Abstract
Circulating tumor cells (CTCs) are tumor cells that have sloughed off the primary tumor and extravasate into and circulate in the blood. Understanding of the metastatic cascade of CTCs has tremendous potential for the identification of targets against cancer metastasis. Detecting these very rare CTCs among the massive blood cells is challenging. However, emerging technologies for CTCs detection have profoundly contributed to deepening investigation into the biology of CTCs and have facilitated their clinical application. Current technologies for the detection of CTCs are summarized herein, together with their advantages and disadvantages. The detection of CTCs is usually dependent on molecular markers, with the epithelial cell adhesion molecule being the most widely used, although molecular markers vary between different types of cancer. Properties associated with epithelial-to-mesenchymal transition and stemness have been identified in CTCs, indicating their increased metastatic capacity. Only a small proportion of CTCs can survive and eventually initiate metastases, suggesting that an interaction and modulation between CTCs and the hostile blood microenvironment is essential for CTC metastasis. Single-cell sequencing of CTCs has been extensively investigated, and has enabled researchers to reveal the genome and transcriptome of CTCs. Herein, we also review the clinical applications of CTCs, especially for monitoring response to cancer treatment and in evaluating prognosis. Hence, CTCs have and will continue to contribute to providing significant insights into metastatic processes and will open new avenues for useful clinical applications.
Similar content being viewed by others
Introduction
Metastasis is the most lethal feature of cancer1. Despite significant developments in cancer diagnosis and treatment over the past centuries, metastasis remains a major obstacle to improving clinical outcomes of cancer patients2. Nevertheless, we have witnessed significant progress over the past two hundred years in revealing fundamental concepts underlying the development of metastasis and in creating new technologies to facilitate cancer metastasis research. Fig 1 highlights the key discoveries and milestones in the study of cancer metastasis. The ‘seed and soil’ hypothesis by Steven Paget3 in the 1830s vividly clarified the progress of cancer metastasis. With the advances of science and technologies, particularly since the 2000s, a plethora of new technologies have been advanced, such as high-throughput sequencing4,5, transgenic mouse models6, CRISPER/Cas9 editing tools7, and single-cell sequencing8. With these powerful technologies, the biological phenomena underlying metastasis, such as epithelial-mesenchymal transition (EMT) of tumor cells9, the role of exosomes in supporting metastasis10, circulating tumor cells (CTCs) and CTC clusters in seeding metastatic colonies11, and complex interactions between tumor cells and microenvironment12, have gradually been unmasked, along with the discovery of numerous metastasis-related driver genes. The ‘black box’ of metastasis is gradually being unveiled, and effective metastasis-targeting agents are believed to be on the horizon in the near future.
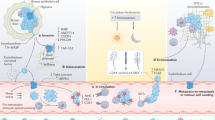
Cancer metastasis is a complex multistep process involving cancer cell invasion in the primary site, intravasation into circulation, survival in the circulation, extravasation from the circulation, and attachment to and colonization of the metastatic site (Fig. 2). CTCs are defined as tumor cells that have been sloughed from the primary tumor and are swept away by the circulatory or lymphatic systems. To date, most CTC research has focused on CTCs in the blood circulation. CTCs were first described in 1869 by Ashworth who observed “some cells” in the blood of a metastatic cancer patient with an appearance similar to tumor cells in the primary tumors13. CTCs have been assumed to be the substrate of metastasis. Although CTCs originate from the primary tumor, they are distinct from primary tumor cells14, with EMT transition properties that help them break free from the primary tumor and facilitate intravasation into the bloodstream, dissemination in clusters of CTCs to increase metastatic potential, and exhibit stemness features that enhance their ability to initiate metastasis (Fig. 2). However, most CTCs perish in the circulation, and only limited CTCs survive and infiltrate distant organs. Interactions between CTCs and the blood environment (Fig. 2), including how CTCs escape immune surveillance in the blood, have been widely implicated in the metastatic mechanisms of CTCs. It has taken more than a century for researchers to recognize the critical role of CTCs in cancer metastasis, due to the unique technical challenges required to isolate these very rare CTCs from the massive pool of circulating blood cells15. However, in the past two decades, emerging technologies for CTC isolation have allowed research on the biology of CTCs and have facilitated the clinical applications of CTCs in cancer screening, treatment response monitoring, and prognosis evaluation.
Multistep process of cancer metastasis. The complex metastatic process includes tumor cell invasion in the primary site, intravasation into circulation, survival in the circulation as CTCs and interaction with blood cells, extravasation from the circulation, attachment to and colonization of the metastatic site. EMT: epithelial to mesenchymal transition, CAF: cancer-associated fibroblast, TAM: tumor-associated macrophage
In this review, the biology of CTCs, as well as their interaction with the blood microenvironment, is fully reviewed. In addition, the growing number of highly sophisticated CTC enrichment and isolation technologies will be summarized. Finally, we also discuss the tremendous potential of CTCs in clinical applications.
The biology of CTCs
Molecular characterization of CTCs
Experimental evidence has supported the notion that tumor cells can spread even during the early stages of tumor evolution16,17. The molecular characterization of CTCs will add knowledge to the underlying mechanism of metastatic processes, contributing to early diagnosis and prevention of metastasis.
Molecular markers of CTCs
A panel of molecular markers has been used to detect CTCs in various cancers. CTC-associated markers used for different cancers are summarized in Table 118,19. As most cancers are of epithelial origin, the most common marker used for CTCs is EpCAM, a “universal” epithelial marker of cancers20. EpCAM expression varies among different cancer types21, and EpCAM-based CTC detection technologies are widely applied for cancers that strongly express EpCAM, such as breast and prostate cancer. Many studies have shown that CTCs in breast and prostate cancer are EpCAM-positive, and have validated their prognostic value in either early or metastatic stage cases22,23. Other epithelial-derived cancer types, such as pancreatic24, colorectal25, and hepatocellular cancers26, also have a considerable detection rate of EpCAM-positive CTCs. Similarly, the presence of these EpCAM-positive CTCs predicts early distant metastasis and poorer survival of patients25,27,28. However, using EpCAM as a CTC marker has limitations. It cannot be used in tumors that are EpCAM-negative or with low expression, such as neurogenic cancers. CTCs can undergo EMT, and epithelial markers, including EpCAM, are down-regulated during EMT, which affects the detection rate of EpCAM-positive CTCs. Although there are doubts as to whether EpCAM-based technologies are appropriate to detect all CTCs, numerous studies have illustrated the potential value of EpCAM-positive CTCs in clinical applications29. To some extent, EpCAM-positive CTCs are a substantial subgroup of all CTCs, thus EpCAM-positive CTCs still could be a reliable biomarker if the cancer prognosis and therapeutic efficacy is relevant to EpCAM-positive CTCs.
Due to the EMT activity of some epithelial cancer cells, detecting only EpCAM-positive CTCs probably underestimates the actual total CTC population and misses important biological information of EpCAM-negative CTCs. In some cancer types, such as non-small-cell lung cancer (NSCLC) patients, it was even found that the quantity of EpCAM-negative CTCs was significantly larger than EpCAM-positive CTCs30. Nevertheless, the poor isolation of CTCs by EpCAM-based technologies can be rescued by using both epithelial and mesenchymal cancer markers, as well as by marker-independent detection methods. For example, in breast cancer, the use of fluorescent-magnetic nanoparticles consisting of a dual-antibody interface targeting both EpCAM and N-cadherin has contributed to high-efficiency isolation and rapid identification of CTCs31,32. In biliary tract cancer, a single-cell assay for detecting CTCs allowed identification of both epithelial CTCs and non-conventional CTCs which lacked epithelial and leukocyte markers, and therefore led to an increase of CTC positivity rate33. The EMT program of cancer cells exhibits molecular alterations, including decreased expression of epithelial markers (E-cadherin, ZO-1, claudins, and occludins) and increased expression of mesenchymal markers (vimentin, N-cadherin, fibroblast-specific protein1, and fibronectin)34. EMT is executed by EMT-related transcription factors, mainly belonging to the SNAIL, TWIST, and ZEB families34. All these EMT-related molecules can theoretically be used for EMT-CTCs targeting methods. However, many EMT-related molecules are cytoplasmic or nuclear proteins, precluding their usage in the currently available membrane molecular-based technologies of CTC detection. Proteins such as E-cadherin, vimentin, and twist were most often used in the past35(Table 1), probably because of their accessibility of detection in traditional CTC detection technologies including flow cytometry sorting, immunostaining, and fluorescence in situ hybridization (FISH) staining. However, the emergence of single-cell CTC sequencing technologies36 will make it possible to unmask the EMT status of CTCs more comprehensively, and can cover all the EMT-related molecular alternations at the RNA level.
Other biomarkers, such as human epidermal growth factor receptor-2 (HER2)37,38,39,40,41,42,43,44,45,46,47, estrogen receptor39,48,49,50, prostate-specific membrane antigen51,52,53, folate receptor54,55,56, and survivin57, have been described as CTCs markers in different cancers, with different clinical significance. These cancer-specific CTC markers are listed in Table 1. Most of these cancer-specific CTC markers are in accordance with the specific molecular markers of the primary tumor. However, there is discordance in the expression of specific markers between the primary tumor and CTCs. For example, the rates of discordance of HER2 gene amplification between CTCs and primary breast tumor were around 15%58, suggesting a clonal selection of CTCs or clonal acquisition, probably due to genetic instability. It should be mentioned that for melanoma, a skin cancer that begins in melanocytes, the detection technologies of CTCs are based on several melanoma cell adhesion molecules, such as HMW-MAA59,60,61, MART-162,63,64, CD14661,65, and MAGE A362,63,66, which are very specific molecular markers for melanoma.
The variety of CTCs markers indicates the heterogeneity of CTCs among different cancer types. Even in one patient, CTCs are spatio-temporally heterogenous, which may be the result of a spatially different microenvironment in the blood and temporal changes in therapy response. Thus, it is difficult to define the entire CTC population using the very limited molecular markers currently available. In addition, CTC markers should not be constant among different stages of cancer and treatment periods.
Genome analysis of CTCs
Genomic instability contributes to tumor evolution and the emergence of resistant tumor subclones. Monitoring tumor genomic instability, especially in terms of tumor resistance and metastases, greatly contributes to the evaluation of treatment response and precision medicine. The evaluation of CTCs assessment using noninvasive liquid biopsy is accessible for serial sampling to detect the genomic instability of the tumor.
Determining the status of EGFR and KRAS mutations is crucial for guiding treatment in NSCLC patients receiving EGFR tyrosine kinase inhibitors and colorectal cancer patients treated with anti-EGFR therapy respectively. The concordance of mutations between CTCs and matched primary or metastatic tumor tissue has attracted much attention. Using a microfluidic technique to capture CTC, Maheswaran et al. found that only two of 31 patients with mutations were overlooked from their detection assay67. They identified the EGFR activating mutation in CTCs in 92% of metastatic patients with NSCLC and detected the drug-resistant mutation T790M in CTCs of 33% of patients who responded to tyrosine kinase inhibitor therapy and in 64% of patients who exhibited clinical progression67. For the analysis of the KRAS gene mutation, the mutational concordance rate between CTCs and matched primary tumors ranged from 37% to 90% in colorectal cancer cases68,69,70,71. This difference in the concordance rate may be due to the different CTC selection protocols used in these studies. KRAS mutations are also common in pancreatic ductal adenocarcinoma (PDAC), present in 90% of PDAC cases. However, Kulemann et al. found that the discordance rate of KRAS mutation in CTCs and corresponding PDAC tumors was 42%72. Studies of gene mutation analysis in CTCs were also conducted in many cancers such as prostate cancer73,74, breast cancer75,76, hepatocellular carcinomas77, and a mutational discordance between CTCs and corresponding tumors were often found. The discordance rate was probably attributed to the different detection efficiency of CTC mutations, or the heterogeneity between CTCs and primary tumor cells. Genomic assessment of tumor tissue and CTCs can be complementary. So, a combination of mutational testing of CTCs and tumor specimens would guide treatment more precisely.
Determining copy number alternations (CNA) of CTCs helps analyze and track cancer profiles as tumors evolve. In lung cancer, Ni et al.78 found that CTCs exhibit reproducible CNAs patterns, similar to those of metastatic tumors, and different patients shared similar CNAs patterns. In small-cell lung cancer, a CNA-based classifier for CTCs correctly assigned 83.3% of patients as chemorefractory or chemosensitive79. In breast cancer, the assessment of CNAs in archived CTCs is feasible. Paoletti et al.80 found that the CNAs of CTCs and paired metastatic tumor tissue in breast cancer patients were highly concordant, although CTCs and matched tumor tissue harbored several discordant copy number alterations, suggesting that CTCs were the subclone cells of tumor tissues. In triple-negative breast cancer, CTCs with chromosome 10 and 21q CNAs are predictive of clinical progression, and their network analysis presented connected modules including HER/phosphatidylinositol-4,5-bisphosphate 3-kinase/RAS/JAK signaling81. In prostate cancer, Lambro et al.82 revealed that CNAs of CTCs were interpatient and intercell heterogeneous, and could be missed in bulk biopsy analyses. In metastatic castration-resistant prostate cancer, whole genomic copy number analysis of CTCs showed that common genomic gains in CTCs involved genes such as androgen receptor (AR), mesenchymal-to-epithelial transition (MET), ERG, and cyclin-dependent kinase 12, while common genomic losses were observed in genes such as phosphatase and tensin homolog (PTEN), RAF1, and GATA283. Similarly, Malihi et al. also observed that CNAs in genes including PTEN, RB1, TP53, and AR closely associated with genomic instability and survival in aggressive variant prostate cancer84.
Other genome analyses have also been conducted in CTCs. FISH testing was adopted in CTCs to detect biomarkers for treatment sensitivity, such as ALK FISH testing in CTCs of NSCLC patients85 and HER2 FISH testing in CTCs of breast cancer patients86,87. Recently, based on the technique of single-cell resolution DNA methylation analysis, the DNA methylome of single CTCs and CTC clusters was revealed for breast cancer patients, and indicated that the CTC cluster hypomethylation profile obtained was associated with a poor prognosis and that treatment with Na+/K+-ATPase inhibitors to dissociate CTC clusters could revert the methylation profile of CTC clusters and suppress metastasis88.
