Abstract
Gastrointestinal (GI) cancer is a formidable malignancy with significant morbidity and mortality rates. Recent studies have shed light on the complex interplay between the nervous system and the GI system, influencing various aspects of GI tumorigenesis, such as the malignance of cancer cells, the conformation of tumor microenvironment (TME), and the resistance to chemotherapies. The discussion in this review first focused on exploring the intricate details of the biological function of the nervous system in the development of the GI tract and the progression of tumors within it. Meanwhile, the cancer cell-originated feedback regulation on the nervous system is revealed to play a crucial role in the growth and development of nerve cells within tumor tissues. This interaction is vital for understanding the complex relationship between the nervous system and GI oncogenesis. Additionally, the study identified various components within the TME that possess a significant influence on the occurrence and progression of GI cancer, including microbiota, immune cells, and fibroblasts. Moreover, we highlighted the transformation relationship between non-neuronal cells and neuronal cells during GI cancer progression, inspiring the development of strategies for nervous system-guided anti-tumor drugs. By further elucidating the deep mechanism of various neuroregulatory signals and neuronal intervention, we underlined the potential of these targeted drugs translating into effective therapies for GI cancer treatment. In summary, this review provides an overview of the mechanisms of neuromodulation and explores potential therapeutic opportunities, providing insights into the understanding and management of GI cancers.
Similar content being viewed by others
Introduction
With the growing understanding of tumorigenesis, there has been an increasing focus on the role of the tumor microenvironment (TME) in this process. The TME consists of not only the tumor itself but also the intricate niche surrounding it. This niche includes nerve cells, surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and the extracellular matrix (ECM) [1]. Recent research has shed light on the influence of not only the immune microenvironment but also the neural microenvironment on the development and occurrence of various solid tumors. Studies have indicated an upsurge in nerve density in these tumors [2], and it has been observed that tumors with higher innervation tend to exhibit increased rates of invasion and metastasis [3]. Although the overall significance and mechanisms of tumor-related neuroplasticity are not yet fully comprehended, these discoveries suggest that the nervous system actively contributes to tumor development, thereby offering new avenues for the treatment of tumor development through the modulation of nerve-cancer cross-talk.
Gastrointestinal (GI) cancers are a group of cancers characterized by a high incidence and affecting various sites within the GI tract. Common types of GI cancers include esophageal cancer (ESCC), gastric cancer (GC), colorectal cancer (CRC), liver cancer (LC), and pancreatic cancer (PDAC) [4]. Projections for the year 2023 in the United States show that GI cancers are expected to have the highest number of new cases and estimated deaths (348,840 new cases and 172,010 deaths). These figures account for 17.81% and 28.21% of the total number of cancer cases, respectively. Among the mentioned cancers, CRC has the highest mortality rate and is relatively easier to diagnose. According to data from the American Cancer Society, CRC ranks third among both male and female cancer patients in the United States [5]. PDAC has the second highest fatality rate among GI cancers, with a 5-year survival rate of only 12% between 2012 and 2018. LC and ESCA also have low survival rates, at only 21% [5]. In comparison, GC and CRC have relatively higher 5-year relative survival rates, at 33% and 65%, respectively. However, GC exhibits significant geographic disparities, primarily affecting low- and middle-income countries, which may correlate with living standards. Reports suggest that in China, GC was the leading cause of cancer deaths until 2004, when it was replaced by lung cancer (ranking as the third leading cause in 2016). Although the overall mortality rate of GC is declining in China, the death rate among GC patients in rural areas remains higher than in urban areas [6].
Research has shown that gut microbiota dysbiosis is associated with the development of various GI cancers. For example, Helicobacter pylori (H. pylori) is classified as a Group I carcinogen, and its infection is a major cause of GC [7]. An increased relative abundance of Desulfovibrio vulgaris has also been linked to promoting CRC [8]. Additionally, factors such as smoking, obesity, genetic predisposition, and poor dietary habits contribute to an increased risk of various GI cancers, including GC, LC, PDAC, and CRC [9, 10]. Despite significant advancements in cancer treatment in recent decades, challenges persist in the diagnosis, drug resistance, and recurrence of tumors. ESCC patients often have a poor prognosis, as they are frequently diagnosed with local invasion or lymph node metastasis [11].
In this review, our objective was to explore the connections between the nervous system and GI cancer. To start, we provided a comprehensive overview of the components that comprise the nervous system, offering insights into its neurogenic organizational structure. Subsequently, the intricate relationship between GI tumors and the nervous system was discussed from multiple perspectives. Valuable insights into potential therapeutic targets were obtained by elucidating the specific neural pathways that innervate the GI tract and their involvement in cancer development. Ultimately, we aspire for this review to advance the field, resulting in improved outcomes and a better quality of life for patients affected by GI cancer.
The interplay between the nervous system and GI system
Classifications of the nervous system
In mammals, the nervous system primarily comprises the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS, consisting of the brain and spinal cord, serves as the central information processing center, while neurons located outside the brain and spinal cord are classified as part of the PNS (Fig. 1) [12].
The diagram depicts the intricate interaction between the central nervous system (colored in yellow) and the peripheral nervous system (colored in purple), showcasing their extensive exchange of information. Both the vagus nerve and the enteric nervous system are composed of various neural components. While the enteric nervous system operates as a remarkably comprehensive neural network, it is important to note that it is not entirely autonomous. This neural network proves proficient in facilitating localized intestinal reflexes through intrinsic nerve ganglia or plexuses, notably in the small and large intestines, but not in the stomach and esophagus. Moreover, it serves as a conduit for relaying signals, employing pathways that involve the sympathetic ganglia, the vagus nerve, and other routes to establish connections with the central nervous system. Reciprocally, neural signals from the central nervous system are also conveyed through these nerves to the enteric nervous system.
The brain functions as the central hub for processing information in the CNS, while the spinal cord plays a vital role in signal transmission and coordinating reflex actions. Situated within the spinal column, the spinal cord connects various organs and tissues through distinct segments. The upper thoracic spinal cord segments innervate both the head and thoracic regions, whereas the lower segments innervate the abdominal and pelvic regions [13].
The PNS is regarded as an extension of the CNS. For instance, the preganglionic neurons of the parasympathetic nervous system (PSNS) emerge from specific segments of the cranial and sacral nerves. Originating from the sacral spinal cord, the PSNS sends preganglionic fibers that extend to the pelvic viscera, controlling the function of the distal colon and the genitourinary system [14]. Within the PNS, the autonomic nervous system (ANS) is responsible for possessing self-regulatory capabilities and has a wide distribution, consisting of numerous pre and postganglionic regions. Different divisions of the ANS respond rapidly to local changes through specific neurotransmitters, aiding the CNS in regulating functions such as blood pressure, heart rate, vascular reactivity, and GI function.
The enteric nervous system (ENS) functions predominantly in the digestive system
The enteric nervous system (ENS), which is unique from other internal organs, is an independent nervous system that has evolved within the GI tract to handle complex behaviors. It has been classified as one of the three major branches of ANS [15]. The ENS is capable of integrating neuronal activity and controlling various GI activities, such as intestinal peristalsis (movement of the intestines), mucosal fluid movement, immune response, and mucosal secretions, without depending on CNS [16].
The ENS is a highly intricate neural network comprising over 100 million neurons and over 400 million enteric glial cells (EGCs). These neurons and glial cells are interconnected in various ways, forming an independent system mainly concentrated in interconnected ganglia found beneath the mucosa and within the layers of the muscles [12]. In addition to assisting CNS in regulating GI functions, the ENS also allows the GI tract to possess a certain level of self-mediated reflex responses and self-regulation, thereby maintaining GI stability [12]. Often referred to as the “second brain” of humans, the ENS is capable of orchestrating the processes of intestinal tissue movement without requiring direct input from the brain or spinal cord [17]. Within the GI tract, each nerve fiber within the ENS consists of a diverse group of neurons with distinct neurochemical encoding and functions. Interstitial chains connect these nerve fibers with neighboring neurons, facilitating signal transmission within the submucosal and myenteric plexuses [18].
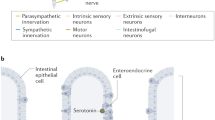
The submucosal plexus and myenteric plexus are responsible for regulating blood vessels and intestinal movements, respectively [16]. When the GI environment changes, the ENS transmits signals to target cells downstream through neural conduction and the release of neurotransmitters. This enables the ENS to control the motility, secretion, and sensory functions of the intestine, ultimately achieving local regulation of intestinal homeostasis. Additionally, intestinal epithelial cells also play a role in regulating the ENS. For example, DCLK1-positive tuft cells exhibit characteristics similar to neurons in terms of their gene expression. These cells are capable of secreting neurotransmitters, which can influence the function of the ENS [11].
Communications between CNS and ENS in normal GI tract development
While the ENS is capable of functioning independently, it still maintains bidirectional communication with CNS. This communication is facilitated by neural connections between the ENS, CNS, and sympathetic ganglia. These connections can be broadly categorized as the vagus nerve, spinal thoracolumbar, and spinal lumbosacral pathways. Generally, local reflexes in the GI tract are transmitted to the CNS through the sympathetic nervous system and ENS. These systems provide feedback signals to GI cells by neurotransmitters (acetylcholine (ACh), nitric oxide, and serotonin) and neuropeptides (neuropeptide Y, substance P (SP), and vasoactive intestinal peptide).
