Abstract
Maintenance of gastrointestinal health is challenging as it requires balancing multifaceted processes within the highly complex and dynamic ecosystem of the gastrointestinal tract. Disturbances within this vibrant environment can have detrimental consequences, including the onset of gastrointestinal cancers. Globally, gastrointestinal cancers account for ~19% of all cancer cases and ~22.5% of all cancer-related deaths. Developing new ways to more readily detect and more efficiently target these malignancies are urgently needed. Whereas members of the tumour microenvironment, such as immune cells and fibroblasts, have already been in the spotlight as key players of cancer initiation and progression, the importance of the nervous system in gastrointestinal cancers has only been highlighted in the past few years. Although extrinsic innervations modulate gastrointestinal cancers, cells and signals from the gut’s intrinsic innervation also have the ability to do so. Here, we shed light on this thriving field and discuss neural influences during gastrointestinal carcinogenesis. We focus on the interactions between neurons and components of the gastrointestinal tract and tumour microenvironment, on the neural signalling pathways involved, and how these factors affect the cancer hallmarks, and discuss the neural signatures in gastrointestinal cancers. Finally, we highlight neural-related therapies that have potential for the management of gastrointestinal cancers.
Key points
-
The gut is a highly innervated, dynamic ecosystem wherein nerves are key for intestinal functioning and homeostasis by communicating with a variety of cell types and the gut microbiome.
-
Neural contributions to gastrointestinal cancers represent a flourishing area of investigation as both intrinsic and extrinsic nerves influence gastrointestinal tumorigenesis via their interplay with cancer cells.
-
Neural-related signals and pathways can influence the cancer hallmarks, interfering with several cancer cell characteristics (metabolism and (epi)genomic stability) and/or supporting a cancer-promoting microenvironment (immune infiltration, extracellular matrix).
-
While neoneurogenesis and axonogenesis are emerging within the gastrointestinal cancer field, both topics require in-depth investigation to identify their exact origin and driving mechanisms.
-
Cancer cells are able to hijack (embryonic) neural pathways to promote their own fitness.
-
Targeting neural cell-derived messengers and their respective receptors holds great promise in the treatment of gastrointestinal cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Anderson, N. M. & Simon, M. C. The tumor microenvironment. Curr. Biol. 30, R921–R925 (2020).
Quante, M., Varga, J., Wang, T. C. & Greten, F. R. The gastrointestinal tumor microenvironment. Gastroenterology 145, 63–78 (2013).
Zahalka, A. H. & Frenette, P. S. Nerves in cancer. Nat. Rev. Cancer 20, 143–157 (2020). Prominent review highlighting the importance of nerves within the TME in a variety of cancer types.
Mancino, M., Ametller, E., Gascon, P. & Almendro, V. The neuronal influence on tumor progression. Biochim. Biophys. Acta 1816, 105–118 (2011).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022). Timely revisited view on the hallmarks of cancer, building on the original hallmarks.
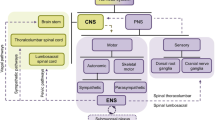
Furness, J. B., Callaghan, B. P., Rivera, L. R. & Cho, H. J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
Langley, J. N. The autonomic nervous system. Brain 26, 1–26 (1903).
Furness, J. B. The Enteric Nervous System 1st edn, 288 (Blackwell Publishing, 2006).
Bon-Frauches, A. C. & Boesmans, W. The enteric nervous system: the hub in a star network. Nat. Rev. Gastroenterol. Hepatol. 17, 717–718 (2020).
Rao, M. & Gershon, M. D. The bowel and beyond: the enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 13, 517–528 (2016).
Niesler, B., Kuerten, S., Demir, I. E. & Schafer, K. H. Disorders of the enteric nervous system - a holistic view. Nat. Rev. Gastroenterol. Hepatol. 18, 393–410 (2021).
Holland, A. M., Bon-Frauches, A. C., Keszthelyi, D., Melotte, V. & Boesmans, W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol. Life Sci. 78, 4713–4733 (2021).
Rademakers, G. et al. The role of enteric neurons in the development and progression of colorectal cancer. Biochim. Biophys. Acta 1868, 420–434 (2017). The first review summarizing the importance of enteric neurons in colorectal carcinogenesis, focusing on the link between the ENS, inflammation and CRC.
Schledwitz, A., Xie, G. & Raufman, J. P. Exploiting unique features of the gut-brain interface to combat gastrointestinal cancer. J. Clin. Invest. https://doi.org/10.1172/JCI143776 (2021).
Griffin, N. et al. Clinicopathological significance of nerves in esophageal cancer. Am. J. Pathol. 190, 1921–1930 (2020).
Xu, G. et al. Prognosis and progression of ESCC patients with perineural invasion. Sci. Rep. 7, 43828 (2017).
Deng, J. et al. Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS ONE 9, e88907 (2014).
Tianhang, L., Guoen, F., Jianwei, B. & Liye, M. The effect of perineural invasion on overall survival in patients with gastric carcinoma. J. Gastrointest. Surg. 12, 1263–1264 (2008).
Knijn, N., Mogk, S. C., Teerenstra, S., Simmer, F. & Nagtegaal, I. D. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am. J. Surg. Pathol. 40, 103–112 (2016). Systematic review depicting the prominence of perineural invasion by summarizing all evidence for its prognostic value for CRC.
Liebig, C. et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 27, 5131–5137 (2009).
Zhao, C. M. et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.3009569 (2014). Leading research article uncovering the functional importance of parasympathetic nerves in gastric cancer and their highly valuable potential as therapeutic targets. This article paved the way to gain more insights into the role of the nervous system in (gastric) cancer.
Albo, D. et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 117, 4834–4845 (2011).
Mauffrey, P. et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678 (2019).
Lu, R. et al. Neurons generated from carcinoma stem cells support cancer progression. Signal. Transduct. Target. Ther. 2, 16036 (2017).
Weinstein, V. A., Druckerman, L. J., Lyons, A. S. & Colp, R. Gastroenterostomy and vagotomy in the treatment of duodenal ulcer. Ann. Surg. 141, 482–487 (1955).
