Abstract
There are substantial sex differences in drug abuse, and a key feature of cocaine addiction is pathologically high motivation for drug. We investigated the role of ovarian hormones on cocaine demand in female rats using a within-session threshold behavioral economics (BE) procedure, which allows us to compare motivation for drug across hormonal states and sex while controlling for differences in dose and intake. This approach quantifies demand elasticity (α) and free consumption (Q0, consumption at null effort) to determine motivation for cocaine. Overall, female rats showed greater motivation for cocaine compared to males. However, this difference was cycle phase-dependent - motivation for cocaine when females were in proestrus was lower compared to the same animals across cycle phases, and overall similar to that of males. Hormonal cycle phase accounted for 70% of the within-subject variance in demand elasticity, obscuring other individual differences in female demand. High serum progesterone (P4; e.g., in proestrus) predicted decreased cocaine motivation (high demand elasticity), whereas serum estradiol (E2) correlated to greater intake at null effort (Q0). However, individual differences were revealed across OVX females, who displayed a range of demand elasticity, as seen in males. E2 replacement in OVX females increased motivation for cocaine, whereas P4 replacement decreased motivation. We also found that as few as 4 weeks of cocaine self-administration accelerated estropause in female rats as young as 12 weeks old. By 13 weeks of self-administration, proestrus epochs were no longer observed, and cocaine demand was potentiated by persistent estrus in all females. Thus, P4 signaling is a key modulator of cocaine demand in females that may underlie previously observed sex differences in addiction phenotypes.
Similar content being viewed by others
Introduction
There is growing evidence for sex differences in substance abuse with clear treatment implications. In particular, there is a relationship between stressful life events, stress reactivity and substance use disorders that differ by gender [1,2,3,4]. Abundant evidence indicates that women progress more quickly from casual drug use to dependence [5, 6] have greater difficulty quitting [7, 8] and shorter periods of abstinence [9, 10].
Similarly, sex differences in drug seeking behavior have been noted in animal models of addiction and relapse. Female rats exhibit enhanced behavioral sensitization to cocaine [11] and acquire cocaine-induced conditioned place preference (CPP) more rapidly and at lower doses than males [12]. In self-administration studies, female rats more readily and rapidly acquire cocaine self-administration [13, 14]. In animal models of relapse, female rats demonstrate greater cocaine-primed [15, 16], and stress-induced [17] reinstatement than males. Female rats also respond more than male rats on the first day of extinction (ED1) after cocaine self-administration [18, 19]. Thus, many studies indicate that females have greater propensity to cocaine abuse than males. We recently showed that demand elasticity (an inverse measure of motivation in behavioral economics, BE) for cocaine predicts multiple addiction behaviors [20], but possible sex differences in this important biomarker have not been reported.
Hormonal milieu alters the subjective experience of cocaine. In particular, progesterone (P4) may decrease, whereas estradiol (E2) may enhance, cocaine’s rewarding properties. During the luteal phase of the menstrual cycle, endogenous P4 levels are high and women report attenuated subjective responses to cocaine and decreased desire to smoke cocaine, compared to during a low P4 (follicular) phase [21,22,23]. Female rats self-administering cocaine respond more, and satiate at higher doses, when they are in the low P4 phase of their cycle than in other phases [24,25,26]. Further, OVX attenuates cocaine self-administration [8] and, as observed in people, cocaine administration can disrupt estrous cyclicity of rodents [27]. Oral administration of P4 decreases subjective effects of cocaine in women and decreases the desire to self-administer cocaine [28,29,30]. Furthermore, plasma P4 levels negatively correlate with cocaine self-administration but E2 levels do not [24]. In contrast, E2 administration to OVX rats enhances cocaine-induced psychomotor behavior and sensitizes rats to future cocaine administration compared to OVX rats administered vehicle, P4, or P4 + E2 [31,32,33]. Also, responsivity to cocaine-associated cues is increased during estrous cycle phases when E2 is high [34], and blockade of E2 formation attenuates cocaine self-administration in female rats [35]. Notably, these effects do not appear to be due to hormone-mediated changes in the pharmacokinetics of cocaine, as peak plasma levels of cocaine and elimination half-life do not differ in follicular versus luteal phases of the menstrual cycle [30]. Therefore, female steroids substantially influence cocaine addiction behaviors.
