and growth. In conclusion, m5C is involved in various processes in digestive system cancer development. Current studies supported the cancer-promoting role of m5C in HCC, GC, ESCC, colon carcinoma, and GBC. The protective effect of the tumor suppressor was demonstrated only in PC. In addition, among m5C-related regulatory genes, the writer is currently the most studied and reported, and NUSN2 among writers is the most extensively studied and the most clearly described in function. More and more relevant studies in the future will further reveal the role and possible molecular mechanisms of m5C in digestive system cancers, providing as many clues as possible for cancer diagnosis, treatment, and prognosis evaluation.
m5C and nondigestive system cancers
Glioma
The construction of the prognostic prediction model of RNA m5C methyltransferase in glioma has also been completed. Data from existing databases were obtained, processed, and evaluated using bioinformatics. There is a certain link between the abnormal expression of m5C methyltransferase and the clinicopathological features of glioma. Except for NSUN6, the expression of other methyltransferases was upregulated with the increase of the WHO grade. Expression levels were also positively correlated with the malignant progression of glioma.413 This study deepens the understanding of the molecular mechanisms by which m5C is involved in the occurrence and development of gliomas and provides possible ideas for finding new biomarkers and targeted therapies. GBM is the most malignant type of glioma.414 Cheray et al.415 investigated the biological role and underlying mechanism of m5C modification of miRNAs in GBM. Specifically, DNMT3A/AGO4 inhibits miR-181a-5p/mRNA duplex formation by mediating cytosine methylation of miR-181a-5p. The original function of miRNA to inhibit mRNA gene expression is lost. The methylation status of miR-181a-5p also affects its interaction with antiapoptotic proteins, resulting in enhanced cancer cell proliferation and invasion and reduced apoptosis. Furthermore, cytosine methylation of miR-181a-5p was also associated with poor prognosis in GBM patients. Epigenetic deletion of NSUN5 affects protein synthesis, on the one hand, and targets the ribosome to activate alternative translation programs involved in stress-adaptive responses, on the other. Epigenetic inactivation of NSUN5 is a marker of long-term survival in glioma patients.416
Lung cancer
Nucleolar protein p120 is also known as NSUN1. High p120 expression predicted poor clinical outcomes compared to LUAD patients with low p120 expression. Multivariate analysis showed that p120 is the independent and strongest prognostic factor for resected LUAD (P = 0.033).417 Bioinformatic analysis methods were also applied to explore the characterization and impact of m5C modification on RNA in LUSC. Most m5C regulators were upregulated in LUSC, with only DNMT2 and NSUN7 having decreased expression levels compared to normal tissues. Among them, NSUN3 and NSUN4 were associated with clinicopathological features and survival of LUSC. In addition, they were also closely associated with the p53, cell cycle, and mTOR signaling pathway. In exploring the relationship between m5C regulators in LUSC and the tumor immune microenvironment, NSUN3 was mainly closely linked to CD8+ T cells, whereas NSUN4 was closely linked to neutrophils. In conclusion, m5C regulators have a major role in predicting the prognosis of LUSC patients and regulating the immune microenvironment.418 In addition, Pan and Chen also constructed prognostic prediction models of m5C modification in LUAD patients, respectively.48,66
Breast cancer (BRC)
Some studies reported the role of m5C modification-related lncRNAs in BRC. Risk models based on three m5C-lncRNAs (AP005131.2, AL121832.2, and LINC01152) can be used to predict the survival and prognosis of BRC patients. These three lncRNAs are expected to be novel markers and therapeutic targets for BRC. Yi et al.419 revealed that NSUN2 is involved in the metastatic progression of BRC. NSUN2 content in BRC was higher than in adjacent normal tissues. NSUN2 expression was closely related to many pathological features, including estrogen receptor (ER) and progesterone receptor. In addition, as in other cancers, NSUN2 also played a cancer-promoting effect in BRC, promoting BRC cell proliferation in vitro and in vivo and cell migration and invasion in vitro. Proliferation-associated nucleolar antigen p120, also as an RNA methyltransferase, also affects BRC prognosis, but the specific mechanism remains to be further explored.420 In addition to the m5C writer, the reader protein plays a role in BRC. Campbell et al.421 found that YBX1 interacted with the ER receptor and inhibited its activity, altering the estrogen dependence of BRC cells. FGFR2 signaling enhances this interaction, collectively transforming BRC into an estrogen-negative form. In conclusion, m5C-related regulators are expected to be new targets for BRC therapy.
Others
Models of m5C-related regulators for predicting prognosis in cancers, including PCa, HNSCC, OC, oral squamous cell carcinoma (OSCC), papillary thyroid carcinoma, and ccRCC, are continuously being established.422,423,424,425,426,427,428 In addition, some studies are based on the establishment of models and in-depth in vitro and in vivo experiments to further verify the role of m5C. For example, m5C promoted BLC progression. Specifically, the m5C reader YBX1 promoted the stability of its targeted mRNAs by recruiting ELAVL1. NSUN2 and YBX1 exerted oncogenic roles by targeting m5C in the 3′-UTR of HDGF. The proposal of the NSUN2/YBX1/m5C-HDGF signaling axis, to a certain extent, revealed the potential molecular mechanism in promoting the development of BLC.371 In leukemia, RNA m5C modifications and methyltransferases affected the chromatin structure, affecting patient response to drugs. NSUN3 and DNMT2 bind hnRNPK, which interacts with the transcription factor GATA1, and SPI1/PU.1, and with CDK9/P-TEFb to recruit RNA polymerase II at nascent RNAs to generate the 5-AZA-sensitive chromatin structure. In contrast, NSUN1 forms an active, 5-AZA-insensitive chromatin structure mainly with BRD4 and RNA polymerase II.429 High p120 (NSUN1) expression in PCa was related to increased tumor aggressiveness and poor prognosis.430 Similarly, NSUN2 was upregulated in HNSCC, and high NSUN2 expression was closely associated with poor clinical outcomes. NSUN2 can be used as a potential prognostic marker for HNSCC, providing a reference for finding new treatments.431 Interestingly, in OC, studies reported for the first time that NSUN2 and insulin-like growth factor-II (IGF-II) synergistically affected patient survival and prognosis. The NSUN2lowIGF-IIhigh subgroup had the worst survival, whereas the NSUN2highIGF-IIlow subgroup had the best overall and progression-free survival.432
Ψ and Ψ synthase
Ψ is the C5-glycoside isomer of uridine. The normal pyrimidine nucleoside is the N-1 atom of the heterocyclic ring bonded to the C-1′ atom of the pentose to form a glycosidic bond, whereas the pseudouracil nucleoside is the C-5 atom of the heterocyclic ring bonded to the C-1′ atom of the pentose.433 Ψ is the first discovered and currently the most abundant modified nucleoside in RNA, known as the “fifth nucleoside” in RNA.434,435,436 As early as 1951, Cohn et al.437 first obtained the complete 5′ nucleotide by enzymatic hydrolysis of calf liver ribonucleic acid. It was later named Ψ (psi, Ψ).438,439 Initially, Ψ was mainly reported in ncRNAs, such as rRNAs, tRNAs, and small nuclear RNAs (snRNAs). With the update of detection methods and technologies, Ψ modified almost all RNAs, including mRNA.440,441 The current commonly used detection methods for Ψ mainly include traditional reverse transcriptase and gel electrophoresis methods and methods based on mass spectrometry (MS; matrix-assisted laser desorption/ionization-MS combined with chemical derivatization).442,443,444
The conversion of uridine to Ψ is catalyzed by Ψ synthase, also called pseudouridine synthase (PUSs) in eukaryotes, the writer of Ψ. So far, six Ψ synthase families have been identified, namely TruA, TruB, TruD, RsuA, RluA, and Pus10p. Although these enzymes differ greatly in sequence, they all share a conserved core structure and active site.445,446,447 In yeast, the TruA family mainly includes Pus1, Pus2, and Pus3. Pus4 is the only member of the TruB family. The RluA family of enzymes includes Pus5, Pus6, Pus8, and Pus9. There is only one member of the TruD family, Pus7. In addition, the human homologs of PUSs are PUS1-4, PUS6, PUS7, PUS7L, PUS9, PUS10, RPUSD1-4.75,448,449 Another writer is dyskerin (DCK1), a H/ACA small ribonucleoprotein guide RNA-dependent enzyme that catalyzes the formation of Ψ using a guide RNA and protein (Cbf5 in yeast and DKC1 in humans) complementary to the sequence to be modified.436,450,451
As mentioned above, there are two main ways of pseudouracilylation of RNA substrates in eukaryotes. One is an RNA-independent mechanism whereby PUSs directly recognize and catalyze substrates. The other is an RNA-dependent mechanism that requires catalysis by box H/ACA RNPs.452 Each RNP contains a unique guide RNA component and a set of evolutionarily conserved four core proteins. The latter mainly includes dyskerin (DKC1; NAP57 in rats and Nop60B in Drosophila), nonhistone 2, nucleolar protein 10, and glycine-arginine-rich protein 1. The box H/ACA small RNPs complex recognizes the substrate and plays a role in the base pairing of the substrate RNA, of which DKC1 has catalytic activity. The RNA-dependent mechanism of pseudouracilylation occurs mainly on ncRNAs.453
No specific “readers” and “erasers” for Ψ have been found. The absence of eraser protein is explained by the fact that the C–C bond formed between the base and the ribose sugar in Ψ is much more inert than the C–N bond, making the pseudouridylation process irreversible (Fig. 5).454

Image created with BioRender (https://biorender.com/)
The mechanism of Ψ regulation. There are two main ways of pseudouracilylation of RNA substrates in eukaryotes. One is an RNA-independent mechanism whereby PUSs directly recognize and catalyze substrates. The other is an RNA-dependent mechanism that requires catalysis by box H/ACA RNPs. Each RNP contains a unique guide RNA component and a set of evolutionarily conserved four core proteins. Among them, only DCK1 has catalytic activity. Nhp2, Nop10, and Gar1 are regulatory units.
Functional consequences of Ψ in coding RNA and ncRNAs
As mentioned above, Ψ was originally reported mainly in tRNA, rRNA, and snRNA. In 2014, Schwartz et al.440 and Carlile et al.441 performed whole-genome sequencing of Ψ. They detected numerous pseudouridylation sites on mRNA and ncRNAs. Schwartz et al. reported 328 unique Ψ sites in yeast mRNA and ncRNA. Carlile et al. revealed ~260 Ψ sites in 238 protein-coding transcripts in yeast and 96 Ψ sites in 89 mRNAs in humans. Furthermore, they revealed that Pus1, Pus2, Pus4, and Pus7 were involved in mRNA pseudouridylation. More importantly, they also found that this pseudouridylation modification was affected by factors, such as the environment, which affected the subsequent regulation of the targeted RNA. In the Ψ structure, the typical N-C bond between the ribose and the base is replaced by a C–C bond, resulting in the retention of the H at the N1 position, which is equivalent to creating an additional hydrogen bond donor. When incorporated into RNA, Ψ can affect RNA thermodynamic stability and spatial conformation by increasing base stacking, improving base pairing, and sclerosing the sugar-phosphate backbone, making RNA more stable.454,455,456,457,458,459 In addition to the above effects, Ψ is involved in tRNA codon-anticodon base pairing, rRNA folding, snRNP biogenesis, pre-mRNA splicing, mRNA encoding, and translation, stress response, translation fidelity, and peptide bond formation rate.435,440,449,460,461
Role of Ψ in tRNA
In tRNA, there are a large number of pseudouridylation sites. Ψ can exist not only in the stem and loop of tRNA anticodon but also in TΨC loop and D stem, etc. For example, position 55 of the TΨC stem-loop structure, position 13 on the D stem, and positions 38 to 40 on the ASL structure.462,463 All these help stabilize the spatial structure of tRNA, coordinate codon and anticodon recognition pairings, and improve translation efficiency and accuracy.447,449,464 Huang et al.465 found that Asp60 mutations, conserved in nearly all Ψ synthases, affect catalytic activity. Furthermore, Asp residues are involved in the nucleophilic catalytic mechanism. However, the specific molecular mechanism is still being explored. A missense mutation in the PUS1 gene affecting highly conserved amino acids affects Ψ synthase 1 (Pus1p) production and depletes the tRNA for pseudouridylation. This eventually leads to mitochondrial myopathy and sideroblastic anemia. A yeast strain with disrupted Pus3p (Deg1p) enzymatic activity fails to form Ψ at tRNA 38 and 39, whereas other sites are unaffected. Ψ in tRNA ALSs are important for regulating translation processes in yeast.466 In addition, Ψ can stabilize the ASL of tRNALys,3.467 The product of the yeast YNL292w (PUS4) gene was identified as an enzyme that catalyzes pseudouracil formation at position 55 in the tRNA molecule. As mentioned earlier, Pus4 is the only member of the TruB family, which is generally conserved among tRNAs.468 Another study demonstrated that the RNA Ψ synthase TruB catalyzes Ψ formation at U55 in tRNA. This modification mainly occurs in the T-arm of tRNA.469
Role of Ψ in rRNA
Ψ is especially widely distributed in rRNA and present on almost all rRNAs. Ψ is distributed in different functional regions, mainly including the interface of large and small ribosomal subunits, the stem-loop structure of tRNA, the site of interaction with mRNA, the decoding center, and the peptidyl transferase center. Ψ affects ribosome production and protein synthesis. Box H/ACA small nucleolar ribonucleoproteins (snoRNAs) play a nonnegligible role in rRNA pseudouridylation.461,470,471 snoRNA can also regulate rRNA folding.472,473 In addition, Cbf5p (dyskerin) depletion also causes pre-rRNA processing defects similar to snR30 depletion.474 One study found that dyskerin deficiency in human cells results in defective rRNA uridine modification, altering ribosome activity.475,476 The gene encoding dyskerin is well-known as DKC1, and mutations in this gene can lead to Ψ deficiency and cause X-linked keratosis congenital.