The vagus nerve connects the brainstem to the GI tract, serving as a communication pathway between the brain and the gut. It transmits sensory information from receptors in the GI tract to CNS. The vagus nerve also plays a crucial role in regulating GI functions, including the control of appetite, GI contractions, and the secretion of acid, hormones, enzymes, and other substances. Interestingly, the bidirectional information exchange between the brain and the gut is unbalanced, with ~90% of the vagus nerve fibers being afferent, transmitting signals from the gut to the brain [19]. This indicates that the brain receives input from receptors along the GI tract.
Cholinergic signaling primarily regulates target cells within the GI tract through the vagus nerve. Notably, the release of ACh extends beyond nerve-related cells. As mentioned earlier, DCLK1+ tuft cells act as a non-neuronal source of ACh within the intestinal tract [20]. These tuft cells have an extended lifespan and possess unique stem cell-like abilities, allowing them to facilitate the repair of damaged intestinal epithelial cells [21]. Additionally, the cytokines produced by these cells enhance intestinal contractility and influence inflammatory responses in the GI mucosa [11, 22]. These studies underscore the crucial importance of cross-regulation between the epithelial cell and neuronal system in maintaining intestinal homeostasis.
The CNS and the ENS exhibit both similarities and differences. As integral nervous system components, they share the capacity to recognize and respond to a wide range of neurotransmitters, including ACh, adrenaline, and catecholamines. However, the CNS and ENS are situated in different anatomical locations and serve distinct functions, while collectively contributing to the overall homeostasis and coordination of the body through bidirectional communication.
Regulating functions of CNS in GI cancer progression
Generally speaking, the mutual regulation between CNS and GI cancers primarily occurs through the influence on immune responses, endocrine functions, vascular formation, or disruption of nervous system functionality. Several decades ago, it was observed that certain behaviors in organisms, such as stress, chronic depression, and lack of social support, can influence the development and progression of tumors [23]. Survey reports showed that individuals with an optimistic mindset had a 16% lower risk of cancer-related mortality than pessimistic individuals. In recent years, researchers have made progress in explaining the impact of mental stress on tumor progression beyond initial clinical phenotype observations. In GI cancers, increased work-related stress and insufficient sleep have been associated with a higher incidence of CRC and ESCC [24].
Current research on molecular mechanisms suggests that stress can disrupt multiple physiological axes, including the brain-adipocyte brain-derived neurotrophic factor (BDNF) /leptin axis, the sympathetic-adrenal axis, and the hypothalamic-pituitary-adrenal axis. This disruption would lead to a reduction in cellular immunity, thereby promoting the development of CRC, PDAC, and tumor resistance [25]. Conversely, positive emotions were found capable of lowering the concentration of neurotrophic factors in the bloodstream, increasing the proportion of CD8+ cytotoxic T lymphocytes, and inducing microglia/macrophage activation, thereby inhibiting the progression of GI cancers [26]. The intricate relationship between the CNS and GI tumors is briefly illustrated in Fig. 2.
The CNS exercises regulatory influence over downstream cellular and molecular transformations linked to GI tumors, achieved through the modulation of pertinent neurotransmitter secretion. This regulatory grasp extends to encompass factors like nerve density, vitality of immune cells, and the activation of factors associated with angiogenesis. CNS resonates GI tumorigenesis through various aspects, including the modulation of drug resistance within GI tumors, the intensity of immune responses, and the potential for vascular growth. Meanwhile, the tumors themselves possess the capability to shape the configuration of the CNS reciprocally.
In addition to stress stimuli, various emotional stimuli can influence the development of GI cancers through the hypothalamus. Oxytocin, a neuropeptide secreted by oxytocin neurons in the hypothalamus, plays a role in regulating anxiety and depression [27]. In addition to stress stimuli, various emotional stimuli can influence the development of GI cancers through the hypothalamus [28]. These findings suggest that chemical stimulation of oxytocin neurons, such as with cephalotin, may serve as a promising therapeutic approach for treating CRC [28]. Dopamine (DA), a catecholamine (also known as the stress neurohormone) and a precursor to epinephrine, is secreted by the CNS. Dopamine has been shown to inhibit tumor angiogenesis and impede the invasion and migration of GC by suppressing VEGFR-2 phosphorylation and the EGFR/AKT/MMP-13 signaling pathway [29]. Dopamine receptors are a class of G protein-coupled receptors with five subtypes (DRD1-DRD5), and are associated with the growth and prognosis of a variety of GI cancers. High expression of DRD2 is associated with poor prognosis in CRC patients. Depletion of DRD2 in CRC can down-regulate β-catenin/ZEB1 signaling and inhibit tumor cell growth [30]. In addition, several neurotransmitters widely present in the CNS have been detected in GI tumors.
Various patterns of the PNS-mediated regulation on GI tumorigenesis
The abnormal phenotypic alterations of PNS have been universally observed in GI cancers at the histological level. It has been found that as cancer progresses from precancerous lesions to overt cancer, there is a significant increase not only in blood vessel density but also in nerve density. Subsequently, the relationship between nerve density and cancer invasiveness has been confirmed in various tumors, including prostate, colon and rectal, head and neck, breast, pancreatic, gastric, and lung cancer. This suggests that the PNS may play a crucial role in tumorigenesis.
The PNS can be functionally divided into the sensory (afferent) nervous system and the motor (efferent) nervous system. As our understanding of the relationship between PNS and tumors advances, it has been found that tumor progression can be influenced by both electrochemical signaling and the secretion of first messengers like neurotransmitters or n eurotrophins factors. Enabled by these regulatory functions, PNS is identified as a crucial participator in GI TME remodeling and GI cancer progression. The interaction between neural components and the TME of GI cancers is depicted in Fig. 3.
The regulation between nerve cells and tumors operates in both directions, forming a bidirectional relationship that is significantly influenced by the tumor microenvironment. Transformations in nerve cells induced by tumor-secreted factors encompassing (i) Conversion of nerve cell types; (ii) Hypertrophy of neurons or axonal elongation; (iii) Transformation of non-neuronal cells into nerve cells; (iv) Infiltration, envelopment, and penetration of tumor cells into nerves. The nerve cell-derived regulation on cancer cells is divided into three modes: paracrine-dependent regulation, electrochemical signals, and peripheral circulatory factor-dependent regulation. The interplay between neural and tumor components involves various cells and molecules, including those from the immune system, gut microbiota, and exosomes. These multifaceted contributors collectively engage in the interplay process, ultimately giving rise to a spectrum of biological phenomena.
Sensory nervous system-mediated sensory innervation
The cell bodies of primary sensory neurons are located in the cranial and spinal ganglia, and their primary function is to transmit information from target organs to the CNS through nerve fibers [14]. Among various types of sensory neurons, visceral sensory nerve fibers play a significant role in regulating GI cancers. Their cell bodies are primarily located in the tuberosity and jugular ganglia and are mainly carried by the vagus nerve. By detecting changes in homeostasis and conveying sensory information to the brainstem, the visceral sensory nervous system controls essential bodily functions, such as circulation, temperature regulation, digestion, respiration, and immune responses [31]. As for GI cancer progression, existing studies mainly focus on their associations with capsaicin and neuropeptide SP.
Capsaicin, a compound found in chili peppers, selectively activates sensory neurons that are sensitive to it. The response of these neurons varies with the concentration of capsaicin: it stimulates unmyelinated axons at low concentrations and desensitizes unmyelinated fibers at higher concentrations [14, 32]. The precise mechanisms of regulating tumors are not yet fully understood. However, several studies have suggested that capsaicin treatment or vagus nerve-mediated denervation can lead to the inactivation of unmyelinated C fibers, thereby increasing the risk of PDAC, GC, CRC, and bile duct cancer (CHOL).
SP, released by sensory neurons, plays a role in pain perception. Its receptor, the neurokinin-1 receptor, is widely expressed on immune cells, neurons, and tumor cell surfaces [33]. Studies have revealed that sensory axons can be recruited by pancreatic intraepithelial neoplasia (PanIN) lesions. Through SP/neurokinin-1 receptor signaling and the JAK-STAT pathway, this recruitment can activate and promote the proliferation of neuroendocrine PanIN cells, thereby facilitating the progression of PanIN lesions in PDAC.
ENS innervation: mediated by various first messengers
The interaction between ENS and GI cancer is intricate and diverse. Neurotransmitters or related substances secreted by neurons or EGCs can regulate the progression of tumors [34]. Correspondingly, those or other neural–related substances from GI cancer or TME can also affect neural reprogramming, new neural recruitment, and axonogenesis. This process is facilitated by a range of neurotrophic factors, axon guidance molecules, as well as neurotransmitters. Table 1 shows the target cells express specific receptors for various neural factors, activating downstream signaling pathways.
Axon guidance molecules
Axon guidance molecules, such as Netrins, Ephrins, Semaphorins, and Slit, have been demonstrated to play a role in the tumorigenesis and development of various GI cancers.
