Abstract
Background
Adverse neurodevelopmental outcomes and MRI alterations are reported in infants born after fetal growth restriction (FGR). This study evaluates the additional role of FGR over prematurity in determining brain impairment.
Methods
Retrospective observational study comparing 48 FGR and 36 appropriate for gestational age infants born between 26 and 32 weeks’ gestation who underwent a cerebral MRI at term equivalent age. Exclusion criteria were twins, congenital anomalies, and findings of overt brain lesions. Main outcomes were total maturation score (TMS) and cerebral areas independently measured by two neuro-radiologists and Griffiths or Bayley scale III scores at median age of 2 years.
Results
TMS was not significantly different between the groups. Inner calvarium and parenchyma’s areas were significantly smaller in FGR cases. There were no significant differences in the average quotient scores. A positive correlation between parenchyma area and cognitive score was found (r = 0.372, p = 0.0078) and confirmed after adjusting for sex, gestational age, and birth weight (p = 0.0014). Among FGR, the subgroup with umbilical arterial Doppler velocimetry alterations had significantly worse gross motor scores (p = 0.005).
Conclusions
FGR plays additional role over prematurity in determining brain impairment. An early structural dimensional MRI evaluation may identify infants who are at higher risk.
Impact
-
Fetal growth-restricted infants showed smaller cerebral parenchymal areas than preterm controls.
-
There is a positive correlation between the parenchyma area and the cognitive score.
-
These results highlight the already known link between structure and function and add importance to the role of a structural dimensional MRI evaluation even in the absence of overt brain lesions.
Similar content being viewed by others
Introduction
Fetal growth restriction (FGR) is currently defined as the inability of the fetus to reach its genetic developmental potential in utero. This condition can be identified by ultrasound evaluation according to agreed criteria1 and is associated with an increased risk of mortality and perinatal morbidity. Placental insufficiency is the main cause of FGR, leading to a state of decreased nutrient transfer and impaired oxygen utilization by the fetus.2,3,4,5 In response to the hypoxemic state, the fetus induces an adaptive response with a reduction in oxygen consumption and a redistribution of cardiac output to favor the development of vital organs, in particular of the brain, termed “brainsparing.” This is reflected by the progressive alteration of the ratio between cerebral and umbilical blood flow (cerebro-placental ratio)6 However, this mechanism does not ensure normal brain metabolism and growth. Several studies have shown that brainsparing could predict neurocognitive impairment in children affected by FGR,7 with a decrease in intelligence quotient score at 5 years of age related to this hemodynamic condition.8
FGR children with evidence of placental dysfunction are shown to be at higher risk for neurological deficits,9 especially those with alterations of Doppler velocimetry in umbilical artery (UA) as absent or reversed end diastolic blood flow (UA-ARED).10,11
Although the simple measure of head circumference (HC) is a known determinant of poor neurological outcome,10,12 neuroimaging studies could add more detailed information about brain development in this population. Magnetic resonance imaging (MRI) at term equivalent age (TEA) represents the gold standard for non-invasive structural study of the brain.13 In a setting of sophisticated MRI studies, preterm infants with FGR showed reduced total brain volume, with loss of both white and gray matter14 especially at the level of specific structures particularly sensitive to hypoxia such as the hippocampus,15 amygdala, basal ganglia, thalamus, and cortical areas.16,17 Through quantification of brain surface, Dubois et al. showed an important delay in cortical development, a discordant pattern of gyrification and decreased cortical thickness in FGR preterm newborns.18 A correlation between smaller regional brain volumes and neurocognitive impairment in preterm infants was also attested.19
Recent advances in imaging were able to demonstrate more complex microstructural changes, including an altered pattern of reorganization in specific regions of the brain.20 These alterations in structural brain connectivity were also associated with poorer social skills and cognitive deficit.21 However, these imaging approaches are time consuming and complex to perform on a daily basis in clinical scanning. On the other hand, even conventional MRI alone provides information through the possibility of studying brain structures in depth and defining brain abnormalities.22 In particular, in cases without focal brain injuries, a system of scoring, called total maturation score (TMS), has been validated to evaluate brain maturation in the preterm infant.23 This method is based on the evaluation of four morphological parameters (myelination [M], cortical folding [C], germinal matrix distribution [GM], and glial cell migration pattern [G]).24 Through the application of this scoring, we did not previously find any significant difference between FGR and preterm AGA infants in terms of level of brain maturation.25 However, FGR infants with in utero brainsparing presented a significant reduction in myelination compared to FGR infants without abnormalities of Doppler velocimetry in middle cerebral artery (MCA).