Role of Ψ in mRNA
Although the modification site of Ψ was found in mRNA much later than in other ncRNAs, many studies focused on the interaction and mechanism between Ψ and mRNA. Ψ can affect mRNA stability. In vitro transcribed mRNA contain in exhibited greater stability when transferred to in vivo studies than in vitro transcribed mRNA containing uridine.477 Another study also demonstrated that PUS7 deletion reduced pseudouracilated mRNA levels after heat shock in yeast, suggesting that it was beneficial for enhanced transcript stability.440 The artificial addition of Ψ can mediate nonsense codon transitions in mRNA and inhibit translation termination, which may be a new mechanism for causing protein diversity.452 Ψ also promotes pre-mRNA splicing. Specifically, snRNAs are involved in the maturation of eukaryotic mRNAs. They are mainly responsible for the splicing of pre-mRNA. Prp5 is an RNA-dependent ATPase involved in monitoring U2 BSRR-branch site base pairing interactions. Results suggested that Ψ in U2 snRNA promotes pre-mRNA splicing by directly altering Prp5 binding.478,479,480 In addition, Ψ also plays a very important role in mediating mRNA translation. When mice were injected with Ψ-encoded mRNA for erythropoietin, red blood cell production increased, indicating that in vitro transcribed mRNA containing the modified nucleoside Ψ is beneficial for enhanced translation.481 A follow-up study found that this translational enhancement was mediated by reduced PKR activation.482 Based on the above-mentioned role of Ψ in mRNA, many therapeutic approaches based on Ψ-mRNA have emerged.477,481,483 For example, Ψ incorporation into mRNA produces excellent nonimmunogenic vectors. It also enhances the translation ability and stability of mRNA.477
Role of Ψ in other ncRNAs
Ψ is also present on lncRNA. For example, lncRNA ZFAS1, telomerase RNA component (TERC), and small nucleolar RNA host genes 1 and 7 (SNHG1 and SNHG7) contain pseudouracil components, but the specific mechanism remains to be confirmed by further studies.461,484,485,486 In conclusion, Ψ is the most abundant modification in RNA, and exploring the mechanism behind it is a challenge and an opportunity. Further studies will uncover more about the role and significance of Ψ in RNA.
Role of Ψ in cancer
As mentioned above, Ψ is the most prevalent posttranscriptional modification in RNA. The pseudouridylation process is mainly catalyzed by PUSs, and there are two catalytic modes in eukaryotes. One is an RNA-independent way, which is acted independently by the PUSs. However, there are few reports about PUSs in cancer. The other is an RNA-dependent mechanism involving an enzyme called dyskerin encoded by the DKC1 gene. It forms a complex with box H/ACA snRNA, pseudouridylates RNA, and mediates the posttranscriptional modification of RNA. In addition, dyskerin is also associated with human telomerase RNA containing the H/ACA RNA motif.486,487,488,489 DKC1 mutations cause dyskeratosis congenita (DC), a disease characterized by increased tumor susceptibility and a propensity to age.487 It was initially thought that lower pseudouridylation levels were a pervasive feature of cancer, in which DKC1 primarily functions as a tumor suppressor.490 However, more and more studies found that the DKC1 expression level is upregulated in some cancers and can also function as an oncogene (Table 3).491,492
Ψ and digestive system cancers
As early as 1983, Salvatore et al.493 pointed out that Ψ in serum can be used as a tumor marker. In 1988, studies reported that serum Ψ and α-fetoprotein (AFP) could serve as complementary markers for HCC diagnosis. Among seven patients with very small liver cancer, four were negative for AFP but positive for serum Ψ, suggesting that serum Ψ may be a useful marker for early diagnosis of HCC.494 In addition, detection of Ψ in urine also is used as a marker for some tumors, including CRC and HCC.
Turano et al.495 detected DKC1 mRNA expression in cancer and adjacent tissues of eight CRC patients using real-time polymerase chain reaction. Results supported that DKC1 expression can be used as a tumor marker for CRC. In colon cancer, DKC1 is highly expressed and predicts a poor prognosis. DKC1 binds to and increases the expression of some ribosomal proteins in a manner dependent on its Ψ synthase activity. The latter interacts with HRAS to inhibit the downstream RAS/RAF/MEK/ERK pathway. The DKC1 inhibitor pyrazofuran and the MEK1/2 inhibitor trametinib synergistically inhibit CRC growth.496 Studies also found a cancer-specific single nucleotide variant at nucleotide 1248.U in 18S rRNA from CRC patients. Loss of rRNA m1acp3Ψ modification is a hallmark of cancer.497 Furthermore, DKC1 enhances CRC angiogenesis by directly activating hypoxia-inducible factor-1α transcription, promoting CRC cell metastasis.498 In HCC, DKC1 expression is similarly upregulated and exerts a tumor-promoting effect. High DKC1 expression was an independent prognostic factor (hazard risk = 2.912; P = 0.007). Also, DKC1 expression was significantly correlated with MKI67 and MYC mRNA.499 This may involve the molecular mechanism of DKC1 promoting cancer. Of course, further research is needed in the future. Furthermore, oxidatively modified protein disulfide isomerase-related 3 increases DKC1 mRNA levels and tumor cell survival, driving the progression of liver malignancies.500 H/ACA snoRNA SNORA24 mediates the pseudouridylation of rRNA U609 and U863. Translation efficiency and accuracy are reduced in HCC cells lacking SNORA24-directed Ψ modification.501
Ψ and nondigestive system cancers
Ψ in nondigestive cancers is currently focused on breast, lung, and prostate cancers. Studies successfully revealed and summarized the predictive value of Ψ in these cancers, and Ψ is expected to become a novel cancer biomarker.435,502,503,504 Nuclear and nucleolar expression of DKC1 protein was strongly associated with higher tumor grade, high nucleolar score, and poorer Nottingham prognostic index. Furthermore, DKC1 overexpression in BRC predicts a poor prognosis.505 Dyskerin expression is highly variable in sporadic human tumors of various histological origins. Particularly in BRC, low dyskerin expression results in rRNA pseudouridylation and a reduction in the RNA component of telomerase.506 In addition, p53 is a well-known tumor suppressor, and p53 function is also reduced in the presence of low keratin levels, contributing to the tumor phenotype.507 At first glance, the finding that DKC1 is overexpressed in common carcinomas seems contradictory, as keratin-inactivating mutations in DC confer increased tumor susceptibility. In tumors arising from DC, a possible explanation is that keratin mutations would constitute the major event and favorably trigger subsequent cancer development. Conversely, dyskerin overexpression may represent an increase in RNA biosynthesis and telomerase activity in cancer progression, such as in breast cancer.508,509 In NSCLC, lncRNAs PCAT1 and DKC1 act synergistically to promote cancer cell proliferation and invasion and inhibit cancer cell apoptosis. The molecular mechanism involves the vascular endothelial growth factor/Akt/Bcl-2/caspase-9 pathway.510 Similarly, in prostate cancer, DKC1 is also highly expressed and promotes cancer progression. Unlike NSCLC, DKC1 mainly affects prostate cancer cell proliferation without causing apoptosis, highlighting the important role of DKC1 in maintaining protein biosynthesis.511 In another interesting study, an association between dyskerin expression and survival was found in lung cancer only in the absence of amplification of the TERC gene. Overall survival was significantly reduced in patients with higher dyskerin expression. In conclusion, the effect of dyskerin expression on tumor clinical outcome is related to its role in maintaining TERC stability.512 In addition, one study investigated the relationship between single nucleotide polymorphisms in chromatin-interacting regions and lung cancer risk and found four new lung cancer susceptibility loci. rs9309336 may interfere with PUS10 expression, reducing tumor cell sensitivity to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Finally, it promotes tumor cell immortalization and lung cancer occurrence.513 Jana et al.514 previously showed that PUS10 moved to the mitochondria during TRAIL-induced apoptosis, releasing cytochrome c and SMAC. This CRM1-mediated nuclear export of PUS10 needed caspase-3, and the translocated PUS10 reciprocally activated caspase-3 to form an amplification loop. Anything that interfered with HuP10 movement or its interaction with mitochondria reduced tumor cell sensitivity to TRAIL. The effect of the PUS enzyme family in cancer is still less studied, and more research is worth adding in the future.
Other studies on Ψ in glioma,515,516 OSCC,517 and OC518 are not described in detail in the main text and can be found in Table 3.
Impact of RNA methylation modification on cancer therapy
DNA methylation was discovered initially. With the advancement of NGS technology, RNA methylation appeared in people’s field of vision. Over the years, there has been a relatively good understanding of some of the basics of RNA methylation. It mainly includes the common types of RNA methylation modifications, such as m6A, m5C, and Ψ, and the types and functions of the regulators of RNA methylation modification. More attention is paid to the impact of RNA methylation modification on life activities and disease progression in recent years, especially in cancer. However, despite extensive research, the impact on the mechanisms by which RNA modifications and their associated proteins are regulated in cancer remains largely unknown. Growing evidence suggested that RNA modification pathways are also mis regulated in human cancers, which may be ideal targets for cancer therapy. Numerous factors supported the idea that RNA-modifying regulators may be ideal targets for drug discovery, and developing activators or inhibitors of RNA methylation-modifying regulators could add valuable drugs and experience for cancer treatment.
In the following, this review mainly discussed and summarized the role of m6A in cancer therapy. A summary of the impact of m5C and Ψ modifications in cancer therapy is presented, although relevant studies are still relatively few. m5C methyltransferases NSUN3 and DNMT2 can mediate the generation of 5-AZA-sensitive chromatin structures through a series of mechanisms, providing new insights into drug resistance in leukemia treatment.429 In addition to the effects on chemoresistance, therapeutic approaches targeting m5C modulators may also hold great promise. Notably, m5C is the most common form of DNA methylation. However, there is no way to control the specific inhibition of RNA methylase by drugs without affecting DNA methylation. As mentioned earlier, given that Ψ is present in urine, blood, saliva, etc., it is expected to be a potential biomarker for early cancer diagnosis.519,520 Moreover, this noninvasive detection method has huge clinical application prospects. In addition, pyrazoline and 5-fluorouracil are currently two common drugs that inhibit DKC1, opening the understanding of the role of Ψ in cancer therapy. Of course, further in-depth research and the development of new targeted drugs are needed.
m6A is the most common type of RNA methylation modification. m6A modification is regulated by methyltransferases, demethylases, and RNA-binding proteins. m6A modulators can be used as potential therapeutic targets for cancer and in targeted therapy, radiotherapy and chemotherapy, immunotherapy, and other aspects. In terms of targeted therapy, there is no small-molecule inhibitor of RNA methyltransferase, but some inhibitors of RNA demethylase have been developed and used.304,308,521,522,523,524,525,526,527,528 Current inhibitors primarily target FTO, a common demethylase. For example, MO-I-500 can selectively inhibit the m6A demethylase activity of FTO. MO-I-500 is an α-ketoglutarate mimetic that exhibits high specificity for FTO. It inhibits FTO in vitro and in vivo, as manifested by increased overall levels of RNA methylation. After MO-I-500 inhibited FTO, it inhibited the survival of rare pan-drug-resistant triple-negative inflammatory breast cancer cells.521,522 Meclofenamic acid (MA) is a nonsteroidal anti-inflammatory drug approved by the U.S. Food and Drug Administration. Recent studies revealed that MA could specifically inhibit FTO, increasing m6A levels. In GBM, FTO inhibition using MA2, the ethyl ester form of MA, inhibits cancer cell survival and tumor progression.304,523 R-2HG is the major metabolite of mutant isocitrate dehydrogenase 1/2. Also considered an FTO inhibitor, R-2HG exerts a tumor suppressor effect in leukemia and glioma by targeting FTO/m6A/MYC/CEBPA signaling.308 In addition, FB23-2, rhein, etc., are currently known inhibitors of FTO.524,525 In chemoradiotherapy, resistance to therapy is an unresolved bottleneck and challenge in cancer treatment. Several studies showed that RNA modifications affect primary and acquired drug resistance in cancer. m6A modulators also reduce or exacerbate resistance to chemoradiotherapy in cancer patients.168 For example, targeting SNHG3/miR-186-5p reversed platinum treatment-induced elevated m6A levels by modulating METTL3 in esophageal cancer.529 m6A modification of FZD10 mRNA leads to PARPi resistance in BRCA-deficient epithelial ovarian cancer cells by upregulating the Wnt/β-catenin pathway.530 In addition, METTL3 can promote chemoresistance and radioresistance of pancreatic cancer cells.531 In terms of immunotherapy, two studies also revealed that m6A might be related to cancer immunotherapy. Yang et al.327 found that FTO promotes anti-PD-1 resistance, and inhibition of FTO expression can increase melanoma cell sensitivity to immunotherapy. Li et al.532 found that m6A-modifying enzymes can act as key regulators of T cells, regulating T-cell homeostasis. In all, the impact of RNA methylation modification in cancer treatment is very extensive, and there are still many unexplored areas with huge research prospects.