Kraft, R. O., Myers, J., Overton, S. & Fry, W. J. Vagotomy and the gastric ulcer. Am. J. Surg. 121, 122–128 (1971).
Calmels, S., Bereziat, J. C., Ohshima, H. & Bartsch, H. Bacterial formation of N-nitroso compounds from administered precursors in the rat stomach after omeprazole-induced achlorhydria. Carcinogenesis 12, 435–439 (1991).
Moller, H., Nissen, A. & Mosbech, J. Use of cimetidine and other peptic ulcer drugs in Denmark 1977-1990 with analysis of the risk of gastric cancer among cimetidine users. Gut 33, 1166–1169 (1992).
Rabben, H. L., Zhao, C. M., Hayakawa, Y., Wang, T. C. & Chen, D. Vagotomy and gastric tumorigenesis. Curr. Neuropharmacol. 14, 967–972 (2016).
Tatsuta, M., Iishi, H., Yamamura, H., Baba, M. & Taniguchi, H. Effects of bilateral and unilateral vagotomy on gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Int. J. Cancer 42, 414–418 (1988).
Kim, S. J. & Choi, C. W. Common locations of gastric cancer: review of research from the endoscopic submucosal dissection era. J. Korean Med. Sci. 34, e231 (2019).
Cassaro, M. et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am. J. Gastroenterol. 95, 1431–1438 (2000).
Liu, V. et al. Extrinsic intestinal denervation modulates tumor development in the small intestine of ApcMin/+ mice. J. Exp. Clin. Cancer Res. 34, 39 (2015).
Nelson, R. L., Briley, S., Vaz, O. P. & Abcarian, H. The effect of vagotomy and pyloroplasty on colorectal tumor induction in the rat. J. Surg. Oncol. 51, 281–286 (1992).
Bayón Lara, A. M. et al. Colonic carcinogenesis in vagotomyzed rats. Rev. Esp. Enferm. Dig. 93, 576–586 (2001).
Caygill, C. P., Hill, M. J., Hall, C. N., Kirkham, J. S. & Northfield, T. C. Increased risk of cancer at multiple sites after gastric surgery for peptic ulcer. Gut 28, 924–928 (1987).
Bundred, N. J. et al. Gastric surgery and the risk of subsequent colorectal cancer. Br. J. Surg. 72, 618–619 (1985).
Watt, P. C., Patterson, C. C. & Kennedy, T. L. Late mortality after vagotomy and drainage for duodenal ulcer. Br. Med. J. 288, 1335–1338 (1984).
Tatsuta, M., Iishi, H., Baba, M. & Taniguchi, H. Inhibitions by 6-hydroxydopamine and neostigmine singly or together of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Int. J. Cancer 51, 767–771 (1992).
Tatsuta, M., Iishi, H., Baba, M. & Taniguchi, H. Inhibition of azoxymethane-induced experimental colon carcinogenesis in Wistar rats by 6-hydroxydopamine. Int. J. Cancer 50, 298–301 (1992).
Sadighparvar, S., Darband, S. G., Ghaderi-Pakdel, F., Mihanfar, A. & Majidinia, M. Parasympathetic, but not sympathetic denervation, suppressed colorectal cancer progression. Eur. J. Pharmacol. 913, 174626 (2021).
Miyato, H., Kitayama, J., Ishigami, H., Kaisaki, S. & Nagawa, H. Loss of sympathetic nerve fibers around intratumoral arterioles reflects malignant potential of gastric cancer. Ann. Surg. Oncol. 18, 2281–2288 (2011).
Gao, J. & Liu, S. G. Role of sympathetic and parasympathetic nerves in the development of gastric cancer through antagonism. Chin. Med. J. 134, 908–909 (2021).
Bae, G. E. et al. Lower sympathetic nervous system density and beta-adrenoreceptor expression are involved in gastric cancer progression. Anticancer Res. 39, 231–236 (2019).
Borresen, T., Henriksen, O. B. & Christensen, N. J. Decreased norepinephrine concentration in normal tissue neighboring a malignant tumor. Cancer Res. 40, 4320–4321 (1980).
Garcia, S. B., Minto, S. B., Marques, I. D. S. & Kannen, V. Myenteric denervation of the gut with benzalkonium chloride: a review of forty years of an experimental model. Can. J. Gastroenterol. Hepatol. 2019, 3562492 (2019).
Zucoloto, S. et al. Effect of chemical ablation of myenteric neurones on intestinal cell proliferation. Cell Tissue Kinet. 21, 213–219 (1988).
Romanello, L. M. F. Effect of destruction of myenteric neurons of rat stomach by benzalkonium chloride on gastric acid secretion and gastrin release. Gut 32, 594 (1991).
Febronio, L. H., Britto-Garcia, S., de Oliveira, J. S. & Zucoloto, S. Megaesophagus in rats. Res. Exp. Med. 197, 109–115 (1997).
Polli-Lopes, A. C., Zucoloto, S., de Queiros Cunha, F., da Silva Figueiredo, L. A. & Garcia, S. B. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 190, 45–50 (2003).
Vespucio, M. V. et al. Intrinsic denervation of the colon is associated with a decrease of some colonic preneoplastic markers in rats treated with a chemical carcinogen. Braz. J. Med. Biol. Res. 41, 311–317 (2008).
Estofolete, C. F., Zucoloto, S., Oliani, S. M., Polli-Lopes, A. C. & Gil, C. D. Myenteric denervation downregulates galectin-1 and -3 expression in gastric carcinogenesis. Dig. Dis. Sci. 56, 1637–1644 (2011).
Sato, A. et al. Pathophysiology of aganglionic colon and anorectum: an experimental study on aganglionosis produced by a new method in the rat. J. Pediatr. Surg. 13, 399–435 (1978).
Garcia, S. B., Oliveira, J. S. M., Pinto, L. Z., Muccillo, G. & Zucoloto, S. The relationship between megacolon and carcinoma of the colon: an experimental approach. Carcinogenesis 17, 1777–1779 (1996).
