Abstract
Background
Growth factors important for normal brain development are low in preterm infants. This study investigated the link between growth factors and preterm brain volumes at term.
Material/methods
Infants born <28 weeks gestational age (GA) were included. Endogenous levels of insulin-like growth factor (IGF)−1, brain-derived growth factor, vascular endothelial growth factor, and platelet-derived growth factor (expressed as area under the curve [AUC] for serum samples from postnatal days 1, 7, 14, and 28) were utilized in a multivariable linear regression model. Brain volumes were determined by magnetic resonance imaging (MRI) at term equivalent age.
Results
In total, 49 infants (median [range] GA 25.4 [22.9–27.9] weeks) were included following MRI segmentation quality assessment and AUC calculation. IGF-1 levels were independently positively associated with the total brain (p < 0.001, β = 0.90), white matter (p = 0.007, β = 0.33), cortical gray matter (p = 0.002, β = 0.43), deep gray matter (p = 0.008, β = 0.05), and cerebellar (p = 0.006, β = 0.08) volume adjusted for GA at birth and postmenstrual age at MRI. No associations were seen for other growth factors.
Conclusions
Endogenous exposure to IGF-1 during the first 4 weeks of life was associated with total and regional brain volumes at term. Optimizing levels of IGF-1 might improve brain growth in extremely preterm infants.
Impact
-
High serum levels of insulin-like growth factor (IGF)-1 during the first month of life were independently associated with increased total brain volume, white matter, gray matter, and cerebellar volume at term equivalent age in extremely preterm infants.
-
IGF-1 is a critical regulator of neurodevelopment and postnatal levels are low in preterm infants. The effects of IGF-1 levels on brain development in extremely preterm infants are not fully understood.
-
Optimizing levels of IGF-1 may benefit early brain growth in extremely preterm infants. The effects of systemically administered IGF-1/IGFBP3 in extremely preterm infants are now being investigated in a randomized controlled trial (Clinicaltrials.gov: NCT03253263).
Similar content being viewed by others
Background
Infants born preterm are at risk of impaired brain growth and maturation even in the absence of macrostructural brain damage.1,2,3 The third trimester is the peak period for brain maturation and development with incipient myelination, a four-fold increase in cortical folding, and increased dendritic arborization alongside a drastic increase in total brain weight, from less than 90 g in gestational week 22–23 to 400 g at term equivalent age (TEA).4
Technical advancements in high–resolution magnetic resonance imaging (MRI) now enable precise determination of brain growth by implementing volumetric segmentation tools. In preterm infants, brain volumes are typically reduced at TEA and later in life compared to healthy term infants. Volume reduction is linked to life-long impairments, including neurosensory, cognitive, and behavioral deficits.5,6,7,8,9
The growth factor most commonly linked to brain growth and maturation is insulin-like growth factor (IGF)-1. IGF-1 is involved in cell growth, survival, proliferation, and migration with brain-specific effects on synapse formation, myelination, and plasticity.10 Low systemic concentrations of IGF-1 characterize the postnatal period following preterm birth compared to corresponding intrauterine levels.10 Low IGF-1 levels are linked to a poor neurodevelopmental outcome at 2 years of age11 and altered brain volumes in moderately preterm infants born before 31 weeks gestational age (GA).12 In addition, experimental studies show that IGF-1 treatment in neonatal brain injury models in rodents and sheep boosted proliferation, differentiation, and survival of the oligodendrocyte lineage and subsequent myelin production.13,14,15,16 However, the link between IGF-1 and brain volumes has not been explored in the most immature preterm infants.
Numerous studies link other growth factors, including brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), to processes crucial in early brain growth and maturation.17,18,19,20 Despite this, there are limited data on how postnatal endogenous exposure to growth factors affect brain development in preterm infants.
This study investigates associations between postnatal endogenous exposure to growth factors and brain volumes at TEA in extremely preterm infants. We also relate our findings to GA at birth to study how immaturity affects the relation between growth factors and brain volumes.
Methods
Study population
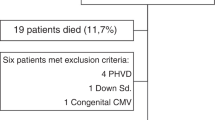
The study included infants born <28 weeks GA, at Sahlgrenska University Hospital, Gothenburg, Sweden from 2013 to 2015 as part of a randomized clinical study investigating the effects of parenteral lipid emulsions on infant morbidities (Clinical trial NCT 02760472). The complete study protocol is available online.21,22 The inclusion flowchart is presented in Fig. 1. Pregnancies were dated by ultrasound at gestational week 17–18. The study was approved by the Regional Ethical Board, Gothenburg (Dnr 303–11; Clinical trial NCT 02760472), and infants were enrolled following written informed parental consent.