Conclusion and prospects
To date, at least 170 different posttranscriptional RNA modifications are known.533,534 These modifications range from methylation to complex chemical structures, with methylation being the most abundant. With the development of high-throughput NGS technology, the improvement of the sensitivity of liquid chromatography, and the update of other sequencing technologies, there is a better understanding and mastery of identifying the overall level of RNA methylation. The discovery and functional studies on the types of RNA methylation and various methylation-related regulators have greatly advanced the understanding of RNA methylation. RNA methylation plays an indispensable role in regulating gene transcription, expression, editing, stability, and degradation.535 RNA modification is a key player in various cellular biological processes, in which RNA modification enzymes play a decisive role.464 As mentioned above, epigenetic modification refers to heritable phenotypic changes without changing the nucleic acid sequence. These changes include DNA methylation, histone modifications, chromatin remodeling, and RNAi. DNA methylation and histone modifications mainly affect transcriptional events, whereas reversible RNA methylation mainly affects the regulation of posttranscriptional gene expression and directly affects protein production.144 RNA modifications can affect not only normal biological processes, including development and cell differentiation, but also abnormal life activities, such as inflammation, infertility, neurological diseases, and cancer. RNA modifications and their regulators are often aberrantly expressed in tumor tissues. Abnormal expression is also closely related to the prognosis of cancer patients. The effects of RNA modifications in cancer are multiple. Both can play a role in promoting cancer. On the one hand, RNA modification can reduce the stability of tumor suppressor genes to eliminate their inhibitory effect, thereby promoting cancer progression; on the other hand, RNA modification can enhance the stability and stability of proto-oncogene transcripts. Conversely, they can also play a tumor suppressor role in cancer, inhibiting cancer cell proliferation, migration, and invasion and inhibiting cancer occurrence and development. In some cases, an enzyme may have opposing effects in different cancer types or even very different effects within the same cancer type. Because RNA modifications are diverse, and the number of modified coding RNAs and ncRNAs is enormous, this paradox seems not surprising. The current hypothesis is based only on individual cohorts with a remarkably limited number of cancer patients whose genetic background and environmental factors influence the results. Based on this, this review affirmed the importance of developing small-molecule inhibitors targeting RNA modification sites and RNA-modifying enzymes, providing new and more targeted approaches for cancer therapy. In addition, more relevant studies are needed to further verify and explain the specific mechanism of RNA methylation in cancer and explain some of the existing contradictory studies more reasonably.
References
Sun, H., Wang, X. & Zhai, S. The rational design and biological mechanisms of nanoradiosensitizers. Nanomaterials 10, 504 (2020).
Hu, S. C., Yang, J., Chen, C., Song, J. R. & Pan, W. D. Design, synthesis of novel tetrandrine-14-l-amino acid and tetrandrine-14-l-amino acid-urea derivatives as potential anti-cancer agents. Molecules 25, 1738 (2020).
Zeng, D. et al. Synergistic photothermal/photodynamic suppression of prostatic carcinoma by targeted biodegradable MnO(2) nanosheets. Drug Deliv. 26, 661–672 (2019).
Rong, Y. et al. DDRS: Detection of drug response SNPs specifically in patients receiving drug treatment. Comput. Struct. Biotechnol. J. 19, 3650–3657 (2021).
Chen, M. H. et al. How may ramucirumab help improve treatment outcome for patients with gastrointestinal cancers? Cancers 13, 3536 (2021).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Zhu, Y. C. et al. Clonally-related primary ALK rearranged adenocarcinoma and associated metastatic lesions. Thorac. Cancer 9, 881–884 (2018).
Li, T. et al. Anlotinib combined with gefitinib can significantly improve the proliferation of epidermal growth factor receptor-mutant advanced non-small cell lung cancer in vitro and in vivo. Transl. Lung Cancer Res. 10, 1873–1888 (2021).
Wan, Y. et al. LncRNA WT1-AS downregulates lncRNA UCA1 to suppress non-small cell lung cancer and predicts poor survival. BMC Cancer 21, 104 (2021).
Wang, T. et al. A randomized multicenter phase II trial of mecapegfilgrastim single administration versus granulocyte colony-stimulating growth factor on treating chemotherapy-induced neutropenia in breast cancer patients. Ann. Transl. Med. 7, 196 (2019).
Naik, N. et al. Deep learning-enabled breast cancer hormonal receptor status determination from base-level H&E stains. Nat. Commun. 11, 5727 (2020).
Lin, Y., Zhang, W., Cao, H., Li, G. & Du, W. Classifying breast cancer subtypes using deep neural networks based on multi-omics data. Genes 11, 888 (2020).
Moens, S. et al. The mitotic checkpoint is a targetable vulnerability of carboplatin-resistant triple negative breast cancers. Sci. Rep. 11, 3176 (2021).
Mizuno, N. et al. Evaluation of robustness in hybrid intensity-modulated radiation therapy plans generated by commercial software for automated breast planning. Sci. Rep. 12, 1418 (2022).
Yu, X. J. et al. Characterization of somatic mutations in air pollution-related lung cancer. EBioMedicine 2, 583–590 (2015).
Palve, V., Mallick, S., Ghaisas, G., Kannan, S. & Teni, T. Overexpression of Mcl-1L splice variant is associated with poor prognosis and chemoresistance in oral cancers. PLoS One 9, e111927 (2014).
Kim, J. A. et al. Serum vitamin levels and their relationships with other biomarkers in Korean breast cancer patients. Nutrients 12, 2831 (2020).
Zheng, L. et al. PBN11-8, a cytotoxic polypeptide purified from marine bacillus, suppresses invasion and migration of human hepatocellular carcinoma cells by targeting focal adhesion kinase pathways. Polymers 10, 1043 (2018).
Vranes, V. et al. Size and shape filtering of malignant cell clusters within breast tumors identifies scattered individual epithelial cells as the most valuable histomorphological clue in the prognosis of distant metastasis risk. Cancers. 11, 1615 (2019).
Yao, D., Wang, Z., Cai, H., Li, Y. & Li, B. Relationship between red cell distribution width and prognosis in patients with breast cancer after operation: A retrospective cohort study. Biosci. Rep. 39, BSR20190740 (2019).
Cheon, H., Paik, J. H., Choi, M., Yang, H. J. & Son, J. H. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci. Rep. 9, 6413 (2019).
Antoun, E. et al. Maternal dysglycaemia, changes in the infant’s epigenome modified with a diet and physical activity intervention in pregnancy: Secondary analysis of a randomised control trial. PLoS Med. 17, e1003229 (2020).
Parira, T. et al. Novel detection of post-translational modifications in human monocyte-derived dendritic cells after chronic alcohol exposure: Role of inflammation regulator H4K12ac. Sci. Rep. 7, 11236 (2017).
Dong, X. et al. Effect of luteolin on the methylation status of the OPCML gene and cell growth in breast cancer cells. Exp. Ther. Med. 16, 3186–3194 (2018).
Zhou, M. et al. miR-181d/RBP2/NF-κB p65 feedback regulation promotes chronic myeloid leukemia blast crisis. Front. Oncol. 11, 654411 (2021).
Liu, X. et al. TET2 is involved in DNA hydroxymethylation, cell proliferation, and inflammatory response in keratinocytes. Mol. Med. Rep. 21, 1941–1949 (2020).
Wang, K. C. et al. Ten-eleven translocation 1 dysfunction reduces 5-hydroxymethylcytosine expression levels in gastric cancer cells. Oncol. Lett. 15, 278–284 (2018).
Ma, Z. et al. Epigenetic drift of H3K27me3 in aging links glycolysis to healthy longevity in Drosophila. Elife 7, e35368 (2018).
Wu, J. et al. Histone methyltransferase SETD1A induces epithelial-mesenchymal transition to promote invasion and metastasis through epigenetic reprogramming of snail in gastric cancer. Front. Cell Dev. Biol. 9, 657888 (2021).
Lin, X., Su, J., Chen, K., Rodriguez, B. & Li, W. Sparse conserved under-methylated CpGs are associated with high-order chromatin structure. Genome Biol. 18, 163 (2017).
Kalin, J. H. et al. Targeting the CoREST complex with dual histone deacetylase and demethylase inhibitors. Nat. Commun. 9, 53 (2018).
Pillai, A., Gungi, A., Reddy, P. C. & Galande, S. Epigenetic regulation in hydra: Conserved and divergent roles. Front. Cell Dev. Biol. 9, 663208 (2021).
Li, W., Shi, Y., Zhang, T., Ye, J. & Ding, J. Structural insight into human N6amt1-Trm112 complex functioning as a protein methyltransferase. Cell Discov. 5, 51 (2019).
Xie, S. Q. et al. N (6)-Methyladenine DNA modification in the woodland strawberry (Fragaria vesca) genome reveals a positive relationship with gene transcription. Front. Genet. 10, 1288 (2019).
Chen, F. et al. Cellular macromolecules-tethered DNA walking indexing to explore nanoenvironments of chromatin modifications. Nat. Commun. 12, 1965 (2021).
Douvlataniotis, K., Bensberg, M., Lentini, A., Gylemo, B. & Nestor, C. E. No evidence for DNA N (6)-methyladenine in mammals. Sci. Adv. 6, eaay3335 (2020).
Fu, T. et al. Thymine DNA glycosylase recognizes the geometry alteration of minor grooves induced by 5-formylcytosine and 5-carboxylcytosine. Chem. Sci. 10, 7407–7417 (2019).
Xu, X., Watt, D. S. & Liu, C. Multifaceted roles for thymine DNA glycosylase in embryonic development and human carcinogenesis. Acta Biochim. Biophys. Sin. 48, 82–89 (2016).
Slyvka, A., Mierzejewska, K. & Bochtler, M. Nei-like 1 (NEIL1) excises 5-carboxylcytosine directly and stimulates TDG-mediated 5-formyl and 5-carboxylcytosine excision. Sci. Rep. 7, 9001 (2017).
Piedra-Aguilera, Á. et al. Integrated single-base resolution maps of transcriptome, sRNAome and methylome of Tomato yellow leaf curl virus (TYLCV) in tomato. Sci. Rep. 9, 2863 (2019).
Du, X. et al. Promoter hypomethylation is responsible for upregulated expression of HAI-1 in hepatocellular carcinoma. Dis. Markers 2019, 9175215 (2019).
Ma, Y. et al. siPRDX2-elevated DNM3 inhibits the proliferation and metastasis of colon cancer cells via AKT signaling pathway. Cancer Manag. Res. 11, 5799–5811 (2019).
Monteiro-Reis, S. et al. A multiplex test assessing MiR663a(me) and VIM(me) in urine accurately discriminates bladder cancer from inflammatory conditions. J. Clin. Med. 9, 605 (2020).
Yuan, M., Yao, L. & Abulizi, G. Tumor-suppressor gene SOX1 is a methylation-specific expression gene in cervical adenocarcinoma. Medicine 98, e17225 (2019).
Marsh, J. W. et al. Bioinformatic analysis of bacteria and host cell dual RNA-sequencing experiments. Brief. Bioinform. 19, 1115–1129 (2018).
Yang, J., Chen, J., Fei, X., Wang, X. & Wang, K. N6-methyladenine RNA modification and cancer. Oncol. Lett. 20, 1504–1512 (2020).
Hopfinger, M. C., Kirkpatrick, C. C. & Znosko, B. M. Predictions and analyses of RNA nearest neighbor parameters for modified nucleotides. Nucleic Acids Res. 48, 8901–8913 (2020).
Chen, H. et al. M(5)C regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in lung adenocarcinoma. Transl. Lung Cancer Res. 10, 2172–2192 (2021).
Aschhoff, H. J. et al. 7-Methylguanine specific tRNA-methyltransferase from Escherichia coli. Nucleic Acids Res. 3, 3109–3122 (1976).
Guo, B. et al. Identification of the signature associated with m(6)A RNA methylation regulators and m(6)A-related genes and construction of the risk score for prognostication in early-stage lung adenocarcinoma. Front. Genet. 12, 656114 (2021).
Shi, Y. et al. Reduced expression of METTL3 promotes metastasis of triple-negative breast cancer by m6A methylation-mediated COL3A1 upregulation. Front. Oncol. 10, 1126 (2020).
Zheng, H., Li, S., Zhang, X. & Sui, N. Functional implications of active N(6)-methyladenosine in plants. Front. Cell Dev. Biol. 8, 291 (2020).
He, J. J. et al. m(6)A Reader YTHDC2 promotes radiotherapy resistance of nasopharyngeal carcinoma via activating IGF1R/AKT/S6 signaling axis. Front. Oncol. 10, 1166 (2020).
Yang, G., Sun, Z. & Zhang, N. Reshaping the role of m6A modification in cancer transcriptome: A review. Cancer Cell Int. 20, 353 (2020).
Song, H. et al. Epitranscriptomics and epiproteomics in cancer drug resistance: therapeutic implications. Signal Transduct. Target Ther. 5, 193 (2020).
Qu, N. et al. Multiple m(6)A RNA methylation modulators promote the malignant progression of hepatocellular carcinoma and affect its clinical prognosis. BMC Cancer 20, 165 (2020).
Feng, Z. Y., Gao, H. Y. & Feng, T. D. Immune infiltrates of m(6)A RNA methylation-related lncRNAs and identification of PD-L1 in patients with primary head and neck squamous cell carcinoma. Front. Cell Dev. Biol. 9, 672248 (2021).
Gu, C. et al. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol. Cancer 18, 168 (2019).
Xu, S. et al. Oxygen glucose deprivation/re-oxygenation-induced neuronal cell death is associated with Lnc-D63785 m6A methylation and miR-422a accumulation. Cell Death Dis. 11, 816 (2020).
Zhang, Z. et al. Genetic analyses support the contribution of mRNA N(6)-methyladenosine (m(6)A) modification to human disease heritability. Nat. Genet. 52, 939–949 (2020).
Wang, Q. et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 11, 911 (2020).
