Abstract
Background
Fetal growth restriction (FGR) is a risk factor for neurodevelopmental problems, yet remains poorly understood. We sought to examine the relationship between intrauterine development and neonatal neurobehavior in pregnancies diagnosed with antenatal FGR.
Methods
We recruited women with singleton pregnancies diagnosed with FGR and measured placental and fetal brain volumes using MRI. NICU Network Neurobehavioral Scale (NNNS) assessments were performed at term equivalent age. Associations between intrauterine volumes and neurobehavioral outcomes were assessed using generalized estimating equation models.
Results
We enrolled 44 women diagnosed with FGR who underwent fetal MRI and 28 infants underwent NNNS assessments. Placental volumes were associated with increased self-regulation and decreased excitability; total brain, brainstem, cortical and subcortical gray matter (SCGM) volumes were positively associated with higher self-regulation; SCGM also was positively associated with higher quality of movement; increasing cerebellar volumes were positively associated with attention, decreased lethargy, non-optimal reflexes and need for special handling; brainstem volumes also were associated with decreased lethargy and non-optimal reflexes; cerebral and cortical white matter volumes were positively associated with hypotonicity.
Conclusion
Disrupted intrauterine growth in pregnancies complicated by antenatally diagnosed FGR is associated with altered neonatal neurobehavior. Further work to determine long-term neurodevelopmental impacts is warranted.
Impact
-
Fetal growth restriction is a risk factor for adverse neurodevelopment, but remains difficult to accurately identify.
-
Intrauterine brain volumes are associated with infant neurobehavior.
-
The antenatal diagnosis of fetal growth restriction is a risk factor for abnormal infant neurobehavior.
Similar content being viewed by others
Introduction
Fetal growth restriction (FGR) increases the risk of perinatal mortality and morbidity, with subsequent long-term neurodevelopmental deficits.1 Occurring in up to 10% of pregnancies, FGR is the second leading cause of perinatal mortality, accounts for 30% of stillborn infants and is the most common cause of premature births and intrapartum asphyxia.2 FGR is a multifactorial syndrome resulting in the fetus not reaching its intrauterine, biological growth and developmental potential. This occurs due to divergence from the normal fetal growth patterns determined through genetic growth potential, along with fetal, placental, and maternal health factors.1,2 The resulting suboptimal brain development that is associated with FGR increases the risk of adverse neurodevelopmental outcomes in infancy, which can subsequently extend into adolescence and adulthood.3,4 The neurological morbidities are broadly categorized by cognitive impairment, behavioral dysfunction, and motor deficits.1,5 Recent literature has consistently shown that school aged children diagnosed with FGR in infancy have diminished memory, academic ability, and overall, a significant reduction in IQ compared to appropriate for gestational age (AGA) peers.1,2,6 Notable neuropsychological dysfunctions associated with FGR include poor attention, hyperactivity, and altered mood.1,6,7,8,9 In addition, there is a 30-fold greater risk of cerebral palsy in FGR infants, and an increased incidence of global fine and gross motor delays.10
Despite the significant mortality and morbidity, the accurate identification of FGR in utero remains difficult.11,12,13,14 Common metrics, such as fetal weight or birth weights falling below the 10th centile for gestational age (small for gestational age, or SGA), provide objective criteria but fail to identify pathologic growth trajectories above the predefined thresholds.15 It is becoming increasingly recognized that alternate measures of placental dysfunction and pathologic fetal growth, even for infants with birth weights appropriate for gestational age (AGA), are associated with adverse pregnancy outcomes.16,17,18,19 These studies highlight the need for more sensitive and specific measures of fetal compromise. We have previously reported the in vivo association between placenta volume and fetal brain volume using quantitative MRI.20 These findings point towards a promising measure for early identification of pathologic growth and an improved understanding of the immediate intrauterine impact on neurodevelopment. However, the relationship between specific in utero volumetric brain growth and short-term neurobehavioral outcomes has not been well established. In this study, we sought to examine the relationship between in utero fetal brain and placental volumes and neonatal neurobehavior in pregnancies complicated by FGR.
Methods
Subjects
Subjects were recruited prospectively into a longitudinal, observational study on placental-fetal development in pregnancies complicated by fetal growth restriction (FGR). MRI was performed between 18 and 39 weeks’ gestation and neonatal neurobehavioral assessments were performed before 44 weeks corrected gestation. The study was approved by the institutional review board of the Children’s National Hospital and written informed consent was obtained from all subjects.