In total, 49 infants were eligible for analysis with complete MRI volume segmentation and growth factor AUC. *In two infants, IGF-1 AUC was available, but not BDNF, VEGF, and PDGF. RCT randomized controlled trial, n number, TEA term equivalent age, IGF Insulin-like growth factor, BDNF brain-derived neurotrophic factor, AUC area under the curve, MRI magnetic resonance imaging.
Data collection and laboratory analysis
Serum aliquots were stored in a freezer at −80 °C until assayed. Blood was drawn from an umbilical or peripheral arterial catheter or venous puncture. For IGF-1 analysis, samples were diluted 1:50, and the IGF-1 concentrations were analyzed using a radioimmunoassay (Mediagnost GmbH, Tubingen, Germany), as described previously.23 For IGF-1, the intraassay coefficients of variation at concentrations of 9, 33, and 179 ng/l were 18, 9, and 7%, respectively, and the interassay coefficients of variation at concentrations of 9, 34, and 194 ng/l were 29, 11, and 8%, respectively, also described previously.24 BDNF, VEGF, and PDGF were analyzed using the ELLA multi-analyte platform (Bio-Techne, Minneapolis, MN), according to the manufacturer-provided protocol, and has been described in detail previously.25 In short, samples were diluted 1:4, and the interassay coefficients of variation for BDNF were at concentrations of 220, 7402, and 10,271 pg/ml, 7, 7, and 5%, respectively; for VEGF at concentrations of 35, 475, and 1667 pg/ml, 7, 7, and 8%, respectively, and for PDGF at concentrations of 7.5, 332, and 945 pg/ml, 5, 4, and 5%, respectively. Intraassay coefficients of variations for BDNF at 7731 pg/ml 6%, for VEGF at 502 pg/ml were 5%, and for PDGF at 983 pg/ml, 5%.
MRI acquisition and evaluation
MRI scanning was performed at TEA on a 3 Tesla system (750W, GE Medical Systems, Waukesha, WI) using a 19- or 32-channel head coil. The scanning protocol matched the clinical routine and included 3D T1–weighted, T1 FLAIR, axial 2D T2–weighted (T2w), 3D T2w fast spin–echo, 3D susceptibility–weighted, and diffusion-weighted imaging. Only T2w images were used for volumetry. Acquisition parameters for 2D T2w were slice thickness 3 mm, repetition time 9278 ms, and echo time 74.5 ms. The 3D T2w were acquired with echo time 81–125 ms, slice thickness 0.8 mm, and repetition time 2740–3000 ms.
Segmentation analysis utilized T2w images. Using the automatic anatomical image segmentation described by Makropoulos et al.,26 volumes were determined for a set of brain regions. An atlas database consisting of expertly segmented reference images27 was applied with the DrawEM (Developing brain Region Annotation With Expectation–Maximization) module of the Medical Image Registration Toolkit.26,28
For segmentation analysis, a 3D image volume was built from each acquisition (2D and direct 3D), referred to as the image stack in the following. An experienced imaging scientist (R.A.H.) reviewed the segmentations and assigned a quality score, following a custom protocol. Image stacks showing quality deficiencies, making volume calculations unreliable, were excluded. If two or more image stacks of sufficient quality were available for the same infant, the best segmentation was selected for further analysis. Merged volumes of brain regions (total brain [i.e., total intracranial volume without cerebrospinal fluid], white matter, cortical gray matter, deep gray matter, and cerebellum) were generated by summation of selected individual regions.
Oral chloral hydrate (35 mg/kg) was used for sedation. A combination of purpose-made in-ear and over-ear sound absorption devices was used for hearing protection. All infants were closely monitored by a trained nurse or physician, including respiratory rate, oxygen saturation, and heart rate throughout the whole procedure.
Statistical analysis and variable definition
Data were analyzed using IBM SPSS 26 (IBM, Armonk, NY). AUC values were retrieved using a trapezoidal method based on serum levels of growth factors from postnatal days (PND) 1, 7, 14, and 28. Both PND 1 and PND 28 values were needed for inclusion in data analysis in the AUC calculations. A p value <0.2 was required in the univariate analysis for inclusion in the final multivariable analysis. Independent variables included in the initial univariate analysis were postmenstrual age at the time of MR scanning (weeks), GA at birth (weeks), development in birth weight standard deviation score from birth until the time of MR scanning, total parenteral and enteral energy intake PND 1–28 (kcal), antenatal steroid treatment, small for gestational age (SGA), sepsis, significant brain injury, and sex. The model utilized was run on total brain volume, following model validation in subregions for eligibility. Included variables in the regression models were checked for multicollinearity (variance inflation factor >1, <2, and eigenvalue, accompanied by visual analysis), normal distribution of residuals, independent observations, and homoscedasticity. The variable significant brain injury was not included in the final model due to violation of multicollinearity diagnostics, in spite of an acceptable VIF score. This was accounted for by both a rerun of the analyzes and the variable significant brain injury and exclusion of infants with severe brain injury. Randomization to treatment was accounted for in multiple linear regression and did not have an association with brain volumes.