Liu, Z., Liu, N., Huang, Z. & Wang, W. METTL14 overexpression promotes osteosarcoma cell apoptosis and slows tumor progression via caspase 3 activation. Cancer Manag. Res. 12, 12759–12767 (2020).
Luo, J., Xu, T. & Sun, K. N6-Methyladenosine RNA modification in inflammation: Roles, mechanisms, and applications. Front. Cell Dev. Biol. 9, 670711 (2021).
Zhang, W. et al. Multifaceted functions and novel insight into the regulatory role of RNA N(6)-methyladenosine modification in musculoskeletal disorders. Front. Cell Dev. Biol. 8, 870 (2020).
Chen, X. et al. m5CPred-SVM: A novel method for predicting m5C sites of RNA. BMC Bioinform. 21, 489 (2020).
Pan, J., Huang, Z. & Xu, Y. m5C-Related lncRNAs predict overall survival of patients and regulate the tumor immune microenvironment in lung adenocarcinoma. Front. Cell Dev. Biol. 9, 671821 (2021).
Liu, L. et al. Bioinformatics approaches for deciphering the epitranscriptome: Recent progress and emerging topics. Comput. Struct. Biotechnol. J. 18, 1587–1604 (2020).
Cheng, M. Y. et al. Novel dual methylation of cytidines in the RNA of mammals. Chem. Sci. 12, 8149–8156 (2021).
Gu, X. et al. Uncovering the association between m(5)C regulator-mediated methylation modification patterns and tumour microenvironment infiltration characteristics in hepatocellular carcinoma. Front. Cell Dev. Biol. 9, 727935 (2021).
Zhang, S. Y., Zhang, S. W., Zhang, T., Fan, X. N. & Meng, J. Recent advances in functional annotation and prediction of the epitranscriptome. Comput. Struct. Biotechnol. J. 19, 3015–3026 (2021).
Gao, Y. et al. NOP2/Sun RNA methyltransferase 2 promotes tumor progression via its interacting partner RPL6 in gallbladder carcinoma. Cancer Sci. 110, 3510–3519 (2019).
Hu, Y. et al. NSUN2 modified by SUMO-2/3 promotes gastric cancer progression and regulates mRNA m5C methylation. Cell Death Dis. 12, 842 (2021).
Wang, J. Z. et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 41, 560–575 (2021).
Addepalli, B. & Limbach, P. A. Pseudouridine in the anticodon of Escherichia coli tRNATyr(QΨA) is catalyzed by the dual specificity enzyme RluF. J. Biol. Chem. 291, 22327–22337 (2016).
Rintala-Dempsey, A. C. & Kothe, U. Eukaryotic stand-alone pseudouridine synthases—RNA modifying enzymes and emerging regulators of gene expression? RNA Biol. 14, 1185–1196 (2017).
Fujikane, R. et al. Contribution of protein Gar1 to the RNA-guided and RNA-independent rRNA:Ψ-synthase activities of the archaeal Cbf5 protein. Sci. Rep. 8, 13815 (2018).
Anderson, B. R. et al. Nucleoside modifications in RNA limit activation of 2’-5’-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 39, 9329–9338 (2011).
Ge, J. & Yu, Y. T. RNA pseudouridylation: New insights into an old modification. Trends Biochem. Sci. 38, 210–218 (2013).
Giofrè, S. V. et al. Synthesis and biological properties of 5-(1H-1,2,3-triazol-4-yl)isoxazolidines: A new class of C-nucleosides. Molecules 20, 5260–5275 (2015).
Desrosiers, R., Friderici, K. & Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA 71, 3971–3975 (1974).
Lee, M., Kim, B. & Kim, V. N. Emerging roles of RNA modification: m(6)A and U-tail. Cell 158, 980–987 (2014).
Meyer, K. D. & Jaffrey, S. R. Rethinking m(6)A readers, writers, and erasers. Annu. Rev. Cell Dev. Biol. 33, 319–342 (2017).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012).
Wei, C. M. & Moss, B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 16, 1672–1676 (1977).
Bokar, J. A., Shambaugh, M. E., Polayes, D., Matera, A. G. & Rottman, F. M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3, 1233–1247 (1997).
Wang, K., Peng, J. & Yi, C. The m(6)A consensus motif provides a paradigm of epitranscriptomic studies. Biochemistry 60, 3410–3412 (2021).
Jia, G. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011).
Jia, G., Fu, Y. & He, C. Reversible RNA adenosine methylation in biological regulation. Trends Genet. 29, 108–115 (2013).
Zhou, Z. et al. Mechanism of RNA modification N6-methyladenosine in human cancer. Mol. Cancer 19, 104 (2020).
Zhao, Y., Shi, Y., Shen, H. & Xie, W. m(6)A-binding proteins: The emerging crucial performers in epigenetics. J. Hematol. Oncol. 13, 35 (2020).
Bujnicki, J. M., Feder, M., Radlinska, M. & Blumenthal, R. M. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m(6)A methyltransferase. J. Mol. Evol. 55, 431–444 (2002).
Wang, Y. et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 16, 191–198 (2014).
Wang, P., Doxtader, K. A. & Nam, Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell. 63, 306–317 (2016).
Ramalingam, H. et al. A methionine-Mettl3-N(6)-methyladenosine axis promotes polycystic kidney disease. Cell Metab. 33, 1234–1247.e1237 (2021).
Wang, X. et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 534, 575–578 (2016).
Schwartz, S. et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 8, 284–296 (2014).
Zhong, S. et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 20, 1278–1288 (2008).
Ping, X. L. et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189 (2014).
Liu, J. et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95 (2014).
Patil, D. P. et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373 (2016).
Knuckles, P. et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 32, 415–429 (2018).
Yue, Y. et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 4, 10 (2018).
Miranda-Gonçalves, V. et al. The component of the m(6)A writer complex VIRMA is implicated in aggressive tumor phenotype, DNA damage response, and cisplatin resistance in germ cell tumors. J. Exp. Clin. Cancer Res. 40, 268 (2021).
Wen, J. et al. Zc3h13 Regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol. Cell. 69, 1028–1038.e1026 (2018).
Warda, A. S. et al. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 18, 2004–2014 (2017).
Shima, H. et al. S-Adenosylmethionine synthesis is regulated by selective N(6)-adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep. 21, 3354–3363 (2017).
Pendleton, K. E. et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169, 824–835.e814 (2017).
van Tran, N. et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 47, 7719–7733 (2019).
Richard, E. M. et al. Bi-allelic variants in METTL5 cause autosomal-recessive intellectual disability and microcephaly. Am. J. Hum. Genet. 105, 869–878 (2019).
Ma, H. et al. Publisher Correction: N(6)-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 15, 549 (2019).
Mathiyalagan, P. et al. FTO-Dependent N(6)-methyladenosine regulates cardiac function during remodeling and repair. Circulation 139, 518–532 (2019).
Wang, J. Y., Chen, L. J. & Qiang, P. The potential role of N6-methyladenosine (m6A) demethylase fat mass and obesity-associated gene (FTO) in human cancers. Onco Targets Ther. 13, 12845–12856 (2020).
Niu, Y. et al. N6-methyl-adenosine (m6A) in RNA: An old modification with a novel epigenetic function. Genomics Proteom. Bioinform. 11, 8–17 (2013).
Imai, Y., Matsuo, N., Ogawa, S., Tohyama, M. & Takagi, T. Cloning of a gene, YT521, for a novel RNA splicing-related protein induced by hypoxia/reoxygenation. Brain Res. Mol. Brain Res. 53, 33–40 (1998).
Hartmann, A. M., Nayler, O., Schwaiger, F. W., Obermeier, A. & Stamm, S. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59(fyn). Mol. Biol. Cell. 10, 3909–3926 (1999).
Li, Q., He, W. & Wan, G. Methyladenosine modification in RNAs: Classification and roles in gastrointestinal cancers. Front. Oncol. 10, 586789 (2020).
Wang, X. et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
Han, D. et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274 (2019).
Du, H. et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 7, 12626 (2016).
Ivanova, I. et al. The RNA m(6)A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and Oocyte competence. Mol. Cell. 67, 1059–1067.e1054 (2017).
Shi, H. et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 27, 315–328 (2017).
Li, A. et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 27, 444–447 (2017).
Chang, G. et al. YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 38, 857–871.e857 (2020).
Roundtree, I. A. et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife 6, e31311 (2017).
Xiao, W. et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol. Cell. 61, 507–519 (2016).
Hsu, P. J. et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 27, 1115–1127 (2017).
Huang, H. et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295 (2018).
Hu, X. et al. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 27, 1782–1794 (2020).
Meyer, K. D. et al. 5’ UTR m(6)A promotes cap-independent translation. Cell 163, 999–1010 (2015).
Liu, N. et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564 (2015).
Zhou, K. I. et al. Regulation of Co-transcriptional Pre-mRNA Splicing by m(6)A through the low-complexity protein hnRNPG. Mol. Cell. 76, 70–81.e79 (2019).
Alarcón, C. R. et al. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell 162, 1299–1308 (2015).
Wu, B. et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat. Commun. 9, 420 (2018).
Chen, M. & Wong, C. M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol. Cancer 19, 44 (2020).
Wiener, D. & Schwartz, S. The epitranscriptome beyond m(6)A. Nat. Rev. Genet. 22, 119–131 (2021).
Yi, Y. C., Chen, X. Y., Zhang, J. & Zhu, J. S. Novel insights into the interplay between m(6)A modification and noncoding RNAs in cancer. Mol. Cancer 19, 121 (2020).
Covelo-Molares, H. et al. The comprehensive interactomes of human adenosine RNA methyltransferases and demethylases reveal distinct functional and regulatory features. Nucleic Acids Res. 49, 10895–10910 (2021).
Merrick, W. C. & Pavitt, G. D. Protein synthesis initiation in eukaryotic cells. Cold Spring Harb. Perspect. Biol. 10, a033092 (2018).
Malone, C. D. et al. The exon junction complex controls transposable element activity by ensuring faithful splicing of the piwi transcript. Genes Dev. 28, 1786–1799 (2014).
Chan, S., Choi, E. A. & Shi, Y. Pre-mRNA 3’-end processing complex assembly and function. Wiley Interdiscip. Rev. Rna. 2, 321–335 (2011).
Zhao, B. S., Roundtree, I. A. & He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 18, 31–42 (2017).
Kierzek, E. & Kierzek, R. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 31, 4472–4480 (2003).
Fu, Y., Dominissini, D., Rechavi, G. & He, C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet. 15, 293–306 (2014).
Zheng, G. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 49, 18–29 (2013).
Bhalala, O. G., Srikanth, M. & Kessler, J. A. The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 9, 328–339 (2013).
Shahid, S. et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 553, 82–85 (2018).
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Guo, H., Ingolia, N. T., Weissman, J. S. & Bartel, D. P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 (2010).
Fabian, M. R. & Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 19, 586–593 (2012).
Wang, F. et al. H19X-encoded miR-424(322)/-503 cluster: emerging roles in cell differentiation, proliferation, plasticity, and metabolism. Cell Mol. Life Sci. 76, 903–920 (2019).
Croce, C. M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10, 704–714 (2009).
Yi, R., Qin, Y., Macara, I. G. & Cullen, B. R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 17, 3011–3016 (2003).
Lund, E., Güttinger, S., Calado, A., Dahlberg, J. E. & Kutay, U. Nuclear export of microRNA precursors. Science 303, 95–98 (2004).
Lee, Y. et al. MicroRNA genes are transcribed by RNA polymerase II. Embo J. 23, 4051–4060 (2004).
Han, J. et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125, 887–901 (2006).
Lee, Y. et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 (2003).
Gregory, R. I. et al. The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 (2004).
Mirihana Arachchilage, G., Dassanayake, A. C. & Basu, S. A potassium ion-dependent RNA structural switch regulates human pre-miRNA 92b maturation. Chem. Biol. 22, 262–272 (2015).
Lee, Y., Jeon, K., Lee, J. T., Kim, S. & Kim, V. N. MicroRNA maturation: Stepwise processing and subcellular localization. Embo J. 21, 4663–4670 (2002).
Sun, L. et al. RNA-binding protein RALY reprogrammes mitochondrial metabolism via mediating miRNA processing in colorectal cancer. Gut 70, 1698–1712 (2021).
He, X. & Shu, Y. RNA N6-methyladenosine modification participates in miR-660/E2F3 axis-mediated inhibition of cell proliferation in gastric cancer. Pathol. Res. Pract. 215, 152393 (2019).
Alarcón, C. R., Lee, H., Goodarzi, H., Halberg, N. & Tavazoie, S. F. N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485 (2015).
Han, B. et al. N(6)-methyladenosine-dependent primary microRNA-126 processing activated PI3K-AKT-mTOR pathway drove the development of pulmonary fibrosis induced by nanoscale carbon black particles in rats. Nanotoxicology 14, 1–20 (2020).
Peng, W. et al. Upregulated METTL3 promotes metastasis of colorectal cancer via miR-1246/SPRED2/MAPK signaling pathway. J. Exp. Clin. Cancer Res. 38, 393 (2019).
Zhang, J. et al. Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat. Commun. 10, 1858 (2019).
Liang, X. et al. Mechanism of methyltransferase like 3 in epithelial-mesenchymal transition process, invasion, and metastasis in esophageal cancer. Bioengineered 12, 10023–10036 (2021).
Sun, Y. et al. N(6)-methyladenosine-dependent pri-miR-17-92 maturation suppresses PTEN/TMEM127 and promotes sensitivity to everolimus in gastric cancer. Cell Death Dis. 11, 836 (2020).