Transcriptome analysis of CTCs
Single-cell sequencing has developed rapidly in recent years and has been applied to investigate the CTC transcriptomes. Single-cell expression profiles can distinguish CTCs from mesothelial cells and blood cells in lung adenocarcinoma, with representative markers including EpCAM for CTCs89. Single-cell sequencing-based transcriptome analysis revealed heterogeneity in the CTC subpopulation. By testing the expression of proliferation-associated genes such as the Ki-67 proliferation marker, Magbanua et al90. found that 65% of CTC in patients with metastatic breast cancer had low proliferation Ki-67 and that the 35% of patients with a high proliferation Ki-67 expression had a poor prognosis. Cheng et al.91 performed a single cell transcriptome analysis of 666 CTCs in patients with metastatic breast cancer. They determined that intra-patient CTCs were heterogeneous with regard to EMT-like and MET-like states, and CTCs were enriched for the stem-like phenotype91. Single-cell sequencing of CTCs also greatly helped in discovering driver signaling pathways that contributed to metastasis and treatment failure. RNA-Seq of single prostate CTC indicated the activation of noncanonical Wnt signaling in antiandrogen resistant patients92. Further, using mouse models, ectopic expression of Wnt5a attenuated the effects of an AR inhibitor and suppression of Wnt5a could partially restore the sensitivity in drug-resistant prostate cancer cells92. CTCs have been associated with a poor prognosis in colorectal cancer. A study of the CTC-specific transcriptome profile93 of six metastatic colorectal cancer patients characterized 410 CTC-specific genes, which were primarily related to cell movement and adhesion, such as VCL ITGB5, bone morphogenetic protein 6, transforming growth factor beta 1, and talin 1, and were related to cell death and proliferation, such as amyloid beta precursor protein, clusterin, and TIMP1.
Epithelial-to-mesenchymal transition of CTCs
EMT is the process by which epithelial tumor cells lose their intercellular adhesion and acquire mesenchymal and invasive properties. During dissemination, tumor cells detach themselves from the basement membrane through EMT activation and directly enter the circulation, serving as CTCs traveling to distant sites. When CTCs extravasate, they then undergo a reverse process termed MET and proliferate to form macro-metastases94,95,96. Herein, metastatic development depends on the delicate balance of the transition between these two phenotypes. The activity of EMT-MET was also proposed to play an important role in the metastatic process of CTCs97. Using mouse models, it was found that epithelial-type CTCs with a restricted mesenchymal transition had the strongest lung metastases formation capacity, whereas mesenchymal-type CTCs showed limited metastatic ability98. In breast cancer, CTCs exhibit dynamic changes in EMT composition, and mesenchymal CTCs were found to be closely associated with cancer progression9,98. A plethora of studies has shown increased EMT of CTCs rather than primary tumor cells in various cancers99,100,101,102,103. In a study based on the bioinformatics analysis of seven sets of gene chips, Guan et al. showed that compared with primary tumors, the main changes in CTCs involved cell adhesion, EMT, and apoptosis104. In a prospective study including 39 patients with invasive breast cancer, Tashireva et al. observed a majority of heterogeneous CTC phenotypes (22 out of 24 detectable samples) exhibiting EMT plasticity105. Interestingly, it was found that fluid shear stress can induce the EMT of CTCs via JNK signaling in breast cancer, which further confirmed the relationship between augmented EMT of CTCs and poor patient survival106.
Clinically, combining the total CTC count and the proportion of mesenchymal CTCs107 can be used to monitor therapeutic resistance and predict prognosis in cancer patients due to the significant survival differences of this criterion108. For example, since the baseline presence of total CTCs in advanced NSCLC conferred poor prognosis109, and the presence of over five EMT-CTCs indicated progressive disease110. Different numbers of total CTCs and EMT CTCs were found to play an important role in determining the prognosis of breast cancer patients. Intriguingly, it was emphasized that a better understanding of EMT-CTC subtypes and their interactions with peripheral blood mononuclear cells could help design better anti-metastatic treatments111. As CTC EMT-positive patients with neutrophil-to-lymphocyte ratios ≥3 had an 8.6 times increased risk of disease recurrence compared with CTC EMT-negative patients with lower neutrophil levels, inflammation-based scores increased the prognostic value of CTCs in primary breast cancer112. Therefore, targeting the EMT pathway may prevent tumor cell spread in early-stage patients and eradicate metastatic cells in advanced stages.
The stemness of CTCs
Many previous studies have indicated a subpopulation of aggressive CTCs with “stemness” traits in different cancers, which refer to their properties for self-renewal and tumor growth induction. In bladder cancer, studies have reported the high expression of OCT4, a crucial stemness maintenance protein113, in a subgroup of CTCs114. In breast cancer, the CD44+/CD24-/low and aldehyde dehydrogenase 1 (ALDH1) + cell phenotypes are reported to be associated with stemness. After detecting the expression of CD44, CD24, and ALDH1 of CTCs in 30 metastatic breast cancer patients, Theodoropoulos et al. found that 35.2% of 1439 CTCs were CD44+/CD24-/low, and 17.7% of 238 CTCs were ALDH1high/CD24-/low, providing evidence of CTCs stemness96. A CTC-3 cell line established from the peripheral blood cells of a breast cancer patient showed more aggressive growth than the widely used MCF-7 breast cancer cell line. Gene profiling revealed higher expression of the stemness markers in the CTC-3 cell line compared to MCF-7 cells115. In hepatocellular carcinoma (HCC), 71.4% of HCC patients were CTC positive for the cancer stem cell marker, CD44; thus, they had a significant population of CTCs with stem properties, which could contribute to tumor cell survival and dissemination116. In glioblastoma, RNA-seq analysis revealed Wnt activation-induced stemness and chemoresistance in CTCs117. In prostate cancer, the stem cell marker CD133 was observed in the majority (> 80%) of CTCs of patients with metastatic castration-resistant prostate cancer97 and a stem-like subpopulation of the C-X-C motif chemokine receptor 4+/CD133+CTCs was more prevalent in EpCAM-negative CTCs than in EpCAM-positive CTCs118.
It is crucial to understand the mechanisms regulating CTCs stemness, and interfering with the CTCs subpopulation stemness properties may more efficiently suppress cancer progression and relapse. CTCs undergo considerable levels of fluid shear flow during their dissemination, and the fluid shear flow itself may have an impact on CTCs. Using a model of breast cancer with brain metastasis, it was suggested that hemodynamic shear flow could upregulate the stemness genes of CTCs in surviving under conditions of shear flow119. EMT-like transition of CTCs by downregulating ERK and GSK3β signaling could promote the conversion of CTCs into stem-like CTCs with high sphere-forming and tumor-initiating capacity120.
CTCs and the blood microenvironment
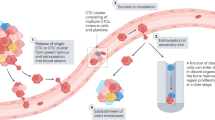
When transported in the bloodstream, a major of CTCs are constrained by detrimental shear stress, or die from anoikis, a programmed cell death mechanism due to loss of cell attachment121,122. Only a small fraction of CTCs interact tightly with platelets, neutrophils, macrophages, myeloid-derived suppressor cells (MDSCs), or cancer-associated fibroblasts (CAFs) to escape the immune system and promote their survival123,124. Recently, accumulating studies suggest that the interaction and modulation between CTCs and hostile blood microenvironment is essential for adhesion to endothelial cells, tissue invasion, and tumor metastasis (Fig. 3).
Interaction of CTCs with neutrophils
Neutrophils are the most abundant circulating leukocytes in humans and have recently been studied to support cancer progression125. An increased number of neutrophils in circulation is associated with poor prognosis in several types of cancers126,127,128. The formation of CTC-white blood cells (WBCs) clusters was previously reported within the bloodstream129. In 2019, Szczerba et al. determined that CTCs were significantly associated with neutrophils in both mouse models and breast cancer patients, exhibiting more metastatic potential with greater expression of genes that involve cell cycle progression compared to CTCs alone130. These observations are consistent with previous findings showing the proliferation role of neutrophils on tumor cells131. CTC and neutrophil binding is mediated by the cell–cell junction and possibly requires the vascular cell adhesion molecule 1130. Furthermore, neutrophils can directly adhere to CTCs through the Mac-1/ICAM-1 interaction and act as a bridge between tumor cells and the liver parenchyma, thus promoting extravasation and liver metastasis132. Thus, CTCs clusters with neutrophils anchor to the vascular endothelium for extravasation while resisting shear stress, and the process is mediated by a series of cell adhesion proteins, such as cadherin, integrin, and surface glycoprotein133,134,135. Intriguingly, Chen et al. found that IL-8 secreted from neutrophils was essential for neutrophil sequestration with arrested tumor cells and for the extravasation behaviors of adjacent tumor cells through the endothelial barrier136.
Neutrophils can also promote metastasis in an indirect manner. Neutrophil extracellular traps (NETs) are web-like structures formed by DNA–histone complexes and proteins released from activated neutrophils, with the ability to impact on CTC biology137. Many studies have found that NETs were able to capture CTCs in the circulation and, in doing so, promoted metastatic dissemination138,139. In vitro and in vivo experiments have shown that CTC adhesion to NET is mediated by β1-integrin expressed on both NET and cancer cells, while this effect was abrogated following DNAse I administration139. In a murine model of surgical stress, the NET formation triggered the release of high-mobility group box 1, which activated TLR9-mediated pathways in CTCs and therefore accelerated the progression of liver metastases140. In addition, NETs can also awaken dormant cancer cells and promote metastasis. Recently, Albrengues et al. elegantly demonstrated that two NET-associated proteases, neutrophil elastase and matrix metalloproteinase 9 (MMP9), concentrate at laminin, provoke its cleavage, by generating an epitope that induced awakening of dormant cancer cells by integrin activation and FAK/ERK/MLCK/YAP signaling141. In turn, tumor-expressed protein, such as protease cathepsin, has been shown to support lung metastasis of breast cancer by promoting NET formation in metastatic niches142. Coiled-coil domain containing protein 25, another protein expressed on the cancer cell membrane, could serve as a specific sensor for the DNA component NETs, which induces migration and adhesion of tumor cells143. In addition, by forming NETs, circulating neutrophils can help CTCs escape immune surveillance by suppressing the activation of peripheral leukocytes144, the function of natural killer (NK) cells145, the antitumor response of effector T cells146, and even cooperation with other immune cells (such as IL17-producing γδT cells)147. Overall, there is evidence for a pro-metastatic role of neutrophils in their interaction with CTCs, but the specific mechanism remains to be elucidated in more detail.
Interaction of CTCs with macrophages
Tumor-associated macrophages (TAMs) not only contribute to metastatic progression within the primary tumor, but also promote later stages of metastasis including dissemination and extravasation of CTCs148. Hamilton et al. tried to investigate the CTC-macrophage interactions by co-culturing peripheral blood mononuclear cells with CTC cell lines obtained from small-cell lung cancer patients. They found that CTCs were able to induce monocyte differentiation to TAMs, which secrete a host of mediators such as osteopontin, MMP9, chitinase-3-like-1, and platelet factor to promote further leukocyte recruitment, migration, and invasion149,150. In another study of colorectal cancer, the feedback loop between TAMs and cancer cells is essential for the EMT program of CTCs and intravasation into the blood stream. Mechanistically, IL6 derived from TAMs regulates invasiveness through the STAT3/miR-506-3p/FoxQ1 pathway, which in turn increases CCL2 expression of TAM-educated tumor cells to help recruit macrophages151. TAMs seem to promote CTCs acquisition of mechanical adhesiveness and endurance, thereby helping them to form protective cell clusters and confer resistance to shear stress152. The fusion of macrophages and tumor cells could be a potential mechanism of immune evasion and invasion. These macrophage-tumor cell hybrids were shown to have M2-like macrophage phenotypes (CD163) as well as epithelial markers (EpCAM)153,154, and have been isolated from the blood of patients with multiple cancers such as PDAC155, melanoma154, breast, ovarian, and colorectal cancer156. Furthermore, when transplanted into mice, they spread widely and formed lesions in distant tissues154,155. A recent study by Gast et al. revealed that fusion hybrids can increase tumor heterogeneity and metastatic behavior, further correlating with disease stage and overall survival among several cancers157. Furthermore, larger hybrid sizes were also associated with worse survival among patients with non-small cell lung cancer158. Understanding the mechanism of direct interaction and molecular fusion between CTCs and macrophages is of great significance for identifying therapeutic targets.
Interaction of CTCs with platelets
The metastasis and progression of cancer are greatly influenced by the recruitment and activation of platelets, which support the survival of CTCs as well as their seeding and outgrowth at secondary sites159,160. Platelets can bind to and form aggregates with CTCs in the blood stream, and CTCs expand the formation of aggregation by releasing prothrombotic and procoagulant microparticles or by expressing tissue factor161,162. Platelet-released mediators, such as TGF-β, have been found to accelerate EMT in CTCs and to promote invasion and metastasis163,164. Xiong et al. recently determined that the expression of the heat shock protein 47 was induced during EMT, which enhanced the cancer cell–platelet interaction through its dependent collagen secretion in breast cancer cells165. Interestingly, platelets are believed to protect CTCs against mechanical stress166 and induce resistance to anoikis, the latter being mediated by the activation of the YAP1 pathway167. Furthermore, platelets promote escape of CTCs from NK cell attack through various mechanisms including (1) platelet aggregates that produce surface shielding to defend the cytolysis effect of NK cells;168 (2) platelet-derived normal MHC-I that are transferred to the surface of the tumor cell, thus preventing the identification of NK cells;169 (3) downregulation of natural killer group 2, member D (NKG2D) in NK cells by platelet-derived TGF-β, as well as platelet-mediated shedding of NKG2D ligands, which contribute to impaired antitumor cytotoxicity;170,171 and (4) platelet-derived glucocorticoid-induced TNF-related ligand which activates GITR in NK cells and reduces their cytotoxicity172. Furthermore, NK cells and platelets can also interfere with neutrophils, T cells, and macrophages and modulate their immune function173,174,175. In addition to safeguard CTCs within the bloodstream, platelets are also involved in the adhesion of endothelial cells. The attachment of platelets and CTCs is mediated by platelet adhesion receptors, such as the integrin αIIbβ3 and P-selectin, thereby supporting the firm adherence of CTCs to the endothelial wall176,177,178. Furthermore, tumor cell-activated platelets release ATP from dense granules, which then induces the activation of the endothelial P2Y2 receptor and allows transendothelial migration of tumor cells by increasing permeability179. One study has also revealed that the interplay between integrin α6β1 on platelets and its receptor, a disintegrin, and metalloprotease 9 on CTCs is necessary for the extravasation process of cancer cells180. Platelets could also increase vascular permeability to help tumor cell extravasation. For example, a preclinical lung metastasis model showed that tumor cell-associated CD97, an G protein-coupled receptor, can initiate platelet activation thereby leading to granule secretion, including both ATP and lysophosphatidic acid release181. Similarly, the interplay between platelet-specific receptor glycoprotein VI and its ligand galectin-3 expressed on colon and breast cancer cells was revealed to promote platelet activation and ATP secretion182. Consequently, these platelet secretions favor a process of tumor metastasis by regulating vascular permeability. Recently, Xu et al. discovered that Ptx@AlbSNO can block tumor-specific platelet functions to suppress tumor EMT as well as prevent platelet adhesion around CTCs. Ptx@AlbSNO can also inhibit TGF-β secretion and enhance intratumoral immune cell infiltration to reverse the immunosuppressive TME, thereby suppressing distant metastasis183. Taken together, the close and complex crosstalk between CTCs and platelets might involve distinct molecule variants and signaling pathways and possibly represent a promising antitumor strategy, particularly attractive for the treatment of several cancers.