Drugs of abuse also alter hormonal milieu. When administered acutely cocaine induces acute hypergonadism, increasing circulating and neural levels of progestogens and androgens in females [36,37,38,39]. Repeated cocaine exposure (non-contingent or self-administered) results in an adaptive hypothalamic-pituitary-gonadal response, such that circulating ovarian steroid hormone production, particularly progesterone, is inhibited [39, 40]. As estrous cyclicity may play an important role in driving cocaine demand, it is imperative to assess if long-term cocaine use influences estrous cycle stability. Herein, we determine the role of estrous cyclicity and ovarian hormones in cocaine demand, and the long-term effects of cocaine self-administration on estrous cyclicity.
Materials and methods
Animals
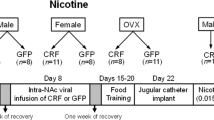
Male (300–350 g on arrival, n = 13) and female (175–200 g on arrival, n = 49) Sprague Dawley rats (Charles River Laboratories, ~55 days of age) were singly housed under a reversed 12 h/12 h light/dark cycle (lights off at 0600 h); all experiments were during the active cycle. Rats had free access to food and water and were housed in the animal facility at Rutgers University (AAALAC accredited). All experiments were approved by the Rutgers University Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals. All rats were implanted with catheters, and ovariectomized or not, at 9 weeks old, and all rats were under 6 months old by the end of the study to avoid reproductive senescence due to aging [41, 42]. Fourteen females were used to determine estrous cycle effects on demand. Of these, 8 that maintained catheter patency for 13 weeks of BE were used to analyze effects of cocaine on estrous cycling. Twenty female rats were used to obtain progesterone and estradiol measures for ELISA following BE stabilization. Twelve additional female rats were ovariectomized to characterize hormone-independent variation in α values, and 10 of those maintained catheter patency to test the effects of individual steroid hormones on demand parameters.
Estrous cycle determination
Estrous cycle phases were recorded daily via vaginal cytology as in prior reports [36, 37, 43]. Vaginal cytology and behavioral testing were performed in the afternoon (1–4 pm) of the reverse light-dark cycle to capture the afternoon peak in P4 levels during proestrus, as per prior reports [19, 36, 43, 44].
Ovariectomy
The removal of endogenous sources of hormones is necessary to assess behavioral control by steroid hormones within rodents. Rats were anesthetized with a mixture of ketamine (56.5 mg/kg) and xylazine (8.7 mg/kg). To remove ovaries [45], small incisions were made in the skin and abdominal wall of the rats’ dorsal sides. The ovaries were located and isolated, the fallopian tube ligated, and the ovaries removed. Following ovariectomy, rats were implanted with indwelling jugular catheters as described below. There was a minimum of 3 weeks between ovariectomy and the initiation of BE testing to allow for complete washout of ovarian steroids.
Indwelling jugular catheterization
Rats were anesthetized with a mixture of ketamine (56.5 mg/kg) and xylazine (8.7 mg/kg). Chronic in-dwelling catheters were constructed in-house and inserted as described previously [19, 20, 46]. Rimadyl (1 mg/kg) was used as a post-operative analgesic for 3 days following surgery. Animals were given cefazolin (10 mg, intravenous (i.v.)) and heparin (10 U, i.v.) starting 3 days following surgery and daily following self-administration sessions. Rats recovered from surgery for at least 1 week before self-administration training.
Hormone replacement
OVX rats received safflower oil-vehicle or hormone replacement with estradiol (E2) and/ progesterone (P4) before BE testing. Administration of E2 (0.09 mg/kg, S.C.) occurred 44–48 h before BE testing, and administration of P4 (4.0 mg/kg S.C.) occurred 4–6 h before BE testing. Rats had 7 days of washout between hormone treatments while continuing BE sessions daily. We used this dosing regimen to investigate intracellular/genomic signaling mechanisms for E2 and P4 rather than rapid non-genomic effects as per prior reports [47,48,49,50,51].