Starting with Netrins, as secreted proteins and membrane-bound proteins, Netrins not only influence neural cell differentiation but also impact tumor progression and stages. Notably, in late-stage GC and most of CRC, defective Netrin-1-dependent receptors DCC and UNC5C result from abnormal gene methylation [35, 36]. Netrin-1 can stimulate YAP signaling, the ERK/MAPK signaling cascades, and the PI3K/AKT pathway, thereby promoting the proliferation and invasion of GC cells [37,38,39]. Hypoxia-induced Netrin-1 activation can also trigger NF-κB and p65 downstream of AKT, promoting epithelial-mesenchymal transition (EMT) in hepatocellular carcinoma (HCC) cells [40]. Furthermore, Netrin-1 can facilitate CRC liver metastasis through the miR-329-3p/Netrin-1-CD146 complex [41]. However, the role of Netrin-1 is intricate and varies across tumorigenesis stages in PDAC. Research reveals low expression in stages I and II PDAC, increasing in stages III and IV. Netrin-1 exhibits both pro- and anti-tumor functions in PDAC [42, 43]. In comparison to Netrin-1, Netrin-4 has garnered less attention in GI cancer research. Nonetheless, some studies suggest that Netrin-4 elicits a similar effect on GC as Netrin-1, activating JAK/STAT, PI3K/Akt, and ERK/MAPK pathways to promote GC cell proliferation [44]. In contrast to Netrin-1, Netrin-4 serves as a tumor suppressor in CRC by inhibiting angiogenesis, hinders primary tumor growth, liver and lung metastases [45, 46].
The Eph-ephrin signal pathway involves Erythropoietin-producing hepatoma (Eph) receptors and their ligands, ephrin [47]. In summary, the majority of members in the Eph-ephrin family function as oncogenes in GI cancers. For instance, upregulation of EphA4, Ephrin-B2, EphA2, and EphrinA-1 is indicative of a poor prognosis in GC and contributes to the migration and invasion of CRC cell lines [48,49,50,51,52]. The EphB/ephrin-B combination serves as a valuable marker for dysplastic/oncogenic transformation in GC [53]. On the molecular level, EphA3 can activate the STAT3/VEGF signaling pathway, while EphA2 mediates CAFs-induced gastric tumorigenesis. The tumor suppressor gene function of Eph-ephrin family members is primarily evident in CRC. In CRC,EphB6, EphA5, and EphA1 are positively correlated with survival time [54, 55]. And EphA1 downregulation enhances the proliferation of HRT18 human rectal adenocarcinoma cells [56].
As for Semaphorins, they are classified into eight classes (classes 1-7 and V-viral), and various subtypes of Semaphorins exhibit abnormal expression patterns in GI tumors. For instance, Sema5A is overexpressed in GC and PDAC [57, 58], whereas Sema4G is down-regulated in CRC [59]. Sema4D is overexpressed in both GC and CRC [60, 61]. Ke Wang et al. comprehensively summarized the roles of Semaphorins in GC: Sema5A/6B/3E/4C/6D/4D/3C act as tumor promoters, facilitating tumor progression by promoting GC invasion, metastasis, or angiogenesis. In contrast, Sema3B/3A functions as GC tumor suppressor [62].
Within the Slit-Robo signaling framework, Slits (Slit1-3) interact with roundabout receptors (Robo1-4) in GI cancers. Among these, Robo1 has been extensively investigated and established as a cancer promoter in various malignancies. In precancerous intestinal lesions and during tumor progression, Slit2/Robo1 signaling triggers the Wnt/β-catenin pathway by down-regulating E-cadherin and induces EMT [61]. In HCC, ESCC, PDAC and GC, the Robo1 signaling axis activation can promotes cancer progression, and metastasis [63,64,65,66,67]. Conversely, deubiquitinating and stabilizing ROBO1 in CRC can inhibit tumor migration [68]. In PDAC, high expression levels of Robo1 and Robo3 are associated with reduced patient survival. It is worth noting that there exists a negative correlation between Robo1 and Robo3 expression, suggesting a potential contradiction within the Slit/Robo pathway [69]. In contrast to Robo1 and Robo3, Robo2 primarily functions as a tumor suppressor. High Robo2 expression often correlates with longer survival in PDAC and HCC patients [70]. In PDAC, Robo2 inhibits the activation of multiple downstream signaling pathways, such as MAPK and PI3K, by disrupting the interaction between TGF-β and HGF-MET, thereby impeding tumor growth [71, 72]. Overall, the Slit-Robo signaling pathway plays a crucial role in GI cancer, with its specific functions being subtype-dependent.
Neurotrophins
Neurotrophins are a class of secreted proteins that play essential roles in the nervous system’s survival, growth, development, and plasticity. Four Neurotrophins: nerve growth factor (NGF), BDNF, NT3, and GDNF, were listed in Table 1. These Neurotrophins exert their effects by binding to their receptor Trk, which belongs to the growth factor receptor (GFR) superfamily and possesses tyrosine kinase activity. In PDAC, CRC, the expression of these neurotrophic factors, including NGF and GFL neurotrophic factors, is upregulated [73, 74]. They can induce the spread of cancer cells around nerves by stimulating the GFRα3/RET receptor or promote neural invasion through activation of the RET-Ras-MAPK pathway, thereby facilitating PDAC metastasis and correlating with a poor prognosis [75, 76]. In ESCC and GC, TrkB is overexpressed and plays a role in tumor growth, metastasis, and EMT via the BDNF/TrkB pathway [77]. In ESCC, the high secretion of NGF is associated with increased expression and activation of TrkA, as well as decreased expression of p75NTR [78, 79]. Neurotrophins play a regulatory role in CRC, primarily through TrkB and TrkC receptors. BDNF/TrkB and BDNF/sortilin co-localize on the plasma membrane of CRC cell, activating downstream AKT signaling pathways and promoting CRC cell proliferation and survival [80, 81]. TrkC is frequently methylated in various CRC cell lines and functions as a conditional tumor suppressor in CRC [82]. There is limited research on the influence of neurotrophins in HCC; however, experimental studies have shown that TrkA acts as an oncogene in HCC, while p75NTR has potential tumor-suppressive functions [83,84,85]. And in PDAC, inhibiting the NCT-3/TrkC signaling pathway can suppress tumor development [86].
Neurotransmitters
Neurotransmitters are released by peripheral and autonomic nerves, which can be classified into three groups according to their chemical structure: (1) amino acids (Such as ACh, glutamate, and γ-aminobutyric acid (GABA)); (2) biogenic amines (Such as dopamine, norepinephrine, epinephrine, and serotonin) (3) neuropeptide (Such as SP, neuropeptide Y, and vasoactive intestinal polypeptide). Here, we focus on the roles of glutamate and GABA in GI cancers.
These two types of neurotransmitters, widely present in CNS and corresponding receptors, have been detected in various tumors. Among them, GABA is the major inhibitory neurotransmitter, while glutamate is the most important excitatory neurotransmitter in CNS. Glutamate is highly expressed in PDAC tissues and promotes tumor migration by activating the KRAS-MAPK signaling pathway [87]. Ionotropic glutamate receptors (iGluRs) show differential expression in CRC and GC, whereas high expression of mGluR4 in CRC leads to resistance to 5-Fluorouracil [88, 89].
There are three different receptors (A, B, and C) of GABA. Generally, GABA-A promotes tumor proliferation, while GABA-B inhibits tumor growth. In GC and PDAC, GABA activates the MAP kinase pathway through GABA-A receptors to promote cell proliferation [90, 91]. In vitro and in vivo experiments have shown that the use of GABA-B agonists can inhibit tumor development in LC [92].
Other ANS-mediated autonomic nerve innervations
Parasympathetic innervation: cholinergic signaling
The PSNS exerts dominant regulation over the GI tract, primarily through the vagus nerve-mediated cholinergic signaling. Nevertheless, the receptors involved in neuro-tumor regulation in response to cholinergic signaling may vary depending on the tumor type. The receptors that receive ACh signals include muscarinic ACh receptors (mAChRs) and nicotinic ACh receptors (nAChRs), with mAChRs being more prevalent in regulating GI cancers. Within the mAChR family, GC samples exhibit high expression of CHRM1 and CHRM3. In CRC, CHRM3 activation is observed, while CHRM1/2/3 is associated with poor prognosis in LC [93].
Recent advancements have shed light on the molecular mechanisms of cholinergic signaling in GI cancer progression. Gastric tumors can release NGF, which fosters nerve recruitment and the proliferation of Dclk1 cells, resulting in increased ACh release [94]. ACh, acting via the CHRM3 receptor, modulates crucial regulatory factors in tumor growth, such as the Wnt and Notch signaling pathways, or utilizes AMPK signaling, thereby subsequently promoting tumor cell proliferation. These findings suggest the potential therapeutic use of anti-cholinergic drugs for GC and related malignancies.
The effects of the PSNS on GI cancer vary considerably and are contingent upon the particular type of tumor. Studies have indicated that in PDAC, the regulatory role of the PSNS contradicts that in GC and LC. Notably, after vagus nerve resection in PDAC patients, a shortened survival time was observed. The CHRM1 receptor, which is closely associated with the prognosis of PDAC, transmits cholinergic signals to inhibit the EGFR/MAPK and PI3K/AKT signaling pathways, thereby extending patient survival (Fig. 4) [95]. Therefore, cholinergic agonists are being considered as potential adjunctive treatments for PDAC. However, studies have demonstrated that activation of ACh receptors, α3, α5, α7 – nAChRs receptors induces PDAC cell proliferation, indicating sophisticated patterns may be involved in PSNS regulation.