Interaction of CTCs with MDSCs
MDSCs are a heterogeneous subset of myeloid cells characterized by immunosuppressive properties that also promote metastatic dissemination. Under a standard protocol for isolating human MDSCs, Cassetta et al. found that polymorphonuclear (PMN)-MDSC were significantly expanded among most cancer types except melanoma compared with infection and inflammation184. CTC-MDSC clusters are thought to evade immune surveillance of the T cell response185. Indeed, a decrease in circulating MDSCs was associated with an increase in activated OX40+PD-1- T cells in patients with diffuse large B-cell lymphoma186. Furthermore, Sprouse et al. found that in vitro co-culture of CTCs derived from melanoma and breast cancer patients and PMN-MDSCs enhanced Notch activation in CTCs through direct interaction between Jagged1 (Notch1 ligands) expressed on MDSCs and the Notch1 receptor expressed on CTCs. The increased production of reactive oxygen species production of MDSCs could upregulate Notch1 receptor expression, therefore, promoting CTC proliferation187. The potential mechanisms underlying the interplay between CTCs and MDSCs remains to be determined.
Interaction of CTCs with CAFs
CAFs are one of the most abundant components in the TME and play a prominent role in tumor initiation, angiogenesis, metastasis, and drug resistance188. Mechanistically, CAFs remodel the extracellular matrix structure, which allows tumor cells to invade through the stroma and communicate with cancer cells by secreting growth factors, chemokines, and cytokines189. However, little is known about the interplay between CAFs and CTCs. Duda et al. first demonstrated that CTCs could carry CAFs from the primary tumor to the metastatic site in mouse models of lung cancer metastasis190. These host-derived CAFs directly enhance tumor cell survival and promote the formation of metastasis, while depleting CAFs from lungs significantly reduces the number of macroscopic metastasis and extends survival rate in mice. Moreover, CAFs can protect CTCs from the fluid shear forces during the dissemination process191. In a three-dimensional co-culture model, CAFs were found to induce shear resistance to prostate tumor cells through stable intercellular contact, as well as soluble factors (such as CXCL5, CCL2, and CCL7), which are associated with cell survival, invasion, and EMT. In addition to experimental models, circulating CAFs (identified by FAP and α-SMA co-expression) have been detected in the peripheral blood of patients with metastatic breast, cancer but not in patients in the early stages192, and exhibit excellent precision in metastatic diagnosis (AUC–ROC, 0.975) when isolated using a novel acoustic microstreaming platform193.
Technologies for CTCs enrichment and isolation
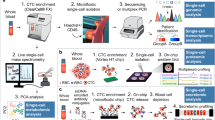
Over the past few years, many methods have been proposed to capture CTCs. Due to the extremely small proportion of CTCs in patients’ blood, it is still a great challenge to accurately isolate CTCs from the numerous blood cells, and especially to invent applicable methods that can efficiently detect viable CTCs for subsequent in-depth analysis. Here, we will discuss the development of CTC-related technologies over the last two decades, as they have experienced tremendous growth. We will emphasize the most innovative methods associated with nanoscale materials or novel microfluidic chips, hoping to provide a useful framework of CTC-related technologies.
Generally, there are three core strategies of CTCs technologies194, which include (1) capture and enrichment, (2) detection and identification, and (3) release. The first strategy of capture and enrichment involves a specific interaction between CTCs and materials through physical interactions or antibody–antigen interactions. The second strategy of detection, which means identifying the CTCs, refers to various methods, such as fluorescence microscopy, fluorescence spectrophotometry, flow cytometry, surface-enhanced Raman scattering, or electrical impedance. In the last strategy, released CTCs are mainly used for downstream analysis, such as genomics, transcriptomics, proteomics, and CTCs culture.
Classic CTC-related technologies based on physical properties
The physical separation enrichment method of CTCs is based on differences between CTCs and blood cells in size, density, deformability, and electrical properties. The Isolation by size of epithelial tumor cells system195 can filter blood samples through an 8-μm diameter polycarbonate TRACK-ETCH-type membrane, but it has low efficiency. An improved method, which consists of a pressure regulating system, the flexible micro spring array device196, reaches a capture efficiency of 90% with a detection of CTCs in 76% of samples. However, there are various trends of CTC counts observed from different samples, making this method not reliable for widespread use. CTCs can also be sorted using the Oncoquick system197, a density-dependent technique that allows red blood cells and WBCs to be filtered, or by Apostream198 that uses dielectric electrophoresis techniques in the microfluidic chamber to capture CTCs. These systems require large volume of blood and cannot collect the CTCs of a similar size as WBCs, which are their main limitations. Overall, the methods based on physical properties are generally inefficient, poor in purity, and lack of specificity, although the vitality is good and the cost is relatively inexpensive.
Classic CTC-related technologies based on biological properties
Biological property-based technology is another important method for CTC detection. Based on antibody–antigen interaction, CTCs are usually positively enriched using epithelial (EpCAM) and mesenchymal (vimentin) markers as well as negatively enriched by using CD45 to deplete unwanted leukocytes199. EpCAM-dependent techniques are most commonly used by researchers. The CellSearch system200, the only FDA-approved device for clinical use, employs EpCAM antibody-coated ferromagnetic beads to enrich CK + /CD45-/DAPI + CTC and remove CK-/CD45 + /DAPI + WBCs. However, CTCs strongly adhere to the surface of the equipment in antibody interaction-based methods, making them difficult to be released. This deficiency can be resolved in another EpCAM-dependent, MagsWeeper system201, which uses a magnetic rod to enrich CTCs and eliminate cells not bound to magnetic beads, allowing the release of CTCs for the following biochemical analysis. CanpatrolTM 202 is another representative of the EpCAM-dependent technique, which provides morphological, cytological, and genetic characterization of individual CTCs. In summary, techniques based EpCAM are extensively-used. However, because the CTC surface antigen has high heterogenicity, CTCs that have low expression of EpCAM may not be enriched, causing inaccurate results, while methods based on physical properties do not have this limitation. Therefore, combining the advantages of different technologies or looking for CTCs with high sensitivity and specific tumor markers have gradually won the attention of researchers.
Recently, with the development of microfluidic chips, nanomaterials, and next-generation sequencing, researchers have many advanced technologies to stimulate progress in CTC-related technologies. Importantly, researchers are trying to reach higher levels of CTC-related technologies in several key parameters: yield, purity, enrichment ratio, throughput, viability, sensitivity, specificity, release rate, accessibility for further analysis, and simplicity of equipment operation).
Recent CTC-related technologies: microfluidic-based and nanotechnology-based techniques
Besides the classic CTC-related technologies discussed above, some newer technologies, such as microfluidic-based and nanotechnology-based techniques have been developed. Microfluidic-based cell sorting approaches use “intrinsic” (e.g., fluid dynamic forces) versus “extrinsic” external forces (e.g., magnetic, electric field, acoustic, and optical forces) to separate cells, and then select target cells from a sample of heterogeneous cells through different physical and biological properties203. The CTC-chip is a silicon microfluidic platform on which the CTCs are captured on the slides of molecular marker coated posts. The CTC-chip204 can separate viable CTCs from whole blood without pre-labeling or processing of samples, resulting in increased cell activity and separation purity. A modified chip-based platform using gold nanoparticles on a herringbone chip (NP-HB CTC-Chip205) easily detaches viable CTCs and safely releases cells for further analysis by utilizing a chemical ligand-exchange reaction with gold nanoparticles on a herringbone chip. Furthermore, the monolithic CTC-iChip206 has high-efficiency WBC depletion and allows the characterization of CTCs with epithelial and mesenchymal characteristics. Although these microfluidic chips have greatly contributed to the development of the detection of CTCs (i.e., improved capture efficiency, viability, and depletion of WBCs), they are not widely applied for clinical use due to limitations, which include long set-up time, high initial cost, bulky instrumentation, and limited ability to perform single-cell molecular analysis.
In an attempt to capture CTCs in an automated manner (Table 2), Zhang et al. successfully sorted MCF-7 cells from a 5 mL volume of diluted blood within 23 m with a recovery rate of 85%207. Even more remarkable, Jia et al. developed a less costly self-driving micro-cavity array chip to achieve cell loading, lysing, isothermal amplification, and signal read-out on a single chip208. This novel chip can perform genetic analysis at the single-cell level, it has great potential in personalized therapy and efficacy monitoring. Furthermore, another automated and integrated microfluidic system proposed by Wang et al. is reported to achieve CTC capture and identification within 90 m. With the advantages of automation, stability, economy, and user-friendly operation, this system provides broad prospects for cancer screening and prognosis, especially in HCC209. Additionally, Lee et al. invented a microfluidic-based integrated system to achieve simultaneous on-chip isolation and characterization of circulating tumors utilizing differences in magnetic field gradient and immune fluorescence. Furthermore, this novel system can differentiate on-chip eight different subtypes of heterogenic CTCs, guiding the diagnosis and prognosis of breast cancer210. By combining the microfluidic technology and in situ molecular profiling techniques, the On-chip Post-processing Enabling chip platform has the ability to perform molecular analyses of single CTC from metastatic breast cancer and metastatic pancreatic cancer patients without any off-chip processes, suggesting its potential implementation of early molecular detection for cancer metastasis211. Taken together, less costly automated and integrated microfluidic systems that allow easy CTCs detection and cell analysis have great clinical value.
With the progress of nanomaterials, nanotechnology-based methods are becoming promising tools for CTC detection at an early-stage disease and for the monitoring of cancer development, as well as in vivo imaging212. Nanomaterials have a large surface-to-volume ratio and allow CTC isolation at high specificity and CTC detection at high sensitivity by adsorbing numbers of targeting ligands to bind specific molecules on cancer cells. At present, studies have reported many types of nanomaterials (Table 2) for CTC detection, including magnetic nanoparticles213,214,215,216,217, gold nanoparticles218,219,220, and quantum dots221,222,223. For example, studies have shown that the utilization of tannic acid-functionalized magnetic nanoparticles214, CoFe2O4@Ag magnetic nanohybrids216, and peptide-based magnetic nanoparticle213, enhances the capture efficiency of CTCs in breast cancer patients. Among them, peptide-based magnetic nanoparticles can distinguish epithelial and mesenchymal CTC subgroups and allow analysis at the single-cell level, the detection effects of which are supported by magnetic nanoparticles and microfluidic-based integrated systems210. Regarding gold nanoparticles, there have been significant developments. For example, the cytosensor proposed by Yang et al.224 showed excellent analytical performance, with a wide linear range, satisfactory CTC release (93.7–97.4%), and good cell viability. Liu et al. reported that gold nanoparticle-modified black phosphorus nanosheets improved the stability in detecting CTC218. Furthermore, the combination of a microfluidic system and gold nanoparticles presents a wider range of applications. Wang et al. synthesize an interfacial zinc oxide coating with a nanostructure on the microsphere surface, which increases the specific surface area and thus leads to an improved capturing efficiency of CTCs225. In addition, the utilization of multicolor magnetic surface-enhanced Raman scattering nanotags and chip-based immunomagnetic separation could detect four different surface protein markers on individual tumor cells in a quantitative and simultaneous manner, thus facilitating the separation of CTC subpopulations217.
Although nanotechnology-based techniques can provide broad prospects for CTC research in various tumors in a cost-effective and simple manner, there are limitations and challenges. First, many factors (e.g., binding of nanoparticle probes, aggregation) can affect nanoparticle-based detections, leading to decreased reliability and reproducibility. Second, most nanoparticle-based assays are prepared for academic studies, and they are still unrealistic for widely clinical translation. Third, there is possible toxicity of nanoparticles.
In the era of precision medicine, CTCs analysis has great clinical value. Tools must be sharpened first if workers are to do their job well. Therefore, CTC-related technologies are the underlying foundation to the application of CTCs in precision medicine. Herein, we reviewed previous CTCs-related technologies based on physical and biological properties, and the most recent development of techniques associated with microfluidic-based and nanoparticle-based approaches. Although there are strengths and weakness between different methods, we believe that an effective combination of these techniques may benefit CTCs research in many ways, especially the in-depth analysis and possibility in clinical applications.
Clinical applications of CTCs
Clinically, CTCs are now used as surrogate biomarkers for many solid cancers. Numerous studies have been carried out, mainly in breast cancer, prostate cancer, lung cancer, liver cancer, pancreatic cancer, gastric cancer, and melanoma. Although the clinical guidelines have not included the clinical use of CTCs, besides the inclusion of CTCs as part of the cM0 tumor classification (i.e., no clinical of overt metastasis but the detection of tumor cells in blood), many studies have predicted the great potential of CTCs in clinical applications. In this section, we will mainly present the role of CTCs as biomarkers for diagnosis, prognostication, and therapy monitoring in different cancers (Fig. 4).