Chen, X. et al. METTL14 suppresses CRC progression via regulating N6-methyladenosine-dependent primary miR-375 processing. Mol. Ther. 28, 599–612 (2020).
Ma, J. Z. et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology 65, 529–543 (2017).
Lin, R. et al. Deoxycholic acid modulates the progression of gallbladder cancer through N(6)-methyladenosine-dependent microRNA maturation. Oncogene 39, 4983–5000 (2020).
Chen, P. et al. N(6)-methyladenosine demethylase ALKBH5 suppresses malignancy of esophageal cancer by regulating microRNA biogenesis and RAI1 expression. Oncogene 40, 5600–5612 (2021).
Zhang, F. et al. Methylation of microRNA-338-5p by EED promotes METTL3-mediated translation of oncogene CDCP1 in gastric cancer. Aging 13, 12224–12238 (2021).
Song, P. et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol. Cancer 19, 129 (2020).
Cui, X. et al. Cross talk between RNA N6-methyladenosine methyltransferase-like 3 and miR-186 regulates hepatoblastoma progression through Wnt/β-catenin signalling pathway. Cell Prolif. 53, e12768 (2020).
He, H., Wu, W., Sun, Z. & Chai, L. MiR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A-caused stabilization of SEC62. Biochem. Biophys. Res. Commun. 517, 581–587 (2019).
Yue, C. et al. microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2-FTO-m6A/MYC axis. J. Exp. Clin. Cancer Res. 39, 240 (2020).
Xue, J., Xiao, P., Yu, X. & Zhang, X. A positive feedback loop between AlkB homolog 5 and miR-193a-3p promotes growth and metastasis in esophageal squamous cell carcinoma. Hum. Cell. 34, 502–514 (2021).
Kim, S. H., Lim, K. H., Yang, S. & Joo, J. Y. Long non-coding RNAs in brain tumors: Roles and potential as therapeutic targets. J. Hematol. Oncol. 14, 77 (2021).
Wang, H., Di, X., Bi, Y., Sun, S. & Wang, T. Long non-coding RNA LINC00649 regulates YES-associated protein 1 (YAP1)/Hippo pathway to accelerate gastric cancer (GC) progression via sequestering miR-16-5p. Bioengineered 12, 1791–1802 (2021).
Wang, S. et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J. Clin. Invest. 127, 4498–4515 (2017).
Collette, J., Le Bourhis, X. & Adriaenssens, E. Regulation of Human Breast Cancer by the Long Non-Coding RNA H19. Int. J. Mol. Sci. 18, 2319 (2017).
Liu, N. et al. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA 19, 1848–1856 (2013).
Moindrot, B. et al. A pooled shRNA screen identifies Rbm15, Spen, and Wtap as factors required for Xist RNA-mediated silencing. Cell Rep. 12, 562–572 (2015).
Yang, X. et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol. Cancer 19, 46 (2020).
Hu, N. & Ji, H. N6-methyladenosine (m6A)-mediated up-regulation of long noncoding RNA LINC01320 promotes the proliferation, migration, and invasion of gastric cancer via miR495-5p/RAB19 axis. Bioengineered 12, 4081–4091 (2021).
Wu, J. et al. m6A-Induced LncRNA MEG3 suppresses the proliferation, migration, and invasion of hepatocellular carcinoma cell through miR-544b/BTG2 signaling. Onco Targets Ther. 14, 3745–3755 (2021).
Zhang, J. et al. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J. Physiol. Biochem. 75, 379–389 (2019).
He, Y. et al. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell Physiol. Biochem. 48, 838–846 (2018).
Cui, Y. et al. RNA m6A demethylase FTO-mediated epigenetic up-regulation of LINC00022 promotes tumorigenesis in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 40, 294 (2021).
Ni, W. et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol. Cancer 18, 143 (2019).
Jeck, W. R. et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157 (2013).
Zhong, Y. et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol. Cancer 17, 79 (2018).
Chen, L. L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490 (2020).
Jeck, W. R. & Sharpless, N. E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 32, 453–461 (2014).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Ebermann, C., Schnarr, T. & Müller, S. Recent advances in understanding circular RNAs. F1000Res 9, 655 (2020).
Chen, L. L. & Yang, L. Regulation of circRNA biogenesis. RNA Biol. 12, 381–388 (2015).
Kristensen, L. S. et al. The biogenesis, biology, and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691 (2019).
Wang, Q. et al. Circ-SLC7A5, a potential prognostic circulating biomarker for detection of ESCC. Cancer Genet. 240, 33–39 (2020).
Sun, H. D. et al. Down-regulation of circPVRL3 promotes the proliferation and migration of gastric cancer cells. Sci. Rep. 8, 10111 (2018).
Zhou, C. et al. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 20, 2262–2276 (2017).
Yang, Y. et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 27, 626–641 (2017).
Di Timoteo, G. et al. Modulation of circRNA metabolism by m(6)A modification. Cell Rep. 31, 107641 (2020).
Hansen, T. B. et al. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. Embo J. 30, 4414–4422 (2011).
Guo, Y. et al. Circ3823 contributes to growth, metastasis, and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol. Cancer 20, 93 (2021).
Chen, Y. G. et al. N6-Methyladenosine modification controls circular RNA immunity. Mol. Cell. 76, 96–109.e109 (2019).
Zhang, L. et al. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol. Cancer 19, 105 (2020).
Chen, R. X. et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 10, 4695 (2019).
Chen, C. et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics 11, 4298–4315 (2021).
Liu, H. et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics 11, 1396–1411 (2021).
Xu, J. et al. N(6)-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol. Cancer 19, 163 (2020).
Rao, X. et al. N(6) -methyladenosine modification of circular RNA circ-ARL3 facilitates Hepatitis B virus-associated hepatocellular carcinoma via sponging miR-1305. IUBMB Life. 73, 408–417 (2021).
Peery, A. F. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187.e1173 (2012).
Pourhoseingholi, M. A., Vahedi, M. & Baghestani, A. R. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol. Hepatol. Bed Bench. 8, 19–27 (2015).
Arnold, M. et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 159, 335–349.e315 (2020).
Kelly, C. M., Gutierrez Sainz, L. & Chi, P. The management of metastatic GIST: Current standard and investigational therapeutics. J. Hematol. Oncol. 14, 2 (2021).
Vallilas, C. et al. Gastrointestinal stromal tumors (GISTs): Novel therapeutic strategies with immunotherapy and small molecules. Int. J. Mol. Sci. 22, 493 (2021).
Abdul-Latif, M., Townsend, K., Dearman, C., Shiu, K. K. & Khan, K. Immunotherapy in gastrointestinal cancer: The current scenario and future perspectives. Cancer Treat. Rev. 88, 102030 (2020).
Xu, L. C., Pan, J. X. & Pan, H. D. Construction and validation of an m6A RNA methylation regulators-based prognostic signature for esophageal cancer. Cancer Manag Res. 12, 5385–5394 (2020).
Li, Y. et al. Expression of demethylase genes, FTO and ALKBH1, is associated with prognosis of gastric cancer. Dig. Dis. Sci. 64, 1503–1513 (2019).
Zhao, Y. et al. Decreased nuclear expression of FTO in human primary hepatocellular carcinoma is associated with poor prognosis. Int. J. Clin. Exp. Pathol. 12, 3376–3383 (2019).
Liu, S. et al. FTO promotes cell proliferation and migration in esophageal squamous cell carcinoma through up-regulation of MMP13. Exp. Cell Res. 389, 111894 (2020).
Li, H. et al. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J. Cell Mol. Med. 24, 4452–4465 (2020).
Zhao, X. et al. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 21, 859–868 (2018).
Li, L., Xie, R. & Wei, Q. Network analysis of miRNA targeting m6A-related genes in patients with esophageal cancer. PeerJ 9, e11893 (2021).
Guan, K. et al. Expression status and prognostic value of M6A-associated genes in gastric cancer. J. Cancer 11, 3027–3040 (2020).
Liu, T., Li, C., Jin, L., Li, C. & Wang, L. The prognostic value of m6A RNA methylation regulators in colon adenocarcinoma. Med. Sci. Monit. 25, 9435–9445 (2019).
Li, L., Xie, R. & Lu, G. Identification of m6A methyltransferase-related lncRNA signature for predicting immunotherapy and prognosis in patients with hepatocellular carcinoma. Biosci. Rep. 41, BSR20210760 (2021).
Yuan, Q. et al. Development and validation of a novel N6-methyladenosine (m6A)-related multi- long non-coding RNA (lncRNA) prognostic signature in pancreatic adenocarcinoma. Bioengineered 12, 2432–2448 (2021).
Ying, P. et al. Identification of genetic variants in m(6)A modification genes associated with pancreatic cancer risk in the Chinese population. Arch. Toxicol. 95, 1117–1128 (2021).
Zuo, X. et al. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J. Hematol. Oncol. 13, 5 (2020).
Lan, T. et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol. Cancer 18, 186 (2019).
Chen, Y. et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol. Cancer 18, 127 (2019).
Hou, H. et al. METTL3 promotes the proliferation and invasion of esophageal cancer cells partly through AKT signaling pathway. Pathol. Res. Pract. 216, 153087 (2020).
Chen, H. et al. RNA N(6)-Methyladenosine methyltransferase METTL3 facilitates colorectal cancer by activating the m(6)A-GLUT1-mTORC1 axis and is a therapeutic target. Gastroenterology 160, 1284–1300.e1216 (2021).
Li, T. et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol. Cancer 18, 112 (2019).
Xia, T. et al. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol. Res Pract. 215, 152666 (2019).
Yang, Z., Jiang, X., Li, D. & Jiang, X. HBXIP promotes gastric cancer via METTL3-mediated MYC mRNA m6A modification. Aging. 12, 24967–24982 (2020).
Chen, X. et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol. Cancer 19, 106 (2020).
Tang, X., Liu, S., Chen, D., Zhao, Z. & Zhou, J. The role of the fat mass and obesity-associated protein in the proliferation of pancreatic cancer cells. Oncol. Lett. 17, 2473–2478 (2019).
Han, S. H. & Choe, J. Diverse molecular functions of m(6)A mRNA modification in cancer. Exp. Mol. Med. 52, 738–749 (2020).
Guo, H. et al. m(6)A Reader HNRNPA2B1 promotes esophageal cancer progression via upregulation of ACLY and ACC1. Front. Oncol. 10, 553045 (2020).
Pi, J. et al. YTHDF1 Promotes gastric carcinogenesis by controlling translation of FZD7. Cancer Res. 81, 2651–2665 (2021).
Hou, J. et al. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol. Cancer 18, 163 (2019).
Tanabe, A. et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 376, 34–42 (2016).
Siegel, R. L. et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J. Clin. 65, 457–480 (2015).
Lin, S., Choe, J., Du, P., Triboulet, R. & Gregory, R. I. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell. 62, 335–345 (2016).
Sheng, H. et al. YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis 41, 541–550 (2020).
Wanna-Udom, S. et al. The m6A methyltransferase METTL3 contributes to Transforming Growth Factor-beta-induced epithelial-mesenchymal transition of lung cancer cells through the regulation of JUNB. Biochem. Biophys. Res. Commun. 524, 150–155 (2020).
Wang, H. et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol. Cancer 18, 181 (2019).
Li, J. et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem. Biophys. Res. Commun. 512, 479–485 (2019).
Liu, J. et al. m(6)A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem. Biophys. Res. Commun. 502, 456–464 (2018).
Jin, D. et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J. Hematol. Oncol. 12, 135 (2019).
Chao, Y., Shang, J. & Ji, W. ALKBH5-m(6)A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem. Biophys. Res. Commun. 521, 499–506 (2020).
Jin, D. et al. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. Mol. Cancer 19, 40 (2020).
Antoni, S. et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 71, 96–108 (2017).
Katsila, T., Liontos, M., Patrinos, G. P., Bamias, A. & Kardamakis, D. The new age of -omics in urothelial cancer—re-wording its diagnosis and treatment. EBioMedicine 28, 43–50 (2018).
Svatek, R. S. et al. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 66, 253–262 (2014).
Cheng, M. et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-κB/MYC signaling network. Oncogene 38, 3667–3680 (2019).
Yang, F. et al. Dynamic m(6)A mRNA methylation reveals the role of METTL3-m(6)A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene 38, 4755–4772 (2019).
Jin, H. et al. N(6)-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine 47, 195–207 (2019).
Xie, H. et al. METTL3/YTHDF2 m(6) A axis promotes tumorigenesis by degrading SETD7 and KLF4 mRNAs in bladder cancer. J. Cell Mol. Med. 24, 4092–4104 (2020).
Han, J. et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol. Cancer 18, 110 (2019).
Wen, L., Pan, X., Yu, Y. & Yang, B. Down-regulation of FTO promotes proliferation and migration, and protects bladder cancer cells from cisplatin-induced cytotoxicity. BMC Urol. 20, 39 (2020).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Kim, I. H. & Lee, H. J. The frontline immunotherapy-based treatment of advanced clear cell renal cell carcinoma: Current evidence and clinical perspective. Biomedicines 10, 251 (2022).
De, P. et al. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986–2007. Cancer Causes Control. 25, 1271–1281 (2014).
Miao, D. et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359, 801–806 (2018).
Cavaliere, C. et al. Current and emerging treatments for metastatic renal cell carcinoma. Curr. Cancer Drug Targets 18, 468–479 (2018).
Bielecka, Z. F., Czarnecka, A. M. & Szczylik, C. Genomic analysis as the first step toward personalized treatment in renal cell carcinoma. Front. Oncol. 4, 194 (2014).