Drugs
Cocaine hydrochloride (NIDA, Research Triangle Park, NC) was dissolved in 0.9% sterile saline. Estradiol (Tocris; 0.09 mg/kg/injection) and progesterone (Tocris; 4.0 mg/kg/injection) were dissolved with heat in safflower oil and administered as described above. Mifepristone (RU486, Tocris; 5 mg/kg, S.C.) was dissolved with heat in safflower oil and administered 1 h prior to BE testing.
Cocaine self-administration procedures
Self-administration sessions were conducted in standard operant chambers housed in sound attenuating cubicles and controlled via MED-PC IV software (Med-Associates, St Albans, VT) as described previously [19]. Rats were trained in daily 2 h sessions to press an active lever for iv cocaine for at least 10d on a fixed ratio 1 (FR1) schedule of reinforcement to reach a criterion of >10 cocaine infusions/day (males: 0.20 mg/50 ul cocaine/infusion, females: 0.16 mg/50ul cocaine/infusion). These doses of cocaine produced similar numbers of infusions and cue-cocaine associations in the two sexes [19]. Each cocaine infusion was followed by a 20 s time-out in which lever pressing produced neither cocaine nor cues. An inactive lever was also present; presses on it were tabulated but had no consequence.
Within-session behavioral economics (BE) protocol
Within-session BE training was as described in our previous papers [20, 46, 52, 53]. In the BE paradigm, rats were given access to a descending series of cocaine doses by volume (421, 237, 133, 75, 41, 24, 13, 7.5, 4.1, 2.4, and 1.3 ug/injection for both sexes) on an FR1 schedule during 11 consecutive 10 min bins in a daily session. A compound stimulus (light + tone) was paired with each infusion. Cocaine doses were manipulated by adjusting pump duration [20, 52]. The initial 10 min bin was a ‘loading bin’ wherein rats had received high-dose cocaine (0.4 mg/injection) to allow animals to establish their preferred brain cocaine concentration at low effort (few presses required). In subsequent 10 min bins, the amount of cocaine infused per active lever press decreased on a ¼ log scale so that price (number of lever responses required per mg cocaine) progressively increased, and rats needed to respond more in each 10 min bin to defend their preferred level of cocaine. Rats exerted more effort (more presses; greater price paid) across bins to maintain their desired cocaine consumption until PMax (maximum price paid), the point beyond which the effort exerted decreased and was not sufficient to maintain cocaine concentration (point slope of demand curve = −1); PMax was typically followed by a rapid fall in consumption. An exponential demand equation [54] was used to fit a demand curve to the data and extract important BE parameters, particularly α (demand elasticity) and Q0 (free cocaine consumption) as described in our publications [20, 52]. The parameter k, representing the range of all consumption data in loge units, was held at a value of 7.368 (3.2 in log10 units) across all experiments. OMax was also determined and indicates the maximum amount of lever pressing (consumption) observed at PMax. As OMax and PMax are derived from single points on the curve, the current manuscript focuses primarily on α which incorporates data from all points on the demand curve and is thus a more comprehensive measure of demand. Rats performed daily BE sessions until they exhibited stable behavior, i.e., until α and Q0 values over the last three sessions (3 cycles in females) were within 20% of their means. Rats acclimated rapidly to this procedure and displayed stable (baseline) α and Q0 typically within 6 BE sessions (males) or three cycles (females). We found that after achieving stabilization criteria, BE performance remained stable over weeks of repeated daily BE testing, allowing prolonged within-subjects testing [20, 52]. Male rats herein were tested in parallel with cycling females, such that all rats had similar numbers of BE sessions.