The aim of the present study was to evaluate daily clinical practice tools as MRI at TEA and neurodevelopmental scores at 2 years of age to understand the role of FGR over prematurity in determining early brain impairment, even in the absence of overt brain lesions. Therefore, we chose (1) to investigate those FGR and preterm babies not complicated by any visible clastic cerebral lesion, because they represent the majority of FGR and premature neonates in the developed countries; (2) to approach such neonatal medical issues through the use of a scoring method specifically designed for brains without focal injuries and, most of all, applicable on the routine clinical two-dimensional (2D) cross-sectional MRI and on single case basis. Previous approaches based on sophisticated volumetric three-dimensional (3D)-segmentation strategies are hardly applicable to routine single case clinical setting.
Methods
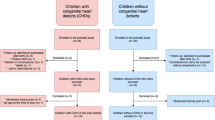
This is a single-center retrospective case–control study of infants born between 26 and 32 weeks’ gestation admitted to our neonatal intensive care unit and undergoing a cerebral MRI study at TEA at Buzzi Children’s Hospital between 2009 and 2018. This study was approved by the medical Ethical Committee of our Institution. Data were retrieved and analyzed after informed parental consent acquisition. Main outcome measures comparing preterm FGR infants and AGA controls were: level of brain maturation, size of the main cerebral areas, and neurodevelopmental score at median age of 2 years.
Subjects
Exclusion criteria were twins, chromosomal anomalies, known fetal malformations, congenital infections, placental abruption, and severe intrapartum events as placental abruption, cord prolapse, and uterine rupture. Neuroradiological exclusion criteria included the presence of serious motion artifacts and finding of focal or diffuse lesions of the brain: intraventricular hemorrhage grade >1, periventricular leukomalacia (PVL; including non-cystic PVL), isolated punctate lesions, and cerebellar hemorrhages (also small hemorrhagic foci within the parenchyma). FGR newborns were defined according to the international consensus definition based on ultrasound criteria.1 These fetuses underwent serial Doppler velocimetry ultrasound examinations of the UA, MCA, and DV (ductus venosus) until delivery. All newborns were studied by brain MRI in natural sleep when they reached term corrected gestational age, according to the local protocol.
MRI data acquisition and analysis
All MRI examinations were acquired by the same 1.5 Tesla scanner, with a dedicated neonatal coil; all studies included axial and coronal T2-weighted sequences (repetition time/echo time (TR/TE) 6000/200 ms, 3 mm slice thickness, interslice gap of 0.3 mm), axial and sagittal T1-weighted sequences (TR/TE 750/12 ms, 3 mm slice thickness, interslice gap of 0.3 mm), and diffusion-weighted sequences on axial plane (3 mm slice thickness, interslice gap of 0.3 mm).
Focal and diffuse lesions were firstly determined from reports found in the radiological information system and then confirmed by a careful scrutiny performed by two pediatric neuro-radiologists, in order to exclude even “minor lesions” as punctate white matter ones. Hemorrhagic foci were ruled out by assessing susceptibility-weighted images available in a fraction of cases or by assessing T2*-b = 0 weighted images from echo-planar diffusion-weighted sequence, when the former was not available.
The analysis of MRI images consisted of two methods performed blindly by two independent pediatric neuro-radiologists, after a consensus trial carried out on ten pilot cases:
(a) TMS scale26 determination: the final score for TMS in each case was the result of the average of the score assigned on the basis of TMS scale by the two operators independently.
(b) 2D area determination according to Fig. 1: in particular, the following areas were measured manually: the inner calvarium (a), the cerebral parenchyma (b), the cerebellar hemispheres (c), and the cerebellar vermis (d). Cerebral parenchyma area was defined as the result of the subtraction of the area defined by the white dotted line and the area of the ventricles (defined by the black dotted line). Both areas were assessed on the same single axial section at Monro foramina level. The area of cerebellar vermis was measured on the mid-sagittal T1-weighted section, while cerebellar hemisphere area was measured on the coronal T2-weighted section encompassing the dentate nuclei. The final value of the areas for each case was given by the average of the results obtained independently by the two raters.