The threshold for the effect of GA on estimated probabilities of total brain volumes and IGF-1 used in the explorative subanalysis was identified using visual analysis of nominal data (weeks). IGF-1high and IGF-1low were defined as AUC values above and below the median, respectively. Development of weight SDS was defined as the difference between weight SDS at the time of MRI and weight SDS at birth.
The Spearman rank test was used for correlations between non-parametric data. For comparisons of non-parametric variables between groups and for categorical variables, the Mann–Whitney U test, χ2 test of independence, or Fisher’s exact test were used as appropriate. In analyzes, p values <0.05 were considered significant. To adjust for multiple hypothesis testing, the Holm–Bonferroni method was used.29 P values ≥0.01 are presented with two decimals, p values in the range 0.001–<0.01 are denoted with three decimals.
Clinical data were collected prospectively, and clinical diagnoses are listed in Table 1. MR-based macrostructural regions used were total brain volume (not including ventricles) and regional volumes (white matter, cortical gray matter, deep gray matter, and cerebellum). Significant brain injury was defined as intraventricular hemorrhage (IVH) grade III (according to Papile et al.30) and periventricular hemorrhagic infarction, white matter lesions (focal signal abnormality score ≥2 according to Kidokoro et al.31), cerebellar hemorrhage (signal abnormality score ≥2 according to Kidokoro et al.31), and/or cystic lesions (cystic lesion score ≥3 according to Kidokoro et al.31). TEA MR scanning was performed between postmenstrual age 39.7 and 49.9 weeks.
Results
In total, 49 infants fulfilled growth factor AUC availability and MRI segmentation image quality criteria, Fig. 1. The clinical characteristics of the included infants are given in Table 1. Infants included in the final data analysis had similar characteristics as infants that did not meet MRI segmentation and AUC availability criteria, Supplementary Table 1.
Larger IGF-1 AUC correlated with increased total brain volume, white matter volume, cortical gray matter volume, deep gray matter volume, and cerebellar volume in univariate correlation analysis, adjusted for multiple testing, Fig. 2a–e. Variables included in the initial univariate data analysis rendering the final statistical model are shown in Supplementary Table 2.
Higher IGF-1 levels were associated with total brain volume; r = 0.49, p < 0.001 (a), white matter volume; r = 0.55, p < 0.001 (b), cortical gray matter volume; r = 0.40, p = 0.004 (c), deep gray matter volume r = 0.45, p = 0.001 (d), and cerebellar volume; r = 0.47, p < 0.001 (e). IGF insulin-like growth factor, AUC area under the curve, PND postnatal day, TEA term equivalent age.
In the full statistical model adjusting for PMA at time of MRI (weeks) and GA at birth (weeks), higher serum levels of IGF-1 expressed as AUC were independently associated with total brain volume (p < 0.001, β = 0.90, 95% CI 0.41–1.39, R2 = 0.64), white matter volume (p = 0.007, β = 0.33, 95% CI 0.09–0.56, R2 = 0.30), cortical gray matter volume (p = 0.002, β = 0.43, 95% CI 0.17–0.70, R2 = 0.72), deep gray matter volume (p = 0.008, β = 0.05, 95% CI 0.01–0.08, R2 = 0.41), and cerebellar volume (p = 0.006, β = 0.08, 95% CI 0.02–0.13, R2 = 0.63), all remaining significant after adjusting for multiple testing. The presence of major cerebral injuries could not explain the associations as results were not affected by the inclusion of significant brain injury as a covariate in the full model or by excluding infants with significant brain injury (n = 18) (data not shown).
Brain volumes in relation to IGF-1high and IGF-1low, defined as above and below IGF-1 AUC median, are demonstrated in Table 2. The relationship between IGF-1 AUC and 84 anatomical subregions is shown in Supplementary Table 3. IGF-1 AUC positively correlated with volume (unadjusted) in 54 subregions of the brain, and ten subregions remained significant following adjustment for multiple testing.