Munari, F. F. et al. The relationship between esophageal cancer, chagasic megaesophagus and HPV: myths, tales or reality? Histol. Histopathol. 33, 1135–1149 (2018).
Kennedy, M. F., Tutton, P. J. & Barkla, D. H. Adrenergic factors involved in the control of crypt cell proliferation in jejunum and descending colon of mouse. Clin. Exp. Pharmacol. Physiol. 10, 577–586 (1983).
Berthoud, H. R., Carlson, N. R. & Powley, T. L. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260, R200–R207 (1991).
Aloe, L. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 149, 175–181 (2011).
Liu, S. M. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroent. Motil. https://doi.org/10.1111/nmo.13446 (2018).
Schonkeren, S. L., Thijssen, M. S., Vaes, N., Boesmans, W. & Melotte, V. The emerging role of nerves and glia in colorectal cancer. Cancers https://doi.org/10.3390/cancers13010152 (2021).
Hayakawa, Y. et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31, 21–34 (2017). Prominent research paper describing that cholinergic signalling affects gastric carcinogenesis in vitro and in vivo.
Wang, L. et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour Biol. 37, 2105–2117 (2016).
Cheng, K. et al. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G591–G597 (2008).
Yang, W. L. & Frucht, H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis 21, 1789–1793 (2000).
Frucht, H., Gazdar, A. F., Park, J. A., Oie, H. & Jensen, R. T. Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res. 52, 1114 (1992).
Cheng, K. R., Zimniak, P. & Raufman, J. P. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of h508 human colon cancer cells. Cancer Res. 63, 6744–6750 (2003).
Yu, H. et al. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 7, 40802–40802 (2017).
Raufman, J. P. et al. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 68, 3573–3578 (2008).
Cai, J., Maitra, A., Anders, R. A., Taketo, M. M. & Pan, D. β-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 29, 1493–1506 (2015).
Rosenbluh, J. et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473 (2012).
Schaak, S. et al. Alpha2 adrenoceptors regulate proliferation of human intestinal epithelial cells. Gut 47, 242–250 (2000).
Peng, Z., Heath, J., Drachenberg, C., Raufman, J. P. & Xie, G. Cholinergic muscarinic receptor activation augments murine intestinal epithelial cell proliferation and tumorigenesis. BMC Cancer 13, 204 (2013).
Xie, G., Cheng, K., Shant, J. & Raufman, J. P. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G755–G763 (2009).
Zhong, L. R., Estes, S., Artinian, L. & Rehder, V. Acetylcholine elongates neuronal growth cone filopodia via activation of nicotinic acetylcholine receptors. Dev. Neurobiol. 73, 487–501 (2013).
Jobling, P. et al. Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res. 75, 1777–1781 (2015).
Buffin-Meyer, B. et al. EGF receptor transactivation and PI3-kinase mediate stimulation of ERK by α2A-adrenoreceptor in intestinal epithelial cells: a role in wound healing. Eur. J. Pharmacol. 574, 85–93 (2007).
Zhang, X. et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 10, 788 (2019).
Ogawa, H. et al. Prognostic significance of β2-adrenergic receptor expression in patients with surgically resected colorectal cancer. Int. J. Clin. Oncol. 25, 1137–1144 (2020).
Shi, M. et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol. Cancer 9, 269 (2010).
Shan, T. et al. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 105, 847–856 (2014).
Lu, Y. J. et al. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol. Cell Biochem. 408, 1–13 (2015).
Njeim, R. & Eid, A. Alpha-2c adrenergic receptor promotes the malignant phenotype of colon cancer cells. FASEB J. 32, 695.5 (2018).
Zhi, X. et al. Adrenergic modulation of AMPKdependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int. J. Oncol. 54, 1625–1638 (2019).
Chin, C. C. et al. Selective β2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J. Cell Physiol. 231, 459–472 (2016).
Griffin, N. et al. The neurotrophic tyrosine kinase receptor 1 (TrkA) is overexpressed in oesophageal squamous cell carcinoma. Pathology 53, 470–477 (2021).
Okumura, T. et al. The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin. Cancer Res. 12, 5096–5103 (2006).
Chen, H. et al. NGFR increases the chemosensitivity of colorectal cancer cells by enhancing the apoptotic and autophagic effects of 5-fluorouracil via the activation of S100A9. Front. Oncol. 11, 652081 (2021).
Yang, Z. et al. Epigenetic inactivation and tumor-suppressor behavior of NGFR in human colorectal cancer. Mol. Cancer Res. 13, 107–119 (2015).
Allen, J. K. et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 78, 3233–3242 (2018).
Akil, H., Perraud, A., Melin, C., Jauberteau, M. O. & Mathonnet, M. Fine-tuning roles of endogenous brain-derived neurotrophic factor, TrkB and sortilin in colorectal cancer cell survival. PLoS ONE 6, e25097 (2011).
Perrone, M. G., Notarnicola, M., Caruso, M. G., Tutino, V. & Scilimati, A. Upregulation of beta3-adrenergic receptor mRNA in human colon cancer: a preliminary study. Oncology 75, 224–229 (2008).
Douma, S. et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430, 1034–1039 (2004).
Fujikawa, H. et al. High TrkB expression levels are associated with poor prognosis and EMT induction in colorectal cancer cells. J. Gastroenterol. 47, 775–784 (2012).
Meng, L. et al. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 17, 2031–2039 (2019).
de Farias, C. B. et al. BDNF/TrkB signaling protects HT-29 human colon cancer cells from EGFR inhibition. Biochem. Biophys. Res. Commun. 425, 328–332 (2012).
Huang, S. M. et al. Brain-derived neurotrophic factor regulates cell motility in human colon cancer. Endocr. Relat. Cancer 22, 455–464 (2015).
Yang, X., Martin, T. A. & Jiang, W. G. Biological influence of brain-derived neurotrophic factor (BDNF) on colon cancer cells. Exp. Ther. Med. 6, 1475–1481 (2013).