In GC progression, ACh is secreted by three distinct sources: neurons, cancer cell-derived choline acetyltransferase, and DCLK1-positive tuft cells. ACh present in the local environment has the capacity to bind to the type 3 muscarinic receptors (M3Rs) situated on the surface of tumor cells. This binding event triggers a cascade of downstream signaling pathways, culminating in the stimulation of NGF production and subsequent release. This NGF-mediated process plays a pivotal role in fostering the proliferation of nerve cells, thereby contributing to the amplification of acetylcholine content within the immediate local environment and completing the positive feedback cycle. TrkA is also present on the surface of GC cells, which is also activated by NGF and influences a variety of phenotypes associated with cell proliferation and metastasis. In PDAC, ACh is also sourced from three distinct origins: neurons, fibroblasts, and T cells. Following release, ACh binds to the type 1 muscarinic receptors (M1Rs) in cancer cells. Notably, this binding event impedes the activation of downstream PI3K/AKT and MAPK pathways that EGFR typically activates. In addition, M3R was highly expressed in PDAC, and LPS and IFN-γ could induce this expression. When M3R is activated, it can induce the expression of COX-2 and NOX in cells, among which COX-2 can induce the formation of chronic pancreatitis and carcinogenesis.
Sympathetic innervation: epinephrine signaling
In most GI cancers, the signaling output of the sympathetic nervous system is mediated through the secretion of neurotransmitters like epinephrine/norepinephrine or isoprenaline, which have an impact on tumor initiation and progression. Through binding to β2-adrenergic receptors (β2-AR), epinephrine/norepinephrine can activate multiple downstream signaling pathways and exert an influence on the progression of GI cancers.
In GC patients, norepinephrine (NE) induces gastric EMT and enhances the invasive and migratory capabilities of GC cells through the activation of multiple pathways, including β2-AR-HIF-1α-Snail signaling, β2-AR-STAT3-CD44 signaling, and β2-AR-MMP7 signaling [96,97,98]. Similarly, activation of the β2-AR by another agonist isoproterenol triggers the initiation of EMT and the expression of associated markers, accelerating the malignancy of GC cells and ultimately tumor progression [97].
Upregulation of NE is also demonstrated in PDAC. Remarkably, PDAC cells demonstrate the capacity to synthesize NE, leading to its localized accumulation and subsequent activation of signaling pathways involved in cell proliferation, growth factor release, and apoptosis inhibition [99, 100]. Meanwhile, increased adrenaline activity can promote the progression of pancreatic diseases from the PanIN stage to adenocarcinoma [101].
The feedback regulation of tumor cells on nerves
Perineural invasion
Peripheral invasion (PNI) is present in various types of GI cancers. According to former reports, the incidence of PNI could reach 98%, 75%, and 33% in PDAC, CHOL, and CRC, respectively [102, 103]. It is characterized by the infiltration and accumulation of tumor cells around peripheral nerves, which is facilitated by the secretion of neurotransmitters and neuropeptides by nerve terminals [104]. PNI plays diverse roles in the progression of GI cancers and is associated with high invasiveness and various clinical features, including the 5-year survival rate, tumor size, and lymph node metastasis. It has been identified as an independent adverse prognostic factor in GC and CRC [105, 106].
Mechanically, the occurrence of PNI is associated with the expression of various cytokines, chemokines, and adhesion molecules. Hepatocyte growth factor (HGF), the ligand of c-Met, has been implicated in promoting PNI in PDAC. Recent studies illustrated the promotion pattern of the HGF/c-Met signaling pathway in PDAC: the overexpression of c-Met leads to activation of the mTOR/NGF axis, resulting in nerve recruitment and enhanced invasion of cancer cells into nerves [107, 108]. Meanwhile, chemokines like CCL21 and CXCL10 facilitate early PNI formation by promoting cancer cell migration towards sensory neurons. The expression of their respective receptors (CCR7 and CXCR3) can also influence patients’ perception of pain [109]. In GC, co-expression of CXCL8 and MMP9 often implies a poor prognosis and serves as a detection marker for PNI [110]. Moreover, in vitro co-culture experiments have revealed that vascular cell adhesion molecule-1 (VCAM1) contributes to PNI and accelerates GC progression by promoting interactions between nerve cells and tumor cells [111]. Furthermore, the connection between extracellular vesicles and PNI has been observed. For instance, extracellular vesicle-derived miR-128-3p has been demonstrated to induce the activation of TGF-β/SMAD and JAK/STAT3 signaling pathways in CRC, thereby promoting perineural infiltration and lymphovascular invasion [112].
Neoneurogenesis, axonogenesis and neural reprogramming
Three predominant biological processes are involved in cancer cell-originated neuron regulation: neoneurogenesis, axonogenesis, and neural reprogramming. Neoneurogenesis, also known as innervation, refers to the formation of new functional neurons promoted by tumor cells through the release of neurotrophic factors. The exact origin of these new neurons is still under debate. Axonogenesis involves inducing axonal growth in surrounding nerve cells by tumor cells. Neural reprogramming, on the other hand, entails converting non-neuronal cells into neurons.
In previous studies, it was mentioned that the removal of PNS, sensory nerves, or vagus nerves, as well as the blockade of related receptors, can impact the progression of GI cancer. However, subsequent studies have shown that this effect cannot be solely attributed to the removal of pre-existing neurons [113]. Further research revealed that nerves appearing in tumors may originate from the CNS or mesenchymal stem cells (MSCs) [114, 115], and the neural density in solid tumors often correlates with prognosis (higher neural density indicating improved survival rates in CRC) [116]. These findings suggest the potential significance of neural reprogramming, axonogenesis, and neoneurogenesis in neuro-tumor regulation. Under appropriate TME conditions, tumor cells recruit suitable cells like MSCs to differentiate into neurons or promote axonal extension and growth [117]. While the molecular mechanisms underlying axonogenesis and neural development are not yet fully understood, it is known that stimulation of acetylcholine-nerve growth factor can induce PNI and increase neural density in GC and PDAC [73]. Recent experimental studies in mice have indicated that tumor cell-released extracellular vesicles play a role in regulating axonogenesis and neurogenesis. Specifically, several studies have shown that tumor cell-released extracellular vesicles can initiate the process of axonogenesis. Furthermore, vesicles containing EphrinB1 protein have been found to enhance the activity of axonogenesis [118].
The cross-talk between the nervous system and TME in GI tumorigenesis
The intricate interplay between the TME and tumor cells results in various effects encompassing various aspects of tumor development and progression. The latest study by Bin He et al. showed that cancer cells can acquire stemness properties by exploiting neural signals in the microenvironment and pointed out that this process is facilitated through the cAMP-responsive element pathway [119]. The indirect modulation of interactions between the nervous system and tumors can be summarized as the TME acting as a bridge, exerting influence on the development of GI cancer. The TME comprises diverse components, including nerve cells, immune cells, microbiota, and tumor-associated fibroblasts. These components can interact and mutually influence one another, thereby directly or indirectly impacting the development of GI cancer (Fig. 5).
The indirect regulation of nerve-tumor interactions is summarized through the TME, acting as the link between nerves and tumors, influencing the development of GI cancer. TME comprises nerve cells, immune cells, microbial populations, and cancer-associated fibroblasts. Dysbiosis of the gut microbiota can influence psychoneurotoxicity and provoke immune responses. Within the TME, fibroblasts, glial cells, and Schwann cells can undergo reprogramming to transform into nerve cells, contributing to nerve repair, regeneration, or peripheral invasion to a certain extent. While immune cells have the potential to transform into neural cells, in most cases, the immune system is influenced by neural signals, leading to alterations in its immune response to tumors.
Gut microbiota
Emerging research suggests that the interaction between the nervous system and tumors offers new insights and potential therapeutic strategies for cancer treatment. Dysregulation of the gut microbiota has been implicated in the development of various cancers. For instance, H. pylori has been identified as a significant factor in developing GC and PDAC. It triggers the accumulation of inflammatory factors in the stomach, leading to abnormal gene methylation in cancers [7]. It may also cause mutations in the KRAS gene and activate tumor-promoting genes (STAT3, c-myc, Bcl-xL) in the pancreas [120]. CRC has been associated with an increased abundance of sulfate-reducing bacteria caused by a high-fat diet, while LC has shown connections with imbalances in Escherichia coli in the GI tract [8, 121].
The Gut Microbiota-brain Axis, which represents the communication between the gut microbiota and the brain, has garnered recognition in recent years. Research has demonstrated that the gut microbiota can interact with the brain through several mechanisms, including neurotransmitter production, immune regulation, inflammation response, and blood-brain barrier permeability [122, 123]. This bidirectional communication has significant implications for human health and the development of neurological disorders, including anxiety disorders, depression, autism spectrum disorders, Parkinson’s disease, and Alzheimer’s disease [123,124,125,126]. Furthermore, imbalances in the gut microbiota have been implicated in emotions, cognitive function, and behavior, resulting in symptoms such as anxiety, depression, and impaired cognitive function [125, 127, 128].
The Gut Microbiota-brain Axis and the interplay between the gut microbiota and tumor regulation imply the potential for indirectly regulating GI cancer development through cross-talk between the nervous system and gut microbiota. Research findings indicate a link between the gut microbiota and psychoneurotoxicity associated with cancer treatment. The gut microbiota can influence the development of psychoneurotoxicity and its associated symptoms through various mechanisms. Dysbiosis of the gut microbiota can disrupt the normal function of the intestinal mucosal barrier, enabling harmful bacteria and metabolites to enter the circulatory system, thereby impacting neurotransmitter production and function [129,130,131]. Additionally, dysbiosis of the gut microbiota can trigger chronic inflammatory responses that can further influence the function of the nervous system and the balance of neurotransmitters [130,131,132].
It is crucial to note that while there exists a link between the gut microbiota, neural regulation, and tumor progression, the specific mechanisms and treatment strategies demand further research and validation. Additional clinical trials and human studies are necessary to assess the safety and efficacy of utilizing gut microbiota in cancer treatment.