Different examples of area measurements: the inner calvarium (a), the cerebral parenchyma (b), the cerebellar hemispheres (c), and the cerebellar vermis (d). Cerebral parenchyma area was defined as the result of the subtraction of the area defined by the white dotted line and the area of the ventricles (defined by the black dotted line). Both areas were assessed on the same single section at the level of Monro foramina.
Neurodevelopmental testing
Developmental tests were performed at 12, 24 months corrected age, and at 36 months of chronological age by a single trained examiner, according to our clinical protocol. The Griffiths rating scale26 was used up to 2014, subsequently the Bayley Scale III27 was used, as for local protocol. In order to compare the Bayley data with the Griffiths data, the composite scores of each scale were transformed into a development quotient, converting the raw score into an equivalent mental age, applying the following formula: equivalent mental age/corrected age at the time of the test × 100, as previously described.28
All analyses were performed using GraphPad Prism 8. FGR cases and appropriate for gestational age (AGA) controls were compared using Fisher exact test for categorical variables and Student’s t tests for continuous variables. Statistical significance was considered as p < 0.05. The correlation between TMS, brain area dimensions, and neurological outcome was analyzed with the Spearman correlation coefficient. A multiple linear regression model was used to evaluate whether parenchyma area was an independent predictor of cognitive score after adjusting for sex, gestational age, and neonatal weight.
As part of the radiological evaluation of the images, an evaluation of the inter-operator agreement was carried out using the intraclass correlation coefficient (ICC) through Mangold ICC Calculator
Results
Characteristics of the population
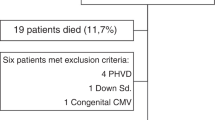
During the study period, a total of 84 newborns (36 AGA and 48 FGR) underwent cerebral MRI at TEA. Twelve cases of overt injuries at MRI were excluded: most of these infants were affected by PVL, which included also minor “punctate” form of PVL, with a significant prevalence among AGA newborns (8/36—22% compared to 1/48—2% of FGR, p = 0.004; with 2 cases of cerebral palsy both belonging to the AGA group). Therefore, 27 AGA and 45 FGR infants were eligible for the study.
Baseline demographic and perinatal characteristics of both groups are presented in Table 1. No significant differences were found for gestational age at birth and postmenstrual age at MRI. FGR neonatal weights and HCs were significantly lower than those of AGA controls. Sex distribution was significantly different between the two groups, showing a prevalence of males in AGA and females in FGR infants.
Neonatal management and infant morbidities are presented in Table 2, without any significant differences between the groups, except for less necrotizing enterocolitis requiring surgical treatment and more patent ductus arteriosus rate among FGR infants.
Flow velocity waveforms were recorded in all FGR fetuses within 48 h before delivery and two subgroups were identified based on UA end diastolic flow (EDF) (21 fetuses with present EDF and 24 with ARED, Supplemental Material). Birth weight was not significantly different between fetuses with present EDF and those with ARED (934 ± 207 versus 864 ± 210 g, respectively, p = 0.285). Indications for delivery in cases with present EDF was mainly maternal, first of all complicated preeclampsia (16/21—76%).
Neuroimaging
No significant differences were found either for TMS (13.60 ± 1.4 versus 13.56 ± 1.1, p = 0.88) or for any single parameter between preterm FGR and AGA children (Fig. 2). No correlation was found between TMS and gestational age (r = 0.19, p = 0.10) or birth weight (r = 0.21, p = 0.08). Even considering only FGR, the scores were similar for the UA-ARED and UA-EDF present subgroups.
Regarding area determination, ICC values were indicative of good inter-operator reliability from 0.72 for ventricles to 0.99 for cerebral parenchyma areas. The absolute dimensions of the inner calvarium and the parenchyma were significantly smaller in FGR cases as shown in Fig. 3, while no significant differences were found for areas of the cerebellar hemispheres, the cerebellar vermis, and the lateral ventricles areas. A correlation was found between parenchyma area and both cortical score (r = 0.267, p = 0.024), birth weight (r = 0.669, p < 0.0001), and HC (r = 0.563, p < 0.0001). Considering only FGR, no differences were found between the UA-ARED and UA-EDF present cases.