De Meerleer, G. et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 15, e170–e177 (2014).
Hakimi, A. A. et al. The impact of metformin use on recurrence and cancer-specific survival in clinically localized high-risk renal cell carcinoma. Can. Urol. Assoc. J. 7, E687–E691 (2013).
Zhou, J. et al. Gene signatures and prognostic values of m6A regulators in clear cell renal cell carcinoma—a retrospective study using TCGA database. Aging. 11, 1633–1647 (2019).
Li, X. et al. The M6A methyltransferase METTL3: Acting as a tumor suppressor in renal cell carcinoma. Oncotarget 8, 96103–96116 (2017).
Gong, D. et al. The m(6)A-suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP-induced Ca(2+) influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J. Exp. Clin. Cancer Res. 38, 233 (2019).
Tang, J. et al. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J. Exp. Clin. Cancer Res. 37, 40 (2018).
Attard, G. et al. Prostate cancer. Lancet 387, 70–82 (2016).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Hudson, S. V., O’Malley, D. M. & Miller, S. M. Achieving optimal delivery of follow-up care for prostate cancer survivors: improving patient outcomes. Patient Relat. Outcome Meas. 6, 75–90 (2015).
Li, E., Wei, B., Wang, X. & Kang, R. METTL3 enhances cell adhesion through stabilizing integrin β1 mRNA via an m6A-HuR-dependent mechanism in prostatic carcinoma. Am. J. Cancer Res. 10, 1012–1025 (2020).
Cai, J. et al. RNA m(6)A methyltransferase METTL3 promotes the growth of prostate cancer by regulating Hedgehog pathway. Onco Targets Ther. 12, 9143–9152 (2019).
Li, J. et al. Downregulation of N(6)-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N(6)-methyladenosine levels. Oncotarget 9, 3752–3764 (2018).
Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 68, 284–296 (2018).
Bowtell, D. D. et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 15, 668–679 (2015).
Oza, A. M. et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 16, 928–936 (2015).
Hua, W. et al. METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecol. Oncol. 151, 356–365 (2018).
Liang, S. et al. METTL3 serves an oncogenic role in human ovarian cancer cells partially via the AKT signaling pathway. Oncol. Lett. 19, 3197–3204 (2020).
Liu, T. et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 48, 3816–3831 (2020).
Zhu, H. et al. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J. Exp. Clin. Cancer Res. 38, 163 (2019).
Jiang, Y. et al. RNA demethylase ALKBH5 promotes ovarian carcinogenesis in a simulated tumour microenvironment through stimulating NF-κB pathway. J. Cell Mol. Med. 24, 6137–6148 (2020).
Small, W. Jr. et al. Cervical cancer: A global health crisis. Cancer 123, 2404–2412 (2017).
LaVigne, A. W., Triedman, S. A., Randall, T. C., Trimble, E. L. & Viswanathan, A. N. Cervical cancer in low and middle income countries: Addressing barriers to radiotherapy delivery. Gynecol. Oncol. Rep. 22, 16–20 (2017).
Waggoner, S. E. Cervical cancer. Lancet 361, 2217–2225 (2003).
Sawaya, G. F., Smith-McCune, K. & Kuppermann, M. Cervical cancer screening: More choices in 2019. Jama 321, 2018–2019 (2019).
Wang, X., Zhang, J. & Wang, Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am. J. Transl. Res. 11, 4909–4921 (2019).
Zou, D. et al. The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 19, 321 (2019).
Zhou, S. et al. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylation. Mol. Carcinog. 57, 590–597 (2018).
Liu, J. et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 20, 1074–1083 (2018).
Johnson, D. R. & O’Neill, B. P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 107, 359–364 (2012).
Lathia, J. D., Mack, S. C., Mulkearns-Hubert, E. E., Valentim, C. L. & Rich, J. N. Cancer stem cells in glioblastoma. Genes Dev. 29, 1203–1217 (2015).
Godlewski, J., Newton, H. B., Chiocca, E. A. & Lawler, S. E. MicroRNAs and glioblastoma; the stem cell connection. Cell Death Differ. 17, 221–228 (2010).
Cui, Q. et al. m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 18, 2622–2634 (2017).
Zhang, S. et al. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 31, 591–606.e596 (2017).
Visvanathan, A. et al. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene 37, 522–533 (2018).
Li, F. et al. N(6)-Methyladenosine modulates nonsense-mediated mRNA decay in human glioblastoma. Cancer Res. 79, 5785–5798 (2019).
Su, R. et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell 172, 90–105.e123 (2018).
Estey, E. & Döhner, H. Acute myeloid leukaemia. Lancet 368, 1894–1907 (2006).
Döhner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152 (2015).
Testa, U. Leukemia stem cells. Ann. Hematol. 90, 245–271 (2011).
Chen, J., Odenike, O. & Rowley, J. D. Leukaemogenesis: More than mutant genes. Nat. Rev. Cancer 10, 23–36 (2010).
Rosenbauer, F. & Tenen, D. G. Transcription factors in myeloid development: Balancing differentiation with transformation. Nat. Rev. Immunol. 7, 105–117 (2007).
Marcucci, G., Mrózek, K. & Bloomfield, C. D. Molecular heterogeneity and prognostic biomarkers in adults with acute myeloid leukemia and normal cytogenetics. Curr. Opin. Hematol. 12, 68–75 (2005).
Bansal, H. et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia 28, 1171–1174 (2014).
Paris, J. et al. Targeting the RNA m(6)A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell. Stem Cell. 25, 137–148.e136 (2019).
Vu, L. P. et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 23, 1369–1376 (2017).
Li, Z. et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 31, 127–141 (2017).
Shen, C. et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell. Stem Cell. 27, 64–80.e69 (2020).
Kwok, C. T., Marshall, A. D., Rasko, J. E. & Wong, J. J. Genetic alterations of m(6)A regulators predict poorer survival in acute myeloid leukemia. J. Hematol. Oncol. 10, 39 (2017).
Weng, H. et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell. Stem Cell. 22, 191–205.e199 (2018).
Wang, H., Xu, B. & Shi, J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene 722, 144076 (2020).
Cai, X. et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 415, 11–19 (2018).
Niu, Y. et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol. Cancer 18, 46 (2019).
Luo, G. et al. RNA m(6) A methylation regulates uveal melanoma cell proliferation, migration, and invasion by targeting c-Met. J. Cell Physiol. 235, 7107–7119 (2020).
Dahal, U., Le, K. & Gupta, M. RNA m6A methyltransferase METTL3 regulates invasiveness of melanoma cells by matrix metallopeptidase 2. Melanoma Res. 29, 382–389 (2019).
Yang, S. et al. m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun. 10, 2782 (2019).
Jia, R. et al. m(6)A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol. Cancer 18, 161 (2019).
Miao, W., Chen, J., Jia, L., Ma, J. & Song, D. The m6A methyltransferase METTL3 promotes osteosarcoma progression by regulating the m6A level of LEF1. Biochem. Biophys. Res. Commun. 516, 719–725 (2019).
Chen, S., Zhou, L. & Wang, Y. ALKBH5-mediated m(6)A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Cancer Cell Int. 20, 34 (2020).
Ban, Y. et al. LNCAROD is stabilized by m6A methylation and promotes cancer progression via forming a ternary complex with HSPA1A and YBX1 in head and neck squamous cell carcinoma. Mol. Oncol. 14, 1282–1296 (2020).
Zheng, Z. Q. et al. Long noncoding RNA FAM225A promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and upregulate ITGB3. Cancer Res. 79, 4612–4626 (2019).
Shriwas, O. et al. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m(6)A-demethylation of FOXM1 and NANOG. Apoptosis 25, 233–246 (2020).
Wyatt, G. R. Occurrence of 5-methylcytosine in nucleic acids. Nature 166, 237–238 (1950).
Dubin, D. T. & Taylor, R. H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 2, 1653–1668 (1975).
Wildenauer, D., Gross, H. J. & Riesner, D. Enzymatic methylations: III. Cadaverine-induced conformational changes of E. coli tRNA fMet as evidenced by the availability of a specific adenosine and a specific cytidine residue for methylation. Nucleic Acids Res. 1, 1165–1182 (1974).
Brzezicha, B. et al. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA). Nucleic Acids Res. 34, 6034–6043 (2006).
Frye, M. & Watt, F. M. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr. Biol. 16, 971–981 (2006).
Blanco, S. et al. The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genet. 7, e1002403 (2011).
Moon, H. J. & Redman, K. L. Trm4 and Nsun2 RNA:m5C methyltransferases form metabolite-dependent, covalent adducts with previously methylated RNA. Biochemistry 53, 7132–7144 (2014).
Khoddami, V. & Cairns, B. R. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat. Biotechnol. 31, 458–464 (2013).
King, M. Y. & Redman, K. L. RNA methyltransferases utilize two cysteine residues in the formation of 5-methylcytosine. Biochemistry 41, 11218–11225 (2002).
Motorin, Y., Lyko, F. & Helm, M. 5-methylcytosine in RNA: Detection, enzymatic formation, and biological functions. Nucleic Acids Res. 38, 1415–1430 (2010).
Liu, Y. & Santi, D. V. m5C RNA and m5C DNA methyl transferases use different cysteine residues as catalysts. Proc. Natl Acad. Sci. USA 97, 8263–8265 (2000).
Tuorto, F. et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat. Struct. Mol. Biol. 19, 900–905 (2012).
Yang, X. et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 27, 606–625 (2017).
Van Haute, L. et al. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 47, 8720–8733 (2019).
Sajini, A. A. et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat. Commun. 10, 2550 (2019).
Li, Y. et al. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett. 430, 57–66 (2018).
Henry, B. A., Kanarek, J. P., Kotter, A., Helm, M. & Lee, N. 5-methylcytosine modification of an Epstein-Barr virus noncoding RNA decreases its stability. RNA 26, 1038–1048 (2020).
Abbasi-Moheb, L. et al. Mutations in NSUN2 cause autosomal-recessive intellectual disability. Am. J. Hum. Genet. 90, 847–855 (2012).
Khan, M. A. et al. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am. J. Hum. Genet. 90, 856–863 (2012).
Martinez, F. J. et al. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. J. Med. Genet. 49, 380–385 (2012).
Bourgeois, G. et al. Eukaryotic rRNA modification by yeast 5-methylcytosine-methyltransferases and human proliferation-associated antigen p120. PLoS One 10, e0133321 (2015).
Sharma, S., Yang, J., Watzinger, P., Kötter, P. & Entian, K. D. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 41, 9062–9076 (2013).
Burgess, A. L., David, R. & Searle, I. R. Conservation of tRNA and rRNA 5-methylcytosine in the kingdom Plantae. BMC Plant Biol. 15, 199 (2015).
Metodiev, M. D. et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 10, e1004110 (2014).
Van Haute, L. et al. Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nat. Commun. 7, 12039 (2016).
Nakano, S. et al. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met). Nat. Chem. Biol. 12, 546–551 (2016).
Haag, S. et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. Embo J. 35, 2104–2119 (2016).
Haag, S. et al. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. RNA 21, 1532–1543 (2015).
Goll, M. G. et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 311, 395–398 (2006).
Schaefer, M. et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 24, 1590–1595 (2010).
Tuorto, F. et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. Embo J. 34, 2350–2362 (2015).
Jeltsch, A. et al. Mechanism and biological role of Dnmt2 in nucleic acid methylation. RNA Biol. 14, 1108–1123 (2017).
Huang, W. et al. Formation and determination of the oxidation products of 5-methylcytosine in RNA. Chem. Sci. 7, 5495–5502 (2016).
Shen, Q. et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 554, 123–127 (2018).
Jin, S. G. et al. Tet3 Reads 5-carboxylcytosine through Its CXXC domain and is a potential guardian against neurodegeneration. Cell Rep. 14, 493–505 (2016).
Shi, M. et al. ALYREF mainly binds to the 5’ and the 3’ regions of the mRNA in vivo. Nucleic Acids Res. 45, 9640–9653 (2017).
Eckwahl, M. et al. 5-Methylcytosine RNA modifications promote retrovirus replication in an ALYREF reader protein-dependent manner. J. Virol. 94, e00544–20 (2020).
Chen, X. et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol. 21, 978–990 (2019).
Squires, J. E. et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 40, 5023–5033 (2012).
Pan, T. Modifications and functional genomics of human transfer RNA. Cell Res. 28, 395–404 (2018).
Mukai, T. et al. Transfer RNAs with novel cloverleaf structures. Nucleic Acids Res. 45, 2776–2785 (2017).
Huang, T. Y., Liu, J. & McLuckey, S. A. Top-down tandem mass spectrometry of tRNA via ion trap collision-induced dissociation. J. Am. Soc. Mass Spectrom. 21, 890–898 (2010).
Motorin, Y. & Grosjean, H. Multisite-specific tRNA:m5C-methyltransferase (Trm4) in yeast Saccharomyces cerevisiae: identification of the gene and substrate specificity of the enzyme. RNA 5, 1105–1118 (1999).
Müller, M. et al. Division of labour: tRNA methylation by the NSun2 tRNA methyltransferases Trm4a and Trm4b in fission yeast. RNA Biol. 16, 249–256 (2019).
García-Vílchez, R., Sevilla, A. & Blanco, S. Post-transcriptional regulation by cytosine-5 methylation of RNA. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 240–252 (2019).
Gkatza, N. A. et al. Cytosine-5 RNA methylation links protein synthesis to cell metabolism. PLoS Biol. 17, e3000297 (2019).
Blanco, S. et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo J. 33, 2020–2039 (2014).