Women with pregnancies complicated by FGR were recruited from regional Maternal-Fetal Medicine practices if the following criteria were met: singleton pregnancy with estimated fetal weight <10th percentile21 and either (A) abnormal Doppler sonography of the umbilical and/or middle cerebral arteries, specifically an umbilical artery pulsatility index >95% or cerebroplacental ratio <1 or (B) evidence of impaired somatic growth where abdominal circumference lagged head circumference >1 week for expected gestational age (GA).22,23 Exclusion criteria included multiple-gestation pregnancy, known or suspected congenital infection, dysmorphic features of the fetus, documented chromosomal abnormalities, uncertain dates or maternal contraindication to MRI. Enrolled subjects found to have dysmorphic structural abnormalities on fetal MRI or postnatal confirmation of a genetic syndrome were subsequently excluded from the analysis.
Demographic and clinical data
GA was calculated based on first-trimester ultrasound measurement or last menstrual period if unavailable; women with uncertain pregnancy dates were excluded. Clinical and demographic data were collected for each subject through medical chart review. Anthropomorphic measures including birthweight, length and head circumference were corrected for GA using the Fenton growth chart calculations.24 Infants with birth weights <10th centile for gestational age were categorized as small for gestational age (SGA), and those with birth weights 10–90th centile were categorized as appropriate for gestational age (AGA) using the Fenton growth chart for weight and sex.25
Fetal MRI
All MRI scans were performed on a 1.5T Discovery MR450 scanner (GE Healthcare, Milwaukee, Wisconsin) using an 8-channel surface receive coil (USAI, Aurora, OH). Single shot fast spin echo (SSFSE) T2-weighted images were performed as follows: for the fetal brain, TE 160 ms, TR 1100 ms, FOV 320 × 320 mm, 2 mm slice thickness and 40–60 consecutive slices for full brain coverage in all three orthogonal plans (axial, coronal, sagittal); for the placenta, fat suppressed with TE 160 ms, TR 1100 ms, FOV 420 × 420 mm, 4 mm slice thickness and 40–60 consecutive slices for full placental coverage in the axial plane.20 No contrast or sedation was used for any of the imaging studies.
Volumetric MRI analysis
Volumetric analysis of the placenta and fetal brain have been previously described.20,26,27,28 In brief, the placenta was manually outlined using ITK-SNAP software,29 while the fetal brain was reconstructed and segmented using a semi-automated approach to include motion correction30,31 and each automated segmentation was visually inspected and manually corrected by a trained expert. Volumes were reported in cm3; cerebral, cerebellar and brainstem volumes were individually calculated, and total brain volume was defined as the sum of the previous three volumes. Placental and regional brain volumes, including cortical gray matter (CGM,) cortical white matter (CWM) and subcortical gray matter (SGCM) were individually calculated.
NICU Network Neurobehavioral Scale (NNNS) assessments
The NICU Networks Neurobehavioral Scale (NNNS) is a quantitative assessment of infant neurobehavior, composed of neurologic, behavioral functioning, stress and abstinence evaluations.32,33,34 The NNNS is a widely used tool that was developed to study both healthy and high-risk infants, including infants born premature, low birthweight, exposed to prenatal stress and substance use, or perinatal injury.32,35,36,37,38 The NNNS includes 128 items that can be summarized into 13 domain summary scores: habituation, attention, arousal, self-regulation, special handling needed from the examiner, quality of movement, excitability, lethargy, non-optimal reflexes, asymmetric reflexes, hypertonicity, hypotonicity and stress/abstinence.34 All assessments were completed by a certified, trained examiner, and summary scores derived for each subject.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or frequency and percent. Our analyses consisted of the following steps. In step one, we assessed the relationship between each NNNS domain and birthweight. Birthweight was classified into SGA or AGA (outlined above) and treated as a binary parameter with AGA infants serving as the referent group. In step two, for each NNNS domain, scores were converted to z-scores using prior work published by Fink et al. to serve as the referent population.39 The developers note that given the directionality of the scales, scores at each extreme are reflective of either an excessively amplified or diminished response.39 As such, we considered z-scores ±2 SD as abnormal, and z-scores ±1.5 SD as at risk, based on common thresholds of referral for early intervention services.39,40,41 The prevalence of abnormality between groups was then compared using Fisher’s exact test. In step three, we used separate generalized mixed models to assess the relationship between each NNNS domain and fetal brain/placental volumes; all models were adjusted for gestational age at time of scan and fetal sex. Lastly, we adjusted for multiple comparisons using the false discovery rate method based on the number of outcomes. All analyses were conducted using SAS (ver. 9.4, Cary, NC) with statistical significance considered for p ≤ 0.05, two-tailed.