Neurodevelopmental outcomes
Twelve of the 45 (27%) FGR infants and 11 of the 27 (41%) AGA controls were lost at neurological follow-up at median age of 2 years. Table 3 presents the median scores of each item of Griffiths and Bayley showing no significant differences between the groups. Among all subjects in our cohort, only 3 (1.5%) infants demonstrated significant impairment with scores <70, while 13 (6.5%) had mild impairment with scores between 70 and 85. Gross motor was the main affected item.
Moreover, considering FGR, we found that the subgroup with UA-ARED had significantly worse gross motor scores than the UA-EDF present subgroup (90.2 ± 9.6 versus 100.7 ± 9.3, p = 0.005) as shown in Fig. 4.
Combining MRI data from the cerebral areas to neurodevelopmental outcome, we found a positive correlation between the parenchyma area and the cognitive score for all subjects in our population (r = 0.333, p = 0.019) and specifically in FGR children (r = 0.443, p = 0.0097) when analysis was performed separately in the two study groups (Fig. 5). Although there is an obvious correlation between parenchyma area and HC (r = 0.555, p = 0.0003), the correlation between HC and cognitive score does not reach significance (r = 0.302, p = 0.065).
No correlation was seen between any of the neurodevelopmental items at a median age of 2 years and both TMS (r = 0.10, p = 0.47) and birth weight (r = 0.26, p = 0.067), while there was a weakly significant correlation between median cognitive score and gestational age (r = 0.32, p = 0.024).
After adjusting for sex, gestational age, and weight at birth, multivariate analysis confirmed the associations between decreased parenchyma areas and worse cognitive scores at a median age of 2 years (p = 0.0014).
Discussion
Our results show a significant dimension discrepancy of the cerebral parenchyma between early preterm FGR and AGA children studied by MRI at TEA and a significant correlation between smaller parenchyma 2D areas and lower cognitive scores at a median age of 2 years for all infants and specifically for those affected by FGR.
However, the correlation between smaller HC and worse cognitive outcome did not reach statistical significance, although it was shown in previous studies.10,12 Indeed, brain parenchyma area measurement is very likely to be more consistent with real brain trophic status of the cerebrum than the simple HC value, since the simple circumference is largely independent from the effect linked to possible cerebrospinal fluid space enlargement, which can be a confounding factor that masks a real brain growth deficit. Previous studies, with different MRI approaches, have reported abnormalities in cortical lobe dimensions and a reduced white and gray matter volume in FGR infants in comparison with AGA controls.14,16,17 More recently, Bruno et al. have shown that, while thalamus and basal ganglia volumes were reduced in growth-restricted preterm infants after adjusting for confounding variables, cerebellar volumes were not affected by FGR.29 These findings are consistent with our results of no differences in cerebellar hemispheres and vermis areas between the FGR and AGA groups. At TEA, Dubois et al. found a correlation between a reduction in cortical volume, surface, and thickness and the functional development among FGR children.18 Considering a small cohort of preterm FGR infants defined by abnormal antenatal Doppler measurements, also Tolsa et al. have reported a correlation between cortical gray matter volumes at term and attention–interaction capacity.14 However, at 12 months corrected age, Padilla et al. failed to register any relationship between global brain volume and developmental outcome but found positive correlations between regional gray matter volumes and motor and adaptive behavior within the FGR group.16
We carried out a study relying on simple clinical handling image analysis in a larger sample size and we could demonstrate an original correlation between parenchyma area and cognitive scores at a median age of 2 years in FGR infants. These results concur with previous studies to underline combined structural and functional consequences on cerebral cortical development caused by FGR in preterm infants.
However, this study failed to demonstrate any impact of FGR on cerebral maturation using TMS and any difference in overall neurodevelopmental outcome between preterm FGR and AGA in the first 2 years of life. The absence of differences in TMS parameters between preterm FGR and AGA children was also previously reported in the study by Ramenghi et al.25 However, although a mild reduced myelination was reported in that study in FGR newborns affected by in utero brainsparing, we did not observe any maturation deficit also comparing FGR cases based on their UA Doppler velocimetry. This difference may be due to the different gestational age selection criteria of the population in the two studies.