Mescher, A. L., Connell, E., Hsu, C., Patel, C. & Overton, B. Transferrin is necessary and sufficient for the neural effect on growth in amphibian limb regeneration blastemas. Dev. Growth Differ. 39, 677–684 (1997).
Wang, L., Marchionni, M. A. & Tassava, R. A. Cloning and neuronal expression of a type III newt neuregulin and rescue of denervated, nerve-dependent newt limb blastemas by rhGGF2. J. Neurobiol. 43, 150–158 (2000).
Smith, M. J., Globus, M. & Vethamany-Globus, S. Nerve extracts and substance P activate the phosphatidylinositol signaling pathway and mitogenesis in newt forelimb regenerates. Dev. Biol. 167, 239–251 (1995).
Boesmans, W. et al. Development, diversity, and neurogenic capacity of enteric glia. Front. Cell Dev. Biol. 9, 775102 (2021).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000). Landmark paper describing the original hallmarks of cancer, that is, the acquired, functional and biological capacities that govern neoplastic transformation.
Senga, S. S. & Grose, R. P. Hallmarks of cancer-the new testament. Open Biol. https://doi.org/10.1098/rsob.200358 (2021). New perspective to add neuronal signalling to the hallmarks of cancer.
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Wang, W. et al. Nerves in the tumor microenvironment: origin and effects. Front. Cell Dev. Biol. 8, 601738 (2020).
Klagsbrun, M. & Eichmann, A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor. Rev. 16, 535–548 (2005).
Gysler, S. M. & Drapkin, R. Tumor innervation: peripheral nerves take control of the tumor microenvironment. J. Clin. Invest. https://doi.org/10.1172/JCI147276 (2021).
Ekstrand, A. J. et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc. Natl Acad. Sci. USA 100, 6033–6038 (2003).
Nuevo-Tapioles, C. et al. Coordinate beta-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat. Commun. 11, 3606 (2020).
Chen, S. H. et al. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 9, 1–21 (2019).
Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017). One of the first studies to indicate that the nervous system affects the angiometabolic switch in prostate cancer cells.
Liu, J. et al. The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 52, 130–142 (2015).
Zamani, A. & Qu, Z. C. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett. 586, 2360–2365 (2012).
Nocito, A. et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 68, 5152–5158 (2008).
Chakroborty, D. et al. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 10, 4349–4356 (2004).
Sarkar, C. et al. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 287, H1554–H1560 (2004).
Sarkar, C., Chakroborty, D., Chowdhury, U. R., Dasgupta, P. S. & Basu, S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 14, 2502–2510 (2008).
Basu, S. et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7, 569–574 (2001).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Hering, N. A. et al. Blockage of cholinergic signaling via muscarinic acetylcholine receptor 3 inhibits tumor growth in human colorectal adenocarcinoma. Cancers 13, 3220 (2021).
Belo, A. et al. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G749–G760 (2011).
Raufman, J. P. et al. Muscarinic receptor agonists stimulate matrix metalloproteinase 1-dependent invasion of human colon cancer cells. Biochem. Biophys. Res. Commun. 415, 319–324 (2011).
Sawayama, H., Ishimoto, T. & Baba, H. Microenvironment in the pathogenesis of gastric cancer metastasis. J. Cancer Metastasis Treat. 4, 10 (2018).
Samantaray, S., Sharma, R., Chattopadhyaya, T. K., Gupta, S. D. & Ralhan, R. Increased expression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 130, 37–44 (2004).
Li, Y. et al. Overexpression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. Dis. Esophagus 22, 664–667 (2009).
Masur, K., Niggemann, B., Zanker, K. S. & Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 61, 2866–2869 (2001).
Lei, Y. et al. Nerve growth factor orchestrates NGAL and matrix metalloproteinases activity to promote colorectal cancer metastasis. Clin. Transl. Oncol. https://doi.org/10.1007/s12094-021-02666-x (2021).
Jin, H. F. et al. P75 neurotrophin receptor inhibits invasion and metastasis of gastric cancer. Mol. Cancer Res. 5, 423–433 (2007).
Okugawa, Y. et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br. J. Cancer 108, 121–130 (2013).
Szpunar, M. J., Burke, K. A., Dawes, R. P., Brown, E. B. & Madden, K. S. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev. Res. 6, 1262–1272 (2013).
Ganguly, D. et al. Cancer-associated fibroblasts: versatile players in the tumor microenvironment. Cancers https://doi.org/10.3390/cancers12092652 (2020).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Ping, Q. R. et al. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther. 28, 984–999 (2021).
Kobayashi, H. et al. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastro Hepat. 16, 282–295 (2019).
Akbareian, S. E. et al. Enteric neural crest-derived cells promote their migration by modifying their microenvironment through tenascin-C production. Dev. Biol. 382, 446–456 (2013).
Nagy, N. et al. Collagen 18 and agrin are secreted by neural crest cells to remodel their microenvironment and regulate their migration during enteric nervous system development. Development https://doi.org/10.1242/dev.160317 (2018).
Vaes, N. et al. Loss of enteric neuronal Ndrg4 promotes colorectal cancer via increased release of Nid1 and Fbln2. EMBO Rep. https://doi.org/10.15252/embr.202051913 (2021). Research paper addressing the role of enteric neural-derived ECM proteins in CRC.
Estofolete, C. F. et al. Effects of myenteric denervation on extracellular matrix fibers and mast cell distribution in normal stomach and gastric lesions. Cancer Cell Int. 10, 18 (2010).
Li, J. et al. Elastin is a key factor of tumor development in colorectal cancer. BMC Cancer https://doi.org/10.1186/s12885-020-6686-x (2020).
Wang, Y., Song, E. C. & Resnick, M. B. in Tumor Microenvironment: Extracellular Matrix Components – Part B (ed. Birbrair, A.) 1–16 (Springer International Publishing, 2020).
Patel, P. R. et al. Norepinephrine reduces reactive oxygen species (ROS) and DNA damage in ovarian surface epithelial cells. J. Bioanal. Biomed. 7, 75–80 (2015).