BDNF AUC correlated positively with total brain volume (r = 0.34, p = 0.02), white matter (r = 0.40, p = 0.006), deep gray matter (r = 0.31, p = 0.03), and cerebellar volumes (r = 0.40, p = 0.005). No associations remained after adjusting for multiple testing or when adjusting for GA at birth and PMA at the time of MRI in the regression model. No associations were found for PDGF or VEGF and brain volumes at TEA.
No correlations were found between IGF-1 serum levels and relative brain region volumes (percentage of total brain volume, adjusted for intracranial volume), Supplementary Fig. 1 a–d. When comparing the relative brain region volumes between the IGF-1high and the IGF-1low group, the volume fraction of the cerebellum was proportionally higher in the IGF-1high group than in the IGF-1low group (p = 0.02), but the difference did not remain after adjusting for multiple testing, Supplementary Table 4.
We exploratively analyzed the role of GA on the link between IGF-1 and brain volumes. This was done by investigating the impact of IGF-1 AUC in the full statistical model adjusting for GA at birth and postmenstrual age at the time of MR scanning on the estimated unstandardized probabilities of total brain volume, illustrated per gestational week in Fig. 3a. Infants born at <25 weeks GA (n = 16) differed in their relationship between IGF-1 AUC and brain volumes when compared to infants born at ≥25 weeks GA (n = 33). Therefore, a threshold of GA of 25 weeks was used in the subanalysis. Infants born at <25 weeks GA had persistently low endogenous IGF-1 serum levels and did not show the increase in serum levels of IGF-1 over time seen in infants born at ≥25 weeks GA, Fig. 3a, b. In a subanalysis of infants born at <25 weeks GA, there was no association between IGF-1 serum levels and brain volumes. In contrast, higher IGF-1 levels corresponded to the larger total brain and larger white matter, cortical gray matter, deep gray matter, and cerebellar volumes in infants born ≥25 weeks GA. Similar results were seen when dichotomizing at median GA (25.4 weeks), Table 3. The postmenstrual age at the time of MR scanning was not significantly different in the infants born at <25 and ≥25 weeks GA, median (minimum–maximum) 43 (40–46.6) and 42.9 (39.7–49.9) weeks, respectively.
a Estimated unstandardized probabilities of total brain volume, retrieved from the full statistical model, illustrated per gestational week. Error bars indicate 95% CI. Colored area: interpolated. b Distribution of serum levels of IGF-1 during postnatal days 1, 7, 14, and 28 in relation to gestational age at birth (below or over 25 weeks). More immature infants show less increase in endogenous IGF-1 levels. Dotted line shows 30 ng/ml. A prolonged period with serum IGF-1 levels below this threshold has been related to morbidities in the neurovascular unit.54 Boxes illustrate interquartile range, whiskers show full range. IGF-1 insulin-like growth factor 1, CI confidence interval, PND postnatal day, GA gestational age.
Discussion
In this study of extremely preterm infants, we show that increasing IGF-1 serum levels are independently associated with increased total brain volume, as well as increased regional white matter, cortical gray matter, deep gray matter, and cerebellar volumes at TEA. At the same time, no associations were found for PDGF, BDNF, or VEGF.