Concerning neurodevelopmental outcomes, we did not find any difference in neurodevelopmental outcome between the FGR and AGA groups. Our results must be discussed in the light of the strict selection criteria chosen in our study design, with the exclusion of infants with even minor MRI findings of focal or diffuse brain lesions. We therefore selected a population with overall expected better neurodevelopmental outcomes. In fact, in both study groups we reported only few cases of mild or severe neurological impairment. In particular, concerning the FGR cohort, the rate of neurological sequelae was less than previously reported by the GRIT and TRUFFLE studies (6.5 and 9.7% of assessed children, respectively).30
In 11 of the 16 papers included in a recent systematic review,31 FGR was associated with poorer scores in the first 3 years of life. Most of these studies found motor, cognitive, or language delays but only half of them included abnormal Doppler parameters in their definitions of FGR. Some developmental studies, however, failed to show clear differences between FGR and AGA children when the control group was composed by early preterm newborns.32,33 Other studies reported a non-significant trend to lower scores at Bayley evaluation in FGR infants compared to preterm AGA but a significant worse performance, mainly for motor scores, in comparison with term AGA subjects.16,18
We hypothesize that in our study the absence of overall differences in neurodevelopmental outcome can be partly explained by the fact that the subjects in the control group were all born preterm and were per se at risk for neurological impairment. Another potential explanation could be the age of clinical assessment that may have been too low to detect possible neurodevelopmental impairment.
Nonetheless, Doppler waveforms analysis added some interesting results to overall findings and allowed us to find a significant deficit in gross motor function in the subgroup of FGR children with ARED. These data are consistent with previous studies and suggest that more severe FGR may show worst neurodevelopmental impairment. Our study has several limitations: it has a retrospective design and no power calculation analysis was performed. The sample size was limited and several infants were lost at follow-up. Due to the small sample size, our analysis was not adjusted for confounder factors such as perinatal complications and maternal education that can affect cognitive outcome. Moreover, two different neurodevelopmental tests were combined to uniform follow-up evaluation during the study period. Although the use of a simple method of 2D area measurement by hand drawing and on a single slice of 3 mm thickness can be considered a limitation of our protocol, the approach we took can be easily applied to any single case in clinical routine time scale. We cannot totally exclude having missed some very small T1-weighted hyperintense punctate white matter lesions since we used 3-mm-thick 2D sections instead of 3D ones. However, such probability is likely to be very low since brain parenchyma was scrutinized on both axial and sagittal T1-weighted sections acquired and with the intrinsic good signal/noise ratio of a neonatal dedicated head-coil.
Among strengths, we underline that Doppler parameters were recorded for each FGR case, providing a stratification of severity within the FGR infants. The lack of clinically relevant differences in infant management and morbidities, in particular for the need of invasive mechanical ventilation, reduced potential confounding factors in evaluating neurodevelopmental outcomes.
In conclusion, the correlation between parenchyma area quantification and cognitive scores highlighted the link between structure and function in the developing human brain. Even in the absence of overt brain lesions, an early MRI evaluation that includes a clinically feasible structural dimensional analysis may help identifying children who are at higher risk of neurodevelopmental impairment to address interventions to improve long-term outcomes. Moreover, attention must be focused on the FGR subgroup with fetal Doppler alterations, whose early worse motor outcomes were reported. Further prospective imaging and follow-up combined studies will help to investigate this issue.
References
Gordijin, S. J. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 48, 333–339 (2016).
Cetin, I. & Alvino, G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30, 77–82 (2009).
Cetin, I. et al. Fetal oxygen and glucose consumption in human pregnancy complicated by fetal growth restriction. Hypertension 75, 748–754 (2020).
Ferrazzi, E. et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet. Gynecol. 19, 140–146 (2002).
Pardi, G. et al. Diagnostic value of blood sampling in fetuses with growth retardation. N. Engl. J. Med. 328, 692–696 (1993).
Figueras, F. & Gratacos, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 36, 86–98 (2014).