Tail vein blood draw and ELISA
Ten rats/cycle were used, and serum was obtained via tail vein blood draw. Within-subjects sampling was taken 90 min post-PMax. Serum was centrifuged (10,000 RPM at 4 °C) and plasma was reserved for processing. Steroid hormones were extracted from serum using ethyl ether [55]. 100 uL of sample was placed in a borosilicate glass culture tube and submerged in acetone/dry ice. The supernatant was collected and ether was allowed to passively evaporate in the hood. Samples were reconstituted in assay buffer per kit instructions. Plasma progesterone (402310, standard range 0.1–100 ng/mL), estradiol (402110, standard range 0.01–10 ng/mL), and testosterone (402510, standard range 0.001–1 ng/mL) levels were measured using enzyme immunoassay kits (Neogen Life Sciences).
Statistical analyses
We used repeated measures one-way analysis of variance (ANOVA) to explore if cocaine demand elasticity (α) varied among cycle phases (estrus, metestrus, diestrus, and proestrus) or if ovarian extirpation and replacement of E2 and/or P4 altered cocaine demand elasticity (α), free consumption (Q0), or consumption at maximal price paid (OMax). Pearsons correlation analyses were used to assess the relationship between cocaine demand parameters and circulating levels of ovarian hormones in intact rats. Linear regressions were performed to determine the rate of decline in estrous cyclicity and corresponding increases in cocaine demand. Wilcoxon matched-paired signed rank tests were used to determine the effects of long-term BE testing on proestrus epochs, and T-tests were used to determine effects of long-term BE testing on average cocaine demand between early and late test weeks.
Results
Demand variation with cycle phase
Following cocaine self-administration, female rats underwent a minimum of three cycle phases of BE testing until stabilization criterion was met. Male rats received the same number of days of BE as females, although males typically met stabilization criterion earlier. We found a significant effect of sex on demand elasticity, wherein females had less cocaine demand elasticity (greater motivation) than males. Also, the within-subject variance in females was high across BE test days, indicating a possible cycle phase effect (Fig. 1A shows example demand curves in a single female across the estrus cycle). On further investigation, we found that female rats in proestrus had higher α values compared to other cycle phases (F(3,55) = 37.48, p < 0.05, and demand elasticity in proestrus was similar to that of male (t = 0.7997, df = 34, p = 0.4295; Fig. 1B) and ovariectomized female rats (t = 0.7912, df = 25, p = 0.4363; Fig. 1B). Analyzing α at the same cycle phase in intact females decreased between-subjects variance, indicating that cycling ovarian hormones had a substantial effect on cocaine demand. To determine the amount of variance accounted for by cycle phase, we used a single factor repeated measures ANOVA across cycles (F(3,24) = 19.01, p < 0.05) and divided the sum of squares of differences in α between cycle phases by the total sum of squares (across individuals + cycle) to determine that 70.4% of the total variance in α in the group was explained by cycle phase, compared to only 23% variance between individual rats within an observed cycle phase. These data indicate that the majority of variance in α values in intact female rats was produced across cycles (within-subjects), and not across individuals (between-subjects).
A Example cocaine demand curves as a function of estrous cycle phase in an individual female. There was a leftward shift in the cocaine demand curve during proestrus, indicating increased demand elasticity (decreased motivation) compared to other cycle phases. B Females in estrus, metestrus, or diestrus have lower α (higher motivation) compared to rats in proestrus **p < 0.05. No significant differences in α were observed when comparing female rats in proestrus, ovariectomized females, or males. C Administering exogenous P4 to rats in diestrus (when circulating P4 levels are low) attenuated cocaine demand, and administering a P4 receptor antagonist (RU486, mifepristone) increased cocaine demand compared to safflower oil vehicle in each cycle phase (Di: diestrus, Pro: proestrus); *p < 0.05 compared to within-subjects vehicle for each cycle phase. D Progesterone (ng/mg) was predictably higher in proestrus rats compared to diestrus rats *p < 0.05. E Progesterone correlated positively with α values (left) but not with Q0 (right). Linear regressions (red lines for collapsed females across cycle, green lines and symbols for females in proestrus, black lines, and symbols for females in diestrus) are plotted. See text and Table 1 for corresponding correlation coefficients, *p < 0.05. F Estradiol (ng/mg) was predictably higher in proestrus rats compared to diestrus rats *p < 0.05. G Estradiol predicted Q0 (free consumption) in diestrus females only (right), but not α (left) Linear regressions (red lines for collapsed females across cycle, green lines and symbols for females in proestrus, black lines and symbols for females in diestrus) are plotted See text and Table 1 for corresponding correlation coefficients, *p < 0.05.