Results
Characteristics of our cohort
Forty-four women with pregnancies complicated by FGR were enrolled in this study. Of these, 10 (22%) were lost to follow-up, 6 (13%) died prior to NNNS examination and therefore, 28 infants were included in this analysis. Infants lost to follow-up, were generally similar to the cohort presented based on available medical record data; 4 (40%) were born SGA, 2 (20%) were born preterm, with an overall GA at birth of 38.1 ± 1.7 weeks, mean BW 2697 ± 478 g (BW percentile of 13.5 ± 10.14%). For the remaining infants included in this analysis, fetal MRI studies were performed at a median gestational age (GA) of 32.32 ± 4.71 weeks (range: 18–37 completed weeks). Mean GA at birth was 36.27 ± 3.93 weeks with birthweight (BW) of 2149 ± 761 g. Nearly half of all subjects were born small for gestational age (SGA, 47%). Additional demographic data are presented in Table 1.
NNNS scores
Mean NNNS summary scores for the cohort are found in Table 2. Twenty-eight infants had complete NNNS scores; of these only 4 (4%) infants were in the correct state to assess habituation and 21 (75%) infants were in the correct state to assess attention; similarly only 9 (32%) infants had asymmetric reflexes noted. Given low rates for habituation and asymmetric reflexes, these were excluded from subsequent analyses. Summary scores were similar between SGA and AGA infants, although SGA infants were noted to have higher arousal scores compared to AGA infants, and also required more special handling to complete the exam (Table 2).
To characterize the number of infants with abnormal neurobehavior, individual infant summary scores were then compared to published norms and scored at-risk if they fell above or below 1.5 SD of the mean and abnormal if they fell above or below 2 SD of the mean.39 Of note, published norms are only available for 12 domains, as too few healthy infants demonstrated signs of hypertonicity in the original report. Therefore, in this work, we only report at-risk infants if the hypertonicity summary score was ≥1 (Table 3). Compared to published norms, FGR infants demonstrate at risk scores in 9 of the 12 domains and abnormal scores in 8 of the 12 domains. Nearly half of neonates scored at-risk in quality of movement and stress-abstinence domains, and a third of neonates were within the abnormal range for quality of movement. While we did not detect significant differences between SGA and AGA infants, it is important to note, nearly a third of antenatally diagnosed FGR infants born AGA also demonstrated abnormal scores for quality of movement, 15% had abnormal scores for excitability and 8% for abnormal self-regulation.
Relationship between placental and fetal brain volumes with NNNS scores
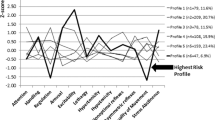
Placental volume, fetal total brain, brainstem, CGM and SGCM volumes were positively associated with higher self-regulation scores (placenta: β = 0.004, p < 0.01; total brain: β = 0.013, p = 0.035; brainstem: β = 0.892, p < 0.01; CGM β = 0.029, p = 0.019; SGCM β = 0.183, p = 0.026); of these the association between brainstem volume and self-regulation was maintained after adjusting for multiple comparisons. SGCM was also associated with improved quality of movement (β = 0.105, p = 0.043). Increasing fetal cerebellar volumes were associated with increased attention (β = 0.396, p = 0.007), decreased need for handling (β = –0.065, p = 0.006), less lethargy (β = –0.321, p = 0.031) and less non-optimal reflexes (β = –0.671, p = 0.001). Greater fetal brainstem volumes also were associated with decreased lethargy (β = –1.250, p = 0.046, and less non-optimal reflexes (β = –1.627, p = 0.044). Cerebral volumes and CWM volumes were positively associated with hypotonicity (cerebrum: β = 0.012, p = 0.012; and CWM: β = 0.023, p = 0.003). Lastly, increasingly placental volume also was associated with less excitability (β = –0.010, p = 0.014) (see Fig. 1 and Tables 4 and 5).
Associations between in utero a placental, b total brain, c cerebellar, d brainstem, e cerebral, f cortical gray matter, g cortical white matter, and h subcortical gray matter volumes with individual NNNS domains. Analyses are adjusted for gestational age at fetal MRI and sex. Lines bars with asterisk denote significant associations with p < 0.05.