Shinoda, S. et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 47, 8734–8745 (2019).
Deutscher, M. P. Maturation and degradation of ribosomal RNA in bacteria. Prog. Mol. Biol. Transl. Sci. 85, 369–391 (2009).
Warner, J. R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24, 437–440 (1999).
Reuveni, S., Ehrenberg, M. & Paulsson, J. Ribosomes are optimized for autocatalytic production. Nature 547, 293–297 (2017).
Gigova, A., Duggimpudi, S., Pollex, T., Schaefer, M. & Koš, M. A cluster of methylations in the domain IV of 25S rRNA is required for ribosome stability. RNA 20, 1632–1644 (2014).
Schosserer, M. et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat. Commun. 6, 6158 (2015).
Cámara, Y. et al. MTERF4 regulates translation by targeting the methyltransferase NSUN4 to the mammalian mitochondrial ribosome. Cell Metab. 13, 527–539 (2011).
Dai, X. et al. YTHDF2 binds to 5-methylcytosine in RNA and modulates the maturation of ribosomal RNA. Anal. Chem. 92, 1346–1354 (2020).
Yang, Y. et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol. Cell. 75, 1188–1202.e1111 (2019).
Li, Q. et al. NSUN2-mediated m5C methylation and METTL3/METTL14-mediated m6A methylation cooperatively enhance p21 translation. J. Cell Biochem. 118, 2587–2598 (2017).
Schumann, U. et al. Multiple links between 5-methylcytosine content of mRNA and translation. BMC Biol. 18, 40 (2020).
Huang, T., Chen, W., Liu, J., Gu, N. & Zhang, R. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat. Struct. Mol. Biol. 26, 380–388 (2019).
Xue, S. et al. Depletion of TRDMT1 affects 5-methylcytosine modification of mRNA and inhibits HEK293 cell proliferation and migration. Biochem. Biophys. Res. Commun. 520, 60–66 (2019).
Amort, T. et al. Long non-coding RNAs as targets for cytosine methylation. RNA Biol. 10, 1003–1008 (2013).
Courtney, D. G. et al. Epitranscriptomic addition of m(5)C to HIV-1 transcripts regulates viral gene expression. Cell Host Microbe 26, 217–227.e216 (2019).
Zhang, Y. et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol. 20, 535–540 (2018).
Okamoto, M. et al. Frequent increased gene copy number and high protein expression of tRNA (cytosine-5-)-methyltransferase (NSUN2) in human cancers. DNA Cell Biol. 31, 660–671 (2012).
Xiang, S. et al. m(5)C RNA methylation primarily affects the ErbB and PI3K-Akt signaling pathways in gastrointestinal cancer. Front. Mol. Biosci. 7, 599340 (2020).
Zhang, Q., Zheng, Q., Yu, X., He, Y. & Guo, W. Overview of distinct 5-methylcytosine profiles of messenger RNA in human hepatocellular carcinoma and paired adjacent non-tumor tissues. J. Transl. Med. 18, 245 (2020).
He, Y., Zhang, Q., Zheng, Q., Yu, X. & Guo, W. Distinct 5-methylcytosine profiles of circular RNA in human hepatocellular carcinoma. Am. J. Transl. Res. 12, 5719–5729 (2020).
He, Y., Shi, Q., Zhang, Y., Yuan, X. & Yu, Z. Transcriptome-wide 5-methylcytosine functional profiling of long non-coding RNA in hepatocellular carcinoma. Cancer Manag. Res. 12, 6877–6885 (2020).
He, Y. et al. Role of m(5)C-related regulatory genes in the diagnosis and prognosis of hepatocellular carcinoma. Am. J. Transl. Res. 12, 912–922 (2020).
Sun, Z. et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene 39, 6906–6919 (2020).
Mei, L. et al. RNA methyltransferase NSUN2 promotes gastric cancer cell proliferation by repressing p57(Kip2) by an m(5)C-dependent manner. Cell Death Dis. 11, 270 (2020).
Vincent, A., Herman, J., Schulick, R., Hruban, R. H. & Goggins, M. Pancreatic cancer. Lancet 378, 607–620 (2011).
Klein, A. P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 18, 493–502 (2021).
Lillemoe, K. D., Yeo, C. J. & Cameron, J. L. Pancreatic cancer: State-of-the-art care. CA Cancer J. Clin. 50, 241–268 (2000).
Yu, X. et al. Predictive value of m5C regulatory gene expression in pancreatic adenocarcinoma. Sci. Rep. 11, 17529 (2021).
Yang, R. et al. The RNA methyltransferase NSUN6 suppresses pancreatic cancer development by regulating cell proliferation. EBioMedicine 63, 103195 (2021).
Yuan, H. et al. Prognostic risk model and tumor immune environment modulation of m5C-related LncRNAs in pancreatic ductal adenocarcinoma. Front. Immunol. 12, 800268 (2021).
Su, J. et al. NSUN2-mediated RNA 5-methylcytosine promotes esophageal squamous cell carcinoma progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene 40, 5814–5828 (2021).
Geng, Q. et al. Comprehensive analysis of the prognostic value and immune infiltrates of the three-m5C signature in colon carcinoma. Cancer Manag. Res. 13, 7989–8002 (2021).
Wang, P. et al. Identification of RNA: 5-methylcytosine methyltransferases-related signature for predicting prognosis in glioma. Front. Oncol. 10, 1119 (2020).
Mondal, I. & Kulshreshtha, R. Potential of microRNA based diagnostics and therapeutics in glioma: A patent review. Expert Opin. Ther. Pat. 31, 91–106 (2021).
Cheray, M. et al. Cytosine methylation of mature microRNAs inhibits their functions and is associated with poor prognosis in glioblastoma multiforme. Mol. Cancer 19, 36 (2020).
Janin, M. et al. Epigenetic loss of RNA-methyltransferase NSUN5 in glioma targets ribosomes to drive a stress adaptive translational program. Acta Neuropathol. 138, 1053–1074 (2019).
Saijo, Y. et al. Expression of nucleolar protein p120 predicts poor prognosis in patients with stage I lung adenocarcinoma. Ann. Oncol. 12, 1121–1125 (2001).
Pan, J., Huang, Z. & Xu, Y. m5C RNA methylation regulators predict prognosis and regulate the immune microenvironment in lung squamous cell carcinoma. Front. Oncol. 11, 657466 (2021).
Yi, J. et al. Overexpression of NSUN2 by DNA hypomethylation is associated with metastatic progression in human breast cancer. Oncotarget 8, 20751–20765 (2017).
Freeman, J. W. et al. Prognostic significance of proliferation associated nucleolar antigen P120 in human breast carcinoma. Cancer Res. 51, 1973–1978 (1991).
Campbell, T. M., Castro, M. A. A., de Oliveira, K. G., Ponder, B. A. J. & Meyer, K. B. ERα binding by transcription factors NFIB and YBX1 enables FGFR2 signaling to modulate estrogen responsiveness in breast cancer. Cancer Res. 78, 410–421 (2018).
Wang, K. et al. 5-Methylcytosine RNA methyltransferases-related long non-coding RNA to develop and validate biochemical recurrence signature in prostate cancer. Front. Mol. Biosci. 8, 775304 (2021).
Xue, M. et al. Gene signatures of m5C regulators may predict prognoses of patients with head and neck squamous cell carcinoma. Am. J. Transl. Res. 12, 6841–6852 (2020).
Wang, L. & Gao, S. Identification of 5-methylcytosine-related signature for predicting prognosis in ovarian cancer. Biol. Res. 54, 18 (2021).
Gao, L. et al. The RNA methylation modification 5-methylcytosine impacts immunity characteristics, prognosis, and progression of oral squamous cell carcinoma by bioinformatics analysis. Front. Bioeng. Biotechnol. 9, 760724 (2021).
Li, F. et al. m(5)C Regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in papillary thyroid carcinoma. Front. Oncol. 11, 729887 (2021).
Li, H., Jiang, H., Huang, Z., Chen, Z. & Chen, N. Prognostic value of an m(5)C RNA methylation regulator-related signature for clear cell renal cell carcinoma. Cancer Manag. Res. 13, 6673–6687 (2021).
Xu, W. et al. Integrative 5-methylcytosine modification immunologically reprograms tumor microenvironment characterizations and phenotypes of clear cell renal cell carcinoma. Front. Cell Dev. Biol. 9, 772436 (2021).
Cheng, J. X. et al. RNA cytosine methylation and methyltransferases mediate chromatin organization and 5-azacytidine response and resistance in leukaemia. Nat. Commun. 9, 1163 (2018).
Bantis, A. et al. Expression of p120, Ki-67, and PCNA as proliferation biomarkers in imprint smears of prostate carcinoma and their prognostic value. Cytopathology 15, 25–31 (2004).
Lu, L., Zhu, G., Zeng, H., Xu, Q. & Holzmann, K. High tRNA transferase NSUN2 gene expression is associated with poor prognosis in head and neck squamous carcinoma. Cancer Invest. 36, 246–253 (2018).
Yang, J. C. et al. Association of tRNA methyltransferase NSUN2/IGF-II molecular signature with ovarian cancer survival. Future Oncol. 13, 1981–1990 (2017).
Ofengand, J., Del Campo, M. & Kaya, Y. Mapping pseudouridines in RNA molecules. Methods 25, 365–373 (2001).
Cohn, W. E. Some results of the applications of ion-exchange chromatography to nucleic acid chemistry. J. Cell Physiol. Suppl. 38, 21–40 (1951).
Stockert, J. A., Weil, R., Yadav, K. K., Kyprianou, N. & Tewari, A. K. Pseudouridine as a novel biomarker in prostate cancer. Urol. Oncol. 39, 63–71 (2021).
Kiss, A. M., Jády, B. E., Bertrand, E. & Kiss, T. Human box H/ACA pseudouridylation guide RNA machinery. Mol. Cell Biol. 24, 5797–5807 (2004).
Cohn, W. E. 5-Ribosyl uracil, a carbon-carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim. Biophys. Acta 32, 569–571 (1959).
Cohn, W. E. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J. Biol. Chem. 235, 1488–1498 (1960).
Davis, F. F. & Allen, F. W. Ribonucleic acids from yeast which contain a fifth nucleotide. J. Biol. Chem. 227, 907–915 (1957).
Schwartz, S. et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159, 148–162 (2014).
Carlile, T. M. et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515, 143–146 (2014).
Mengel-Jørgensen, J. & Kirpekar, F. Detection of pseudouridine and other modifications in tRNA by cyanoethylation and MALDI mass spectrometry. Nucleic Acids Res. 30, e135 (2002).
Zhang, W., Eckwahl, M. J., Zhou, K. I. & Pan, T. Sensitive and quantitative probing of pseudouridine modification in mRNA and long noncoding RNA. RNA 25, 1218–1225 (2019).
Lei, Z. & Yi, C. A radiolabeling-free, qPCR-based method for locus-specific pseudouridine detection. Angew. Chem. Int. Ed. Engl. 56, 14878–14882 (2017).
Hamma, T. & Ferré-D’Amaré, A. R. Pseudouridine synthases. Chem. Biol. 13, 1125–1135 (2006).
Hur, S., Stroud, R. M. & Finer-Moore, J. Substrate recognition by RNA 5-methyluridine methyltransferases and pseudouridine synthases: A structural perspective. J. Biol. Chem. 281, 38969–38973 (2006).
Spenkuch, F., Motorin, Y. & Helm, M. Pseudouridine: Still mysterious, but never a fake (uridine)! RNA Biol. 11, 1540–1554 (2014).
Gilbert, W. V., Bell, T. A. & Schaening, C. Messenger RNA modifications: Form, distribution, and function. Science 352, 1408–1412 (2016).
Li, X., Ma, S. & Yi, C. Pseudouridine: The fifth RNA nucleotide with renewed interests. Curr. Opin. Chem. Biol. 33, 108–116 (2016).
Yu, Y. T. & Meier, U. T. RNA-guided isomerization of uridine to pseudouridine-pseudouridylation. RNA Biol. 11, 1483–1494 (2014).
Torsin, L. I. et al. Editing and chemical modifications on non-coding RNAs in cancer: A new tale with clinical significance. Int. J. Mol. Sci. 22, 581 (2021).
Karijolich, J. & Yu, Y. T. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 474, 395–398 (2011).
Sánchez-Vásquez, E., Alata Jimenez, N., Vázquez, N. A. & Strobl-Mazzulla, P. H. Emerging role of dynamic RNA modifications during animal development. Mech. Dev. 154, 24–32 (2018).
Charette, M. & Gray, M. W. Pseudouridine in RNA: What, where, how, and why. IUBMB Life. 49, 341–351 (2000).
Lovejoy, A. F., Riordan, D. P. & Brown, P. O. Transcriptome-wide mapping of pseudouridines: Pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS One 9, e110799 (2014).
Davis, D. R. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 23, 5020–5026 (1995).
Newby, M. I. & Greenbaum, N. L. A conserved pseudouridine modification in eukaryotic U2 snRNA induces a change in branch-site architecture. RNA 7, 833–845 (2001).
Arnez, J. G. & Steitz, T. A. Crystal structure of unmodified tRNA(Gln) complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudo-uridines in stabilization of RNA structure. Biochemistry 33, 7560–7567 (1994).
Newby, M. I. & Greenbaum, N. L. Investigation of Overhauser effects between pseudouridine and water protons in RNA helices. Proc. Natl Acad. Sci. USA 99, 12697–12702 (2002).
Rong, D. et al. Epigenetics: Roles and therapeutic implications of non-coding RNA modifications in human cancers. Mol. Ther. Nucleic Acids 25, 67–82 (2021).