Expression of IGF-1 in the brain is found in the cortex, cerebellum, hypothalamus, hippocampus, and spinal cord,32 with a peak in the perinatal period and a decrease when neuronal proliferation ceases.33 IGF-1 plays a crucial regulatory role for early neuronal maturational and differentiation processes, mainly via the PI3-K–Akt pathway.34,35 It affects vital neurodevelopmental processes such as the development of astrocytes and oligodendrocytes, synapse formation, myelination, and production of neurotransmitters.36 The main proportion of circulating IGF-1 is synthesized in the liver, but IGF-1 is secreted by almost all fetal tissues at some developmental stage and exerts its actions in endocrine, paracrine, and autocrine manners by binding to the IGF-1 receptor.37 In addition to endogenous brain expression, IGF-1 is also actively transported via the choroid plexus from the circulation into the nervous system and acts together with brain-derived IGF-1.38,39 Systemic levels of circulating IGF-1 may thus reflect IGF-1-mediated actions within the nervous system, as suggested by the findings in our study.32
The effect of IGF-1 on oligodendrocytes has been demonstrated in several studies. Our findings harmonize with a study of more mature infants, <31 weeks GA at birth, and with less pronounced depression of serum IGF-1. Pupp et al.12 observed an association between IGF-1 levels and total brain, unmyelinated white matter, and cerebellar volumes using a lower anatomical resolution MRI method. The association was restricted to cerebellar volume when adjusting for SGA.12 Carson et al.40 observed that overexpression of IGF-1 in a mouse model was associated with a 55% increase in brain size and an increase in myelin content by 130%, suggesting a specific effect of IGF-1 on white matter development.40 Following these findings, an increased percentage of myelinated axons and increased thickness of the myelin sheath have been found following increased expression of IGF-1 in transgenic mice.41 Similarly, in a preterm rabbit pup model, low IGF-1 levels were linked to decreased cerebellar external granular layer proliferation and decreased Purkinje cell maturation.42 IGF-1 thus seems to be of specific importance from a preterm clinical perspective, as early white matter abnormalities, as well as cerebellar hypoplasia, have been linked to later neurodevelopmental impairments.43,44 More specifically, white matter abnormalities at TEA have been associated with neurosensory impairments, cognitive delay, motor delay, and cerebral palsy at 2 years of age and motor impairment, cognitive impairments, special assistance requirements at school, and cerebral palsy later in childhood.43,45,46 Early quantitative measurements of the cerebellum have been associated with motor behavior during the first 2 years of life, as well as cognitive development; however, further studies are required to elucidate the exact role of a reduced size of the cerebellum.47,48,49,50
To explore the association between IGF-1 and brain regions of specific functional importance in the preterm infant, we investigated the association between IGF-1 and the relative volume (percentage adjusted for intracranial volume) of particular brain regions. No clear associations were found, and the tendency towards a larger relative volume of the cerebellum in infants with high levels of systemic IGF-1 did not remain significant after correction for multiple comparisons. This suggests that systemic IGF-1 levels are related to global brain growth rather than the growth and maturation of specific regions. However, this does not exclude that the reduced volume may be of particular importance in areas such as white matter or cerebellum that are commonly linked to adverse outcomes in the preterm infant.
The third trimester is a critical period in brain development, encompassing myelination, synaptogenesis, and neuronal organization, alongside the development of functional capacity. Preterm birth results in morphological brain alterations, including reduced brain growth, that persist until adulthood even in the absence of macrostructural brain injury. These changes may in turn be associated with life-long cognitive and behavioral consequences.3,4,5,6,7,8,9,10,51 Several other perinatal risk factors, including sex, focal brain injury, and SGA at birth, affect brain volumes in these vulnerable infants, with GA possibly the most prominent factor affecting both brain volumes and later outcome.52 In our study, the association between brain volumes and IGF-1 persisted when corrected for GA at birth and when infants with significant focal brain injury were removed or corrected for. In addition, and somewhat surprisingly, neither total energy intake nor SGA or extrauterine growth development, as measured by development in weight SDS from birth to time of MRI significantly contributed to brain volume at term. The influence of nutrition on the IGF-1-axis in extremely preterm infants has been suggested to mainly occur at 30–33 weeks PMA, thus after reaching a certain degree of maturity.53 Taken together, these results suggest that IGF-1 has an independent role in early postnatal brain development that could not be explained by general body growth or focal brain injuries.
An interesting finding in our study was that the most immature infants had the lowest serum levels of IGF-1, with a less pronounced increase with advancing postnatal age, and lacked the association with brain volumes. A possible explanation is that IGF-1 levels above a threshold value may be needed to promote brain development. A prolonged period with serum IGF-1 levels below 30 ng/ml has been related to retinal neurovascular morbidity in the preterm infant.54 A link between the angiogenic function of VEGF and IGF-1 has been shown, where IGF-1 acts as a permissive factor for VEGF in the neonate.55,56,57 In our study, median IGF-1 levels were below this value at all time points in the most immature group. In addition, adverse clinical events occur more frequently in the most immature infants and may, together with immaturity itself, have a more prominent role in brain growth and maturation than low IGF-1. It is also possible that the small number of infants in the subanalysis prevented any differences from reaching statistical significance.
