Abstract
Tumor lysis syndrome (TLS) is a common and fatal complication of childhood hematologic malignancies, especially acute lymphoblastic leukemia (ALL). The clinical features, therapeutic regimens, and outcomes of TLS have not been comprehensively analyzed in Chinese children with ALL. A total of 5537 children with ALL were recruited from the Chinese Children’s Cancer Group, including 79 diagnosed with TLS. The clinical characteristics, treatment regimens, and survival of TLS patients were analyzed. Age distribution of children with TLS was remarkably different from those without TLS. White blood cells (WBC) count ≥ 50 × 109/L was associated with a higher risk of TLS [odds ratio (OR) = 2.6, 95% CI = 1.6–4.5]. The incidence of T-ALL in TLS children was significantly higher than that in non-TLS controls (OR = 4.7, 95% CI = 2.6–8.8). Hyperphosphatemia and hypocalcemia were more common in TLS children with hyperleukocytosis (OR = 2.6, 95% CI = 1.0–6.9 and OR = 5.4, 95% CI = 2.0–14.2, respectively). Significant differences in levels of potassium (P = 0.004), calcium (P < 0.001), phosphorus (P < 0.001) and uric acid (P < 0.001) were observed between groups of TLS patients with and without increased creatinine. Laboratory analysis showed that older age was associated with a higher level of creatinine. Calcium level was notably lower in males. WBC count, lactate dehydrogenase, and creatinine levels were significantly higher in T-ALL subgroup, whereas procalcitonin level was higher in B-ALL children. Older age, infant, a higher level of WBC and T-ALL were risk factors TLS occurrence. Hyperleukocytosis has an impact on the severity of TLS, while renal injury may be an important feature in the process of TLS.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) is a type of cancer that accounts for 25% of all pediatric malignancies having developed before age of 151,2. About 20% of patients may confront treatment failure, mainly due to relapse, secondary tumor, chemotoxicity, or severe complications3. Among the complications, tumor lysis syndrome (TLS) is common and fatal, especially in newly diagnosed patients. A deep understanding of TLS may benefit the overall outcome of childhood ALL.
TLS refers to a spectrum of disorders resulting from the rapid release of intracellular substances from lysed cells4. It is a potentially fatal clinical condition. TLS is characterized by clinical findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia5,6. Rapid progression of TLS often exerts severe toxic effects on organs, leading to renal impairment, epilepsy, cardiac arrhythmias, pulmonary edema, and even death. Clinical observations suggest that TLS tends to arise from highly proliferative malignancies, such as Burkitt’s lymphoma and ALL, in which tumor burden is heavy, or in response to initial therapy7. Therefore, TLS is a great challenge in childhood ALL for clinical physicians.
Previous studies have reported the risk factors and management standards for TLS8,9. However, few comprehensive analyses have been carried out to illustrate the clinical features, therapeutic regimens, and outcomes of TLS in ALL children10,11. Here, we analyzed the clinical data of TLS in ALL children from the Chinese Children’s Cancer Group (CCCG). The present findings will help to get a better understanding of TLS.
Subjects and methods
Patient recruitment
All patients included in this retrospective study were diagnosed with childhood ALL according to their morphology, immunology, cytogenetic and molecular biology between January 2015 and September 2018. Patient recruitment was conducted in 20 major hospitals or medical centers registered in CCCG, across 10 provinces, 3 municipalities directly under the Central Government in mainland China and Hong Kong (as listed in Authors’ information Section). The last follow-up was made in December 2018. The ages of patients ranged from 0 to 18 years. Laboratory TLS (LTLS) was defined as the metabolic disturbance of hyperkalemia, hyperphosphatemia, hyperuricemia, and hypocalcemia, while clinical TLS as LTLS along with renal injury/cardiac arrhythmias/seizures7. Finally, 5537 childhood ALL patients were included in our present study. A total of 79 TLS cases were reported from 13 institutions (as shown in the Acknowledgments section), among which 35 were diagnosed as clinical TLS. The research protocol was approved by the Medical Ethics Committee of the 20 institutions participating in this study and informed consent was obtained from children’s parents. All methods were performed following relevant guidelines and regulations expounded in Policies of the Nature Research journals.