Li, X. et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat. Chem. Biol. 11, 592–597 (2015).
Nurse, K., Wrzesinski, J., Bakin, A., Lane, B. G. & Ofengand, J. Purification, cloning, and properties of the tRNA psi 55 synthase from Escherichia coli. RNA 1, 102–112 (1995).
Arps, P. J. et al. Structural features of the hisT operon of Escherichia coli K-12. Nucleic Acids Res. 13, 5297–5315 (1985).
Uddin, M. B., Wang, Z. & Yang, C. Dysregulations of functional RNA modifications in cancer, cancer stemness, and cancer therapeutics. Theranostics 10, 3164–3189 (2020).
Huang, L., Pookanjanatavip, M., Gu, X. & Santi, D. V. A conserved aspartate of tRNA pseudouridine synthase is essential for activity and a probable nucleophilic catalyst. Biochemistry 37, 344–351 (1998).
Lecointe, F. et al. Characterization of yeast protein Deg1 as pseudouridine synthase (Pus3) catalyzing the formation of psi 38 and psi 39 in tRNA anticodon loop. J. Biol. Chem. 273, 1316–1323 (1998).
Durant, P. C. & Davis, D. R. Stabilization of the anticodon stem-loop of tRNALys,3 by an A+-C base-pair and by pseudouridine. J. Mol. Biol. 285, 115–131 (1999).
Becker, H. F., Motorin, Y., Planta, R. J. & Grosjean, H. The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalyzing the formation of psi55 in both mitochondrial and cytoplasmic tRNAs. Nucleic Acids Res. 25, 4493–4499 (1997).
Pan, H., Agarwalla, S., Moustakas, D. T., Finer-Moore, J. & Stroud, R. M. Structure of tRNA pseudouridine synthase TruB and its RNA complex: RNA recognition through a combination of rigid docking and induced fit. Proc. Natl Acad. Sci. USA 100, 12648–12653 (2003).
Penzo, M. & Montanaro, L. Turning uridines around: Role of rRNA pseudouridylation in ribosome biogenesis and ribosomal function. Biomolecules 8, 38 (2018).
Ganot, P., Bortolin, M. L. & Kiss, T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell 89, 799–809 (1997).
Maxwell, E. S. & Fournier, M. J. The small nucleolar RNAs. Annu. Rev. Biochem. 64, 897–934 (1995).
Tollervey, D. & Kiss, T. Function and synthesis of small nucleolar RNAs. Curr. Opin. Cell Biol. 9, 337–342 (1997).
Lafontaine, D. L., Bousquet-Antonelli, C., Henry, Y., Caizergues-Ferrer, M. & Tollervey, D. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 12, 527–537 (1998).
Penzo, M. et al. Human ribosomes from cells with reduced dyskerin levels are intrinsically altered in translation. Faseb J. 29, 3472–3482 (2015).
Garus, A. & Autexier, C. Dyskerin: An essential pseudouridine synthase with multifaceted roles in ribosome biogenesis, splicing, and telomere maintenance. RNA 27, 1441–1458 (2021).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Kierzek, E. et al. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res. 42, 3492–3501 (2014).
Wu, G. et al. Pseudouridines in U2 snRNA stimulate the ATPase activity of Prp5 during spliceosome assembly. Embo J. 35, 654–667 (2016).
Chen, C., Zhao, X., Kierzek, R. & Yu, Y. T. A flexible RNA backbone within the polypyrimidine tract is required for U2AF65 binding and pre-mRNA splicing in vivo. Mol. Cell Biol. 30, 4108–4119 (2010).
Karikó, K., Muramatsu, H., Keller, J. M. & Weissman, D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 20, 948–953 (2012).
Anderson, B. R. et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 38, 5884–5892 (2010).
Andries, O. et al. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control Release 217, 337–344 (2015).
Dinescu, S. et al. Epitranscriptomic signatures in lncRNAs and their possible roles in cancer. Genes. 10, 52 (2019).
Grammatikakis, I., Panda, A. C., Abdelmohsen, K. & Gorospe, M. Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging. Aging 6, 992–1009 (2014).
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Ruggero, D. et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science 299, 259–262 (2003).
Ni, J., Tien, A. L. & Fournier, M. J. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell 89, 565–573 (1997).
Yang, Y. et al. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol. Biol. Cell. 11, 567–577 (2000).
Tusup, M., Kundig, T. & Pascolo, S. Epitranscriptomics of cancer. World J. Clin. Oncol. 9, 42–55 (2018).
Kim, M. S., Kim, S. S., Yoo, N. J. & Lee, S. H. Expressional analysis of NOLA1, NOLA2, NOLA3 and DKC1, the core proteins in H/ACA riboproteins, in gastric and colorectal cancers. Pathology 44, 576–577 (2012).
Zhang, M. et al. H/ACA snoRNP gene family as diagnostic and prognostic biomarkers for hepatocellular carcinoma. Pharmgenomics Pers. Med. 14, 1331–1345 (2021).
Salvatore, F. et al. Pseudouridine determination in blood serum as tumor marker. Cancer Detect Prev. 6, 531–536 (1983).
Amuro, Y. et al. Serum pseudouridine as a biochemical marker in patients with hepatocellular carcinoma. Clin. Chim. Acta 178, 151–158 (1988).
Turano, M., Angrisani, A., De Rosa, M., Izzo, P. & Furia, M. Real-time PCR quantification of human DKC1 expression in colorectal cancer. Acta Oncol. 47, 1598–1599 (2008).
Kan, G. et al. Dual inhibition of DKC1 and MEK1/2 synergistically restrains the growth of colorectal cancer cells. Adv. Sci. 8, 2004344 (2021).
Babaian, A. et al. Loss of m(1)acp(3)Ψ ribosomal RNA modification is a major feature of cancer. Cell Rep. 31, 107611 (2020).
Hou, P. et al. DKC1 enhances angiogenesis by promoting HIF-1α transcription and facilitates metastasis in colorectal cancer. Br. J. Cancer 122, 668–679 (2020).
Liu, B., Zhang, J., Huang, C. & Liu, H. Dyskerin overexpression in human hepatocellular carcinoma is associated with advanced clinical stage and poor patient prognosis. PLoS One 7, e43147 (2012).
Ko, E. et al. Oxidatively modified protein-disulfide isomerase-associated 3 promotes dyskerin pseudouridine synthase 1-mediated malignancy and survival of hepatocellular carcinoma cells. Hepatology 68, 1851–1864 (2018).
McMahon, M. et al. A single H/ACA small nucleolar RNA mediates tumor suppression downstream of oncogenic RAS. Elife 8, e48847 (2019).
Rostami, P. et al. Gene panel testing in hereditary breast cancer. Arch. Iran. Med. 23, 155–162 (2020).
Lu, J. Y., Lai, R. S., Liang, L. L., Wang, H. C. & Lin, T. I. Evaluation of urinary pseudouridine as a tumor marker in lung cancer. J. Formos. Med. Assoc. 93, 25–29 (1994).
Stockert, J. A. et al. Predictive value of pseudouridine in prostate cancer. Am. J. Clin. Exp. Urol. 7, 262–272 (2019).
Elsharawy, K. A. et al. The nucleolar-related protein Dyskerin pseudouridine synthase 1 (DKC1) predicts poor prognosis in breast cancer. Br. J. Cancer 123, 1543–1552 (2020).
Montanaro, L. et al. Dyskerin expression influences the level of ribosomal RNA pseudo-uridylation and telomerase RNA component in human breast cancer. J. Pathol. 210, 10–18 (2006).
Montanaro, L. et al. Novel dyskerin-mediated mechanism of p53 inactivation through defective mRNA translation. Cancer Res. 70, 4767–4777 (2010).
Montanaro, L. et al. Relationship between dyskerin expression and telomerase activity in human breast cancer. Cell Oncol. 30, 483–490 (2008).
Gu, B. W., Bessler, M. & Mason, P. J. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc. Natl Acad. Sci. USA 105, 10173–10178 (2008).
Liu, S. Y., Zhao, Z. Y., Qiao, Z., Li, S. M. & Zhang, W. N. LncRNA PCAT1 interacts with DKC1 to regulate proliferation, invasion, and apoptosis in NSCLC cells via the VEGF/AKT/Bcl2/Caspase9 pathway. Cell Transplant. 30, 963689720986071 (2021).
Sieron, P. et al. DKC1 overexpression associated with prostate cancer progression. Br. J. Cancer 101, 1410–1416 (2009).
Penzo, M. et al. Dyskerin and TERC expression may condition survival in lung cancer patients. Oncotarget 6, 21755–21760 (2015).
Ji, P. et al. Systematic analyses of genetic variants in chromatin interaction regions identified four novel lung cancer susceptibility loci. J. Cancer 11, 1075–1081 (2020).
Jana, S., Hsieh, A. C. & Gupta, R. Reciprocal amplification of caspase-3 activity by nuclear export of a putative human RNA-modifying protein, PUS10 during TRAIL-induced apoptosis. Cell Death Dis. 8, e3093 (2017).
Cui, Q. et al. Targeting PUS7 suppresses tRNA pseudouridylation and glioblastoma tumorigenesis. Nat. Cancer 2, 932–949 (2021).
Miao, F. A. et al. Increased DKC1 expression in glioma and its significance in tumor cell proliferation, migration, and invasion. Invest N. Drugs 37, 1177–1186 (2019).
Alawi, F., Lin, P., Ziober, B. & Patel, R. Correlation of dyskerin expression with active proliferation independent of telomerase. Head. Neck. 33, 1041–1051 (2011).
Li, H. et al. The identification of RNA modification gene PUS7 as a potential biomarker of ovarian cancer. Biology 10, 1130 (2021).
Motyl, T. et al. Blood plasma pseudouridine in patients with malignant proliferative diseases. Eur. J. Clin. Chem. Clin. Biochem. 31, 765–771 (1993).
Zeleznik, O. A. et al. A prospective analysis of circulating plasma metabolites associated with ovarian cancer risk. Cancer Res. 80, 1357–1367 (2020).
Zheng, G. et al. Synthesis of a FTO inhibitor with anticonvulsant activity. ACS Chem. Neurosci. 5, 658–665 (2014).
Singh, B. et al. Important role of FTO in the survival of rare panresistant triple-negative inflammatory breast cancer cells facing a severe metabolic challenge. PLoS One 11, e0159072 (2016).
Huang, Y. et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 43, 373–384 (2015).
Huang, Y. et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 35, 677–691.e610 (2019).
Chen, B. et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J. Am. Chem. Soc. 134, 17963–17971 (2012).
Aik, W. et al. Structure of human RNA N6-methyladenine demethylase ALKBH5 provides insights into its mechanisms of nucleic acid recognition and demethylation. Nucleic Acids Res. 42, 4741–4754 (2014).
Xu, C. et al. Structures of human ALKBH5 demethylase reveal a unique binding mode for specific single-stranded N6-methyladenosine RNA demethylation. J. Biol. Chem. 289, 17299–17311 (2014).
Zhang, J. et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut 65, 1482–1493 (2016).
Zhang, M. et al. Targeting SNHG3/miR-186-5p reverses the increased m6A level caused by platinum treatment through regulating METTL3 in esophageal cancer. Cancer Cell Int. 21, 114 (2021).
Fukumoto, T. et al. N(6)-Methylation of adenosine of FZD10 mRNA contributes to PARP inhibitor resistance. Cancer Res. 79, 2812–2820 (2019).
Taketo, K. et al. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int. J. Oncol. 52, 621–629 (2018).
Li, H. B. et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342 (2017).
Ramanathan, A., Robb, G. B. & Chan, S. H. mRNA capping: Biological functions and applications. Nucleic Acids Res. 44, 7511–7526 (2016).
Xu, L. et al. Three distinct 3-methylcytidine (m(3)C) methyltransferases modify tRNA and mRNA in mice and humans. J. Biol. Chem. 292, 14695–14703 (2017).
Nombela, P., Miguel-López, B. & Blanco, S. The role of m(6)A, m(5)C and Ψ RNA modifications in cancer: Novel therapeutic opportunities. Mol. Cancer 20, 18 (2021).
Acknowledgements
This work was funded by the National Key Research and Development Program of China (2021YFC2301800), the National Nature Science Foundation of China (U20A20343), and Zhejiang University Academic Award for Outstanding Doctoral Candidates (2020055).
Author information
Authors and Affiliations
Contributions
L.L. and J.L. designed the study, and reviewed and edited the manuscript; C.X., Q.C., and Q.Z. participated in original draft preparation; S.J., Z.B., and Y.S. collected the references and help with reviewing the manuscript; All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, C., Chu, Q., Zheng, Q. et al. Role of main RNA modifications in cancer: N6-methyladenosine, 5-methylcytosine, and pseudouridine. Sig Transduct Target Ther 7, 142 (2022). https://doi.org/10.1038/s41392-022-01003-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01003-0
This article is cited by
-
Epigenetic regulation of diverse cell death modalities in cancer: a focus on pyroptosis, ferroptosis, cuproptosis, and disulfidptosis
Journal of Hematology & Oncology (2024)
-
Research progress of N1-methyladenosine RNA modification in cancer
Cell Communication and Signaling (2024)
-
Clinical significance of RNA methylation in hepatocellular carcinoma
Cell Communication and Signaling (2024)
-
The interaction between intratumoral bacteria and metabolic distortion in hepatocellular carcinoma
Journal of Translational Medicine (2024)
-
RNA modifications in cellular metabolism: implications for metabolism-targeted therapy and immunotherapy
Signal Transduction and Targeted Therapy (2024)