Despite previous studies associating BDNF as well as VEGF and PDGF to brain development and disease experimentally and in adults,17,18,19,20 we did not find any significant association between these growth factors and preterm brain volumes. BDNF serum levels correlated with brain volumes, but the association did not remain after adjustment for immaturity and PMA at MRI. The lack of association could be due to the more dynamic circadian pattern of BDNF compared to IGF-158 or the strong association of BDNF with GA.59 Another possible explanation for the associations with IGF-1, but not with BDNF, PDGF, and VEGF, might be due to the well-known, pronounced mitogenic role of circulating IGF-1 during the perinatal period extremely preterm infant. It is important to keep in mind that the brain-specific effect of these factors on a cellular level might be orchestrated and conducted via other influencing factors and mechanisms. The AUC in this study, calculated from circulating serum levels of BDNF, VEGF, and PDGF at PNDs 1, 7, 14, and 28, may thus not reflect the exact brain-specific action in extremely preterm infants during this particular phase of development. Furthermore, as recently described by Hellgren et al.,25 the endogenic longitudinal postnatal serum patterns of BDNF, VEGF, and PDGF do not follow the same postnatal pattern as IGF-1. IGF-1 is an agent in the somatotropic axis, and it is, as previously described, mainly produced in the liver and released into the bloodstream, whereas levels of BDNF, VEGF, and PDGF are likely more tightly linked to other circulating factors. For example, there are tight associations between circulating levels of BDNF, VEGF, and PDGF and platelet function25, which is linked to several clinical conditions such as sepsis and oxygen exposure. BDNF, which binds to different receptors, including TrK-B, has been of high interest during the last decades due to its essential roles in neuronal and synaptic properties, especially during the fetal developmental stages. BDNF activates several pathways, including the PI3-K/Akt, the PLC- γ, MAPK, and GTPases.60 Studies have found links between low levels of BDNF in newborns and autism spectrum disorder, and preterm infants with lower levels of BDNF had a higher probability of failing neurodevelopmental outcome tests.61,62 The roles of circulating VEGF, and PDGF in the preterm infant are not fully elucidated; however, they are both mediators of angiogenesis during development. In the preterm infant, potential links between VEGF and PDGF signaling, and dysregulation, inflammation, and altered vascular development have been suggested.63,64 VEGF is also a well-known mediator of neovascularization in the neurovascular disease ROP, which is associated with several outcomes such as brain volumes and poor neurodevelopmental outcome.65 It is important to further elucidate the complex interplay, function, and downstream mechanisms of circulating factors during different developmental phases in the extremely preterm.
Limitations
MRI series not meeting the MRI quality volume acquisition criteria (Fig. 1), and a limited number of infants, especially in subanalysis, may have prevented us from detecting less pronounced associations. In addition, preterm infants constitute a heterogeneous group with numerous confounding factors associated with neonatal morbidity, and clinical interventions may have influenced brain development independent of IGF-1 levels.
Conclusion
In conclusion, this study shows that higher circulating IGF-1 levels during the first four weeks of life are associated with increased total and regional brain volumes at TEA in extremely preterm infants. This effect was more pronounced in more mature infants with higher levels of IGF-1. Our findings suggest that IGF-1 promotes brain growth, which may protect the developing brain. The effects of systemically administered IGF-1/IGF-1BP3 on brain morphology and cognitive outcomes in extremely preterm infants are now being investigated in a randomized controlled trial (Clinicaltrials.gov: NCT03253263).
Data availability
Deidentified data analyzed in the current study are available from the corresponding author on reasonable request, in accordance with the jurisdiction of personal integrity.
References
Ball, G. et al. Development of cortical microstructure in the preterm human brain. Proc. Natl Acad. Sci. USA. 110, 9541–9546 (2013).
Smyser, C. D. et al. Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb. Cortex. 26, 322–333 (2016).
Scheinost, D. et al. Preterm birth alters neonatal, functional rich club organization. Brain Struct. Funct. 221, 3211–3222 (2016).
Guihard-Costa, A. M. & Larroche, J. C. Differential growth between the fetal brain and its infratentorial part. Early Hum. Dev. 23, 27–40 (1990).
Lind, A. et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr. Radiol. 41, 953–961 (2011).
Thompson, D. K. et al. Characterisation of brain volume and microstructure at term-equivalent age in infants born across the gestational age spectrum. NeuroImage Clin. 21, 101630 (2019).
Storbeck, T., Bruns, N., Weiss, C., Felderhoff-Müser, U. & Müller, H. Correlation of lateral ventricular size and deep gray matter volume in MRI at term equivalent age with neurodevelopmental outcome at a corrected age of 24 months and with handedness in preterm infants. Eur. J. Pediatr. 179, 271–278 (2020).
Peterson, B. S. et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284, 1939–1947 (2000).
Kelly, C. E. et al. Regional brain volumes, microstructure and neurodevelopment in moderate–late preterm children. Arch. Dis. Child. Fetal Neonatal Ed. 105, 593 (2020).
Hellstrom, A. et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 105, 576–586 (2016).
Hansen-Pupp, I. et al. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr. Res. 74, 564–569 (2013).
Hansen-Pupp, I. et al. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J. Clin. Endocrinol. Metab. 96, 1129–1135 (2011).