Early diagnosis of cancer
As a non-invasive method, CTC detection is attractive in assisting cancer diagnosis. Studies of CTCs used for early diagnosis of cancer in the past three years are listed in Table 3. A tumor lesion already has more than 109 tumor cells by the time they are detectable in patients using current imaging procedures15, such as computed tomography, magnetic resonance imaging, and positron emission tomography. Diagnosis of cancers as early as possible, especially for fast-progressing cancers, is the best way to defeat them. Studies have determined that CTCs are correlated with tumor stage, but the clinical utility of CTCs in cancer detection or even in early cancer diagnosis is still a matter of debate. CTCs are considered a surrogate marker of metastatic activity, but whether metastatic dissemination of CTCs in patients occurs early during tumor formation is still controversial. However, in mouse models, early dissemination seeding metastasis has been found in breast226,227 and pancreatic228,229 carcinogenesis, indicating that CTC circulation is likely to be a very early event in cancer progression. In Barrière et al.’s study230, CTCs were detected in 41% of T1 stage and 47% of axillary lymph node-negative breast cancer patients, both of which are early-stage breast cancer. In the study by Thery et al.231 the CTC positivity rate was 21% and 24% in lymph node-negative and positive breast cancer, respectively. Based on this hypothesis, CTCs can be detected earlier before the primary tumor is visible on imaging studies, while the biggest challenge of the application of CTC application in early cancer diagnosis is indeed their scarcity and isolation. The limited sensitivity of CTC detection methods hinders their use as an effective biomarker in early cancer diagnostics.
Evaluation of the cancer prognosis
The prognostic value of CTCs has been extensively studied. CellSearch is the only FDA-approved system for CTC detection used clinically. Based on CellSearch system46,232,233,234,235,236,237,238,239,240,241,242,243,244,245, CTCs represent an independent prognostic factor. Studies evaluating other CTC detection systems such as CanPatol, CTC-chip obtained the similar results. CTC enumeration is the main target of investigation, with a cut-off value of ≥5 for positivity, which usually indicates a worse prognosis. It is generally considered that increased CTC counts are correlated with higher likelihood of metastasis and cancer aggressiveness. In a meta-analysis pooling 2239 breast cancer patients including 21 studies246, the CTC count before neoadjuvant chemotherapy had a detrimental and decremental impact on patient survival, and patients with one, two, three to four, and five or more CTCs displayed a HR of death (95% CI) of 1.09 (0.65–1.69), 2.63 (1.42–4.54), 3.83 (2.08–6.66), and 6.25 (4.34–9.09), respectively. Furthermore, elevated baseline CTCs levels were associated with inferior survival, the presence of CTC clusters often predicted poor prognosis247,248,249, and increasing CTC counts or failure to clear CTCs during treatment was also a prognostic factor for worse survival234,250,251. Many studies have found that the molecular phenotypes of CTCs have strong prognostic value. EMT and stemness are the main molecular phenotypes of CTCs studied clinically. CTCs with expression of mesenchymal252 or stemness253-related markers associated with inferior survival. The expression of other molecular markers, such as HER246, CD47254, PD-L1254 also have prognostic implications. Most studies investigate the prognostic value of CTCs at a single timepoint, while intriguingly, some studies have taken CTC dynamics into account. Magbanua et al.237 developed a novel latent mixture model to stratify groups with similar CTC trajectory patterns during the treatment course, and they found that analysis of serial CTCs can further stratify the patients of poor prognosis into distinct prognostic subgroups. The dynamic changes of CTCs may act as a surrogate prognosis biomarker over the long course of cancer progression. Given the rapid advancements in the accessibility and strengthening of sequencing technologies at the single-cell level, we may expect that in the future, genomic/transcriptional profiles of CTCs may serve as an outstanding prognostic marker, representing biological information that is more comprehensive and more closely related to prognosis. Studies of CTCs for predicting prognosis in recent three years are summarized in Table 3.
Monitoring of the therapeutic response
In many clinical trials, CTCs have been used as useful biomarkers for monitoring cancer treatment responses234,255,256,257, either combined with imaging examinations, serum biomarkers or alone. Researchers prefer to involve CTCs in the evaluation of therapeutic efficiency, in view of the higher sensitivity of CTCs than imaging examination in some cases9. As a non-invasive method, the detection of CTCs may also contribute to avoiding frequent radiation exposure from imaging studies during the evaluation of treatment response. Most studies found a decrease or clearance in CTC counts was associated with a good therapeutic response, while the increase of CTC counts signified the opposite258,259. The Response Evaluation Criteria in Solid Tumors (RECIST) guidelines are the most often used standard for evaluating therapeutic response in solid tumors. However, in some studies, changes in CTC following therapy were not correlated with RECIST responses in cancer patients260. Indeed, CTCs assessment has not been included in the RECIST guidelines. Some CTCs measurement technologies have been recently developed to achieve genotyping for CTCs, which can also detect crucial gene mutations, such as ER39,49, HER239,49, EGFR261, KRAS262, and TP53263, thus helping clinicians in treatment personalization and resistance options at the time of tumor progression. Studies using CTCs to monitor treatment response in recent three years are summarized in Table 3.
The great potential for CTCs in the clinical application of cancer diagnostics has emerged, although clinically, its use as a surrogate biomarker for cancer screening, treatment monitoring, and prognosis predicting is still limited. Once metastasis occurs, repeat biopsies of metastatic lesions are usually difficult to obtain, and different metastases are heterogenous even in the same patients. CTCs testing using peripheral blood samples is convenient, and may be more representative of the traits of metastatic cells, which are derived from different metastatic lesions in patients. Nevertheless, there is still a lack of guidelines for the clinical use of CTCs, such as a standardized CTCs detecting assay for different cancers, a combination diagnostic scheme with other clinical examinations, and indications for the appropriate timepoints for blood sampling.
Discussion
Studies investigating CTCs have the great potential to reveal the fundamental processes of metastases, including the mechanisms involved in extravasation of CTCs from the primary tumor, how CTCs interact with blood cells to survive in the circulatory microenvironment, and how CTCs intravasate into the distant metastatic site to initiate new lesions. Significant molecular traits of CTCs can greatly contribute to identify targets for anti-metastatic therapies. Only a small proportion of CTCs can finally generate metastases, thus studies focusing on these strongly metastatic CTCs may provide deeper insights into CTCs-related therapeutic targets.
Various CTCs detecting technologies have emerged, however, the sensitivity and specificity of these technologies still need to be further improved. Epithelial marker-based CTC detection technology, such as the CellSearch system has opened a new era for CTC analysis and clinical applications, but their drawbacks are rapidly being acknowledged and appreciated by researchers. EMT is a crucial trait of metastatic cancer cells, indicating insufficient capture efficiency of epithelial marker-based CTC detection technology. However, mesenchymal marker-based detection technologies may also be contaminated by non-CTCs, such as tumor-associated fibroblasts and endothelial clusters, which induce the risk of false positivity. Nevertheless, it is intriguing that recent studies have reported that those non-cancerous tumor-derived cells presented in cancer patients are also important surrogate biomarkers for cancer patients264. Cancer-type-specific molecular markers for CTCs are likely another option, as CTCs of different cancer types possess different molecular markers. However, the sensitivity and specificity of known cancer-type-specific CTC markers are not satisfactory. Physical-property-based CTC detection technologies also have the problem of contamination by non-CTCs, especially for those with similar physical properties as CTCs. Microfluidic-based and nanotechnology-based CTC detection technologies have become popular in recent years, while the efficiency of these technologies still needs further large-scale clinical validation. High cell detection efficiency and contamination removal capability are the two key strengths of a successful CTC detection technology, while substantial technical optimization of CTC detection is urgently needed to achieve these requirements.
Comprehensive characterization of CTCs is lacking. The limited amount of genomic DNA, RNA, and protein content of CTCs is a bottleneck for exploring their genome, transcriptome, epigenome, and proteome properties. Nonetheless, the emerging genome and transcriptome studies of CTCs have recently profited from the fast-evolving technology of single-cell sequencing, while the proteome studies of CTCs are still elusive due to the very limited technologies for proteome exploring at a single-cell level. However, the study of the CTC proteome is imminent, not only because it can provide a picture of the biological characterization of CTCs, but also because it can help to discover CTC-specific membrane proteins which may help optimize CTC detection.
As for the solid tumor microenvironment, the blood microenvironment around CTCs also plays a significant role in tumor survival and invasion capacity. However, knowledge of the underlying mechanisms behind the survival of CTCs is still limited, as it is a complex process that involves not only shear forces and fluid mechanics but also soluble factors and tumor-associated extracellular vesicles, which are not detailed here. Furthermore, it remains to be confirmed whether CTC clusters are more suitable for interacting with other blood components or adapting to shear forces than single migratory CTCs. If combined with specific biomarkers for strategically detecting CTCs and the interaction of CTCs with associated peripheral blood cells, we could improve the clinical practicability and monitoring power of CTCs by obtaining more comprehensive information on tumor burden and immune status of patients.
Although CTCs have shown initial promise in clinical applications, many challenges must still be overcome before CTC analysis can be widely applied in the clinic. Today, the clinical application of CTCs mainly depends on the analysis of CTC cell enumeration and molecular phenotypes. A more comprehensive characterization of CTCs based on their genome, transcriptome, and proteome with high-throughput sequencing will further benefit clinical application, but also add to the complexity and difficulty of data analysis. CTCs will be a crucial component of “Precision medicine” in the future, as the phenotypic, genotypic, and functional characterization can provide an opportunity to study drug susceptibility that is related to metastasis. The genome and transcriptome analysis of CTCs can unveil potential drug targets. Viable CTCs for drug sensitivity/resistance testing over the therapy course can guide precision medication. However, the culture of CTCs is very challenging: (1) limited methods are available to isolate viable CTCs, which also yield low numbers of CTCs and (2) a favorable circulatory microenvironment for CTC survival is difficult to mimic. Very limited CTC-derived cell lines from cancer patients have been established. Optimization of CTC culture conditions will be needed.
Furthermore, CTCs, circulating tumor DNA (ctDNA), and exosomes are all present in liquid biopsy samples. An exploration of the advantages and disadvantages of each substrate present in the liquid biopsies, and how to better incorporate them into clinical application is needed to achieve more precise diagnoses. Among liquid biopsy methods, CTCs have tremendous advantages, as isolated CTCs can be viable, which can optimize CTC-derived explants or three-dimensional organoid cultures for functional testing or for drug-screening assays. The study of CTCs is attractive, and CTC detection may likely become an essential component of cancer management in the future. As the picture becomes clearer, we are fully confident about the promising potentials of CTCs.
References
Sethi, N. & Kang, Y. Unravelling the complexity of metastasis-molecular understanding and targeted therapies. Nat. Rev. Cancer 11, 735–748 (2011).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Jones, S. et al. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl Acad. Sci. USA 105, 4283–4288 (2008).
Ley, T. J. et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456, 66–72 (2008).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Chen, S. et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell 160, 1246–1260 (2015).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Ashworth, T. R. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australas. Med. J. 14, 146–149 (1869).
Pantel, K. & Speicher, M. R. The biology of circulating tumor cells. Oncogene 35, 1216–1224 (2016).
Alix-Panabieres, C. & Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 14, 623–631 (2014).
Harper, K. L. et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 (2016).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Lianidou, E. S. & Markou, A. Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin. Chem. 57, 1242–1255 (2011).
Thanh Huong, P. et al. Emerging role of circulating tumor cells in gastric cancer. Cancers 12, 695–716 (2020).
Gires, O., Pan, M., Schinke, H., Canis, M. & Baeuerle, P. A. Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metastasis Rev. 39, 969–987 (2020).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014).
Criscitiello, C., Sotiriou, C. & Ignatiadis, M. Circulating tumor cells and emerging blood biomarkers in breast cancer. Curr. Opin. Oncol. 22, 552–558 (2010).
Gorin, M. A. et al. Circulating tumour cells as biomarkers of prostate, bladder, and kidney cancer. Nat. Rev. Urol. 14, 90–97 (2017).
Varillas, J. I. et al. Microfluidic isolation of circulating tumor cells and cancer stem-like cells from patients with pancreatic ductal adenocarcinoma. Theranostics 9, 1417–1425 (2019).
Marcuello, M. et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol. Asp. Med 69, 107–122 (2019).
Xia, W. et al. In vivo coinstantaneous identification of hepatocellular carcinoma circulating tumor cells by dual-targeting magnetic-fluorescent nanobeads. Nano Lett. 21, 634–641 (2021).
Ye, Q., Ling, S., Zheng, S. & Xu, X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol. cancer 18, 114 (2019).
Gall, T. M. H., Belete, S., Khanderia, E., Frampton, A. E. & Jiao, L. R. Circulating tumor cells and cell-free DNA in pancreatic ductal adenocarcinoma. Am. J. Pathol. 189, 71–81 (2019).
Eslami, S. Z., Cortes-Hernandez, L. E. & Alix-Panabieres, C. Epithelial cell adhesion molecule: an anchor to isolate clinically relevant circulating tumor cells. Cells 9, 1836–1852 (2020).
Wang, J. et al. Label-free isolation and mRNA detection of circulating tumor cells from patients with metastatic lung cancer for disease diagnosis and monitoring therapeutic efficacy. Anal. Chem. 87, 11893–11900 (2015).
Wang, Z. L. et al. High-efficiency isolation and rapid identification of heterogeneous circulating tumor cells (CTCS) using dual-antibody-modified fluorescent-magnetic nanoparticles. ACS Appl. Mater. Interface 11, 39586–39593 (2019).
Wang, Z. et al. Antifouling hydrogel-coated magnetic nanoparticles for selective isolation and recovery of circulating tumor cells. J. Mater. Chem. B 9, 677–682 (2021).
Reduzzi, C. et al. A novel circulating tumor cell subpopulation for treatment monitoring and molecular characterization in biliary tract cancer. Int J. Cancer 146, 3495–3503 (2020).
Mittal, V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 13, 395–412 (2018).
Okabe, T. et al. Mesenchymal characteristics and predictive biomarkers on circulating tumor cells for therapeutic strategy. Cancers 12, 3588–3610 (2020).