Discussion
Summary of findings
In this exploratory analysis, we relate in utero fetal brain and placental development with neonatal neurobehavior in a cohort of pregnancies complicated by FGR. First, we note that a significant number of subjects identified as FGR were born AGA, highlighting the challenges in the accurate diagnosis of pathologic fetal growth. Second, a significant proportion of infants had altered neonatal neurobehavior across multiple domains of the NNNS assessment, and this was true for antenatally diagnosed FGR infants born both SGA and AGA. Lastly, we found several associations of intrauterine growth with neonatal behavior. Specifically, we found that increasing intrauterine volumes of the placenta and both global and regional brain volumes were associated with increased self-regulation, attention and quality of movement along with decreased excitability, lethargy, non-optimal reflexes and need for special handling in neonates. Previously, we have shown that intrauterine placental volumes were positively associated with total brain, cerebral and cerebellar volumes;20 as improved placental growth supports improved brain growth, the current data suggest improved brain growth is associated with improved neonatal neurobehavior. We also noted an association between fetal cerebral and CWM volumes and hypotonicity; however the significance of this finding is limited, given that overall hypotonicity scores remained low, and all hypotonicity scores fell within normal reference ranges.
NNNS in high-risk infants
The NNNS assessment is a standardized and validated tool for the prediction of motor, cognitive and behavioral outcomes for high-risk infants that can be performed in infants between 32 and 48 weeks corrected gestational age.32,42 In addition, there are well-established normative data that can serve as reference data from over 300 healthy, term neonates.39 The NNNS assessment has also been applied widely to identify and describe a range of neurobehavioral abnormalities across multiple conditions in both high- and low-risk populations.43,44 Despite the need to relate intrauterine growth and exposures with long-term outcomes, there is an increasing body of literature that shows the NNNS assessment can be predictive of long-term medical and developmental outcomes,43,44,45,46,47 and thus serves as a useful neonatal biomarker of later development. Specifically, Liu et al. demonstrated that infants with low self-regulation, attention and quality of movement along with high excitability, hypertonicity and more special handling in the neonatal period were more likely to exhibit low performance on the Bayley Scales of Infant Development, decreased school readiness with lower child IQ and more behavior problems from infancy through age four.44 These are key domains that we show are directly associated with fetal brain volumes. By identifying deviations of typical brain growth patterns in utero, we can better explore the onset and duration of placental insufficiency and subsequent FGR, and the relationship with regional vulnerability of the developing brain.
Challenges in the accurate identification of fetal growth restriction
By definition, FGR is a pathologic condition in which the inability to achieve target growth disrupts normal development;11 however, the clinical identification and diagnosis of FGR remains difficult. Clinically, FGR is often defined when fetal size falls below a predefined threshold, typically the 10th centile for growth for a given gestational age (GA).48 As a result, both FGR and small for gestational age (SGA) infants are risk factors for adverse neurobehavioral outcomes.5,49,50,51 Despite the practical application of using growth cutoffs to identify FGR, this approach remains limited in that it may (a) misclassify SGA fetuses that are constitutionally small but healthy or (b) fail to identify infants above the 10th centile but still below their target growth potential. Relatedly, fetuses that may drop below the percentile criterion and then recover, may still suffer neurodevelopmental consequences from transient nutrient restriction, while limitations in fetal weight estimates may mis-identify SGA fetuses that in fact are developing above the 10th centile. In this cohort, a significant number of infants identified antenatally as FGR were born AGA, and may reflect the known limitation in the accurate measures of fetal growth.52,53 However, current tools remain insufficient to distinguish between limitations in accurate measures of fetal growth and pathologic growth. The significant rates of abnormal neurobehavior detected in the subgroup of AGA infants presented here suggest more precise biomarkers of neurodevelopment are needed that will be more sensitive and specific to identify pathologic fetal growth. In this work, AGA infants diagnosed with antenatal FGR demonstrated abnormal scores for quality of movement and excitability, domains associated with intrauterine volumes of SCGM and the placenta, respectively, as well as self-regulation, which was associated with fetal total brain, cerebral, brainstem, CGM and SCGM volumes.
Fetal brain structure and neurodevelopment
Several studies have reported on abnormal brain structure in infant survivors of FGR and SGA, including the particular vulnerability of the cerebellum.20,54,55,56,57,58,59,60 However, less is known about fetal brain structure and neonatal neurobehavioral outcomes. One study of SGA fetuses evaluated with MRI at 37 weeks’ gestation underwent neonatal neurobehavior assessment with the Neonatal Behavioral Assessment Scale (NBAS).61 The authors reported that cerebellar volume was greater in SGA infants compared to AGA matched controls, and cerebellar volume was associated with neonatal motor scores.61 While this study noted increased cerebellar volume, most fetal studies of brain volume have noted smaller volumes of the cerebellum both by neuro-sonography and MRI;20,60 these differences may reflect differences in the populations studied, the onset and timing of growth restriction, as well as the window studied (37 weeks compared to wider gestational windows). We also found that cerebellar volume was associated with four key NNNS domains, more than any other measured region of the brain. Specifically, we found that fetal cerebellar volume was associated with increased attention, decreased need for special handling (indicating that less input is needed from the examiner to elicit visual and auditory responses from the infant,) decreased lethargy and non-optimal reflexes. This is consistent with emerging work that the developing cerebellum plays a key role in movement, cognition and socio-behavioral function.62 Egana-Ugrinovic et al. also reported decreased insular morphometry in SGA fetuses, which in turn was associated with infant state organization/regulation, autonomic nervous system function, attention and social-interactive functions63 and decreased corpus callosal area that was associated with abnormal NBAS clusters.64 We note that several fetal brain structures, including the cerebrum, and specifically CGM and SCGM, as well as overall brain and brainstem volumes were positively associated with neonatal self-regulation. This work further explored fetal brain volumes across a much wider window of assessment, which will be needed to identify the onset and timing of growth failure in relation to early neurodevelopment.