We ovariectomized a separate group of female rats to eliminate within-subjects variance produced by cycle, so that individual differences (between-subjects variance) in cocaine demand elasticity independent of cycle could be measured. OVX females (F(11,14) = 4.970, p < 0.05) and male rats (F(20,14) = 3.901, p < 0.05) had significantly greater between-subjects variance than females in proestrus [highest co-efficient of variance (CV) across cycles]. An F-test to compare between-subjects variances revealed that OVX females and males did not significantly differ in their variance (F(11,20) = 1.274, p = 0.6132 Fig. 1B). These data indicate that in females, OVX can reveal individual, between-subjects, differences in cocaine demand that are otherwise obscured by ovarian hormone cycling.
Manipulating P4 signaling and demand
In a separate group of rats (n = 7), we investigated if administering exogenous P4 to rats in diestrus (when circulating P4 levels are low) attenuated cocaine demand. Rats were administered P4 (4 mg/kg, I.P.) or safflower oil vehicle 4 h before BE testing. We found that female rats administered P4 in diestrus had greater demand elasticity (decreased motivation) for cocaine compared to those administered vehicle (paired t = 3.489, df = 6, p < 0.05; Fig. 1C). In these same rats, we administered a P4 receptor antagonist (RU486, mifepristone) during proestrus to determine if decreased motivation for cocaine during proestrus was due to actions at P4 receptors. Rats were administered RU486 (5 mg/kg, S.C.) or safflower oil vehicle 1 h before testing in BE. We found that female rats administered RU486 in proestrus had lower demand elasticity (increased motivation) for cocaine compared to those administered safflower oil vehicle (paired t = 4.615, df = 6, p < 0.05; Fig. 1C). These results indicate that P4, acting at the cognate progesterone receptor, may attenuate demand for cocaine.
Correlations of demand with hormone concentrations
A separate group of 20 female rats underwent 10 weeks only of BE testing, and tail vein blood was drawn 90 min post-PMax to measure circulating P4, E2, and T levels in proestrus and diestrus females. Sampling was performed just prior to euthanasia as we found that tail vein draws severely disrupted BE stability in females. Rats were transcardially perfused following tail vein blood draws. We observed high P4/E2 ratios in proestrus (3436 ± 782.2, n = 10) and diestrus (2869 ± 564.3, n = 10) in these rats, inconsistent with reproductive senescence [41]. As expected, proestrus females had greater circulating P4 (t = 4.789, df = 18, p < 0.05; Fig. 1D), E2 (t = 4.066, df = 18, p < 0.05; Fig. 1F), and T (t = 2.103, df = 18, p < 0.05; proestrus: 0.03329 ng/mL ± 0.006, n = 10, diestrus: 0.01894 ng/mL ± 0.002, n = 10) compared to their diestrus counterparts.
When hormonal milieu was compared to demand parameters on the day of sampling, P4 significantly correlated to α, but not Q0 (Fig. 1E). E2 correlated to Q0 only, and only in diestrus rats (R2 = 0.68, p < 0.05; Fig. 1G). Additional correlations between behavioral economics measures and circulating hormone levels are found in Table 1. These results indicate that hormonal milieu may strongly influence cocaine demand state in female rats.