Immune cells
The intestine is widely acknowledged as the largest immune organ. As mentioned earlier, the immune system is involved in diverse regulatory processes, including neuro-tumor regulation. In recent years, there has been growing evidence suggesting that disrupted neuro-immune interactions contribute to the development of cancer and metastasis. Notable examples of this are the neuropeptides SP and calcitonin gene-related peptide (CGRP). These neuropeptides demonstrate tumor characteristics and play a significant role in regulating the immune system in sensory neurons.
For example, research studies indicate that high levels of SP have been shown to facilitate the migration of natural killer cells (NK cells), stimulate the secretion of immune-related factors such as IFN-γ and IL-12, and boost the cytotoxic activity of immune cells, including cytotoxic cells and NK cells [133, 134]. SP can activate cell-mediated cytotoxic immunity against pathogens and immune responses against precancerous cells during the immune response to pathogenic agents [135]. In summary, the release of SP by sensory neurons can bolster the cytotoxic immune response against advanced cancer, thereby modulating tumor development.
Moreover, the interaction between neuro-tumor-immune processes extends beyond the aforementioned examples. ACh can also be synthesized by T cells, specifically those that express β2-AR. Upon stimulation by adrenergic neural signals, these T cells secrete ACh, which subsequently plays a role in the immune regulation of tumors. For instance, ACh has the ability to facilitate tumor cell proliferation and inhibit the expression of tumor necrosis factor in macrophages [136, 137].
Fibroblasts
In solid tumors, the tumor stroma constitutes more than 50% of the tumor composition. Alongside endothelial cells, immune cells, and ECM, cancer-associated fibroblasts (CAFs) play a significant role. CAFs, a distinct cell type within the TME, can either promote or suppress tumor characteristics through ECM remodeling or cytokine secretion. The functions of CAFs encompass various roles, including stimulating angiogenesis, controlling immune suppression, regulating cancer cell metabolism, and promoting or inhibiting cancer cell proliferation and metastasis [138].
In GC, CAFs serve as a source of IL-17, which activates the JAK2/STAT3 signaling pathway and thereby enhances the invasion and migration of GC cells [139]. In a study conducted by Shen et al., YAP1 expression level was upregulated when co-culturing CAFs and PDAC cells. Furthermore, this study confirmed that CAFs can enhance perineural invasion in PDAC by activating the YAP1/TEAD1/NGF pathway [140]. CAFs also exert an influence on CRC. Although the maturation of these cells does not significantly correlate with the prognosis of CRC patients, they have the ability to impact the invasion pattern of the cancer [141]. In PDAC, there is an upregulation of CCL26 expression in CAFs. This upregulation activates the PI3K/AKT/mTOR pathway, consequently fostering perineural invasion and facilitating lymph node metastasis, particularly in advanced tumor stages [142].
Several studies have attempted to develop new cancer treatment strategies based on the interaction between CAFs and TME. Interestingly, efforts to deplete CAFs have resulted in unexpected outcomes in the advancement of PDAC. Instead of inhibiting tumor growth as anticipated, CAF depletion has led to the promotion of immunosuppression and, consequently, a decrease in overall survival [143]. Recent studies have identified the presence of CAFs with contrasting functions in PDAC. Notably, depleting fibroblast activation protein (FAP)+ CAFs has been found to reduce the survival rates of PDAC patients. Conversely, the depletion of α-smooth muscle actin (α-SMA)+ CAFs has shown promise in improving the survival rates of individuals with PDAC [144]. These findings emphasize the importance of precisely targeting specific subtypes of CAFs and highlight the complex nature of CAF involvement in cancer therapy.
Non-neurocyte-originated neurocyte transformation
The impact of various tissue and cell components within the TME on regulating neural tumors extends beyond indirect regulation via molecule release. Some specialized cells, such as macrophages, T cells, fibroblasts, and glial cells, can also actively engage in generating new nerves through direct reprogramming. This direct reprogramming also referred to as transdifferentiation, has been extensively investigated, particularly for transforming fibroblasts into neurons. Studies have demonstrated that specific transcription factors (OCT4, SOX2, c-MYC, p53) [145, 146], RNA molecules (such as miR-9 and miR-124) [147], and recombinant proteins [148] can induce the direct reprogramming of fibroblasts into neurons or dopamine neurons. Most of these processes are related to molecular mechanisms such as the JAK/STAT signaling pathway and the Wnt signaling pathway. However, there is still insufficient research on the transformation of CAFs into functional neurons in the TME. Previous studies have shown that glial cells can be reprogrammed and converted into neuronal-like cells (for example, astrocytes can differentiate into functional neurons). In neuro-tumor regulation, much research has been done on Schwann cells (SCs). SCs mainly participate in neuro-tumor regulation in two ways. One way is through involvement in PNI. One way is through involvement in PNI. As the main source of various cell movement and adhesion-related proteins, SCs promote the migration of SCs to cancer cells and release chemical molecules to facilitate tumor proliferation in PDAC with low expression of SLIT2. They also enhance the interaction between cancer cells and nerves, ultimately promoting the PNI process [149]. Another way is through reprogramming to participate in nerve repair and regeneration. When the peripheral nervous system is damaged, some SCs differentiate into repair SCs (rSCs) and guide the process of axonal growth and nerve regeneration [150]. In 2022, Philip Tang et al. revealed a process called macrophage-to-neuronal cell transformation. The study showed that under the induction of tumor secretions, a specific subset of tumor-associated macrophages that express Tubb3 and lose macrophage markers is generated in the TME. This process is dependent on Smad3 and is associated with cancer pain [151].
Neuroendocrine tumors (NETs) in the gastrointestinal system
Neuroendocrine cells regulate body growth, development, and internal homeostasis by secreting hormones and peptides. In the GI system, over 14 types of neuroendocrine cells have been identified, and mutations in these cells contribute to the development of NETs [152]. The specific gene mutations promoting NET development remain unclear. Studies indicate that elevated expression of somatostatin receptors (SSTRs) and angiogenesis-related genes, including vascular endothelial growth factor (VEGF) and fibroblast growth factor, is characteristic of NETs [153]. Higher tumor differentiation correlates with increased gene expression. Additionally, the pathogenesis of NETs varies across different sites. For instance, hereditary small intestinal neuroendocrine neoplasms (NENs) result from IPMK inactivation, whereas strong PROX1 expression in rectal NENs is likely to promote tumor progression [154, 155]. Clinically, most NENs (e.g., those with MEN1 gene mutations) grow slowly, while some (e.g., those with VHL gene mutations) have malignant potential [156, 157]. Overall, NENs exhibit high heterogeneity.
Therapeutic opportunities
A comprehensive understanding of neuro-tumor regulation provides valuable insights and potential avenues for cancer treatment. The complex interaction between the nervous system and tumors is widely recognized as a crucial factor in tumor progression and treatment response. Investigating neuroregulatory mechanisms can unveil novel targets and pathways, forming the basis for developing innovative treatment strategies.
Tumor resistance to neurological intervention
Chemotherapy is a primary therapeutic approach for treating GI cancers. However, the effectiveness of chemotherapy can vary among individuals, and the development of drug resistance poses a significant clinical challenge in cancer treatment. Mouse experiments have shown that the activation of adrenaline can influence the sensitivity of tumor cells to chemotherapy by modulating apoptotic pathways. In the case of breast cancer (BC), the relationship between the β2-AR and resistance to the anti-HER2 monoclonal antibody trastuzumab has been elucidated. Upregulation of β2-AR by HER2 can activate the PI3K/AKT/mTOR pathway, leading to tumor resistance to trastuzumab, a first-line treatment for both GC and BC [158]. Subsequent experiments combining β-blockers with trastuzumab have improved BC patients’ survival rates [159]. These findings highlight the significance of adrenergic signaling in modulating chemotherapy response and drug resistance in cancer. Understanding the underlying mechanisms and identifying therapeutic strategies to overcome drug resistance are crucial research areas for enhancing chemotherapy’s effectiveness in GI cancers.
Moreover, ACh inhibitors have been studied to improve the sensitivity of GC to chemotherapy [160]. Both clinical and mouse experiments have shown that administering botulinum toxin, an inhibitor of the ACh pathway, can effectively suppress GC recurrence, enhance patient survival, and optimize chemotherapy outcomes [3]. These findings suggest a potential association between neuroregulation and tumor drug resistance, indicating that targeting neuro-related pathways could enhance tumor sensitivity to drugs. Further investigation into adrenergic and cholinergic signaling pathways may lead to novel therapeutic strategies, ultimately improving treatment outcomes and patient survival rates in GI cancers.
New neuromodulation-targeted therapy
Therapeutic approaches targeting neurotrophic factor receptors have emerged due to the increasing understanding of their role in neuro-tumor regulation. Ongoing clinical investigations mainly focus on NGF and its associated receptor TRKA. Larotrectinib and entrectinib, two small molecule inhibitors of Trk receptors, have been suggested as effective treatment options [161]. Ongoing research focuses on using these inhibitors to block NGF-TrkA or BDNF-TrkB signaling pathways, thus inhibiting tumor development [94]. These drugs have already received FDA approval for treating Trk fusion-positive tumors [161].
Furthermore, neuroregulation therapy shows promise in the management of cancer-related pain. Tumor-related pain is a prevalent challenge for cancer patients, and neuroregulation techniques such as spinal cord stimulation, dorsal root ganglion stimulation, and peripheral nerve stimulation provide non-pharmacological and non-surgical alternatives for pain relief [162]. Several studies suggest that these neuroregulation techniques can relieve cancer-related pain and improve patients’ quality of life [163].