Placental development and neurodevelopment
While placental insufficiency is a leading cause of fetal growth restriction, there remain significant gaps in understanding the pathophysiology of placental failure and its effect on early brain development.65 Placental disease can result in nutrient restriction, chronic hypoxia, hypoperfusion and inflammation, disrupted neuroendocrine functions, as well as epigenetic placental changes, which in turn, can adversely influence early brain development.51,66,67,68 Animal models of uteroplacental insufficiency have demonstrated both structural changes in brain development as well as neurodevelopmental outcomes,69 while molecular studies have identified several key neurotrophins and neurosteroids that can influence placental development and neurodevelopmental outcomes.70,71,72,73 In vivo studies rely primarily on Doppler sonography to detect placental pathology. Clinical studies suggest that the primary advantage of these evaluations is in the reduction of perinatal death, with limited data on the specificity and sensitivity of these measures in predicting neurodevelopmental outcomes.74 Within very low birthweight preterm infants, abnormal fetal Doppler studies consistent with placental insufficiency were associated with adverse neurocognition, mediated in large part by decreased brain volumes.75 We have previously shown that in vivo placental volume was positively associated with fetal cerebral and cerebellar volumes.20 A recent study on placental allopregnanolone found that decreased levels of this neurosteroid led to cerebellar microstructural abnormalities and autistic like behavioral abnormalities in mice, further linking placental changes with brain development and behavior.73 In this work, we demonstrate that placental volume was positively associated with self-regulation, presumably through improved overall growth of several brain regions. Increasing placental weight was also associated with lower excitability, or lower levels of motor, state and physiologic reactivity.44 In a cohort of high-risk infants in the NICU, lower excitability was also found in infants of family-centered care and higher parental satisfaction. Further work to elucidate mechanisms of parental stress, placental growth, brain development and neurodevelopmental outcomes is warranted.
Strengths and limitations
The strengths of this work include the accuracy and rigor of quantitative fetal MRI with comprehensive neonatal neurobehavioral assessments. While there are controversies regarding optimal definition and identification of FGR, the classification scheme in this study was implemented to exclude fetuses with chromosomal abnormalities, genetic syndromes or intrauterine infections that can independently and adversely influence neurodevelopment. Despite these strengths, there are several limitations that deserve mention. First, there is no control group of otherwise healthy pregnancies with normal birth outcomes to measure fetal brain volumes and neurodevelopmental outcomes. It also worth noting that the infants lost to follow-up may have introduced bias into the sample. Similarly, given the dynamic nature of early brain development and neurobehavior, it is important to recognize that while we adjusted for gestational age at study timepoints, it is likely that there are specific periods of vulnerability in brain development. Prospective studies that include both healthy and high-risk pregnancies over discrete gestational ages are needed to identify optimal windows of both risk and subsequent intervention. Second, additional risk factors for growth restriction that may also confound with adverse neurodevelopment must be considered, including parental genetics, socio-economic status, stress and lifestyle factors, including smoking, substance or environmental toxin exposure. Third, while we identified several regions of fetal brain and placental volumes that were associated across multiple NNNS domains, there was no consistent pattern of fetal brain volume and neurodevelopmental outcomes. This may be due to other significant contributors of placental insufficiency, such as the timing or duration, that were unaccounted for in this analysis. Adjustments for multiple comparisons also narrowed the number of significant associations, highlighting the association between brainstem volume and neonatal self-regulation. Nonetheless, given the exploratory nature of this work, the described findings are consistent with known developmental and behavioral functions of key brain areas, and worth validating in larger populations. Similarly, further studies are warranted to determine if these differences in the newborn period persist throughout childhood. These critical long-term studies are currently underway.