Effects of cocaine self-administration on estrous cyclicity
Acute or chronic administration of cocaine substantially alters female hormone levels [36, 37, 39, 40]. Thus, we hypothesized that over time cocaine may disrupt estrous cyclicity, which may contribute to the higher cocaine demand seen in females. We administered BE tests 5 days per week for 13 weeks and measured estrous cycling throughout those times. Repeated measures two-way ANOVAs comparing cycle phase epochs (diestrus, proestrus, estrus, metestrus) across weeks of BE indicated that estrous cyclicity was disrupted such that fewer proestrus epochs were observed in weeks 7–13 compared to earlier weeks (F (45,360) = 4.001, p < 0.05; Fig. 2A). Post-hoc comparisons confirmed that cycle stability decreased by BE week 6, indicating that even those rats that did not enter this “perma-estrous” state remained in other cycle phases (e.g., diestrus; Fig. 2A), longer than the normal ~24 h periods per cycle phase (F (1126) = 146.3, p < 0.05; Fig. 2B). Repeated measures one-way ANOVAs indicated that sequential days between estrus epochs significantly increased over the 13 weeks of BE testing (F (1,126) = 42.14, p < 0.05; Fig. 2C). Proestrus-to-estrus transitions were nearly eliminated by week 13 (F(3,31) = 8.765, p < 0.05; Fig. 2D). These data together indicate that female rats show periods of decreased demand for cocaine during initial drug exposure when P4 levels are high (e.g., during proestrus); however, the cycle-related P4 peak is inhibited by long-term cocaine exposure (during BE testing) and the addiction-protective proestrus phase is nearly eliminated, producing a positive feedback loop that promotes elevated cocaine demand.
A Proestrus epochs decreased over time and were significantly reduced in weeks 7–13 of daily cocaine self-administration (BE sessions) compared to pre-cocaine (no cocaine) estrous cycling; *p < 0.05 indicates significant values in post-hoc analyses for individual days. B Number of cycle events decreased over weeks of BE sessions. Each week, the number of phase changes observed (out of four possible phases) was recorded. As expected, prior to cocaine exposure, our rats showed, on average, 3.672 ± 0.11 cycle phase changes per 5-day period. Cycle phase changes observed significantly decreased (F(1,14) = 292.2, p < 0.05) over 16 weeks to 0.50 ± 0.27 days between estrus epochs. C Days between estrus phases were substantially reduced by cocaine. As expected, prior to cocaine exposure, rats exhibited 3.34 ± 0.33 days between estrus epochs (3–4 days between epochs indicate a normal 4–5 day cycle). The time between estrus epochs significantly decreased (F(1,14) = 33.85, p < 0.05)) over 13 weeks of BE sessions to 0.12 ± 0.09 days between estrus epochs. D Proestrus to estrus events were reduced by daily cocaine sessions, reaching significance by cycles 13–16 (cocaine exposure weeks 11–13) *p < 0.05.
We also investigated effects of cocaine on the frequency of proestrus epochs and associated average demand for cocaine. There was a significant effect of time on incidence of proestrus epochs, wherein fewer proestrus epochs were observed during weeks 11–13 of BE compared to weeks 1–3 (Wilcoxon matched-paired signed rank test, Z = −2.52, N = 8, p = 0.0078; Fig. 3A). Comparing the first 3 weeks of BE to weeks 11–13, we found that average demand elasticity was decreased (motivation was increased) in weeks 11–13 compared to weeks 1-3 (α: t = 4.027, df = 7, p < 0.05; Fig. 3B, OMax: t = 4.016, df = 7, p < 0.05; Fig. 3D) and correlated to a reduced number of proestrus epochs observed (α: R2 = 0.40, p < 0.05; Fig. 3B, OMax: R2 = 0.45, p < 0.05; Fig. 3D). Q0 (t = 0.459, df = 7, p = 0.6602; R2 = 0.13, p = 0.1778; Fig. 3C) was unchanged. Thus, cocaine-induced suppression of proestrus epochs may contribute to higher motivation for cocaine over time.