Rizzi, E. et al. β1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophy. Free Radic. Biol. Med. 73, 308–317 (2014).
Sakita, J. Y. et al. Serotonin synthesis protects the mouse colonic crypt from DNA damage and colorectal tumorigenesis. J. Pathol. 249, 102–113 (2019).
Eckerling, A., Ricon-Becker, I., Sorski, L., Sandbank, E. & Ben-Eliyahu, S. Stress and cancer: mechanisms, significance and future directions. Nat. Rev. Cancer 21, 767–785 (2021).
Hara, M. R. et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011).
Sun, F. et al. Adrenergic DNA damage of embryonic pluripotent cells via β2 receptor signalling. Sci. Rep. 5, 15950 (2015).
Lamboy-Caraballo, R. et al. Norepinephrine-induced DNA damage in ovarian cancer cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21062250 (2020).
Dialynas, G. K., Vitalini, M. W. & Wallrath, L. L. Linking heterochromatin protein 1 (HP1) to cancer progression. Mutat. Res. 647, 13–20 (2008).
Ruginis, T., Taglia, L., Matusiak, D., Lee, B. S. & Benya, R. V. Consequence of gastrin-releasing peptide receptor activation in a human colon cancer cell line: a proteomic approach. J. Proteome Res. 5, 1460–1468 (2006).
Zeng, W., Ball, A. R. Jr. & Yokomori, K. HP1: heterochromatin binding proteins working the genome. Epigenetics 5, 287–292 (2010).
Cornelio, D. B., Roesler, R. & Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 18, 1457–1466 (2007).
Tell, R. et al. Gastrin-releasing peptide signaling alters colon cancer invasiveness via heterochromatin protein 1Hsβ. Am. J. Pathol. 178, 672–678 (2011).
Schwartsmann, G. et al. A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Invest. New Drugs 24, 403–412 (2006).
Brunetto de Farias, C. et al. BDNF/TrkB content and interaction with gastrin-releasing peptide receptor blockade in colorectal cancer. Oncology 79, 430–439 (2010).
Rick, F. G. et al. Combination of gastrin-releasing peptide antagonist with cytotoxic agents produces synergistic inhibition of growth of human experimental colon cancers. Cell Cycle 11, 2518–2525 (2012).
Zhao, S. et al. Histone H3Q5 serotonylation stabilizes H3K4 methylation and potentiates its readout. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2016742118 (2021).
Ye, D. et al. Targeting SERT promotes tryptophan metabolism: mechanisms and implications in colon cancer treatment. J. Exp. Clin. Cancer Res. 40, 173 (2021).
Rabben, H. L. et al. Neural signaling modulates metabolism of gastric cancer. iScience 24, 102091 (2021). Important study identifying a metabolic signature in gastric cancer as well as the reversibility of this metabolic reprogramming by vagotomy.
Song, Z., Wei, B., Lu, C., Li, P. & Chen, L. Glutaminase sustains cell survival via the regulation of glycolysis and glutaminolysis in colorectal cancer. Oncol. Lett. 14, 3117–3123 (2017).
Hao, Y. et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat. Commun. 7, 11971 (2016).
Qie, S. et al. Targeting glutamine-addiction and overcoming CDK4/6 inhibitor resistance in human esophageal squamous cell carcinoma. Nat. Commun. 10, 1296 (2019).
Zhao, Y. et al. Colorectal cancers utilize glutamine as an anaplerotic substrate of the TCA cycle in vivo. Sci. Rep. 9, 19180 (2019).
Shi, M., Liu, D., Yang, Z. & Guo, N. Central and peripheral nervous systems: master controllers in cancer metastasis. Cancer Metastasis Rev. 32, 603–621 (2013).
Jacobson, A., Yang, D., Vella, M. & Chiu, I. M. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 14, 555–565 (2021).
Hodo, T. W., de Aquino, M. T. P., Shimamoto, A. & Shanker, A. Critical neurotransmitters in the neuroimmune network. Front. Immunol. 11, 1869 (2020).
Qiao, G. et al. β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. 68, 11–22 (2019).
Salmon, H., Remark, R., Gnjatic, S. & Merad, M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer 19, 215–227 (2019).
Wang, H. et al. Role of the nervous system in cancers: a review. Cell Death Discov. 7, 76 (2021).
Rihawi, K. et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: novel translational implications. Int. J. Mol. Sci. 22, 3805 (2021).
Kitamura, T., Qian, B. Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Kuol, N., Stojanovska, L., Apostolopoulos, V. & Nurgali, K. Crosstalk between cancer and the neuro-immune system. J. Neuroimmunol. 315, 15–23 (2018).
Qin, J.-F. et al. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 48, 295–300 (2015).
Lamkin, D. M. et al. β-Adrenergic-stimulated macrophages: comprehensive localization in the M1-M2 spectrum. Brain Behav. Immun. 57, 338–346 (2016).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Matheis, F. et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78.e16 (2020).
Park, J. Y. et al. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin. Res. Hepatol. Gastroenterol. 40, 357–365 (2016).
Nakayama, Y. et al. Relationships between tumor-associated macrophages and clinicopathological factors in patients with colorectal cancer. Anticancer Res. 22, 4291–4296 (2002).
Herrera, M. et al. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 104, 437–444 (2013).
Koelzer, V. H. et al. Phenotyping of tumor-associated macrophages in colorectal cancer: impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology https://doi.org/10.1080/2162402X.2015.1106677 (2016).
Zhong, X. M., Chen, B. & Yang, Z. W. The role of tumor-associated macrophages in colorectal carcinoma progression. Cell Physiol. Biochem. 45, 356–365 (2018).
Pages, F. et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018).
Zhang, Z. et al. Development of a prognostic signature for esophageal cancer based on nine immune related genes. BMC Cancer 21, 113 (2021).
Zhang, N. et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis and experimental validation. Arch. Med. Sci. 16, 1092–1103 (2020).