Treatment protocol
All patients were treated according to CCCG-ALL 2015 protocol (ChiCTR-IPR-14005706). Chemotherapy regimens were described in previous studies12,13. After the establishment of ALL diagnosis, the patients received dexamethasone for 4 days, as upfront window therapy, followed by remission induction from day 5 to day 28 with prednisone, vincristine, daunorubicin, and PEG-asparaginase. Bone marrow cell morphology and flow cytometric MRD were used to assess treatment response at day 19 and day 46. The risk level of these cases was determined according to CCCG-ALL 2015 protocol. Basis of risk escalation included T-ALL, age < 1 or > 10 years, gene fusion, chromosome abnormality, high white blood cell count at diagnosis, CNS involvement, etc. TLS was treated with hydration with about 1/4–1/3 dextrose normal saline (2–3 L/m2/day), diuretic therapy, or allopurinol.
Data collection
The clinical characteristics, including age, gender, immunophenotype, risk category, time of diagnosis, highest white blood cell (WBC) count and the most abnormal value of blood biochemical test, special findings of clinical manifestation, treatment regimens, and survival were collected from all 20 institutions.
Statistical analyses
Statistical analyses were performed using PASW Statistics 18. Continuous variables with normal distribution were summarized by mean ± standard deviation and compared by using two-tailed Student’s t-test. Continuous variables with non-normal distribution were expressed as median ± interquartile range (25th–75th percentile) and analyzed by Nonparametric test. Categorical variables were described with the number of subjects and compared using the Chi-square test. Multivariable logistic analysis was used to assess the association of clinical characteristics with TLS, as measured by the estimated odds ratio (OR) with the 95% confidence interval (95% CI). We performed logistic regression analyses to detect variables significantly associated with the prognosis of TLS. P value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Children's Hospital of each participating institution. All the guardians of participants signed an informed consent for participation in this study.
Consent for publication
All the authors have reviewed and approved this manuscript and consented to publish this paper.
Results
Basic information of study subjects
A total of 5537 patients were recruited from 13 Chinese hospitals in the CCCG group between 2015 to 2018. After their data were confirmed, 79 TLS patients were included in this retrospective study.
Children older than 10 years and younger than 1 year had a higher risk of TLS than ALL children of 1–10 years old (OR = 2.2, 95% CI = 1.3–3.8 for the older group and OR = 8.6, 95% CI = 3.0–22.2 for the younger group). Children with WBC count ≥ 50 × 109/L were more susceptible to TLS (OR = 2.6, 95% CI = 1.6–4.5). In addition, the incidence of T-ALL in TLS patients was significantly higher than that in other patients (OR = 4.7, 95% CI = 2.6–8.8). Multivariable logistic analysis demonstrated no significant association between TLS risk and other clinical characteristics (i.e., gene fusion, chromosome karyotype, and treatment branch) (Table 1).
In the subgroup analysis of TLS children divided according to WBC count (Table 2), the results showed a higher proportion of T-ALL children in the high WBC count group (≥ 50 × 109/L) (OR = 7.3, 95% CI = 2.6–19.9). In addition, the incidences of hyperphosphatemia and hypocalcemia were significantly higher in TLS children with hyperleukocytosis. It also seemed that proportion of ALL children aged < 1 year was higher in the high WBC count group than in the low WBC count group, but the difference was insignificant.
Clinical features and treatment data
Clinical features were summarized in Fig. 1A. Among the 79 patients, arrhythmia was observed in 6 cases (7.5%), epilepsy in 3 cases (3.8%), high creatinine in 33 cases (41.7%), and oliguresis in 8 cases (10.1%). As to the distribution of abnormal laboratory indexes, hyperphosphatemia occurred with the highest frequency (68.8%) and hyperkalemia occurred with the lowest frequency (35.4%) (Fig. 1B). It should be noted that a few clinical data were missing.