In conclusion, a deep understanding of the interaction between nerves and tumors has provided new insights and methodologies for cancer treatment. This progress has guided the development of clinical interventions, transitioning from the historically problematic denervation surgeries of the 19th century to modern adjunctive therapies that target tumor innervation. This transformation has not only increased the effectiveness of cancer treatment but has also provided new therapeutic options for clinical implementation. Additionally, the use of neuromodulation techniques for pain management adds another dimension. These techniques have the potential to alleviate moderate to severe cancer-related pain and improve the quality of life for individuals undergoing cancer treatment. With the deepening research in this field, neuromodulation is positioned to provide patients with more effective treatment options, thereby improving the overall prognosis for individuals with cancer.
Conclusions and perspectives
In summary, the regulation of GI cancers by the nervous system is primarily mediated through ANS. Neuro-related cells, mediators, and components of the TME actively participate in the complex interplay between the nervous system and digestive tract tumors. This bidirectional communication and influence highlight how tumors can exploit neural pathways to their advantage in the pursuit of growth and progression. While this field of research has garnered significant attention and provided valuable clinical guidance, several unresolved issues persist.
Further research is necessary to deepen our understanding of the effectiveness and underlying mechanisms of gut neuroregulation. Despite some progress, the treatment outcomes for gut diseases such as inflammatory bowel disease, obesity, nausea, and gastroparesis remain inconsistent [164]. Numerous factors, including individual differences, disease types, and disease severity, may impact the efficacy of treatments for these complex conditions. Exploring novel treatment modalities and identifying personalized approaches will be crucial for improving therapeutic outcomes. Additionally, careful consideration must be given to the safety and long-term effects of neuroregulation techniques. While certain neuroregulation techniques, like gastric electrical stimulation for upper GI disorders, have been utilized, understanding the potential risks and evaluating the long-term effects is paramount [165]. Ensuring both the safety and effectiveness of these treatments poses a significant challenge in advancing neuroregulation techniques for clinical application.
Moving forward, future studies should prioritize enhancing our understanding of neuroregulation in the TME. This would encompass the exploration of innovative treatment strategies and the fostering of interdisciplinary collaborations. By doing so, we can optimize treatment outcomes and substantially enhance the quality of life for patients suffering from GI cancer. It is through these endeavors that we can hope to unlock the full potential of neuroregulation techniques and improve patient care in this challenging disease landscape.
References
Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166.
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115.
Rabben HL, Zhao CM, Hayakawa Y, Wang TC, Chen D. Vagotomy and gastric tumorigenesis. Curr Neuropharmacol. 2016;14:967–72.
Tong Y, Gao HR, Qi QC, Liu XY, Li J, Gao J, et al. High fat diet, gut microbiome, and gastrointestinal cancer. Theranostics. 2021;11:5889–910.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca-Cancer J Clin. 2023;73:17–48.
Feng RM, Su QL, Huang XY, Basnet T, Xu X, Ye WM. Cancer situation in China: what does the China cancer map indicate from the first national death survey to the latest cancer registration? Cancer Commun. 2023;43:75–86.
Takayama S, Takahashi H, Matsuo Y, Okada Y, Manabe T. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepatogastroenterology. 2007;54:2387–91.
Wells JE, Hylemon PB. Identification and characterization of a bile acid 7-alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7-alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66:1107–13.
Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022;163:386–402.
Aqel B, DiBaise JK. Role of the gut microbiome in nonalcoholic fatty liver disease. Nutr Clin Pr. 2015;30:780–6.
Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–80.
Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–28.
Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–39.
Sternini C. Organization of the peripheral nervous system: autonomic and sensory ganglia. J Investig Dermatol Symp Proc. 1997;2:1–7.
Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6:1239–78.
Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17:338–51.
Holland AM, Bon-Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci. 2021;78:4713–33.
Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18.
Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014;817:115–33.
Schutz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6:87.
Gerbe F, Jay P. Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol. 2016;9:1353–9.
McLean L, Smith A, Cheung LM, Desai N, Grinchuk V, Zhao AP, et al. Type 3 muscarinic receptors (M3R) contribute to expulsion of Nippostrongylus brasiliensis through induction of Th2 cytokines. Am J Gastroenterol. 2014;109:S502.
Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. Opinion—The influence of bio-behavioral factors on tumor biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8.
Yang TT, Qiao Y, Xiang SY, Li WZ, Gan Y, Chen YC. Work stress and the risk of cancer: a meta-analysis of observational studies. Int J Cancer. 2019;144:2390–400.
Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64.
Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest tumors. 2016;3:25–36.
Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–59.
Pan S, Yin K, Tang Z, Wang S, Chen Z, Wang Y, et al. Stimulation of hypothalamic oxytocin neurons suppresses colorectal cancer progression in mice. Elife. 2021;10:e67535.
Ganguly S, Basu B, Shome S, Jadhav T, Roy S, Majumdar J, et al. Dopamine, by acting through Its D-2 receptor, inhibits insulin-like growth factor-I (IGF-I)-induced gastric cancer cell proliferation via up-regulation of kruppel-like factor 4 through down-regulation of IGF-IR and AKT phosphorylation. Am J Pathol. 2010;177:2701–7.
Lee H, Shim S, Kong JS, Kim MJ, Park S, Lee SS, et al. Overexpression of dopamine receptor D2 promotes colorectal cancer progression by activating the beta-catenin/ZEB1 axis. Cancer Sci. 2021;112:3732–43.
Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev. 2016;96:975–1024.
Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218.
Johnson MB, Young AD, Marriott I. The therapeutic potential of targeting substance P/NK-1R interactions in inflammatory CNS disorders. Front Cell Neurosci. 2016;10:296.
Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012;1826:112–20.
Toda K, Nagasaka T, Umeda Y, Tanaka T, Kawai T, Fuji T, et al. Genetic and epigenetic alterations of netrin-1 receptors in gastric cancer with chromosomal instability. Clin Epigenetics. 2015;7:73.
Shin SK, Nagasaka T, Jung BH, Matsubara N, Kim WH, Carethers JM, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133:1849–57.
Yin K, Shang M, Dang S, Wang L, Xia Y, Cui L, et al. Netrin‑1 induces the proliferation of gastric cancer cells via the ERK/MAPK signaling pathway and FAK activation. Oncol Rep. 2018;40:2325–33.
Yin K, Dang S, Cui L, Fan X, Wang L, Xie R, et al. Netrin-1 promotes metastasis of gastric cancer by regulating YAP activity. Biochem Biophys Res Commun. 2018;496:76–82.
Yin K, Wang LJ, Zhang X, He ZY, Xia YW, Xu JH, et al. Netrin-1 promotes gastric cancer cell proliferation and invasion via the receptor neogenin through PI3K/AKT signaling pathway. Oncotarget. 2017;8:51177–89.
Yan W, Han P, Zhou ZZ, Tu W, Liao JZ, Li PY, et al. Netrin-1 induces epithelial-mesenchymal transition and promotes hepatocellular carcinoma invasiveness. Dig Dis Sci. 2014;59:1213–21.
Fang X, Xu Y, Li K, Liu P, Zhang H, Jiang Y, et al. Exosomal lncRNA PCAT1 promotes tumor-circulating cell-mediated colorectal cancer liver metastasis by regulating the activity of the miR-329-3p/Netrin-1-CD146 complex. J Immunol Res. 2022;2022:9916228.
An XZ, Zhao ZG, Luo YX, Zhang R, Tang XQ, Hao D, et al. Netrin-1 suppresses the MEK/ERK pathway and ITGB4 in pancreatic cancer. Oncotarget. 2016;7:24719–33.
Wang LJ, Zhi XF, Zhu Y, Zhang Q, Wang WZ, Li Z, et al. MUC4-promoted neural invasion is mediated by the axon guidance factor netrin-1 in PDAC. Oncotarget. 2015;6:33805–22.
Lv B, Song CH, Wu LJ, Zhang Q, Hou DS, Chen P, et al. Netrin-4 as a biomarker promotes cell proliferation and invasion in gastric cancer. Oncotarget. 2015;6:9794–806.
Eveno C, Broqueres-You D, Feron JG, Rampanou A, Tijeras-Raballand A, Ropert S, et al. Netrin-4 delays colorectal cancer carcinomatosis by inhibiting tumor angiogenesis. Am J Pathol. 2011;178:1861–9.
Eveno C, Contreres JO, Hainaud P, Nemeth J, Dupuy E, Pocard M. Netrin-4 overexpression suppresses primary and metastatic colorectal tumor progression. Oncol Rep. 2013;29:73–8.
Arora S, Scott AM, Janes PW. Eph receptors in cancer. Biomedicines. 2023;11:314.
Oki M, Yamamoto H, Taniguchi H, Adachi Y, Imai K, Shinomura Y. Overexpression of the receptor tyrosine kinase EphA4 in human gastric cancers. World J Gastroenterol. 2008;14:5650–6.
Lv JH, Xia QY, Wang JJ, Shen Q, Zhang J, Zhou XJ. EphB4 promotes the proliferation, invasion, and angiogenesis of human colorectal cancer. Exp Mol Pathol. 2016;100:402–8.
Yuan WJ, Ge J, Chen ZK, Wu SB, Shen H, Yang P. et al. Over-expression of EphA2 and EphrinA-1 in human gastric adenocarcinoma and its prognostic value for postoperative patients. Dig Dis Sci. 2009;54:2410–7.
Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–47.