Conclusions
In this work, we identify regional impairments in fetal brain and placental growth detected across the second half of pregnancy and report significant associations with altered neonatal neurobehavior in pregnancies complicated by the antenatal diagnosis of FGR. While only 45% of these pregnancies delivered SGA infants, rates of abnormal neurobehavior remained high for both SGA and AGA groups, highlighting the limitations in identifying high-risk groups, and the need for more precise measures of intrauterine neurodevelopment. Advances in the field also will require more precise measures of placental function, along with more specific detection methods of pathologic growth. Interestingly, the cerebellum was a key brain region associated with several neurobehavioral domains. This finding coincides with previous work identifying the cerebellum as one of the fastest and largest growing regions of the fetal brain in the second half of pregnancy, and thus one of the most vulnerable to disturbed antenatal growth. Further work to explore the onset and duration of placental insufficiency and subsequent FGR and the relationship with regional vulnerability of the developing brain is warranted.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Malhotra, A. et al. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. (Lausanne) 10, 55 (2019).
Nardozza, L. M. et al. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet. 295, 1061–1077 (2017).
Meher, S., Hernandez-Andrade, E., Basheer, S. N. & Lees, C. Impact of cerebral redistribution on neurodevelopmental outcome in small-for-gestational-age or growth-restricted babies: a systematic review. Ultrasound Obstet. Gynecol. 46, 398–404 (2015).
Monteith, C. et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am. J. Obstet. Gynecol. 221, 273 e271–273 e279 (2019).
Miller, S. L., Huppi, P. S. & Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 594, 807–823 (2016).
Leitner, Y. et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J. Child Neurol. 22, 580–587 (2007).
Geva, R., Eshel, R., Leitner, Y., Fattal-Valevski, A. & Harel, S. Memory functions of children born with asymmetric intrauterine growth restriction. Brain Res. 1117, 186–194 (2006).
Low, J. A. et al. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am. J. Obstet. Gynecol. 167, 1499–1505 (1992).
Fischi-Gomez, E. et al. Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cereb. Cortex 25, 2793–2805 (2015).
MacLennan, A. H., Thompson, S. C. & Gecz, J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am. J. Obstet. Gynecol. 213, 779–788 (2015).
Gordijn, S. J. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 48, 333–339 (2016).
Lindqvist, P. G. & Molin, J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet. Gynecol. 25, 258–264 (2005).
Sharma, D., Shastri, S. & Sharma, P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 10, 67–83 (2016).
Crovetto, F. et al. Differential performance of first-trimester screening in predicting small-for-gestational-age neonate or fetal growth restriction. Ultrasound Obstet. Gynecol. 49, 349–356 (2017).
McCowan, L. M., Figueras, F. & Anderson, N. H. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 218, S855–S868 (2018).
Kennedy, L. M. et al. Reduced growth velocity from the mid-trimester is associated with placental insufficiency in fetuses born at a normal birthweight. BMC Med. 18, 395 (2020).
Dieste Perez, P. et al. Reduced growth in non-small for gestational age fetuses from 35 weeks of gestation to birth and perinatal outcomes. Fetal Diagn. Ther. 48, 768–777 (2021).
Khalil, A. et al. Is cerebroplacental ratio a marker of impaired fetal growth velocity and adverse pregnancy outcome? Am. J. Obstet. Gynecol. 216, 606 e601–606 e610 (2017).
Hendrix, M. L. E. et al. Maternal vascular malformation in the placenta is an indicator for fetal growth restriction irrespective of neonatal birthweight. Placenta 87, 8–15 (2019).
Andescavage, N. et al. In vivo assessment of placental and brain volumes in growth-restricted fetuses with and without fetal Doppler changes using quantitative 3D MRI. J. Perinatol. 37, 1278–1284 (2017).
Hadlock, F. P., Deter, R. L., Harrist, R. B. & Park, S. K. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology 152, 497–501 (1984).
Poljak, B., Agarwal, U., Jackson, R., Alfirevic, Z. & Sharp, A. Diagnostic accuracy of individual antenatal tools for prediction of small-for-gestational age at birth. Ultrasound Obstet. Gynecol. 49, 493–499 (2017).
Stirnemann, J. et al. International estimated fetal weight standards of the intergrowth-21st project. Ultrasound Obstet. Gynecol. 49, 478–486 (2017).
Fenton, T. R. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 3, 13 (2003).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Andescavage, N. et al. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta 36, 1024–1030 (2015).
Andescavage, N. N. et al. Complex trajectories of brain development in the healthy human fetus. Cereb. Cortex 27, 5274–5283 (2017).