Cai, Z., Fan, L. W., Lin, S., Pang, Y. & Rhodes, P. G. Intranasal administration of insulin-like growth factor-1 protects against lipopolysaccharide-induced injury in the developing rat brain. Neuroscience 194, 195–207 (2011).
Lin, S. et al. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 1063, 15–26 (2005).
Brywe, K. G. et al. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur. J. Neurosci. 21, 1489–1502 (2005).
Cao, Y. et al. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J. Cereb. Blood Flow. Metab. 23, 739–747 (2003).
Miranda M., Morici J. F., Zanoni M. B. & Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 13, 363 (2019).
Ogunshola, O. O. et al. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res. Dev. Brain Res. 119, 139–153 (2000).
Feng, Y., Rhodes, P. G. & Bhatt, A. J. Neuroprotective effects of vascular endothelial growth factor following hypoxic ischemic brain injury in neonatal rats. Pediatr. Res. 64, 370–374 (2008).
Funa, K. & Sasahara, M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J. Neuroimmune Pharmacol. 9, 168–181 (2014).
ClinicalTrials.gov. Fatty Acids Study in Preventing Retinopathy of Prematurity. NCT02760472. https://clinicaltrials.gov/ct2/show/NCT02760472. Accessed November 2019.
Najm, S. et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial. Clin. Nutr. Espen. 20, 17–23 (2017).
Blum, W. F. & Breier, B. H. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. 4(Suppl 1), 11–19 (1994).
Löfqvist, C. et al. A pharmacokinetic and dosing study of intravenous insulin-like growth factor-I and IGF-binding protein-3 complex to preterm infants. Pediatr. Res. 65, 574–579 (2009).
Hellgren, G. et al. Decreased platelet counts and serum levels of VEGF-A, PDGF-BB, and BDNF in extremely preterm infants developing severe ROP. Neonatology 118, 18–27 (2021).
Makropoulos, A. et al. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. NeuroImage 173, 88–112 (2018).
Gousias, I. S. et al. Magnetic resonance imaging of the newborn brain: automatic segmentation of brain images into 50 anatomical regions. PLoS One 8, e59990 (2013).
Makropoulos, A. et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans. Med. Imaging 33, 1818–1831 (2014).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am. J. Neuroradiol. 34, 2208–2214 (2013).
Bach, M. A., Shen-Orr, Z., Lowe, W. L., Roberts, C. T. & Leroith, D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Mol. Brain Res. 10, 43–48 (1991).
Bartlett, W. P., Li, X. S., Williams, M. & Benkovic, S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev. Biol. 147, 239–250 (1991).
Aburto, M. R., Magariños, M., Leon, Y., Varela-Nieto, I. & Sanchez-Calderon, H. AKT signaling mediates IGF-I survival actions on otic neural progenitors. PLoS One 7, e30790 (2012).
Arsenijevic, Y., Weiss, S., Schneider, B. & Aebischer, P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J. Neurosci. 21, 7194–7202 (2001).
Ye, P. et al. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J. Neurosci. Res. 78, 472–484 (2004).
Han, V. K., D’Ercole, A. J. & Lund, P. K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science 236, 193–197 (1987).
Carro, E., Nuñez, A., Busiguina, S. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933 (2000).
Santi, A., Genis, L. & Torres Aleman, I. A coordinated action of blood-borne and brain insulin-like growth factor I in the response to traumatic brain injury. Cereb. Cortex. 28, 2007–2014 (2018).
Carson, M. J., Behringer, R. R., Brinster, R. L. & McMorris, F. A. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 10, 729–740 (1993).
Ye, P., Carson, J. & D’Ercole, A. J. In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. J. Neurosci. 15, 7344–7356 (1995).
Sveinsdóttir, K. et al. Impaired cerebellar maturation, growth restriction, and circulating insulin-like growth factor 1 in preterm rabbit pups. Dev. Neurosci. 39, 487–497 (2017).
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355, 685–694 (2006).
Volpe, J. J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 24, 1085–1104 (2009).
Iwata, S. et al. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics 129, e1138–e1147 (2012).
Spittle, A. J. et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev. Med. Child Neurol. 53, 1000–1006 (2011).
Katušić, A., Raguž, M. & Žunić Išasegi, I. Brain tissue volumes at term-equivalent age are associated with early motor behavior in very preterm infants. Int. J. Dev. Neurosci. 80, 409–417 (2020).
Shah, D. K. et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr. Res. 60, 97–102 (2006).