Xu, J. et al. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol. Cancer 20, 104 (2021).
Wulfing, P. et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin. Cancer Res. 12, 1715–1720 (2006).
Hayashi, N. et al. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J. Clin. Oncol. 17, 96–104 (2012).
Beije, N. et al. Prognostic impact of HER2 and ER status of circulating tumor cells in metastatic breast cancer patients with a HER2-negative primary tumor. Neoplasia 18, 647–653 (2016).
Wang, C. H., Chang, C. J., Yeh, K. Y., Chang, P. H. & Huang, J. S. The prognostic value of HER2-positive circulating tumorcells in breast cancer patients: a systematic review and meta-analysis. Clin. Breast Cancer 17, 341–349 (2017).
Jaeger, B. A. S. et al. The HER2 phenotype of circulating tumor cells in HER2-positive early breast cancer: a translationalresearch project of a prospective randomized phase III trial. PLoS One 12, e0173593 (2017).
Brouwer, A. et al. HER-2 status of circulating tumor cells in a metastatic breast cancer cohort: a comparative study oncharacterization techniques. PLoS One 14, e0220906 (2019).
Chen, W. et al. Detection of HER2-positive circulating tumor cells using the liquidbiopsy system in breast cancer. Clin. Breast Cancer 19, e239–e246 (2019).
Jacot, W. et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCeT-DM1 trial. Breast Cancer Res. 21, 121 (2019).
Nanou, A., Zeune, L. L., Bidard, F. C., Pierga, J. Y. & Terstappen, L. HER2 expression on tumor-derived extracellularvesicles and circulating tumor cells in metastatic breast cancer. Breast Cancer Res. 22, 86 (2020).
Wang, C. et al. Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients withHER2-negative tumors. Breast Cancer Res. Treat. 181, 679–689 (2020).
Mishima, Y. et al. Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target Oncol. 12, 341–351 (2017).
Gradilone, A. et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance andphenotypic characterization. Ann. Oncol. 22, 86–92 (2011).
Somlo, G. et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary andmetastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res. Treat. 128, 155–163 (2011).
Forsare, C. et al. Evolution of estrogen receptor status from primary tumors to metastasis and serially collected circulating tumor cells. Int. J. Mol. Sci. 21, 2885–2897 (2020).
Todenhofer, T. et al. Preliminary experience on the use of the adnatest (R) system for detection of circulating tumor cells inprostate cancer patients. Anticancer Res. 32, 3507–3513 (2012).
Friedlander, T. W. et al. Detection and characterization of invasive circulating tumor cells derived from men with metastaticcastration-resistant prostate cancer. Int. J. Cancer 134, 2284–2293 (2014).
Yin, C. et al. Molecular profiling of pooled circulating tumor cells from prostate cancer patients using a dual-antibodyfunctionalizedmicrofluidic device. Anal. Chem. 90, 3744–3751 (2018).
Chen, L. et al. Combined use of EpCAM and FRalpha enables the high-efficiency capture of circulating tumor cells in nonsmallcell lung cancer. Sci. Rep. 8, 1188 (2018).
Chen, X. et al. Folate receptor-positive circulating tumor cells as a predictive biomarker for the efficacy of first-linepemetrexed-based chemotherapy in patients with non-squamous non-small cell lung cancer. Ann. Transl. Med. 8, 631 (2020).
Wei, S. et al. Effect of vein-first vs artery-first surgical technique on circulating tumor cells and survival in patients with nonsmallcell lung cancer: a randomized clinical trial and registry-based propensity score matching analysis. JAMA Surg. 154, e190972 (2019).
Cao, W. et al. Using detection of survivin-expressing circulating tumor cells in peripheral blood to predict tumor recurrencefollowing curative resection of gastric cancer. J. Surg. Oncol. 103, 110–115 (2011).
Krishnamurthy, S. et al. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients withoperable breast cancer. Cancer Med. 2, 226–233 (2013).
Bidard, F. C. et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastaticuveal melanoma. Int. J. Cancer 134, 1207–1213 (2014).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra224 (2014).
Lucci, A. et al. Circulating tumor cells and early relapse in node-positive melanoma. Clin. Cancer Res. 26, 1886–1895 (2020).
Hoshimoto, S. et al. Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy aftercomplete resection of stage IV melanoma. Ann. Surg. 255, 357–362 (2012).
Hoshimoto, S. et al. Association between circulating tumor cells and prognosis in patients with stage III melanoma withsentinel lymph node metastasis in a phase III international multicenter trial. J. Clin. Oncol. 30, 3819–3826 (2012).
Kiniwa, Y. et al. Usefulness of monitoring circulating tumor cells as a therapeutic biomarker in melanoma with BRAFmutation. BMC Cancer 21, 287 (2021).
Hall, C. S. et al. Circulating tumor cells in stage IV melanoma patients. J. Am. Coll. Surg. 227, 116–124 (2018).
Lin, S. Y. et al. Prospective molecular profiling of circulating tumor cells from patients with melanoma receivingcombinatorial immunotherapy. Clin. Chem. 66, 169–177 (2020).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Fabbri, F. et al. Detection and recovery of circulating colon cancer cells using a dielectrophoresis-based device: KRASmutation status in pure CTCs. Cancer Lett. 335, 225–231 (2013).
Buim, M. E. et al. Detection of KRAS mutations in circulating tumor cells from patients with metastatic colorectal cancer. Cancer Biol. Ther. 16, 1289–1295 (2015).
Kalikaki, A. et al. KRAS genotypic changes of circulating tumor cells during treatment of patients with metastatic colorectalcancer. PloS One 9, e104902 (2014).
Kondo, Y. et al. KRAS mutation analysis of single circulating tumor cells from patients with metastatic colorectal cancer. BMC Cancer 17, 311 (2017).
Kulemann, B. et al. Pancreatic cancer: circulating tumor cells and primary tumors show heterogeneous KRAS mutations. Sci. Rep. 7, 4510 (2017).
Lohr, J. G. et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 32, 479–484 (2014).
Faugeroux, V. et al. An accessible and unique insight into metastasis mutational content through whole-exome sequencing ofcirculating tumor cells in metastatic prostate cancer. Eur. Urol. Oncol. 3, 498–508 (2020).
Deng, G. et al. Single cell mutational analysis of PIK3CA in circulating tumor cells and metastases in breast cancer reveals heterogeneity, discordance, and mutation persistence in cultured disseminated tumor cells from bone marrow. BMC Cancer 14, 456–467 (2014).
Fernandez, V. et al. TP53 mutations detected in circulating tumor cells present in the blood of metastatic triple negative breastcancer patients. Breast Cancer Res. 16, 445 (2014).
Kelley, R. K. et al. Circulating tumor cells in hepatocellular carcinoma: a pilot study of detection, enumeration, and nextgenerationsequencing in cases and controls. BMC Cancer 15, 206 (2015).
Ni, X. et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc. Natl Acad. Sci. USA 110, 21083–21088 (2013).
Carter, L. et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients withchemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 23, 114–119 (2017).
Paoletti, C. et al. Comprehensive mutation and copy number profiling in archived circulating breast cancer tumor cellsdocuments heterogeneous resistance mechanisms. Cancer Res. 78, 1110–1122 (2018).
Ortolan, E. et al. Blood-based genomics of triple-negative breast cancer progression in patients treated with neoadjuvantchemotherapy. ESMO Open 6, 100086 (2021).
Lambros, M. B. et al. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin. Cancer Res.: . J. Am. Assoc. Cancer Res. 24, 5635–5644 (2018).
Gupta, S. et al. Whole genomic copy number alterations in circulating tumor cells from men with abiraterone orenzalutamide-resistant metastatic castration-resistant prostate cancer. Clin. Cancer Res.: . J. Am. Assoc. Cancer Res. 23, 1346–1357 (2017).
Malihi, P. D. et al. Single-cell circulating tumor cell analysis reveals genomic instability as a distinctive feature of aggressiveprostate cancer. Clin. Cancer Res.: . J. Am. Assoc. Cancer Res. 26, 4143–4153 (2020).
Pailler, E. et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-celllung cancer. J. Clin. Oncol. 31, 2273–2281 (2013).
Mayer, J. A. et al. FISH-based determination of HER2 status in circulating tumor cells isolated with the microfluidic CEEplatform. Cancer Genet. 204, 589–595 (2011).
Frithiof, H., Aaltonen, K. & Ryden, L. A FISH-based method for assessment of HER-2 amplification status in breast cancercirculating tumor cells following CellSearch isolation. OncoTargets Ther. 9, 7095–7103 (2016).
Gkountela, S. et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112.e114 (2019).
Dong, Y., Wang, Z. & Shi, Q. Liquid biopsy based single-cell transcriptome profiling characterizes heterogeneity ofdisseminated tumor cells from lung adenocarcinoma. Proteomics 20, e1900224 (2020).
Magbanua, M. J. M. et al. Expanded genomic profiling of circulating tumor cells in metastatic breast cancer patients to assessbiomarker status and biology over time (CALGB 40502 and CALGB 40503, Alliance). Clin. Cancer Res.: . J. Am. Assoc. Cancer Res. 24, 1486–1499 (2018).
Cheng, Y.-H. et al. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat. Commun. 10, 2163–2173 (2019).
Miyamoto, D. et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 349, 1351–1356 (2015).
Barbazan, J. et al. Molecular characterization of circulating tumor cells in human metastatic colorectal cancer. PloS One 7, e40476 (2012).
Kallergi, G. et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastaticbreast cancer patients. Breast Cancer Res. 13, R59 (2011).
Balasubramanian, P. et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples frompatients with squamous cell carcinoma of the head and neck. PLoS One 7, e42048 (2012).
Theodoropoulos, P. A. et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients withbreast cancer. Cancer Lett. 288, 99–106 (2010).
Armstrong, A. J. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelialand mesenchymal markers. Mol. Cancer Res. 9, 997–1007 (2011).
Liu, X. et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source ofmetastasis. Sci. Adv. 5, eaav4275 (2019).
Thiery, J. P. & Lim, C. T. Tumor dissemination: an EMT affair. Cancer Cell 23, 272–273 (2013).
Pecot, C. V. et al. A novel platform for detection of CK+ and CK- CTCs. Cancer Discov. 1, 580–586 (2011).
Sun, Y. F. et al. Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial andmesenchymal composition and distinct clinical significance in hepatocellular carcinoma. Clin. Cancer Res.: . J. Am. Assoc. Cancer Res. 24, 547–559 (2018).
Xu, L. et al. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 23, 5112–5122 (2017).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications forcancer. Nat. Rev. Mol. Cell Bio 20, 69–84 (2019).
Guan, Y. et al. Identification of key genes and functions of circulating tumor cells in multiple cancers through bioinformaticanalysis. BMC Med. Genom. 13, 140 (2020).
Tashireva, L. A. et al. Heterogeneous manifestations of epithelial-mesenchymal plasticity of circulating tumor cells in breast cancer patients. Int. J. Mol. Sci. 22, 2504–2521 (2021).
Xin, Y., Li, K., Yang, M. & Tan, Y. Fluid shear stress induces EMT of circulating tumor cells via jnk signaling in favor of their survival during hematogenous dissemination. Int. J. Mol. Sci. 21, 8115–8130 (2020).
Horimoto, Y. et al. Analysis of circulating tumour cell and the epithelial mesenchymal transition (EMT) status duringeribulin-based treatment in 22 patients with metastatic breast cancer: a pilot study. J. Transl. Med. 16, 287 (2018).
Guan, X. W. et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. CancerCommun 39, 1–10 (2019).
Lindsay, C. R. et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecularsubgroups. Ann. Oncol. 28, 1523–1531 (2017).
Satelli, A. et al. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin. Cancer Res. 21, 899–906 (2015).
Brechbuhl, H. M. et al. Analysis of circulating breast cancer cell heterogeneity and interactions with peripheral bloodmononuclear cells. Mol. Carcinog. 59, 1129–1139 (2020).
Miklikova, S. et al. Inflammation-based scores increase the prognostic value of circulating tumor cells in primary breast cancer. Cancers 12, 1134–1148 (2020).
Wu, Y. et al. SUMOylation represses Nanog expression via modulating transcription factors Oct4 and Sox2. PloS One 7, e39606 (2012).
Zhang, R. et al. Co-expression of stem cell and epithelial mesenchymal transition markers in circulating tumor cells ofbladder cancer patients. OncoTargets Ther. 13, 10739–10748 (2020).
Zhao, P. et al. Establishment and characterization of a CTC cell line from peripheral blood of breast cancer patient. J. Cancer 10, 6095–6104 (2019).
Wan, S. et al. New labyrinth microfluidic device detects circulating tumor cells expressing cancer stem cell marker andcirculating tumor microemboli in hepatocellular carcinoma. Sci. Rep. 9, 18575 (2019).
Liu, T. et al. Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 78, 6632–6642 (2018).
Yin, J. et al. Circulating tumor cells enriched by the depletion of leukocytes with bi-antibodies in non-small cell lung cancer:potential clinical application. PloS One 10, e0137076 (2015).
Jin, J., Tang, K., Xin, Y., Zhang, T. & Tan, Y. Hemodynamic shear flow regulates biophysical characteristics and functionsof circulating breast tumor cells reminiscent of brain metastasis. Soft Matter 14, 9528–9533 (2018).
Choi, H. et al. Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3?activities. Breast Cancer Res. 21, 6 (2019).
Follain, G. et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat. Rev. Cancer 20, 107–124 (2020).
Strilic, B. & Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer cell 32, 282–293 (2017).
Rejniak, K. A. Circulating tumor cells: when a solid tumor meets a fluid microenvironment. Adv. Exp. Med. Biol. 936, 93–106 (2016).
Garrido-Navas, C. et al. Cooperative and escaping mechanisms between circulating tumor cells and blood constituents. Cells 8, 1382–1391 (2019).
Shaul, M. E. & Fridlender, Z. G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16, 601–620 (2019).
Aliustaoglu, M. et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancerbefore treatment. Med. Oncol. 27, 1060–1065 (2010).
Hu, S. et al. The preoperative peripheral blood monocyte count is associated with liver metastasis and overall survival incolorectal cancer patients. PloS One 11, e0157486 (2016).