Andescavage, N. N. et al. Cerebrospinal fluid and parenchymal brain development and growth in the healthy fetus. Dev. Neurosci. 38, 420–429 (2016).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31, 1116–1128 (2006).
Tustison, N. J. et al. N4itk: improved N3 bias correction. IEEE Trans. Med. imaging 29, 1310–1320 (2010).
Serag, A. et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. NeuroImage 59, 2255–2265 (2012).
Lester, B. M., Andreozzi-Fontaine, L., Tronick, E. & Bigsby, R. Assessment and evaluation of the high risk neonate: the NICU Network Neurobehavioral Scale. J. Vis. Exp. 3368 (2014).
Lester, B. M. & Tronick, E. Z. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113, 634–640 (2004).
Lester, B. M., Tronick, E. Z. & Brazelton, T. B. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 113, 641–667 (2004).
Massaro, A. N. et al. Neonatal neurobehavior after therapeutic hypothermia for hypoxic ischemic encephalopathy. Early Hum. Dev. 91, 593–599 (2015).
Provenzi, L. et al. NICU Network Neurobehavioral Scale: 1-month normative data and variation from birth to 1 month. Pediatr. Res. 83, 1104–1109 (2018).
Tronick, E. Z. et al. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113, 676–678 (2004).
Coleman, M. B. et al. Neonatal neurobehavioral abnormalities and MRI brain injury in encephalopathic newborns treated with hypothermia. Early Hum. Dev. 89, 733–737 (2013).
Fink, N. S., Tronick, E., Olson, K. & Lester, B. Healthy newborns’ neurobehavior: norms and relations to medical and demographic factors. J. Pediatr. 161, 1073–1079 (2012).
Tronick, E. & Lester, B. M. Grandchild of the NBAS: The NICU Network Neurobehavioral Scale (NNNS): a review of the research using the NNNS. J. Child Adolesc. Psychiatr. Nurs. 26, 193–203 (2013).
The Program for Infants and Toddlers with Disabilities. https://ectacenter.org/partc/partc.asp.
de Souza Perrella, V. V., Marina Carvalho de Moraes, B., Sanudo, A. & Guinsburg, R. Neurobehavior of preterm infants from 32 to 48 weeks post-menstrual age. J. Perinatol. 39, 800–807 (2019).
Sucharew, H., Khoury, J. C., Xu, Y., Succop, P. & Yolton, K. NICU Network Neurobehavioral Scale profiles predict developmental outcomes in a low-risk sample. Paediatr. Perinat. Epidemiol. 26, 344–352 (2012).
Liu, J. et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics 125, e90–e98 (2010).
Wouldes, T. A. & Woodward, L. J. Neurobehavior of newborn infants exposed prenatally to methadone and identification of a neurobehavioral profile linked to poorer neurodevelopmental outcomes at age 24 months. PLoS One 15, e0240905 (2020).
Spittle, A. J. et al. Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Dev. Med. Child Neurol. 59, 207–215 (2017).
Meether, M., Bush, C. N., Richter, M. & Pineda, R. Neurobehaviour of very preterm infants at term equivalent age is related to early childhood outcomes. Acta Paediatr. 110, 1181–1188 (2021).
Fetal growth restriction: ACOG Practice Bulletin, Number 227. Obstet. Gynecol. 137, e16–e28 (2021).
de Bie, H. M., Oostrom, K. J. & Delemarre-van de Waal, H. A. Brain development, intelligence and cognitive outcome in children born small for gestational age. Horm. Res Paediatr. 73, 6–14 (2010).
Baschat, A. A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 37, 501–514 (2011).
Arcangeli, T., Thilaganathan, B., Hooper, R., Khan, K. S. & Bhide, A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet. Gynecol. 40, 267–275 (2012).
Dudley, N. J. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet. Gynecol. 25, 80–89 (2005).
Dudley, N. J. The management of error in ultrasound fetal growth monitoring. Ultrasound 29, 4–9 (2021).
Limperopoulos, C. et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121, 26–33 (2010).
Hansen, T., Henriksen, T. B., Bach, C. C. & Matthiesen, N. B. Congenital heart defects and measures of prenatal brain growth: a systematic review. Pediatr. Neurol. 72, 7–18.e11 (2017).
Husen, S. C. et al. Three-dimensional ultrasound imaging of fetal brain fissures in the growth restricted fetus. PLoS One 14, e0217538 (2019).
Egana-Ugrinovic, G., Sanz-Cortes, M., Figueras, F., Bargallo, N. & Gratacos, E. Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am. J. Obstet. Gynecol. 209, 126 e121–126 e128 (2013).