Spittle, A. J. et al. Reduced cerebellar diameter in very preterm infants with abnormal general movements. Early Hum. Dev. 86, 1–5 (2010).
Van Kooij, B. J. et al. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev. Med Child Neurol. 54, 260–266 (2012).
Anderson, P. & Doyle, L. W. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 289, 3264–3272 (2003).
Keunen, K. et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J. Matern. Fetal Neonatal Med. 25, 89–100 (2012).
Yumani, D. F. J., Calor A. K. & van Weissenbruch M. M. The course Of IGF-1 levels and nutrient intake in extremely and very preterm infants during hospitalisation. Nutrients 12, 675 (2020).
Hellstrom, A. et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc. Natl Acad. Sci. USA. 98, 5804 (2001).
Smith, L. E. et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 5, 1390–1395 (1999).
Hellström, A. et al. IGF-I is critical for normal vascularization of the human retina. J. Clin. Endocrinol. Metab. 87, 3413–3416 (2002).
Hellstrom, A. et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc. Natl Acad. Sci. USA. 98, 5804–5808 (2001).
Begliuomini, S. et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J. Endocrinol. 197, 429–435 (2008).
Chouthai, N. S., Sampers, J., Desai, N. & Smith, G. M. Changes in neurotrophin levels in umbilical cord blood from infants with different gestational ages and clinical conditions. Pediatr. Res. 53, 965–969 (2003).
Kowiański, P. et al. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 38, 579–593 (2018).
Skogstrand, K. et al. Reduced neonatal brain-derived neurotrophic factor is associated with autism spectrum disorders. Transl. Psychiatry 9, 252 (2019).
Ghassabian, A. et al. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev. Psychopathol. 29, 1499–1511 (2017).
Oak, P. & Hilgendorff, A. The BPD trio? Interaction of dysregulated PDGF, VEGF, and TGF signaling in neonatal chronic lung disease. Mol. Cell. Pediatr. 4, 11 (2017).
Oak, P. et al. Attenuated PDGF signaling drives alveolar and microvascular defects in neonatal chronic lung disease. EMBO Mol. Med. 9, 1504–1520 (2017).
Sveinsdóttir, K. et al. Relation of retinopathy of prematurity to brain volumes at term equivalent age and developmental outcome at 2 years of corrected age in very preterm infants. Neonatology 114, 46–52 (2018).
Niklasson, A., Ericson, A., Fryer, J. G., Karlberg, J., Lawrence, C., Karlberg, P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981). Acta Paediatr Scand 80, 756–62 (1991).
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 123, 991–9 (2005).
Funding
This study was supported by the Swedish Medical Research Council #2016–01131, The Gothenburg Medical Society, Government grants, under the ALF agreement ALFGBG–717971. Wallenberg Clinical Scholars, and Region Västra Götaland, Regional Collaboration Grant, VGRFOUREG573581 and 932182. L.M.H. received funding from the Athena grant, “Utrecht Center for Food and Health – research program specialized nutrition”, subsidy from the Dutch Ministry of Economic Affairs, Utrecht Province, and the municipality of Utrecht. Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
All authors have met the Pediatric Research authorship requirements and made substantial contributions to study design, acquisition of data, or analysis and interpretation of data and participated in drafting and/or critically revising the article for important intellectual content. W.H., L.M.H., R.A.H., C.L., G.H., M.J.N.L.B., A.H., and K.S. conceptualized and designed the study, carried out the initial analyzes, reviewed and drafted the initial manuscript, and reviewed and revised the manuscript. I.M.B.–B. supervised, was part of conceptualizing and designing the study and coordinated data collection, and critically reviewed the manuscript for important intellectual content. M.L.T., and D.L. were part of conceptualizing the study and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
A.H. and C.L. hold stock/stock options in Premalux AB. In addition, A.H. and D.L. have received consulting fees from Shire, a Takeda company. The rest of the authors declare no competing interests.
Consent to participate
Written informed parental consent was required prior to the study enrollment of the infant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hellström, W., Hortensius, L.M., Löfqvist, C. et al. Postnatal serum IGF-1 levels associate with brain volumes at term in extremely preterm infants. Pediatr Res 93, 666–674 (2023). https://doi.org/10.1038/s41390-022-02134-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02134-4
This article is cited by
-
Insulin-like growth factor-1 and insulin-like growth factor binding protein-3 as early predictors of growth, body composition, and neurodevelopment in preterm infants
Journal of Perinatology (2024)
-
An exploratory study of clinical factors associated with IGF-1 and IGFBP-3 in preterm infants
Pediatric Research (2024)