Distribution of clinical manifestation (A) and abnormal laboratory values (B) in TLS patients. It should be noted that the uric acid and phosphorus data were missing in 2 patients. Elevated creatinine: increased serum creatinine level of > 0.3 mg/dL (26.5 μmol/L) (or a single value > 1.5 times the upper limit of the age-appropriate normal range); oliguria: an average urine output of < 0.5 mL/kg/h for 6 h; hyperuricemia: uric acid > 8.0 mg/dL (475.8 μmol/L); hyperkalemia: potassium > 6.0 mmol/L; hyperphosphatemia: phosphorus > 6.5 mg/dL (2.1 mmol/L); hypocalcemia: corrected calcium < 7.0 mg/dL (1.75 mmol/L).
After the initiation of the chemotherapy, TLS developed in 43 patients (54.4%) at 1–3 days, and in 28 patients (35.5%) at 4 days or later. Only 8 patients (10.1%) showed TLS before the chemotherapy. As shown in Supplementary Figure 1A–C, hydration lasted for less than 7 days in 28 patients, 7–13 days in 32 patients, and 14 days or even longer in another 17 patients. The median hydration treatment duration was 8 days (range 1–30 days). Diuresis was also applied in 60 TLS children for less than 7 days, and for a longer duration in the remaining 17 children, with a median of 3 days and a range of 0–15 days. In addition, allopurinol treatment was applied in 30 TLS children for 0–7 days, in 33 patients for 7–13 days, and in 14 patients for longer than 14 days, with a median of 8 days and a range of 0–30 days.
Laboratory indexes
The relationship among abnormal biochemical indexes is illustrated in the Venn diagram in Fig. 2. Hyperphosphatemia was found to be the most common abnormality, often accompanied by hyperkalemia (25/53) and frequently causing hypocalcemia (32/53). That is, only three patients (10.7%) with hyperkalemia and seven patients (17.9%) with hypocalcemia had normal levels of phosphorus. To identify features associated with clinical symptoms, we analyzed biochemical abnormalities among patients with/without arrhythmias, seizures, and renal injury (including increased creatinine level and oliguria). We found significant differences in levels of potassium, calcium, phosphorus, and uric acid between groups of TLS patients with or without high creatinine (Table 3). Besides, patients with oliguria had a higher level of potassium and a lower level of calcium.
Subsequently, all the 79 TLS patients were grouped by gender, age, immunophenotype, chromosome abnormality, gene fusion, and laboratory results. As shown in Supplementary Table 1, older age was associated with a higher level of creatinine. In addition, calcium level was remarkably lower in male. Subsequently, we found that WBC count, lactate dehydrogenase (LDH), and creatinine levels were significantly higher in T-ALL subgroup, while the procalcitonin (PCT) level was higher in B-ALL children (Supplementary Table 2).
Outcomes
All the 79 patients received CCCG-2015 chemotherapy regimens after the diagnosis of ALL. Only one case lost to follow-up. Among the others, 4 cases died within 1–2 months after the diagnosis of TLS; 3 cases experienced a recurrence of ALL (within half a year in 1 case relapsed, after 1–2 years in 2 cases). The remaining of TLS children were cured and are surviving event-free at the time of writing (Month/Date/Year).
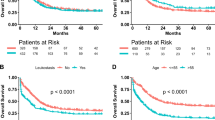
The 78 children with complete follow-up data were grouped by their survival status. No statistically significant association was observed between important lab indexes and TLS survival (Table 4). Furthermore, we investigated the value of immunophenotype, chromosome karyotype, gene fusion, and treatment duration in the prognosis of TLS, and found no significant association between event-free survival and clinical features as well as treatment duration (Fig. 3). Nevertheless, a poorer prognosis was observed in TLS children with abnormal karyotype and a hydration treatment of shorter than 7 days.
Discussion
In the present study, we made a comprehensive analysis on the clinical features and risk factors of TLS. The incidence of TLS in our study was 1.4%, lower than previously reported 20%7. The difference may be attributed to the preventive measures in CCCG-ALL 2015 protocol, such as sufficient hydration and controlling biochemical indexes. In addition, the clinical data reported in CCCG were complete. Therefore, we believe that the incidence of 1.4% was accurate.