Figueras, F. et al. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet. Gynecol. 38, 288–294 (2011).
Scherjon, S., Briet, J., Oosting, H. & Kok, J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic sign of fetal brain sparing. Pediatrics 105, 385–391 (2000).
Baschat, A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 37, 501–514 (2011).
Bashat, A. A., Viscardi, R. M., Hussey-Gardner, B., Hashmi, N. & Harman, C. Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet. Gynecol. 33, 44–50 (2009).
Morsing, E., Asard, M., Ley, D., Stjernqvist, K. & Marsàl, K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics 127, e874–e882 (2011).
Gale, C. R., O’Callaghan, F. J., Bredow, M. & Martyn, C. N. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 118, 1486–1492 (2006).
Malhotra, A. et al. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr. Res. 82, 184–193 (2017).
Tolsa, C. B. et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr. Res. 56, 132–138 (2004).
Lodygensky, G. A. et al. Intrauterine growth restriction affects the preterm infant’s hippocampus. Pediatr. Res. 63, 438–443 (2008).
Padilla, N. et al. Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res. 1382, 98–108 (2011).
Padilla, N. et al. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 1545, 1–11 (2014).
Dubois, J. et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131, 2028–2041 (2008).
Peterson, B. S. et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111, 939–948 (2003).
Batalle, D. et al. Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage 60, 1352–1366 (2012).
Fischi-Gomez, E. et al. Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cereb. Cortex 25, 2793–2805 (2014).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR Imaging assessment tool to define brain abnormalities in very preterm infants at term. Am. J. Neuroradiol. 34, 2208–2214 (2013).
Childs, A. M. et al. Cerebral maturation in premature infants: quantitative assessment using MR imaging. Am. J. Neuroradiol. 22, 1577–1582 (2001).
Ramenghi, L. A. et al. Magnetic resonance imaging assessment of brain maturation in preterm neonates with punctate white matter lesions. Neuroradiology 49, 161–167 (2007).
Ramenghi, L. A. et al. Cerebral maturation in IUGR and appropriate for gestational age preterm babies. Reprod. Sci. 18, 469–475 (2011).
Griffiths, R. The Abilities of Young Children (The Test Agency, High Wycombe, 1970).
Bayley, N. Bayley Scales of Infant and Toddler Development (The Psychological Corporation, San Antonio, TX, 2006).
Milne, S. L. et al. Alternate scoring of the Bayley-III improves prediction of performance on Griffiths Mental Development Scales before school entry in preschoolers with developmental concerns. Child. Care Health Dev. 41, 203–212 (2014).
Bruno, C. J. et al. MRI differences associated with intrauterine growth restriction in preterm infants. Neonatology 111, 317–323 (2017).
Ganzevoort, W. et al. Comparative analysis of the 2-year outcomes in the GRIT and TRUFFLE trials. Ultrasound Obstet. Gynecol. 55, 68–74 (2020).
Levine, T. A. et al. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics 135, 126–141 (2015).
Padilla, N. et al. Twelve-month neurodevelopmental outcome in preterm infants with and without intrauterine growth restriction. Acta Paediatr. 99, 1498–1503 (2010).
Procianoy, R. S., Koch, M. S. & Silveira, R. C. Neurodevelopmental outcome of appropriate and small for gestational age very low birth weight infants. J. Child Neurol. 24, 788–79 (2009).
Acknowledgements
We would like to thank all the hospital staff and the patients who agreed to participate making this study possible. No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
G.B., E.C., F.M.C., E.T., M.B., M.D.S., C.C., and E.L. gave substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data. G.B., I.C., A.R., G.L., and B.S. contributed to draft the article and to revise it critically for important intellectual content. I.C. gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of patient consent
Appropriate patient consent was obtained for the publication of this manuscript by G.B.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brembilla, G., Righini, A., Scelsa, B. et al. Neuroimaging and neurodevelopmental outcome after early fetal growth restriction: NEUROPROJECT—FGR. Pediatr Res 90, 869–875 (2021). https://doi.org/10.1038/s41390-020-01333-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01333-1
This article is cited by
-
Early Fetal Growth Restriction with or Without Hypertensive Disorders: a Clinical Overview
Reproductive Sciences (2024)