Wang, Y. et al. Circulating neutrophils predict poor survival for HCC and promote HCC progression through p53 andSTAT3 signaling pathway. J. Cancer 11, 3736–3744 (2020).
Stott, S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl Acad. Sci. USA 107, 18392–18397 (2010).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019).
Hattar, K. et al. Interactions between neutrophils and non-small cell lung cancer cells: enhancement of tumor proliferationand inflammatory mediator synthesis. Cancer Immunol., Immunother. 63, 1297–1306 (2014).
Spicer, J. D. et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 72, 3919–3927 (2012).
Strell, C., Lang, K., Niggemann, B., Zaenker, K. S. & Entschladen, F. Surface molecules regulating rolling and adhesion toendothelium of neutrophil granulocytes and MDA-MB-468 breast carcinoma cells and their interaction. Cell. Mol. life Sci. 64, 3306–3316 (2007).
Huh, S. J., Liang, S., Sharma, A., Dong, C. & Robertson, G. P. Transiently entrapped circulating tumor cells interact withneutrophils to facilitate lung metastasis development. Cancer Res. 70, 6071–6082 (2010).
Rowson-Hodel, A. R. et al. Membrane Mucin Muc4 promotes blood cell association with tumor cells and mediates efficientmetastasis in a mouse model of breast cancer. Oncogene 37, 197–207 (2018).
Chen, M. B. et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumorcell extravasation. Proc. Natl Acad. Sci. USA 115, 7022–7027 (2018).
Masucci, M. T., Minopoli, M., Del Vecchio, S. & Carriero, M. V. The emerging role of neutrophil extracellular traps (NETs)in tumor progression and metastasis. Front. Immunol. 11, 1749 (2020).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 123, 3446–3458 (2013).
Najmeh, S. et al. Neutrophil extracellular traps sequester circulating tumor cells via ?1-integrin mediated interactions. Int. J. Cancer 140, 2321–2330 (2017).
Tohme, S. et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgicalstress. Cancer Res. 76, 1367–1380 (2016).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, 1353–1367 (2018).
Xiao, Y. et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophilextracellular trap formation. Cancer Cell 39, 423–437.e427 (2021).
Yang, L. et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138 (2020).
Zhang, J. et al. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressingperipheral leukocyte activation. Tumour Biol.: J. Int. Soc. Oncodev. Biol. Med. 37, 5397–5404 (2016).
Spiegel, A. et al. Neutrophils suppress intraluminal nk cell-mediated tumor cell clearance and enhance extravasation ofdisseminated carcinoma cells. Cancer Discov. 6, 630–649 (2016).
Kumagai, Y. et al. Surgical stress increases circulating low-density neutrophils which may promote tumor recurrence. J. Surg. Res. 246, 52–61 (2020).
Coffelt, S. B. et al. IL-17-producing ?? T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Doak, G. R., Schwertfeger, K. L. & Wood, D. K. Distant relations: macrophage functions in the metastatic niche. TrendsCancer 4, 445–459 (2018).
Hamilton, G., Rath, B., Klameth, L. & Hochmair, M. J. Small cell lung cancer: recruitment of macrophages by circulatingtumor cells. Oncoimmunology 5, e1093277 (2016).
Hamilton, G. & Rath, B. Circulating tumor cell interactions with macrophages: implications for biology and treatment. Transl. Lung Cancer Res. 6, 418–430 (2017).
Wei, C. et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulatingtumor cell-mediated colorectal cancer metastasis. Mol. Cancer 18, 64 (2019).
Osmulski, P. A. et al. Contacts with macrophages promote an aggressive nanomechanical phenotype of circulating tumorcells in prostate cancer. Cancer Res. 81, 4110–4123 (2021).
Shabo, I. et al. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained bycellular interaction. BMC Cancer 15, 922 (2015).
Clawson, G. A. et al. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PloS One 10, e0134320 (2015).
Clawson, G. A. et al. “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients withpancreatic ductal adenocarcinoma. PloS One 12, e0184451 (2017).
Manjunath, Y. et al. Tumor-cell-macrophage fusion cells as liquid biomarkers and tumor enhancers in cancer. Int. J. Mol. Sci. 21, 1872 (2020).
Gast, C. E. et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage andsurvival. Sci. Adv. 4, eaat7828 (2018).
Manjunath, Y. et al. Circulating giant tumor-macrophage fusion cells are independent prognosticators in patients withNSCLC. J. Thorac. Oncol.: . Publ. Int. Assoc. Study Lung Cancer 15, 1460–1471 (2020).
Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 11, 125 (2018).
Gaertner, F. & Massberg, S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat. Rev. Immunol. 19, 747–760 (2019).
Thomas, G. M. et al. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases theincidence of deep vein thrombosis in mice. J. thrombosis Haemost. 13, 1310–1319 (2015).
Stark, K. et al. Distinct pathogenesis of pancreatic cancer microvesicle-associated venous thrombosis identifies newantithrombotic targets in vivo. Arteriosclerosis, thrombosis, Vasc. Biol. 38, 772–786 (2018).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelialmesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011).
Guo, Y., Cui, W., Pei, Y. & Xu, D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovariancancer cells by TGF-? signaling pathway. Gynecol. Oncol. 153, 639–650 (2019).
Xiong, G. et al. Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell-platelet interaction. Proc. Natl Acad. Sci. USA 117, 3748–3758 (2020).
Egan, K., Cooke, N. & Kenny, D. Living in shear: platelets protect cancer cells from shear induced damage. Clin. Exp. Metastasis 31, 697–704 (2014).
Haemmerle, M. et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun. 8, 310 (2017).
Nieswandt, B., Hafner, M., Echtenacher, B. & Männel, D. N. Lysis of tumor cells by natural killer cells in mice is impededby platelets. Cancer Res. 59, 1295–1300 (1999).
Placke, T. et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumorreactivity of natural killer immune cells. Cancer Res. 72, 440–448 (2012).
Kopp, H. G., Placke, T. & Salih, H. R. Platelet-derived transforming growth factor-beta down-regulates NKG2D therebyinhibiting natural killer cell antitumor reactivity. Cancer Res. 69, 7775–7783 (2009).
Maurer, S. et al. Platelet-mediated shedding of NKG2D ligands impairs NK cell immune-surveillance of tumor cells. Oncoimmunology 7, e1364827 (2018).
Placke, T., Salih, H. R. & Kopp, H. G. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J. Immunol. 189, 154–160 (2012).
Ren, J. et al. Platelet TLR4-ERK5 axis facilitates net-mediated capturing of circulating tumor cells and distant metastasisafter surgical stress. Cancer Res. 81, 2373–2385 (2021).
Rachidi, S. et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci. Immunol. 2, 7911–7937 (2017).
Xiang, B. et al. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via thecyclooxygenase 1 signalling pathway. Nat. Commun. 4, 2657 (2013).
Bendas, G. & Borsig, L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins.Int. J. Cell Biol. 2012, 676731 (2012).
Lonsdorf, A. S. et al. Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3 integrin mediates interaction of melanoma cells with platelets: a connection to hematogenous metastasis. J. Biol. Chem. 287, 2168–2178 (2012).
Coupland, L. A., Chong, B. H. & Parish, C. R. Platelets and P-selectin control tumor cell metastasis in an organ-specificmanner and independently of NK cells. Cancer Res. 72, 4662–4671 (2012).
Schumacher, D., Strilic, B., Sivaraj, K. K., Wettschureck, N. & Offermanns, S. Platelet-derived nucleotides promote tumorcelltransendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24, 130–137 (2013).
Mammadova-Bach, E. et al. Platelet integrin ?6?1 controls lung metastasis through direct binding to cancer cell-derivedADAM9. JCI Insight 1, e88245 (2016).
Ward, Y. et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinatestransendothelial migration. Cell Rep. 23, 808–822 (2018).
Mammadova-Bach, E. et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derivedgalectin-3. Blood 135, 1146–1160 (2020).
Xu, Y. et al. Blockade of platelets using tumor-specific NO-releasing nanoparticles prevents tumor metastasis and reversestumor immunosuppression. ACS Nano 14, 9780–9795 (2020).
Cassetta, L. et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer 8, 1223–1235 (2020).
Liu, Q., Liao, Q. & Zhao, Y. Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies byshielding circulating tumor cells (CTC) from immune surveillance. Med. Hypotheses 87, 34–39 (2016).
Jiménez-Cortegana, C. et al. Circulating myeloid-derived suppressor cells and regulatory T cells as immunological biomarkers in refractory/relapsed diffuse large B-cell lymphoma: translational results from the R2-GDP-GOTEL trial. J. Immunother. Cancer 9, 2323–2334 (2021).
Sprouse, M. L. et al. PMN-MDSCs enhance CTC metastatic properties through reciprocal interactions via ROS/Notch/nodal signaling. Int. J. Mol. Sci. 20, 1916–1935 (2019).
Chen, X. & Song, E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 18, 99–115 (2019).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading andfollowing cells. Nat. Cell Biol. 9, 1392–1400 (2007).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Ortiz-Otero, N. et al. Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancermetastatic progression. Oncotarget 11, 1037–1050 (2020).
Ao, Z. et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 75, 4681–4687 (2015).
Jiang, R. et al. Rapid isolation of circulating cancer associated fibroblasts by acoustic microstreaming for assessing metastatic propensity of breast cancer patients. Lab. Chip 969-981 (2020).
Shen, Z., Wu, A. & Chen, X. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 46, 2038–2056 (2017).
Vona, G. et al. Isolation by size of epithelial tumor cells-a new method for the immunomorphological and molecularcharacterization of circulating tumor cells. Am. J. Pathol. 156, 57–63 (2000).
Harouaka, R. A. et al. Flexible micro spring array device for high-throughput enrichment of viable circulating tumor cells. Clin. Chem. 60, 323–333 (2014).
Rosenberg, R. et al. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumorcells in blood. Cytometry 49, 150–158 (2002).
Gupta, V. et al. ApoStream(), a new dielectrophoretic device for antibody independent isolation and recovery of viablecancer cells from blood. Biomicrofluidics 6, 24133 (2012).
Sajay, B. N. et al. Microfluidic platform for negative enrichment of circulating tumor cells. Biomed. Microdevices 16, 537–548 (2014).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects orpatients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904 (2004).
Talasaz, A. H. et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood usinga magnetic sweeper device. Proc. Natl Acad. Sci. USA 106, 3970–3975 (2009).
Wu, S. et al. Enrichment and enumeration of circulating tumor cells by efficient depletion of leukocyte fractions. Clin. Chem. Lab. Med. 53, 337 (2015).
Nasiri, R. et al. Microfluidic-based approaches in targeted cell/particle separation based on physical properties: fundamentalsand applications. Small 16, e2000171 (2020).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Park, M. H. et al. Enhanced isolation and release of circulating tumor cells using nanoparticle binding and ligand exchangein a microfluidic chip. J. Am. Chem. Soc. 139, 2741–2749 (2017).
Fachin, F. et al. Monolithic chip for high-throughput blood cell depletion to sort rare circulating tumor cells. Sci. Rep. 7, 10936–10946 (2017).
Zhang, X., Zhu, Z., Xiang, N., Long, F. & Ni, Z. Automated microfluidic instrument for label-free and high-throughput cellseparation. Anal. Chem. 90, 4212–4220 (2018).
Jia, Z. et al. Single-cell genetic analysis of lung tumor cells based on self-driving micro-cavity array chip. Talanta 226, 122172 (2021).
Wang, J. et al. A fully automated and integrated microfluidic system for efficient ctc detection and its application inhepatocellular carcinoma screening and prognosis. ACS Appl. Mater. Interfaces 13, 30174–30186 (2021).
Lee, J. & Kwak, B. Simultaneous on-chip isolation and characterization of circulating tumor cell sub-populations. Biosens. Bioelectron. 168, 112564 (2020).
Lee, A. C. et al. OPENchip: an on-chip in situ molecular profiling platform for gene expression analysis and oncogenicmutation detection in single circulating tumour cells. Lab Chip 20, 912–922 (2020).
Zhang, Y., Li, M., Gao, X., Chen, Y. & Liu, T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J. Hematol. Oncol. 12, 137 (2019).
Jia, F. et al. Novel peptide-based magnetic nanoparticle for mesenchymal circulating tumor cells detection. Anal. Chem. 93, 5670–5675 (2021).
Ding, P. et al. Tannic acid (TA)-functionalized magnetic nanoparticles for EpCAM-independent circulating tumor cell(CTC) isolation from patients with different cancers. ACS Appl. Mater. Interfaces 13, 3694–3700 (2021).
Li, F. et al. Nondestructive capture, release, and detection of circulating tumor cells with cystamine-mediated folic aciddecorated magnetic nanospheres. J. Mater. Chem. B 8, 9971–9979 (2020).
Vajhadin, F. et al. MXene-based cytosensor for the detection of HER2-positive cancer cells using CoFe2O4@Ag magneticnanohybrids conjugated to the HB5 aptamer. Biosens. Bioelectron. 195, 113626 (2021).
Wilson, R. E. et al. Immunomagnetic capture and multiplexed surface marker detection of circulating tumor cells withmagnetic multicolor surface-enhanced Raman scattering nanotags. ACS Appl. Mater. Interfaces 12, 47220–47232 (2020).
Liu, S., Luo, J., Jiang, X., Li, X. & Yang, M. Gold nanoparticle-modified black phosphorus nanosheets with improvedstability for detection of circulating tumor cells. Mikrochim. Acta 187, 397 (2020).
Dou, B., Xu, L., Jiang, B., Yuan, R. & Xiang, Y. Aptamer-functionalized and gold nanoparticle array-decorated magneticgraphene nanosheets enable multiplexed and sensitive electrochemical detection of rare circulating tumor cells in whole blood. Anal. Chem. 91, 10792–10799 (2019).
Sheng, W., Chen, T., Tan, W. & Fan, Z. H. Multivalent DNA nanospheres for enhanced capture of cancer cells inmicrofluidic devices. ACS Nano 7, 7067–7076 (2013).
Zhang, Y. et al. Gold nanorods-mediated efficient synergistic immunotherapy for detection and inhibition of postoperativetumor recurrence. Acta Pharm. Sin. B 11, 1978–1992 (2021).