A panel of TLS experts has developed guidelines for risk identification of TLS in adults and children with malignant diseases8. They put forward that ALL patients were at intermediate risk or high risk of TLS, depending on WBC and lactate dehydrogenase (LDH) levels at diagnosis. Another evidence-based review also pointed out that elevated circulating tumor cells were a risk factor of TLS in ALL patients9. In the present study, we compared demographic characteristics of 79 ALL children with TLS and 5458 ALL children without TLS. As expected, our results showed that older age and being an infant were risk factors of TLS. This was consistent with a previous study listing the risk factor of TLS8. Similarly, Gopakumar et al. reported children with TLS were likely to be older than those without TLS, although the difference was not significant11. Furthermore, ALL patients with TLS had remarkably higher WBC count, which is also aligned with the results in previous studies8,14. The proportion of T-ALL children was higher in the TLS group, indicating T-ALL cells may exert a heavier tumor burden on leukemia cells. Although these findings have been well documented in the literature, they are still valuable considering the large sample size of our study.
We have made several interesting observations by dividing TLS patient into two subgroups based on their WBC count. We found a higher proportion of T-ALL children in the high WBC count group,consistent with the literature. We also found that the incidences of hyperphosphatemia and hypocalcemia were significantly higher in TLS patients with hyperleukocytosis, indicating its significant impact on the severity of TLS. This can be interpreted by the fact that more phosphorus is released from leukemia cells. It was also interesting that the WBC count moderate in some patients. The reason could be elucidated with more clinical data of low-WBC-count ALL children without TLS. In addition, our results were consistent with previous reports that TLS commonly occurred within the first 3 days after chemotherapy was initiated8,15.
We summarized the effectiveness of chemotherapy regimens. It should be noted that rasburicase may be an effective therapy for hyperuricemia. However, it was just approved to be used in TLS by the National Medical Products Administration (NMPA) of China at the end of 2018. Therefore, we did not collect the data on this drug. More clinical data on using urate oxidase are needed to enhance our findings.
The Venn diagram showed that hypocalcemia and hyperphosphatemia were almost concomitant, which was in line with metabolic characteristics. To identify the association between clinical symptoms and laboratory indexes, we analyzed the biochemical profiles of patients with/without arrhythmias, seizures, and renal injury. The results suggested that renal injury might be an important feature in the process of TLS. It should be noted that these data were collected in the absence of urate oxidase administration, which is now considered a standard treatment for high-risk patients.
We next divided the subjects into subgroups according to their demographic feature and the basic feature of their disease (i.e. immunophenotype, chromosome abnormality, and gene fusion). The results showed that the mean calcium level was lower in males than in females. Clinically, phosphorus and calcium are physiologically linked, and hypocalcemia is often secondary to hyperphosphatemia caused by tumor cell lysis6,16. Hyperphosphatemia may cause soft tissue calcification17, while severe hypocalcemia can lead to arrhythmia and convulsion. In our present data, male patients had a significantly lower calcium level than females, indicating the critical role of calcium in the TLS mechanism. However, no obvious disparity was found in phosphorus levels between subgroups.
Another characteristic of TLS was hyperkalemia. Hyperkalemia is considered a life-threatening consequence of TLS due to the possibility of causing cardiac arrhythmia and cardiac arrest4. Hyperuricemia is also a manifestation of metabolic disturbances of TLS. When leukemia cells are disrupted, intracellular substances such as purine and their metabolites, are released in the serum. Uric acid is an end product of purine derivatives18. In our present study, there was no notable difference in kalium and uric acid levels between subgroups, indicating a relatively concordant metabolic change of these chemicals in males and females, older and younger, as well as B-ALL and T-ALL TLS patients.
Darmon et al. reported that about 64% of TLS patients experienced acute kidney injury (AKI)19. In the condition of kidney failure, the clearance of phosphorus, potassium, and uric acid is curbed20. However, in our 79 subjects, only 41.8% (33 patients) confronted significantly elevated serum creatinine. This discrepancy is partly due to the difference in disease types (i.e. Darmon et al. recruited acute myeloid leukaemia (AML), acute lymphoblastic leukemia (ALL), and aggressive NHL). We found serum creatinine was significantly higher in older age, as well as in T-ALL patients, indicating that these populations are more susceptible to kidney injury. However, we found no association of lab indexes with chromosome abnormality as well as gene fusion, which should be verified in future studies.