Businelli, C., de Wit, C., Visser, G. H. A. & Pistorius, L. R. Ultrasound evaluation of cortical brain development in fetuses with intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 28, 1302–1307 (2015).
Polat, A., Barlow, S., Ber, R., Achiron, R. & Katorza, E. Volumetric MRI study of the intrauterine growth restriction fetal brain. Eur. Radio. 27, 2110–2118 (2017).
Benavides-Serralde, A. et al. Three-dimensional sonographic calculation of the volume of intracranial structures in growth-restricted and appropriate-for-gestational age fetuses. Ultrasound Obstet. Gynecol. 33, 530–537 (2009).
Sanz-Cortes, M., Egana-Ugrinovic, G., Zupan, R., Figueras, F. & Gratacos, E. Brainstem and cerebellar differences and their association with neurobehavior in term small-for-gestational-age fetuses assessed by fetal MRI. Am. J. Obstet. Gynecol. 210, 452 e451–452 e458 (2014).
Limperopoulos, C. The vulnerable immature cerebellum. Semin. Fetal. Neonatal Med. 21, 293–294 (2016).
Egana-Ugrinovic, G., Sanz-Cortes, M., Figueras, F., Couve-Perez, C. & Gratacos, E. Fetal MRI insular cortical morphometry and its association with neurobehavior in late-onset small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 44, 322–329 (2014).
Egana-Ugrinovic, G., Sanz-Cortes, M., Couve-Perez, C., Figueras, F. & Gratacos, E. Corpus callosum differences assessed by fetal MRI in late-onset intrauterine growth restriction and its association with neurobehavior. Prenat. Diagn. 34, 843–849 (2014).
Sun, C. et al. The placenta in fetal growth restriction: what is going wrong? Placenta 96, 10–18 (2020).
Bangma, J. T., Hartwell, H., Santos, H. P. Jr, O’Shea, T. M. & Fry, R. C. Placental programming, perinatal inflammation, and neurodevelopment impairment among those born extremely preterm. Pediatr. Res. 89, 326–335 (2021).
Lester, B. M. & Marsit, C. J. Epigenetic mechanisms in the placenta related to infant neurodevelopment. Epigenomics 10, 321–333 (2018).
Marsit, C. J., Maccani, M. A., Padbury, J. F. & Lester, B. M. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One 7, e33794 (2012).
Pla, L. et al. Structural brain changes during the neonatal period in a rabbit model of intrauterine growth restriction. Dev. Neurosci. 42, 217–229 (2020).
Sahay, A., Kale, A. & Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 83, 102075 (2020).
Sahay, A. S., Sundrani, D. P. & Joshi, S. R. Neurotrophins: role in placental growth and development. Vitam. Horm. 104, 243–261 (2017).
Dhobale, M. Neurotrophins: role in adverse pregnancy outcome. Int. J. Developmental Neurosci. 37, 8–14 (2014).
Vacher, C. M. et al. Placental endocrine function shapes cerebellar development and social behavior. Nat. Neurosci. 24, 1392–1401 (2021).
Conde-Agudelo, A., Villar, J., Kennedy, S. H. & Papageorghiou, A. T. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 52, 430–441 (2018).
Leppanen, M. et al. Abnormal antenatal doppler velocimetry and cognitive outcome in very-low-birth-weight infants at 2 years of age. Ultrasound Obstet. Gynecol. 36, 178–185 (2010).
Funding
This work was supported by the National Institutes of Health (1U54HD090257, R01-HL116585, K23HD092585-01A1).
Author information
Authors and Affiliations
Contributions
N.A. contributed to conception, design, data acquisition and analysis, drafting the article and final approval of the version submitted. T.B. contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. M.L. contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. S.D.B. contributed to data analysis, critical review of the manuscript and final approval of the version submitted. A.K. contributed to data acquisition and critical review of the manuscript and final approval of the version submitted. K.K. contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. H.A. contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. G.V. contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. J.Q. contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. C. Lopez contributed to data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. A.d.P. contributed to study conception, data acquisition and analysis, critical review of the manuscript and final approval of the version submitted. C. Limperopoulos contributed to study conception and design, data acquisition and analysis, critical review of the manuscript and final approval of the version submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Written informed consent was obtained from all subjects enrolled in this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andescavage, N., Bullen, T., Liggett, M. et al. Impaired in vivo feto-placental development is associated with neonatal neurobehavioral outcomes. Pediatr Res 93, 1276–1284 (2023). https://doi.org/10.1038/s41390-022-02340-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02340-0
This article is cited by
-
Band aids for Medicaid: preserving the high numbers of child health coverage during the pandemic
Pediatric Research (2023)