A The number of proestrus epochs was significantly lower in weeks 11–13 of BE sessions compared to weeks 1–3 *p < 0.05. B α was significantly higher (lower motivation) in early weeks of cocaine self-administration/BE (normal cycling; higher number of proestrus epochs) compared to later weeks, when proestrus was rarely observed *p < 0.05. Inset: α significantly correlated to the number of proestrus epochs observed (R2 = 0.49, p < 0.05). C Q0 was unchanged over time of chronic cocaine self-administration. Inset: Q0 did not correlate to the number of proestrus epochs observed. D OMax was significantly lower in early weeks of BE (normal cycling; higher number of proestrus epochs) compared to later weeks, when proestrus was rarely observed *p < 0.05. Inset: OMax significantly correlated to the number of proestrus epochs observed (R2 = 0.32, p < 0.05).
Ovariectomy and hormone replacement
We next investigated the effects of administering physiologically relevant levels of E2 and P4 on cocaine demand in OVX rats. Statistical analyses were performed compared to an average baseline measurement, allowing for individual variability assessment at in BE pretests (See Fig. 4A for timeline of injection conditions relative to baseline, BE pre-test, BE testing, and BE post-test). Injections of E2 were made 44–48 h prior to BE testing, and injections of P4 were made 4–6 h prior to testing. These injection schedules and doses produce physiologically relevant levels of steroid hormones and behavioral endocrine responses (e.g., lordosis) when combined that reflect proestrus. One-way ANOVAs for vehicle, E2, P4, and E2 + P4 treatments indicate that E2 alone increased, whereas P4 alone decreased, demand behavior in the BE task (α: F(3,39) = 23.72, p < 0.05; p < 0.05; Fig. 4C, OMax: F(3,39) = 10.93, p < 0.05; Fig. 4E); Q0 was unaffected by these treatments. When P4 and E2 were co-administered, there was no change from baseline demand measurements, indicating that these two steroids may have opposing effects on demand, and that P4 negates the effects of E2.
A Timeline of injection conditions indicating when injections of estradiol, progesterone, and safflower oil vehicle occurred with respect to baseline measures (for percent change comparisons), BE pre-test (black lines preceding symbols on B–D), BE test (symbols on B–D), and BE Post-test (black lines succeeding symbols on B–D). B Example cocaine demand curves as a function of exogenous hormone administration in an individual OVX female. Progesterone produced a leftward shift in the cocaine demand curve, indicating increased demand elasticity (decreased motivation) compared to vehicle conditions. Estradiol produced a rightward shift in the cocaine demand curve, indicating decreased demand elasticity (increased motivation) compared to vehicle conditions. Estradiol + progesterone produced a demand curve similar to vehicle administration. C. Changes in α induced by estradiol or progesterone plotted for individual female rats. Progesterone increased, and estradiol decreased, α compared to rats given vehicle. Notably, elasticity after estradiol+progesterone co-administration was not different from vehicle; *p < 0.05 compared to baseline/veh. D Changes in Q0 induced by estradiol or progesterone plotted for individual female rats. There were no effects of progesterone or estradiol on Q0 in the same animals with significant effects in α (compare B and C). E Changes in OMax induced by estradiol or progesterone plotted for individual female rats. Progesterone decreased, and estradiol increased, OMax compared to rats given vehicle or estradiol+progesterone together; *p < 0.05 compared to baseline/veh.
Discussion
Our studies indicate that female rats show higher motivation for cocaine than males in a hormone-dependent manner. P4 lowered cocaine demand of females, supporting prior results that P4 lowered several core addiction-like behaviors in rodents and correlated to lower addiction phenotypes in women [29, 30, 56]. Our results also indicate that although extrinsic E2 increased motivation (decreased α) for drug in ovariectomized rats, intrinsic E2 only correlated to free consumption (Q0) in diestrus females. Similarly, administration of E2 and P4 together did not alter addiction phenotypes in ovariectomized rats; thus, variation of E2 and P4 levels may competitively influence motivation for cocaine in females.