Dunne PD, Dasgupta S, Blayney JK, McArt DG, Redmond KL, Weir JA, et al. EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin Cancer Res. 2016;22:230–42.
Uchiyama S, Saeki N, Ogawa K. Aberrant EphB/ephrin-B expression in experimental gastric lesions and tumor cells. World J Gastroentero. 2015;21:453–64.
Peng L, Tu P, Wang X, Shi S, Zhou X, Wang J. Loss of EphB6 protein expression in human colorectal cancer correlates with poor prognosis. J Mol Histol. 2014;45:555–63.
Gu SD, Feng J, Jin Q, Wang W, Zhang S. Reduced expression of EphA5 is associated with lymph node metastasis, advanced TNM stage, and poor prognosis in colorectal carcinoma. Histol Histopathol. 2017;32:491–7.
Wu BO, Jiang WG, Zhou D, Cui YX. Knockdown of EPHA1 by CRISPR/CAS9 promotes adhesion and motility of HRT18 colorectal carcinoma cells. Anticancer Res. 2016;36:1211–9.
Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, et al. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion, and metastasis. Int J Cancer. 2010;127:1373–83.
Pan G, Lv H, Ren H, Wang Y, Liu Y, Jiang H, et al. Elevated expression of semaphorin 5A in human gastric cancer and its implication in carcinogenesis. Life Sci. 2010;86:139–44.
Wang XL, Zhou CZ, Qiu GQ, Fan JW, Tang HM, Peng ZH. Screening of new tumor suppressor genes in sporadic colorectal cancer patients. Hepato-Gastroenterol. 2008;55:2039–44.
Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. P Natl Acad Sci USA. 2006;103:9017–22.
Li H, Wang JS, Mu LJ, Shan KS, Li LP, Zhou YB. Promotion of Sema4D expression by tumor-associated macrophages: significance in gastric carcinoma. World J Gastroenterol. 2018;24:593–601.
Wang K, Zhao XH, Liu J, Zhang R, Li JP. Nervous system and gastric cancer. Biochim Biophys Acta Rev Cancer. 2020;1873:188313.
Basha S, Jin-Smith B, Sun C, Pi L. The SLIT/ROBO pathway in liver fibrosis and cancer. Biomolecules. 2023;13:785.
He H, Hao SJ, Yao L, Yang F, Di Y, Li J, et al. MicroRNA-218 inhibits cell invasion and migration of pancreatic cancer via regulating ROBO1. Cancer Biol Ther. 2014;15:1333–9.
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6:e1000879.
Jiang LX, Wang YF, Rong YX, Xu LH, Chu Y, Zhang Y, et al. miR-1179 promotes cell invasion through SLIT2/ROBO1 axis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:319–27.
Lu GF, Du R, Dong JQ, Sun Y, Zhou FL, Feng F, et al. Cancer associated fibroblast derived SLIT2 drives gastric cancer cell metastasis by activating NEK9. Cell Death Dis. 2023;14:421.
Huang Z, Wen P, Kong R, Cheng H, Zhang B, Quan C, et al. USP33 mediates Slit-Robo signaling in inhibiting colorectal cancer cell migration. Int J Cancer. 2015;136:1792–802.
Han SL, Cao CW, Tang T, Lu CH, Xu JC, Wang S, et al. ROBO3 promotes growth and metastasis of pancreatic carcinoma. Cancer Lett. 2015;366:61–70.
Song QQ, Zhang HY, He JN, Kong HY, Tao R, Huang Y, et al. Long non-coding RNA LINC00473 acts as a microRNA-29a-3p sponge to promote hepatocellular carcinoma development by activating Robo1-dependent PI3K/AKT/mTOR signaling pathway. Ther Adv Med Oncol. 2020;12:1758835920937890.
Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hemat. 2005;53:35–69.
Pinho AV, Van Bulck M, Chantrill L, Arshi M, Sklyarova T, Herrmann D, et al. ROBO2 is a stroma suppressor gene in the pancreas and acts via TGF-beta signaling. Nat Commun. 2018;9:5083.
Ceyhan GO, Schafer KH, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–31.
Wang SY, Nie LM, Song YX, Zhang F, Chen XZ, Shi WJ, et al. Neurturin promotes tumor cell motility and angiogenesis in colorectal cancer. Exp Cell Res. 2022;413:113049.
Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, et al. The neurotrophic factor Artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–81.
Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol. 2008;23:1852–9.
Okugawa Y, Tanaka K, Inoue Y, Kawamura M, Kawamoto A, Hiro J, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108:121–30.
Tsunoda S, Okumura T, Ito T, Mori Y, Soma T, Watanabe G, et al. Significance of nerve growth factor overexpression and its autocrine loop in oesophageal squamous cell carcinoma. Br J Cancer. 2006;95:322–30.
Zhu ZW, Kleeff J, Kayed H, Wang L, Korc M, Buchler MW, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35:138–47.
Brunetto de Farias C, Rosemberg DB, Heinen TE, Koehler-Santos P, Abujamra AL, Kapczinski F, et al. BDNF/TrkB content and interaction with gastrin-releasing peptide receptor blockade in colorectal cancer. Oncology. 2010;79:430–9.
Mazouffre C, Geyl S, Perraud A, Blondy S, Jauberteau MO, Mathonnet M, et al. Dual inhibition of BDNF/TrkB and autophagy: a promising therapeutic approach for colorectal cancer. J Cell Mol Med. 2017;21:2610–22.
Genevois AL, Ichim G, Coissieux MM, Lambert MP, Lavial F, Goldschneider D, et al. Dependence receptor TrkC is a putative colon cancer tumor suppressor. P Natl Acad Sci USA. 2013;110:3017–22.
Oakley F, Trim N, Constandinou CM, Ye W, Gray AM, Frantz G, et al. Hepatocytes express nerve growth factor during liver injury: evidence for paracrine regulation of hepatic stellate cell apoptosis. Am J Pathol. 2003;163:1849–58.
Yuanlong H, Haifeng J, Xiaoyin Z, Jialin S, Jie L, Li Y, et al. The inhibitory effect of p75 neurotrophin receptor on growth of human hepatocellular carcinoma cells. Cancer Lett. 2008;268:110–9.
Rasi G, Serafino A, Bellis L, Lonardo MT, Andreola F, Zonfrillo M, et al. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J Gastroenterol. 2007;13:4986–95.
Xu XQ, Lu XM, Chen LP, Peng K, Ji FH. Downregulation of MMP1 functions in preventing perineural invasion of pancreatic cancer through blocking the NT-3/TrkC signaling pathway. J Clin Lab Anal. 2022;36:e24719.
Herner A, Sauliunaite D, Michalski CW, Erkan M, De Oliveira T, Abiatari I, et al. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int J Cancer. 2011;129:2349–59.
Watanabe K, Kanno T, Oshima T, Miwa H, Tashiro C, Nishizaki T. The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem Bioph Res Co. 2008;367:487–90.
Chang HJ, Yoo BC, Lim SB, Jeong SY, Kim WH, Park JG. Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin Cancer Res. 2005;11:3288–95.
Maemura K, Shiraishi N, Sakagami K, Kawakami K, Inoue T, Murano M, et al. Proliferative effects of gamma-aminobutyric acid on the gastric cancer cell line are associated with extracellular signal-regulated kinase 1/2 activation. J Gastroenterol Hepatol. 2009;24:688–96.
Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, et al. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67:9704–12.
Wang T, Huang W, Chen F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. 2008;82:536–41.
Calaf GM, Crispin LA, Munoz JP, Aguayo F, Bleak TC. Muscarinic receptors associated with cancer. Cancers. 2022;14:2322.
Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34.
Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang ZY, Macchini M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8:1458–73.
Shan T, Cui XJ, Li W, Lin WR, Li YM, Chen X, et al. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014;105:847–56.
Lu YJ, Geng ZJ, Sun XY, Li YH, Fu XB, Zhao XY, et al. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol Cell Biochem. 2015;408:1–13.
Shi M, Liu D, Duan HJ, Han CL, Wei B, Qian L, et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer. 2010;9:269.
Qian WK, Lv SF, Li J, Chen K, Jiang ZD, Cheng L, et al. Norepinephrine enhances cell viability and invasion and inhibits apoptosis of pancreatic cancer cells in a Notch-1-dependent manner. Oncol Rep. 2018;40:3015–23.
Zhang D, Ma Q, Wang Z, Zhang M, Guo K, Wang F, et al. beta2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathway. Mol Cancer. 2011;10:146.
Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. beta 2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75–90.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74.
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91.
Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77–82.
Aurello P, Berardi G, Tierno SM, Vinciguerra GLR, Socciarelli F, Laracca GG, et al. Influence of perineural invasion in predicting overall survival and disease-free survival in patients With locally advanced gastric cancer. Am J Surg. 2017;213:748–53.
Zhang FX, Chen HX, Luo DD, Xiong ZZ, Li XZ, Yin S, et al. Lymphovascular or perineural invasion is associated with lymph node metastasis and survival outcomes in patients with gastric cancer. Cancer Med. 2023;12:9401–8.
Wang J, Chen Y, Li X, Zou X. Perineural invasion and associated pain transmission in pancreatic cancer. Cancers. 2021;13:4594.
Qin T, Xiao Y, Qian W, Wang X, Gong M, Wang Q, et al. HGF/c-Met pathway facilitates the perineural invasion of pancreatic cancer by activating the mTOR/NGF axis. Cell Death Dis. 2022;13:387.
Hirth M, Gandla J, Hoper C, Gaida MM, Agarwal N, Simonetti M, et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology. 2020;159:665–81.e613.