Ronald et al. from Mayo Clinic have evaluated the outcomes in hospitalized patients with TLS, using the National Inpatient Sample (NIS)5. They identified a series of prognostic factors of TLS, including age, cancer type, etc. In our present study, of the seven patients encountering relapse or death, four patients died within 1–2 months after TLS onset. We found no significant association between major laboratory indexes and TLS survival, which is inconsistent with the previous finding that a high WBC count was related to a poor prognosis21. This discrepancy may be explained by the small number of cases with relapse and death, and more data should be collected to explore the effect of lab values on TLS outcomes.
We found no significant association between event-free survival and other clinical features, such as immunophenotype, karyotype, and treatment regimen. Nevertheless, we found that abnormal karyotype and hydration < 7 days might indicate a worse prognosis of TLS patients, indicating the mechanistic role of chromosome karyotype and the obvious effectiveness of hydration treatment. It should be noted that death within 1–2 months was more likely a direct consequence of TLS. However, only four deaths were reported in the present study. Further studies with more death cases should be conducted to analyze prognostic factors of TLS. We showed the survival analysis to provide a reference for other studies and provide some potential clues for clinical treatment.
It should be stated that, for some of the TLS patients, we only recorded their normal data for a certain laboratory index and did not track down all the definite abnormal data. Therefore, they may not have two abnormal laboratory indicators in our dataset. However, there was a definite medical record showingthat they had met diagnostic criteria of TLS and most of them have obvious TLS symptoms (arrhythmia or renal injury). In addition, all of those patients accepted the treatment of TLS and were categorized as TLS patients in our CCCG dataset. Therefore, their data were also incorporated into our analysis. Although limitations exist in our recruitment, the real-world nature of our data is of significant value.
Conclusion
Older age (≥ 10 years), younger age (≤ 1 year), higher WBC count and T-ALL are risk factors of TLS. Hyperleukocytosis can increase the severity of TLS. Hyperphosphatemia is the most frequent laboratory abnormality. Renal injury may be an important feature in the progression of TLS. Our results provided strong evidence for understanding and managing TLS complicated with childhood ALL.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary files.
References
Hunger, S. P. et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J. Clin. Oncol. 30(14), 1663–1669 (2012).
Bhojwani, D., Yang, J. J. & Pui, C. H. Biology of childhood acute lymphoblastic leukemia. Pediatr. Clin. North Am. 62(1), 47–60 (2015).
Oskarsson, T. et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. Haematologica 101(1), 68–76 (2016).
Burns, R. A., Topoz, I. & Reynolds, S. L. Tumor lysis syndrome: risk factors, diagnosis, and management. Pediatr. Emerg. Care 30(8), 571–576 (2014) (quiz 577–9).
Durani, U., Shah, N. D. & Go, R. S. In-hospital outcomes of tumor lysis syndrome: a population-based study using the national inpatient sample. Oncologist 22(12), 1506–1509 (2017).
Criscuolo, M., Fianchi, L., Dragonetti, G. & Pagano, L. Tumor lysis syndrome: review of pathogenesis, risk factors and management of a medical emergency. Expert Rev. Hematol. 9(2), 197–208 (2016).
Howard, S. C., Jones, D. P. & Pui, C. H. The tumor lysis syndrome. N. Engl. J. Med. 364(19), 1844–1854 (2011).
Cairo, M. S., Coiffier, B., Reiter, A., Younes, A. & T.L.S.E. Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br. J. Haematol. 149(4), 578–586 (2010).
Coiffier, B., Altman, A., Pui, C. H., Younes, A. & Cairo, M. S. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J. Clin. Oncol. 26(16), 2767–2778 (2008).
Saeed, F., Ali, M. S., Ashraf, M. S., Vadsaria, K. & Siddiqui, D. E. Tumour lysis syndrome in children with haematological cancers: experience at a tertiary care hospital in Karachi. J. Pak. Med. Assoc. 68(11), 1625–1630 (2018).