Pages, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Costa, P. A. C. et al. Chemogenetic modulation of sensory neurons reveals their regulating role in melanoma progression. Acta Neuropathol. Commun. 9, 183 (2021).
Dai, S. et al. Chronic stress promotes cancer development. Front. Oncol. 10, 1492 (2020).
Bucsek, M. J. et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 77, 5639–5651 (2017).
Nakai, A., Hayano, Y., Furuta, F., Noda, M. & Suzuki, K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med. 211, 2583–2598 (2014).
Tavazoie, M. F. et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell 172, 825–840.e18 (2018).
Guo, W. et al. Prognostic value of PD-L1 in esophageal squamous cell carcinoma: a meta-analysis. Oncotarget 9, 13920–13933 (2018).
Li, Y. et al. The prognostic and clinicopathological roles of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. Front. Pharmacol. 10, 139 (2019).
Gu, L. et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS ONE 12, e0182692 (2017).
Wang, L. et al. Immune sculpting of norepinephrine on MHC-I, B7-1, IDO and B7-H1 expression and regulation of proliferation and invasion in pancreatic carcinoma cells. PLoS ONE 7, e45491 (2012).
Mo, R. J. et al. Expression of PD-L1 in tumor-associated nerves correlates with reduced CD8+ tumor-associated lymphocytes and poor prognosis in prostate cancer. Int. J. Cancer 144, 3099–3110 (2019).
Kuol, N. et al. Cholinergic signalling influences the expression of immune checkpoint inhibitors, PD-L1 and PD-L2, in human colorectal cancer tissues and cell lines. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-665274/v1 (2021).
Hou, N. et al. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem. Biophys. Res. Commun. 439, 471–476 (2013).
Qiao, G. X. et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol. Res. 9, 651–664 (2021).
Mitsui, T. et al. Truncal vagotomy temporarily decreases the pro- and anti-inflammatory cytokine levels in the small intestine. Surg. Today 44, 1123–1127 (2014).
Costa, A. C., Santos, J. M. O., Gil da Costa, R. M. & Medeiros, R. Impact of immune cells on the hallmarks of cancer: a literature review. Crit. Rev. Oncol. Hematol. 168, 103541 (2021).
Saloman, J. L. et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl Acad. Sci. USA 113, 3078–3083 (2016).
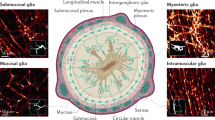
Barres, B. A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008).
Seguella, L. & Gulbransen, B. D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 18, 571–587 (2021). Prominent review discussing enteric glial heterogeneity and their functional roles in gastrointestinal pathophysiology.
Rosenberg, H. J. & Rao, M. Enteric glia in homeostasis and disease: from fundamental biology to human pathology. iScience 24, 102863 (2021).
Boesmans, W. et al. Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 67, 1167–1178 (2019).
Ahmadzai, M. M., Seguella, L. & Gulbransen, B. D. Circuit-specific enteric glia regulate intestinal motor neurocircuits. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2025938118 (2021).
Vergnolle, N. & Cirillo, C. Neurons and glia in the enteric nervous system and epithelial barrier function. Physiology 33, 269–280 (2018).
Neunlist, M. et al. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 90–100 (2013). Renowned review summarizing the importance of the ENS for maintenance of intestinal epithelial barrier integrity, thereby putting forward the digestive 'neuronal–glial–epithelial unit'.
Vales, S. et al. Tumor cells hijack enteric glia to activate colon cancer stem cells and stimulate tumorigenesis. EBioMedicine 49, 172–188 (2019). This paper is one of the first functional studies describing glia as part of the TME.
Neunlist, M. et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G231–G241 (2007).
Liu, Y. A. et al. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol. Motil. 25, e324–e338 (2013).
Godlewski, J. & Kmiec, Z. Colorectal cancer invasion and atrophy of the enteric nervous system: potential feedback and impact on cancer progression. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21093391 (2020).
Yuan, R. et al. Enteric glia play a critical role in promoting the development of colorectal cancer. Front. Oncol. https://doi.org/10.3389/fonc.2020.595892 (2020).
Baghdadi, M. B. et al. Enteric glial cell heterogeneity regulates intestinal stem cell niches. Cell Stem Cell 29, 86–100.e6 (2022).
Progatzky, F. & Pachnis, V. Enteric glia bring fresh WNT to the intestinal stem cell niche. Cell Stem Cell 29, 3–4 (2022).
Boesmans, W. et al. Imaging neuron-glia interactions in the enteric nervous system. Front. Cell Neurosci. https://doi.org/10.3389/fncel.2013.00183 (2013).
Rolig, A. S. et al. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. https://doi.org/10.1371/journal.pbio.2000689 (2017).
Ge, Y. et al. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 40, 42 (2021).
De Vadder, F. et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl Acad. Sci. USA 115, 6458–6463 (2018).
Collins, J., Borojevic, R., Verdu, E. F., Huizinga, J. D. & Ratcliffe, E. M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroent. Motil. 26, 98–107 (2014).
Vicentini, F. A. et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 9, 210 (2021).
Kabouridis, P. S. et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85, 289–295 (2015).
Obata, Y. et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 578, 284–289 (2020).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science https://doi.org/10.1126/science.abc4552 (2021). A detailed overview of the known links between the microbiome and various types of cancer, including the microbiome–cancer–immune crosstalk.
Lofgren, J. L. et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140, 210–220 (2011).
Zhan, Y. et al. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res. 73, 7199–7210 (2013).
Wong, S. H. & Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 16, 690–704 (2019). Summary of the evidence linking CRC to the gut microbiome, with specific attention to the clinical applicability of this knowledge.
Takiishi, T., Fenero, C. I. M. & Camara, N. O. S. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers https://doi.org/10.1080/21688370.2017.1373208 (2017).
Karin, M. & Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315 (2016).
Zhou, J. & Boutros, M. JNK-dependent intestinal barrier failure disrupts host-microbe homeostasis during tumorigenesis. Proc. Natl Acad. Sci. USA 117, 9401–9412 (2020).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Ha, C. W. Y. et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 183, 666–683.e17 (2020).