Zhang, J. et al. Sensitive signal amplifying a diagnostic biochip based on a biomimetic periodic nanostructure for detectingcancer exosomes. ACS Appl. Mater. Interfaces 12, 33473–33482 (2020).
Cui, H. et al. Rapid and efficient isolation and detection of circulating tumor cells based on ZnS:Mn(2+) quantum dots andmagnetic nanocomposites. Talanta 202, 230–236 (2019).
Yang, W. et al. Reversible capturing and voltammetric determination of circulating tumor cells using two-dimensionalnanozyme based on PdMo decorated with gold nanoparticles and aptamer. Mikrochim. Acta 188, 319 (2021).
Su, Y. et al. Antibody-functional microsphere-integrated filter chip with inertial microflow for size-immune-capturing anddigital detection of circulating tumor cells. ACS Appl Mater. Interfaces 11, 29569–29578 (2019).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Rhim, A. D. et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 146, 647–651 (2014).
Yamaguchi, J. et al. Premalignant pancreatic cells seed stealth metastasis in distant organs in mice. Oncogene 40, 2273–2284 (2021).
Barrière, G., Riouallon, A., Renaudie, J., Tartary, M. & Rigaud, M. Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer 12, 114–120 (2012).
Thery, L. et al. Circulating tumor cells in early breast. Cancer JNCI Cancer Spectr. 3, pkz026 (2019).
Paoletti, C. et al. Circulating tumor cell clusters in patients with metastatic breast cancer: a SWOG S0500 translationalmedicine study. Clin. Cancer Res 25, 6089–6097 (2019).
Magbanua, M. J. M. et al. Synchronous detection of circulating tumor cells in blood and disseminated tumor cells in bonemarrow predicts adverse outcome in early breast cancer. Clin. Cancer Res. 25, 5388–5397 (2019).
Magbanua, M. J. M. et al. Clinical significance of circulating tumor cells in hormone receptor-positive metastatic breastcancer patients who received letrozole with or without Bevacizumab. Clin. Cancer Res. 26, 4911–4920 (2020).
Bortolini Silveira, A. et al. Multimodal liquid biopsy for early monitoring and outcome prediction of chemotherapy inmetastatic breast cancer. NPJ Breast Cancer 7, 115 (2021).
Paoletti, C. et al. Circulating tumor cell number and endocrine therapy index in ER positive metastatic breast cancerpatients. NPJ Breast Cancer 7, 77 (2021).
Magbanua, M. J. M. et al. Serial analysis of circulating tumor cells in metastatic breast cancer receiving first-linechemotherapy. J. Natl Cancer Inst. 113, 443–452 (2021).
de Kruijff, I. E. et al. Circulating tumor cell enumeration and characterization in metastatic castration-resistant prostate cancer patients treated with cabazitaxel. Cancers 11, 1212–1225 (2019).
Cieslikowski, W. A. et al. Circulating tumor cells as a marker of disseminated disease in patients with newly diagnosed high-risk prostate cancer. Cancers 12, 160–175 (2020).
Basso, U. et al. Prognostic role of circulating tumor cells in metastatic renal cell carcinoma: a large, multicenter, prospectivetrial. Oncologist 26, 740–750 (2021).
Chemi, F. et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat. Med 25, 1534–1539 (2019).
Messaritakis, I. et al. Characterization of DLL3-positive circulating tumor cells (CTCs) in patients with small cell lungcancer (SCLC) and evaluation of their clinical relevance during front-line treatment. Lung Cancer 135, 33–39 (2019).
Sun, Y. F. et al. Postoperative circulating tumor cells: an early predictor of extrahepatic metastases in patients withhepatocellular carcinoma undergoing curative surgical resection. Cancer Cytopathol. 128, 733–745 (2020).
Matsushita, D. et al. Clinical significance of circulating tumor cells in the response to trastuzumab for HER2-negativemetastatic gastric cancer. Cancer Chemother. Pharm. 87, 789–797 (2021).
Sastre, J. et al. Association between baseline circulating tumor cells, molecular tumor profiling, and clinical characteristics ina large cohort of chemo-naive metastatic colorectal cancer patients prospectively collected. Clin. Colorectal Cancer 19, e110–e116 (2020).
Bidard, F. C. et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J. Natl Cancer Inst. 110, 560–567 (2018).
Murlidhar, V. et al. Poor prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancers. CancerRes 77, 5194–5206 (2017).
Jansson, S., Bendahl, P. O., Larsson, A. M., Aaltonen, K. E. & Ryden, L. Prognostic impact of circulating tumor cellapoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 16, 433 (2016).
Wang, C. et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. BreastCancer Res. Treat. 161, 83–94 (2017).
Wang, C. et al. Improved prognostic stratification using circulating tumor cell clusters in patients with metastatic castration-resistant prostate cancer. Cancers 13, 268–280 (2021).
Lozano, R. et al. Value of early circulating tumor cells dynamics to estimate docetaxel benefit in metastatic castration-resistant prostate cancer (mCRPC) patients. Cancers 13, 2334–2344 (2021).
Su, P. et al. Mesenchymal and phosphatase of regenerating liver-3 status in circulating tumor cells may serve as a crucialprognostic marker for assessing relapse or metastasis in postoperative patients with colorectal cancer. Clin. Transl. Gastroenterol. 11, e00265 (2020).
Strati, A., Nikolaou, M., Georgoulias, V. & Lianidou, E. S. Prognostic significance of TWIST1, CD24, CD44, and ALDH1 transcript quantification in EpCAM-positive circulating tumor cells from early stage breast cancer patients. Cells 8, 652–667 (2019).
Papadaki, M. A. et al. Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers 12, 376–396 (2020).
Wang, X. Q., Liu, B., Li, B. Y., Wang, T. & Chen, D. Q. Effect of CTCs and INHBA level on the effect and prognosis ofdifferent treatment methods for patients with early breast cancer. Eur. Rev. Med. Pharm. Sci. 24, 12735–12740 (2020).
Graf, R. P. et al. Clinical utility of the nuclear-localized AR-V7 biomarker in circulating tumor cells in improving physiciantreatment choice in castration-resistant prostate cancer. Eur. Urol. 77, 170–177 (2020).
Wei, T. et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patientswith pancreatic cancer. Cancer Lett. 452, 237–243 (2019).
Economos, C., Morrissey, C. & Vessella, R. L. Circulating tumor cells as a marker of response: implications for determiningtreatment efficacy and evaluating new agents. Curr. Opin. Urol. 22, 190–196 (2012).
Li, Y., Wu, S. & Bai, F. Molecular characterization of circulating tumor cells-from bench to bedside. Semin. Cell Dev. Biol. 75, 88–97 (2018).
Massard, C. et al. RECIST response and variation of circulating tumour cells in phase 1 trials: a prospective multicentricstudy. Eur. J. Cancer 83, 185–193 (2017).
Ntzifa, A., Kotsakis, A., Georgoulias, V. & Lianidou, E. Detection of EGFR mutations in plasma cfDNA and paired CTCs of NSCLC patients before and after osimertinib therapy using crystal digital PCR. Cancers 13, 2376–2394 (2021).
Pan, X. & Zhang, X. Utility of circulating tumor cells and DNA in the management of advanced colorectal cancer. FutureOncol 16, 1289–1299 (2020).
Garrido-Navas, M. C. et al. The polemic diagnostic role of tp53 mutations in liquid biopsies from breast, colon and lung cancers. Cancers 12, 3343–3359 (2020).
Mong, J. & Tan, M. H. Size-based enrichment technologies for non-cancerous tumor-derived cells in blood. TrendsBiotechnol 36, 511–522 (2018).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Liu, M. C. et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J. Clin. Oncol. 27, 5153–5159 (2009).
Pestrin, M. et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib inpatients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. BreastCancer Res. Treat. 134, 283–289 (2012).
Pierga, J. Y. et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumormarkers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann. Oncol. 23, 618-624
Giordano, A. et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of predictionin HER2-positive disease treated with targeted therapy. Ann. Oncol. 23, 1144–1150 (2012).
Jiang, Z. F. et al. Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastaticbreast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann. Oncol. 24, 2766–2772 (2013).
Pierga, J. Y. et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: theLANDSCAPE trial. Ann. Oncol. 24, 2999–3004 (2013).
Smerage, J. B. et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 32, 3483–3489 (2014).
Hall, C. et al. Circulating tumor cells after neoadjuvant chemotherapy in stage I-III triple-negative breast cancer. Ann. Surg. Oncol. 22, S552–S558 (2015). Suppl 3.
Hall, C. S. et al. Prognostic value of circulating tumor cells identified before surgical resection in nonmetastatic breastcancer patients. J. Am. Coll. Surg. 223, 20–29 (2016).
Riethdorf, S. et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant“geparquattro” trial. Clin. Cancer Res. 23, 5384–5393 (2017).
Sparano, J. et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: asecondary analysis of a randomized clinical trial. JAMA Oncol. 4, 1700–1706 (2018).
Rossi, G. et al. Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin. Cancer Res. 24, 560–568 (2018).
Trapp, E. et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J. NatlCancer Inst. 111, 380–387 (2019).
Radovich, M. et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy withdisease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12-158 randomizedclinical trial. JAMA Oncol. 6, 1410–1415 (2020).
Zhou, J. et al. Epithelial-mesenchymal transition status of circulating tumor cells in breast cancer and its clinical relevance. Cancer Biol. Med. 17, 169–180 (2020).
Le, Du,F. et al. EpCAM-independent isolation of circulating tumor cells with epithelial-to-mesenchymal transition andcancer stem cell phenotypes using ApoStream(R) in patients with breast cancer treated with primary systemic therapy. PLoS One 15, e0229903 (2020).
Zhang, S. R. et al. Mesenchymal phenotype of circulating tumor cells is associated with distant metastasis in breast cancerpatients. Cancer Manag. Res. 9, 691–700 (2017).
Bulfoni, M. et al. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-tomesenchymaltransition is associated with a poor prognosis. Breast Cancer Res. 18, 30 (2016).
Papadaki, M. A. et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features arechemoresistant and predictive of poor outcome in metastatic breast Cancer. Mol. Cancer Ther. 18, 437–447 (2019).
de Kruijff, I. E. et al. Androgen receptor expression in circulating tumor cells of patients with metastatic breast cancer. Int. J. Cancer 145, 1083–1089 (2019).
Bock, C. et al. Distinct expression of cytokeratin, N-cadherin and CD133 in circulating tumor cells of metastatic breastcancer patients. Future Oncol. 10, 1751–1765 (2014).
Goodman, O. B. et al. Circulating tumor cells in patients with castration-resistant prostate cancer baseline values andcorrelation with prognostic factors. Cancer Epidemiol. Biomark. Prev. 18, 1904–1913 (2009).
Goldkorn, A. et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase iii trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 32, 1136–113 (2014).
Satelli, A. et al. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as aprognostic marker in cancer patients. Sci. Rep. 6, 28910 (2016).
Chen, J. et al. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J. Exp. Clin. Cancer Res. 37, 127 (2018).
Liu, H., Ding, J., Wu, Y., Wu, D. & Qi, J. Prospective study of the clinical impact of epithelial and mesenchymal circulatingtumor cells in localized prostate cancer. Cancer Manag. Res. 12, 4549–4560 (2020).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Okegawa, T. et al. AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate andenzalutamide treatment in castration-resistant prostate cancer patients. Prostate 78, 576–582 (2018).
Tagawa, S. T. et al. Expression of AR-V7 and ARv(567es) in circulating tumor cells correlates with outcomes to taxanetherapy in men with metastatic prostate cancer treated in TAXYNERGY. Clin. Cancer Res. 25, 1880–1888 (2019).
Markou, A. et al. PIM-1 Is Overexpressed at a high frequency in circulating tumor cells from metastatic castration-resistant prostate cancer patients. Cancers 12, 1188–1201 (2020).
Liu, S. et al. Combined cell surface carbonic anhydrase 9 and CD147 antigens enable high-efficiency capture of circulatingtumor cells in clear cell renal cell carcinoma patients. Oncotarget 7, 59877–59891 (2016).
Naoe, M. et al. Detection of circulating urothelial cancer cells in the blood using the CellSearch System. Cancer 109, 1439–1445 (2007).
Gallagher, D. J. et al. Detection of circulating tumor cells in patients with urothelial cancer. Ann. Oncol. 20, 305–308 (2009).
Rink, M. et al. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladdercancer. BJU Int. 107, 1668–1675 (2011).
Gazzaniga, P. et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: a CellSearch analysis. Ann. Oncol. 23, 2352–2356 (2012).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival inpatients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Cohen, S. J. et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 20, 1223–1229 (2009).
Wang, W. et al. Mesenchymal marker and LGR5 expression levels in circulating tumor cells correlate with colorectal cancerprognosis. Cell Oncol. 41, 495–504 (2018).
Wu, F. et al. Associations between the epithelial-mesenchymal transition phenotypes of circulating tumor cells and theclinicopathological features of patients with colorectal cancer. Dis. Markers 2017, 9474532 (2017).
Zhao, R. et al. Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells fromcolorectal cancer. Oncotarget 8, 9293–9302 (2017).
Ning, Y. et al. Clinical relevance of EMT and stem-like gene expression in circulating tumor cells of metastatic colorectalcancer patients. Pharmacogenom. J. 18, 29–34 (2018).
Bao, H. et al. High expression of carcinoembryonic antigen and telomerase reverse transcriptase in circulating tumor cells isassociated with poor clinical response to the immune checkpoint inhibitor nivolumab. Oncol. Lett. 15, 3061–3067 (2018).
Shen, C., Hu, L., Xia, L. & Li, Y. Quantitative real-time RT-PCR detection for survivin, CK20 and CEA in peripheral bloodof colorectal cancer patients. Jpn. J. Clin. Oncol. 38, 770–776 (2008).
Shimada, R., Iinuma, H., Akahane, T., Horiuchi, A. & Watanabe, T. Prognostic significance of CTCs and CSCs of tumordrainage vein blood in Dukes' stage B and C colorectal cancer patients. Oncol. Rep. 27, 947–953 (2012).