Jia XZ, Lu MJ, Rui C, Xiao Y. Consensus-expressed CXCL8 and MMP9 identified by meta-analyzed perineural invasion gene signature in gastric cancer microarray data. Front Genet. 2019;10:851.
Xia QJ, Bai QR, Dong MS, Sun XC, Zhang HH, Cui JX, et al. Interaction between gastric carcinoma cells and neural cells promotes perineural invasion by a pathway involving VCAM1. Dig Dis Sci. 2015;60:3283–92.
Bai J, Zhang X, Shi D, Xiang Z, Wang S, Yang C, et al. Exosomal miR-128-3p promotes epithelial-to-mesenchymal transition in colorectal cancer cells by targeting FOXO4 via TGF-beta/SMAD and JAK/STAT3 signaling. Front Cell Dev Biol. 2021;9:568738.
Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578:449–54.
Lu R, Fan C, Shangguan W, Liu Y, Li Y, Shang Y, et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct Target Ther. 2017;2:16036.
Mauffrey P, Tchitchek N, Barroca V, Bemelmans AP, Firlej V, Allory Y, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569:672–8.
Olar A, He D, Florentin D, Ding Y, Ayala G. Biologic correlates and significance of axonogenesis in prostate cancer. Hum Pathol. 2014;45:1358–64.
Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, Tredici G. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther. 2011;6:82–92.
Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, et al. Cancer exosomes induce tumor innervation. Nat Commun. 2018;9:4284.
He B, Gao R, Lv S, Chen A, Huang J, Wang L, et al. Cancer cell employs a microenvironmental neural signal trans-activating nucleus-mitochondria coordination to acquire stemness. Signal Transduct Target Ther. 2023;8:275.
Sitaraman R. Helicobacter pylori DNA methyltransferases and the epigenetic field effect in cancerization. Front Microbiol. 2014;5:115.
Grat M, Wronka KM, Krasnodebski M, Masior L, Lewandowski Z, Kosinska I, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transpl Proc. 2016;48:1687–91.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013.
Liu L, Huh JR, Shah K. Microbiota and the gut-brain-axis: implications for new therapeutic design in the CNS. EBioMedicine. 2022;77:103908.
Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17:322–32.
Suganya K, Koo BS. Gut-brain axis: role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int J Mol Sci. 2020;21:7551.
Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behavior and brain disorders. Nat Rev Microbiol. 2021;19:241–55.
Chakrabarti A, Geurts L, Hoyles L, Iozzo P, Kraneveld AD, La Fata G, et al. The microbiota-gut-brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate, and how to put knowledge into practice. Cell Mol Life Sci. 2022;79:80.
Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J. 2016;129:2373–80.
Bai J, Bruner DW, Fedirko V, Beitler JJ, Zhou C, Gu J, et al. Gut microbiome associated with the psychoneurological symptom cluster in patients with head and neck cancers. Cancers. 2020;12:2531.
Li Q, Jin M, Liu Y, Jin L. Gut microbiota: its potential roles in pancreatic cancer. Front Cell Infect Microbiol. 2020;10:572492.
Song BC, Bai J. Microbiome-gut-brain axis in cancer treatment-related psychoneurological toxicities and symptoms: a systematic review. Support Care Cancer. 2021;29:605–17.
Wang A, Liu Y, Zeng S, Liu Y, Li W, Wu D, et al. Dietary plant polysaccharides for cancer prevention: role of immune cells and gut microbiota, challenges and perspectives. Nutrients. 2023;15:3019.
Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-gamma production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol. 2005;35:1567–75.
Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–9.
Erin N. Role of sensory neurons, neuroimmune pathways, and transient receptor potential vanilloid 1 (TRPV1) channels in a murine model of breast cancer metastasis. Cancer Immunol Immunother. 2020;69:307–14.
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8.
Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101.
Ping Q, Yan R, Cheng X, Wang W, Zhong Y, Hou Z, et al. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther. 2021;28:984–99.
Zhang J, Li S, Zhao Y, Ma P, Cao Y, Liu C, et al. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling. Ann Transl Med. 2020;8:877.
Shen T, Li Y, Wang D, Su Y, Li G, Shang Z, et al. YAP1-TEAD1 mediates the perineural invasion of prostate cancer cells induced by cancer-associated fibroblasts. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166540.
Shin N, Son GM, Shin DH, Kwon MS, Park BS, Kim HS, et al. Cancer-associated fibroblasts and desmoplastic reactions related to cancer invasiveness in patients with colorectal cancer. Ann Coloproctol. 2019;35:36–46.
Chen XM, An Y, Zhang Y, Xu D, Chen TB, Yang Y, et al. CCL26 is upregulated by nab-paclitaxel in pancreatic cancer-associated fibroblasts and promotes PDAC invasiveness through activation of the PI3K/AKT/mTOR pathway. Acta Bioch Bioph Sin. 2021;53:612–9.
Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng XF, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34.
McAndrews KM, Chen Y, Darpolor JK, Zheng XF, Yang SJ, Carstens JL, et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discov. 2022;12:1580–97.
Zhou D, Zhang Z, He LM, Du J, Zhang F, Sun CK, et al. Conversion of fibroblasts to neural cells by p53 depletion. Cell Rep. 2014;9:2034–42.
Masip M, Veiga A, Belmonte JCI, Simon C. Reprogramming with defined factors: from induced pluripotency to induced transdifferentiation. Mol Hum Reprod. 2010;16:856–68.
Cates K, McCoy MJ, Kwon JS, Liu YJ, Abernathy DG, Zhang B, et al. Deconstructing Stepwise Fate Conversion of Human Fibroblasts to Neurons by MicroRNAs. Cell Stem Cell. 2021;28:127–40.
Ryu J, Hwang NS, Park HH, Park TH. Protein-based direct reprogramming of fibroblasts to neuronal cells using 30Kc19 protein and transcription factor Ascl1. Int J Biochem Cell Biol. 2020;121:105717.
Gohrig A, Detjen KM, Hilfenhaus G, Korner JL, Welzel M, Arsenic R, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74:1529–40.
Jessen KR, Arthur-Farraj P. Repair Schwann cell update: adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019;67:421–37.
Tang PC, Chung JY, Liao J, Chan MK, Chan AS, Cheng G, et al. Single-cell RNA sequencing uncovers a neuron-like macrophage subset associated with cancer pain. Sci Adv. 2022;8:eabn5535.
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol. 2008;9:61–72.
Cives M, Strosberg J. The expanding role of somatostatin analogs in gastroenteropancreatic and lung neuroendocrine tumors. Drugs. 2015;75:847–58.
Sei Y, Zhao X, Forbes J, Szymczak S, Li Q, Trivedi A, et al. A hereditary form of small intestinal carcinoid associated with a germline mutation in inositol polyphosphate multikinase. Gastroenterology. 2015;149:67–78.
Jernman J, Kallio P, Hagström J, Välimäki M, Haapasalo H, Alitalo K, et al. PROX1 is involved in progression of rectal neuroendocrine tumors, NETs. Virchows Arch. 2015;467:279–84.
Ebeling T, Vierimaa O, Kytölä S, Leisti J, Salmela PI. Effect of multiple endocrine neoplasia type 1 (MEN1) gene mutations on premature mortality in familial MEN1 syndrome with founder mutations. J Clin Endocr Metab. 2004;89:3392–6.
Kaelin WG. The von Hippel-Lindau tumor suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–73.
Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, et al. The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351–62.
Liu D, Yang Z, Wang T, Yang Z, Chen H, Hu Y, et al. beta2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene. 2016;35:47–58.
Ali O, Tolaymat M, Hu S, Xie G, Raufman JP. Overcoming obstacles to targeting muscarinic receptor signaling in colorectal cancer. Int J Mol Sci. 2021;22:716.
Jin W. Roles of TrkC signaling in the regulation of tumorigenicity and metastasis of cancer. Cancers. 2020;12:147.
D’Souza RS, Her YF, Jin MY, Morsi M, Abd-Elsayed A. Neuromodulation therapy for chemotherapy-induced peripheral neuropathy: a systematic review. Biomedicines. 2022;10:1909.
Magee DJ, Schutzer-Weissmann J, Pereira EAC, Brown MRD. Neuromodulation techniques for cancer pain management. Curr Opin Support Palliat Care. 2021;15:77–83.
Payne SC, Furness JB, Stebbing MJ. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol. 2019;16:89–105.
Abell TL, Chen J, Emmanuel A, Jolley C, Sarela AI, Tornblom H. Neurostimulation of the gastrointestinal tract: review of recent developments. Neuromodulation. 2015;18:221–7.
Acknowledgements
We also appreciate the technical support from Core Utilities of Cancer Genomics and Pathobiology of the Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong.
Funding
This study is supported by the National Natural Science Foundation of China (NSFC) (No. 82272990), Health and Medical Research Fund (HMRF, 08190586), CUHK direct research grant (2022.001, 2020.004), and Cheng Yue Pui Charity Foundation.
Author information
Authors and Affiliations
Contributions
WK offered directions on this manuscript. YL, FX and BC reviewed the literature and drafted this manuscript. WSS, WC, YH, KTL, GMKT, JY and KFT reviewed the manuscript and gave comments. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lyu, Y., Xie, F., Chen, B. et al. The nerve cells in gastrointestinal cancers: from molecular mechanisms to clinical intervention. Oncogene 43, 77–91 (2024). https://doi.org/10.1038/s41388-023-02909-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02909-x