Piscione, M., Mazzone, M., Di Marcantonio, M. C., Muraro, R. & Mincione, G. Eradication of helicobacter pylori and gastric cancer: a controversial relationship. Front. Microbiol. 12, 630852 (2021).
Butt, J. & Epplein, M. Helicobacter pylori and colorectal cancer — a bacterium going abroad? PLoS Pathog. https://doi.org/10.1371/journal.ppat.1007861 (2019).
Sticlaru, L. et al. Dangerous liaison: helicobacter pylori, ganglionitis, and myenteric gastric neurons: a histopathological study. Anal. Cell Pathol. https://doi.org/10.1155/2019/3085181 (2019).
Gorle, N., Bauwens, E., Haesebrouck, F., Smet, A. & Vandenbroucke, R. E. Helicobacter and the potential role in neurological disorders: there is more than Helicobacter pylori. Front. Immunol. https://doi.org/10.3389/fimmu.2020.584165 (2021).
Ramirez, V. T. et al. T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog. 15, e1007719 (2019).
Colosimo, D. A. et al. Mapping interactions of microbial metabolites with human G-protein-coupled receptors. Cell Host Microbe 26, 273–282.e7 (2019).
Chen, H. W. et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell 177, 1217–1231.e18 (2019).
Choudhry, H. The microbiome and its implications in cancer immunotherapy. Molecules https://doi.org/10.3390/molecules26010206 (2021).
Wang, T. T. et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 6, 320–329 (2012).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Tropepe, V. et al. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30, 65–78 (2001).
Smukler, S. R., Runciman, S. B., Xu, S. & van der Kooy, D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J. Cell Biol. 172, 79–90 (2006).
Przyborski, S. A. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cell 23, 1242–1250 (2005).
Germain, N. D. et al. Teratocarcinoma formation in embryonic stem cell-derived neural progenitor hippocampal transplants. Cell Transpl. 21, 1603–1611 (2012).
Xu, L. et al. Neural stemness contributes to cell tumorigenicity. Cell Biosci. 11, 21 (2021).
Nagy, N. & Goldstein, A. M. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 66, 94–106 (2017).
Munro, M. J., Wickremesekera, S. K., Peng, L., Tan, S. T. & Itinteang, T. Cancer stem cells in colorectal cancer: a review. J. Clin. Pathol. 71, 110–116 (2018).
Sakakibara, S. et al. Mouse-musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230–242 (1996).
Ji, G. et al. PCGF1 promotes epigenetic activation of stemness markers and colorectal cancer stem cell enrichment. Cell Death Dis. 12, 633 (2021).
Guan, A. et al. MiR-330-3p inhibits gastric cancer progression through targeting MSI1. Am. J. Transl. Res. 8, 4802–4811 (2016).
Gao, C. et al. Downregulation of Msi1 suppresses the growth of human colon cancer by targeting p21cip1. Int. J. Oncol. 46, 732–740 (2015).
Yang, W. et al. Identification of hub genes and therapeutic drugs in esophageal squamous cell carcinoma based on integrated bioinformatics strategy. Cancer Cell Int. 19, 142 (2019).
Yang, Y. et al. Identification of regulatory role of DNA methylation in colon cancer gene expression via systematic bioinformatics analysis. Medicine 96, e8487 (2017).
Fan, J. et al. Genome-wide DNA methylation profiles of low- and high-grade adenoma reveals potential biomarkers for early detection of colorectal carcinoma. Clin. Epigenetics 12, 56 (2020).
Rademakers, G. et al. Identification of DNA methylation markers for early detection of CRC indicates a role for nervous system-related genes in CRC. Clin. Epigenetics 13, 80 (2021).
Zhang, Z. et al. Similarity in gene-regulatory networks suggests that cancer cells share characteristics of embryonic neural cells. J. Biol. Chem. 292, 12842–12859 (2017). One of the earliest reports that highlight neural stemness in cancer cells.
Lei, A. et al. EZH2 regulates protein stability via recruiting USP7 to mediate neuronal gene expression in cancer cells. Front. Genet. 10, 422 (2019).
Chang, P. Y. et al. Propranolol reduces cancer risk: a population-based cohort study. Medicine 94, e1097 (2015).
Ahl, R. et al. Effects of beta-blocker therapy on mortality after elective colon cancer surgery: a Swedish nationwide cohort study. BMJ Open 10, e036164 (2020).
Reda, S. et al. Pre-operative beta-blocker therapy does not affect short-term mortality after esophageal resection for cancer. BMC Surg. 20, 333 (2020).
Fiala, O. et al. Incidental use of beta-blockers is associated with outcome of metastatic colorectal cancer patients treated with bevacizumab-based therapy: a single-institution retrospective analysis of 514 patients. Cancers https://doi.org/10.3390/cancers11121856 (2019).
Deng, Y. et al. Effects of antihypertensive drugs use on risk and prognosis of colorectal cancer: a meta-analysis of 37 observational studies. Front. Pharmacol. 12, 670657 (2021).
Liao, X. et al. Effects of propranolol in combination with radiation on apoptosis and survival of gastric cancer cells in vitro. Radiat. Oncol. 5, 98 (2010).
MacDonald, C. R. et al. Adrenergic receptor signaling regulates the response of tumors to ionizing radiation. Radiat. Res. 191, 585–589 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03245554 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04005365 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04682158 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03919461 (2019).
Felton, J., Hu, S. & Raufman, J. P. Targeting M3 muscarinic receptors for colon cancer therapy. Curr. Mol. Pharmacol. 11, 184–190 (2018).
Ali, O., Tolaymat, M., Hu, S., Xie, G. & Raufman, J. P. Overcoming obstacles to targeting muscarinic receptor signaling in colorectal cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22020716 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01822210 (2018).
Jin, W. Roles of TrkC signaling in the regulation of tumorigenicity and metastasis of cancer. Cancers https://doi.org/10.3390/cancers12010147 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03556228 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02650401 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02219711 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02568267 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02576431 (2022).