Barbazan, J. et al. A multimarker panel for circulating tumor cells detection predicts patient AQ5 outcome and therapyresponse in metastatic colorectal cancer. Int. J. Cancer 135, 2633–2643 (2014).
Yokobori, T. et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transitionand is associated with colorectal cancer prognosis. Cancer Res. 73, 2059–2069 (2013).
Bian, J., Yan, K., Liu, N. & Xu, X. Correlations between circulating tumor cell phenotyping and 18F-fluorodeoxyglucosepositron emission tomography uptake in non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 146, 2621–2630 (2020).
Dong, J. et al. Detection of circulating tumor cell molecular subtype in pulmonary vein predicting prognosis of stage I-IIInon-small cell lung cancer patients. Front Oncol. 9, 1139 (2019).
Manjunath, Y. et al. PD-L1 Expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer. Cancers 11, 806–816 (2019).
Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lungcancer. J. Clin. Oncol. 29, 1556–1563 (2011).
Punnoose, E. A. et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer:association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin. Cancer Res. 18, 2391–2401 (2012).
Liang, N. et al. Efficient isolation and quantification of circulating tumor cells in non-small cell lung cancer patients usingpeptide-functionalized magnetic nanoparticles. J. Thorac. Dis. 12, 4262–4273 (2020).
Tamminga, M. et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumorresponse to checkpoint inhibitors. J. Immunother. Cancer 7, 173 (2019).
Tamminga, M. et al. Analysis of released circulating tumor cells during surgery for non-small cell lung cancer. Clin. CancerRes. 26,, 1656–1666 (2020).
Frick, M. A. et al. Circulating tumor cells are associated with recurrent disease in patients with early-stage non-small celllung cancer treated with stereotactic body radiotherapy. Clin. Cancer Res. 26, 2372–2380 (2020).
Hiltermann, T. J. N. et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann. Oncol. 23, 2937–2942 (2012).
Hou, J. M. et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patientsundergoing chemotherapy. Am. J. Pathol. 175, 808–816 (2009).
Hou, J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumormicroemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532 (2012).
Huang, C. H. et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker inpatients with extensive small-cell lung cancer. Front Oncol. 4, 271 (2014).
Naito, T. et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J. Thorac. Oncol. 7, 512–519 (2012).
Normanno, N. et al. Prognostic value of circulating tumor cells' reduction in patients with extensive small-cell lung cancer. Lung Cancer 85, 314–319 (2014).
Messaritakis, I. et al. Phenotypic characterization of circulating tumor cells in the peripheral blood of patients with small celllung cancer. PLoS One 12, e0181211 (2017).
Bidard, F. C. et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study tothe LAP 07 trial. Ann. Oncol. 24, 2057–2061 (2013).
Court, C. M. et al. Circulating tumor cells predict occult metastatic disease and prognosis in pancreatic cancer. Ann. Surg. Oncol. 25, 1000–1008 (2018).
Gall, T. M. et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients withpancreatic cancer. JAMA Surg. 149, 482–485 (2014).
Hugenschmidt, H. et al. Circulating tumor cells are an independent predictor of shorter survival in patients undergoingresection for pancreatic and periampullary adenocarcinoma. Ann. Surg. 271, 549–558 (2020).
Okubo, K. et al. Clinical impact of circulating tumor cells and therapy response in pancreatic cancer. Eur. J. Surg. Oncol. 43, 1050–1055 (2017).
Dong, X. et al. Spatial heterogeneity in epithelial to mesenchymal transition properties of circulating tumor cells associatedwith distant recurrence in pancreatic cancer patients. Ann. Transl. Med. 8, 676 (2020).
Zhao, X. H. et al. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells frompancreatic cancer patients: Potential role in clinical practice. World J. Gastroenterol. 25, 138–150 (2019).
Zhu, P. et al. Circulating tumor cells expressing Kruppel-like factor 8 and vimentin as predictors of poor prognosis inpancreatic cancer patients. Cancer Control 28, 10732748211027163 (2021).
Ou, H. et al. Circulating tumor cell phenotype indicates poor survival and recurrence after surgery for hepatocellularcarcinoma. Dig. Dis. Sci. 63, 2373–2380 (2018).
Wang, P. X. et al. Circulating tumor cells are an indicator for the administration of adjuvant transarterial chemoembolizationin hepatocellular carcinoma: a single-center, retrospective, propensity-matched study. Clin. Transl. Med. 10, e137 (2020).
Yu, J. J. et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMCCancer 18, 835 (2018).
Zhou, J. et al. Preoperative circulating tumor cells to predict microvascular invasion and dynamical detection indicate theprognosis of hepatocellular carcinoma. BMC Cancer 20, 1047 (2020).
Zhou, K. Q. et al. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status inhepatocellular carcinoma. EBioMedicine 62, 103107 (2020).
Liu, Y. K. et al. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells inhepatocellular carcinoma patients. Hepatol. Int 10, 640–646 (2016).
Qi, L. N. et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients withhepatocellular carcinoma. Cancer Res. 78, 4731–4744 (2018).
Wang, Z. et al. Correlation between postoperative early recurrence of hepatocellular carcinoma and mesenchymal circulatingtumor cells in peripheral blood. J. Gastrointest. Surg. 22, 633–639 (2018).
Court, C. M. et al. A novel multimarker assay for the phenotypic profiling of circulating tumor cells in hepatocellularcarcinoma. Liver Transpl. 24, 946–960 (2018).
Yi, B. et al. The clinical significance of CTC enrichment by GPC3-IML and its genetic analysis in hepatocellular carcinoma. J. Nanobiotechnol. 19, 74 (2021).
Sun, Y. F. et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq inhepatocellular carcinoma. Nat. Commun. 12, 4091 (2021).
Yin, L. C. et al. Twist expression in circulating hepatocellular carcinoma cells predicts metastasis and prognoses. Biomed. Res. Int 2018, 3789613 (2018).
Sun, Y. F. et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis ofhepatocellular carcinoma after curative resection. Hepatology 57, 1458–1468 (2013).
Lee, S. J. et al. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int. J. Biol. Markers 30, e382–e386 (2015).
Uenosono, Y. et al. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer 119, 3984–3991 (2013).
Zhang, Q. et al. A prospective study on the changes and clinical significance of pre-operative and post-operative circulatingtumor cells in resectable gastric cancer. J. Transl. Med. 16, 171 (2018).
Liu, M. et al. Prognostic significance of PD-L1 expression on cell-surface vimentin-positive circulating tumor cells in gastriccancer patients. Mol. Oncol. 14, 865–881 (2020).
Hatakeyama, K. et al. A novel splice variant of XIAP-associated factor 1 (XAF1) is expressed in peripheral bloodcontaining gastric cancer-derived circulating tumor cells. Gastr. Cancer 18, 751–761 (2015).
Vaiopoulos, A. G. et al. Detection of circulating tumor cells in colorectal and gastric cancer using a multiplex PCR assay. Anticancer Res. 34, 3083–3092 (2014).
Ishiguro, Y. et al. Prognostic significance of circulating tumor cells with mesenchymal phenotypes in patients with gastriccancer: a prospective study. Ann. Surg. Oncol. 28, 1178–1186 (2021).
Mimori, K. et al. A large-scale study of MT1-MMP as a marker for isolated tumor cells in peripheral blood and bonemarrow in gastric cancer cases. Ann. Surg. Oncol. 15, 2934–2942 (2008).
Li, Y. et al. Prognostic value of circulating tumor cells detected with the CellSearch system in esophageal cancer patients: asystematic review and meta-analysis. BMC Cancer 20, 581 (2020).
Tewari, K. S. et al. Circulating tumor cells in advanced cervical cancer: NRG Oncology-Gynecologic Oncology Group study240 (NCT 00803062). Mol. Cancer Ther. 19, 2363–2370 (2020).
Anand, K. et al. Pilot study of circulating tumor cells in early-stage and metastatic uveal melanoma. Cancers 11, 856–863 (2019).
Kim, D. D., Yang, C. S., Chae, H. D., Kwak, S. G. & Jeon, C. H. Melanoma antigen-encoding gene family member A1-6and hTERT in the detection of circulating tumor cells following CD45(-) depletion and RNA extraction. Oncol. Lett. 14, 837–843 (2017).
Mego, M. et al. Circulating tumor cells with epithelial-to-mesenchymal transition phenotypes associated with inferioroutcomes in primary breast cancer. Anticancer Res. 39, 1829–1837 (2019).
Zhang, Y. et al. Utility of circulating tumor cells for detection of early-stage luminal a breast cancer. Am. J. Med Sci. 360, 543–551 (2020).
Stefanovic, S. et al. The lack of evidence for an association between cancer biomarker conversion patterns and CTC-status in patients with metastatic breast cancer. Int. J. Mol. Sci. 21, 2161–2171 (2020).
Jin, L. et al. Evaluation of the diagnostic value of circulating tumor cells with CytoSorter((R)) CTC capture system inpatients with breast cancer. Cancer Med. 9, 1638–1647 (2020).
Shliakhtunou, Y. A. CTCs-oriented adjuvant personalized cytostatic therapy non-metastatic breast cancer patients:continuous non-randomized prospective study and prospective randomized controlled study. Breast Cancer Res. Treat. 186, 439–451 (2021).
Sieuwerts, A. M. et al. AR splice variants in circulating tumor cells of patients with castration-resistant prostate cancer:relation with outcome to cabazitaxel. Mol. Oncol. 13, 1795–1807 (2019).
Schonhoft, J. D. et al. Morphology-predicted large-scale transition number in circulating tumor cells identifies achromosomal instability biomarker associated with poor outcome in castration-resistant prostate cancer. Cancer Res. 80, 4892–4903 (2020).
Armstrong, A. J. et al. Prospective multicenter study of circulating tumor cell AR-V7 and taxane versus hormonal treatment outcomes in metastatic castration-resistant prostate cancer. JCO Precis. Oncol. 4, 1285–1301 (2020).
Xu, L. et al. Noninvasive detection of clinically significant prostate cancer using circulating tumor cells. J. Urol. 203, 73–82 (2020).
Sperger, J. M. et al. Prospective evaluation of clinical outcomes using a multiplex liquid biopsy targeting diverse resistancemechanisms in metastatic prostate cancer. J. Clin. Oncol. 39, 2926–2937 (2021).
Zhang, P. et al. The significance of detection of circulating tumor cells and BECLIN1 in peripheral blood of patients withrenal cell carcinoma. Crit. Rev. Eukaryot. Gene Expr. 30, 483–492 (2020).
Li, Z. et al. Circulating tumor cells can predict the prognosis of patients with non-small cell lung cancer after resection: aretrospective study. Transl. Lung Cancer Res 10, 995–1006 (2021).
Wang, P. P. et al. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J. Cancer 11, 2113–2122 (2020).
Ha, Y. et al. Circulating tumor cells are associated with poor outcomes in early-stage hepatocellular carcinoma: aprospective study. Hepatol. Int. 13, 726–735 (2019).
Chen, Y. et al. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrencein hepatocellular carcinoma. Sci. Rep. 9, 7084 (2019).
Cheng, Y. et al. Diagnostic value of different phenotype circulating tumor cells in hepatocellular carcinoma. J. Gastrointest. Surg. 23, 2354–2361 (2019).
Lei, Y. et al. Association of preoperative NANOG-positive circulating tumor cell levels with recurrence of hepatocellularcarcinoma. Front. Oncol. 11, 601668 (2021).
Szczepanik, A. et al. CD44(+) cytokeratin-positive tumor cells in blood and bone marrow are associated with poor prognosisof patients with gastric cancer. Gastr. Cancer 22, 264–272 (2019).
Miki, Y. et al. Circulating CEA-positive and EpCAM-negative tumor cells might be a predictive biomarker for recurrence inpatients with gastric cancer. Cancer Med. 10, 521–528 (2021).
Kuroda, K. et al. Circulating tumor cells with FGFR2 expression might be useful to identify patients with existing FGFR2-overexpressing tumor. Cancer Sci. 111, 4500–4509 (2020).
Wang, D. et al. Prognostic models based on postoperative circulating tumor cells can predict poor tumor recurrence-freesurvival in patients with stage II-III colorectal cancer. J. Cancer 10, 4552–4563 (2019).
Bidard, F. C. et al. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: a prospective ancillary study to the unicancer Prodige-14 Trial. Cells 8, 516–528 (2019).
Wang, L. et al. Circulating tumor cells as an independent prognostic factor in advanced colorectal cancer: a retrospectivestudy in 121 patients. Int. J. Colorectal Dis. 34, 589–597 (2019).
Messaritakis, I. et al. Evaluation of the role of circulating tumor cells and microsatellite instability status in predicting outcome of advanced CRC patients. J. Pers. Med. 10, 235–347 (2020).
Pan, R. J. et al. Detection and clinical value of circulating tumor cells as an assisted prognostic marker in colorectal cancerpatients. Cancer Manag. Res. 13, 4567–4578 (2021).
Funding
This study was supported by the funding of the National Natural Science Foundation of China (Grant No. 82172344, 81702866, Jiaojiao Zhou), Natural Science Foundation of Zhejiang Province (Grant No. LY21H160039, Jiaojiao Zhou), Fundamental Research Funds for the Central Universities (Grant No. 2021FZJD009, Jiaojiao Zhou) and the National Natural Science Foundation of China (Grant No. 82072900, Yiding Chen).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, D., Shen, L., Luo, M. et al. Circulating tumor cells: biology and clinical significance. Sig Transduct Target Ther 6, 404 (2021). https://doi.org/10.1038/s41392-021-00817-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-021-00817-8
This article is cited by
-
Blood-based biomarkers in patients with non-small cell lung cancer treated with immune checkpoint blockade
Journal of Experimental & Clinical Cancer Research (2024)
-
Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation
Journal of Experimental & Clinical Cancer Research (2024)
-
Circulating tumor cells shielded with extracellular vesicle-derived CD45 evade T cell attack to enable metastasis
Signal Transduction and Targeted Therapy (2024)
-
Progression from ductal carcinoma in situ to invasive breast cancer: molecular features and clinical significance
Signal Transduction and Targeted Therapy (2024)
-
Key processes in tumor metastasis and therapeutic strategies with nanocarriers: a review
Molecular Biology Reports (2024)