Gopakumar, K. G. et al. Risk-based management strategy and outcomes of tumor lysis syndrome in children with leukemia/lymphoma: analysis from a resource-limited setting. Pediatr. Blood Cancer 65(12), e27401 (2018).
Cai, J. et al. Treatment abandonment in childhood acute lymphoblastic leukaemia in China: a retrospective cohort study of the Chinese Children’s Cancer Group. Arch. Dis. Child. 104(6), 522–529 (2019).
Shen, S. et al. Effect of dasatinib versus imatinib in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: a randomized clinical trial. JAMA Oncol. 6, 358 (2020).
Rahmani, B. et al. Current understanding of tumor lysis syndrome. Hematol. Oncol. 37, 537–547 (2019).
Cairo, M. S. & Bishop, M. Tumour lysis syndrome: new therapeutic strategies and classification. Br. J. Haematol. 127(1), 3–11 (2004).
Ahn, Y. H. et al. Tumour lysis syndrome in children: experience of last decade. Hematol. Oncol. 29(4), 196–201 (2011).
Abdullah, S. et al. Sevelamer hydrochloride: a novel treatment of hyperphosphatemia associated with tumor lysis syndrome in children. Pediatr. Blood Cancer 51(1), 59–61 (2008).
Micho, H., Mohammed, Y., Hailu, D. & Genet, S. Evaluation and characterization of tumor lysis syndrome before and after chemotherapy among pediatric oncology patients in Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. BMC Hematol. 18, 22 (2018).
Darmon, M. et al. Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Reanimation Respiratoire et Onco-Hematologique. Br. J. Haematol. 162(4), 489–497 (2013).
Wilson, F. P. & Berns, J. S. Tumor lysis syndrome: new challenges and recent advances. Adv. Chronic Kidney Dis. 21(1), 18–26 (2014).
Giammarco, S. et al. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev. Hematol. 10(2), 147–154 (2017).
Acknowledgements
The information of 79 TLS was provided by Shanghai Children’s Medical Center, Children’s Hospital of Chongqing Medical University, Children’s Hospital of Fudan University (Shanghai), The Second Hospital of Anhui Medical University, West China Second University Hospital of Sichuan University, Kunming Children’s Hospital, Tongji Hospital of Tongji Medical College (Wuhan), Children’s Hospital of Nanjing Medical University, Qilu Hospital of Shandong University, Guangzhou Women and Children’s Medical Center, Institute of Hematology and Blood Disease Hospital (Tianjin), Nanfang Hospital of Southern Medical University (Guangzhou), Prince of Wales Hospital (Hong Kong)
Funding
This research was supported by VIVA China Children's Cancer Foundation, the National Natural Science Foundation of China (81602913, 81670155), China Postdoctoral Science Foundation funded project (2019M650118), the Nanjing Medical Science and Technology Development Foundation (QRX17164), Special Fund for Health Science and Technology Development in Nanjing (JQX19008).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: Y.X., J.C. and Y.F. Acquisition of clinical data: X.G., J.C., X.Z., N.W., J.G., Y.L., Y.F., M.Y., Y.Z., H.Z., X.Z., X.W., C.K.L., S.H., C.L., R.J., H.J., M.Y., L.S. and K.P. Data analysis and interpretation: S.G. and J.C. Drafting of the manuscript: Y.X. and X.G. Critical revision of the manuscript: Y.F., J.T., C.K.L. and H.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, Y., Chen, J., Gao, S. et al. Clinical characteristics of tumor lysis syndrome in childhood acute lymphoblastic leukemia. Sci Rep 11, 9656 (2021). https://doi.org/10.1038/s41598-021-88912-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88912-2
This article is cited by
-
Daily serum phosphate increase as early and reliable indicator of kidney injury in children with leukemia and lymphoma developing tumor lysis syndrome
Pediatric Nephrology (2023)
-
Pediatric acute myeloid leukemia and hyperleukocytosis with WBC count greater than 50 × 109/L
International Journal of Hematology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.