Aboody, K. S., Najbauer, J. & Danks, M. K. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 15, 739–752 (2008).
Park, G. T., Kim, S. U. & Choi, K. C. Anti-proliferative effect of engineered neural stem cells expressing cytosine deaminase and interferon-beta against lymph node-derived metastatic colorectal adenocarcinoma in cellular and xenograft mouse models. Cancer Res. Treat. 49, 79–91 (2017).
Yi, B. R. et al. Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-beta via their tumor-tropic effect in cellular and xenograft mouse models. Mol. Oncol. 7, 543–554 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02015819 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03072134 (2022).
Sharma, M. & Flood, P. M. in Neuroprotection Ch. 4 https://doi.org/10.5772/intechopen.81343 (IntechOpen, 2019).
Costa, M., Brookes, S. J. & Hennig, G. W. Anatomy and physiology of the enteric nervous system. Gut 47 (Suppl. 4), iv15–iv19 (2000).
Brookes, S., Chen, N., Humenick, A., Spencer, N. J. & Costa, M. Extrinsic sensory innervation of the gut: structure and function. Adv. Exp. Med. Biol. 891, 63–69 (2016).
Beyak, M. J. et al. in Physiology of the Gastrointestinal Tract 4th edn (ed. Johnsond, L. R.) 685–725 (Academic Press, 2006).
Ratcliffe, E. M. Molecular development of the extrinsic sensory innervation of the gastrointestinal tract. Auton. Neurosci. 161, 1–5 (2011).
Brierley, S. M. & Linden, D. R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 11, 611–627 (2014).
Furness, J. B. Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. Auton. Neurosci. 125, 81–85 (2006).
Saffrey, M. J. in Encyclopedia of Neuroscience (ed. Squire, L. R.) 1097–1102 (Academic Press, 2009).
Campbell, I. Gut motility and its control. Anaesth. Intensive Care Med. 13, 59–61 (2012).
Mohs, F. E. & Lathrop, T. G. Modes of spread of cancer of skin. AMA Arch. Derm. Syphilol. 66, 427–439 (1952).
Batsakis, J. G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 94, 426–427 (1985).
Veness, M. J. Perineural spread in head and neck skin cancer. Australas. J. Dermatol. 41, 117–119 (2000).
Fagan, J. J. et al. Perineural invasion in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 124, 637–640 (1998).
Bockman, D. E., Buchler, M. & Beger, H. G. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 107, 219–230 (1994).
Nagakawa, T. et al. Patterns of neural and plexus invasion of human pancreatic cancer and experimental cancer. Int. J. Pancreatol. 10, 113–119 (1991).
Liebig, C., Ayala, G., Wilks, J. A., Berger, D. H. & Albo, D. Perineural invasion in cancer: a review of the literature. Cancer 115, 3379–3391 (2009).
Ayala, G. E. et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49, 213–223 (2001).
Mirkin, K. A. et al. Impact of perineural invasion on survival in node negative colon cancer. Cancer Biol. Ther. 18, 740–745 (2017).
Hu, G., Li, L. & Hu, K. Clinical implications of perineural invasion in patients with colorectal cancer. Medicine 99, e19860 (2020).
Duchalais, E. et al. Colorectal cancer cells adhere to and migrate along the neurons of the enteric nervous system. Cell Mol. Gastroenterol. Hepatol. 5, 31–49 (2018).
Bakst, R. L. et al. Perineural invasion and perineural tumor spread in head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 103, 1109–1124 (2019).
Chi, A. C., Katabi, N., Chen, H. S. & Cheng, Y. L. Interobserver variation among pathologists in evaluating perineural invasion for oral squamous cell carcinoma. Head. Neck Pathol. 10, 451–464 (2016).
Schmitd, L. B., Scanlon, C. S. & D’Silva, N. J. Perineural invasion in head and neck cancer. J. Dent. Res. 97, 742–750 (2018).
Thursby, E. & Juge, N. Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 (2017).
Metchnikoff, E. The wilde medal and lecture of the manchester literary and philosophical society. Br. Med. J. 1, 1027–1028 (1901).
Hyland, N. P. & Cryan, J. F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 417, 182–187 (2016).
Rodriguez, J. M. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015).
Gagniere, J. et al. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 22, 501–518 (2016).
Natividad, J. M. M. & Verdu, E. F. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol. Res. 69, 42–51 (2013).
Gensollen, T., Iyer, S. S., Kasper, D. L. & Blumberg, R. S. How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016).
Baumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93 (2016).
Acknowledgements
The authors’ work is financially supported by VENI-NWO grant (016.186.124) obtained by V.M., the Kootstra Talent Fellowship grant (Maastricht University) obtained by N.V. and the Hestia – NWO grant (VidW.1154.18.045) obtained by M.I and V.M.
Author information
Authors and Affiliations
Contributions
N.V., M.I. and V.M. researched data for and wrote the article. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Jean-Pierre Raufman, who co-reviewed with Alyssa Schledwitz; Brian Davis; Brian Gulbransen; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vaes, N., Idris, M., Boesmans, W. et al. Nerves in gastrointestinal cancer: from mechanism to modulations. Nat Rev Gastroenterol Hepatol 19, 768–784 (2022). https://doi.org/10.1038/s41575-022-00669-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00669-9
This article is cited by
-
cAMP-PKA/EPAC signaling and cancer: the interplay in tumor microenvironment
Journal of Hematology & Oncology (2024)
-
Differences in enteric neuronal density in the NSE-Noggin mouse model across institutes
Scientific Reports (2024)
-
Promoter hypermethylation of neural-related genes is compatible with stemness in solid cancers
Epigenetics & Chromatin (2023)
-
Systemic sclerosis gastrointestinal dysmotility: risk factors, pathophysiology, diagnosis and management
Nature Reviews Rheumatology (2023)
-
Nanomaterials for visualized tumor surgical navigation and postoperative recurrence inhibition
Nano Research (2023)