It is noteworthy that effects of exogenous hormones vary greatly depending on many factors, including dose, timing, endogenous hormonal status, and substance of abuse. Herein, we focus on the effects of exogenous steroids in psychostimulants. Exogenous E2 has U-shaped dose-dependent effects on psychostimulant dopamine release that are reversed by co-administration of P4 [57,58,59]. We see similar effects that correspond to drug demand, in that the effects of exogenous E2 to increase cocaine demand can be ameliorated by co-administration of P4. E2 and P4 have time-dependent effects that determine which downstream signaling mechanisms contribute to their effects. For example, there are rapid effects of E2 (30 min) to potentiate striatal D2-receptor binding that are reversed by P4 co-administration [60]. Many such rapid effects of steroid hormones are thought to be due to membrane-bound receptors rather than nuclear signaling mechanisms [45]. Interestingly, we did not observe effects of E2 at up to 24 h post-injection; this indicates that the effects of E2 to increase cocaine demand may be dependent on nuclear signaling rather than membrane-bound hormone receptor activation or striatal dopamine release.
Psychostimulants may alter cycling gonadal hormones and fertility in both men and women. Six weeks of 10 mg/kg/day non-contingent cocaine decreases ovulation in rats by >40% [40]; higher doses, e.g., 20 mg/kg/day, produces effects that persist long after cocaine cessation [61]. Similar effects of cocaine to disrupt menstrual cycling in rhesus monkeys have been reported [62, 63], including in self-administration studies [27]. Herein, we show that the estrous cycle is disrupted in female rodents performing our cocaine self-administration/BE procedure, such that proestrus epochs are nearly eliminated, however as the maximal age for intact females in our studies was <6 months, these effects were not consistent with normative reproductive senescence in Sprague Dawley rats [41, 42]. We also show that reduced proestrus epochs in later weeks of BE sessions correlate to increased average cocaine demand. We postulate that over time cocaine disrupts estrous cyclicity, driving female rats into a persistent high-demand estrous phase (e.g., diestrus, estrus), and greatly decreasing the low demand proestrus cycle phase. Although endogenous variability in testosterone in intact females did not seem to contribute to cycle-phase variability in demand, it is possible that we would observe differences in the OVX and/or intact male model, as OVX decreases androgen production. Our results indicate that cocaine’s endocrine disrupting effects, in particular those on P4 and E2, may potentiate addiction vulnerability in females. Future studies may aim to identify the role of androgen steroids (and estradiol) in driving individual trait differences in cocaine demand.
Prior studies show that women have increased drug-seeking behaviors during menstrual cycle phases with high estrogen levels [64]. Our data show that female rats in the high progesterone phase (i.e., proestrus) have decreased demand for cocaine, compared to all other cycle phases. Our data indicate that P4 may primarily regulate motivation for cocaine rather than free consumption, as it predicted α but not Q0. Similarly, we show that exogenous P4 can attenuate motivation in both ovariectomized females and intact females in low P4 cycle phases (e.g., diestrus), and that inhibition of P4 receptors by mifepristone increases demand in high P4 cycle phases (e.g., proestrus). We postulate that fluctuating concentrations of endogenous hormones over longer periods of time (e.g., hours, days) creates a dynamic landscape that impacts patterns of cocaine demand and can be modified with exogenous hormone manipulations. These results are more intriguing when we consider that administration of cocaine itself impacts the endogenous hormonal milieu. Although our results primarily relate to genomic effects of ovarian hormones, they are similar to those observed with rapid non-genomic effects in FR1 and reinstatement experiments wherein E2 and P4 promote and inhibit drug-seeking behaviors in female rats, respectively [11, 56]. Steroid hormones in females may play a more substantial role in cocaine use than previously thought given the relative variability in demand accounted for by cycle phase and that ovariectomized female rats exhibit individual variability in demand similar to that observed in males. Thus, our results indicate that sex differences in motivation for cocaine are driven substantially by underlying hormonal factors.
References
Brady KT, Sinha R. Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–93.
Sinha R. Chronic stress, drug use, and vulnerability to addiction. Addiction Rev. 2008;1141:105–30.
Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacol. 1999;142:343–51.
Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacol. 2000;152:140–8.
McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers - implications for treatment and prognosis. Am J Addictions. 1999;8:300–11.
O'brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N. Y Acad Sci. 1992;654:400–15.
Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47.
Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacol. 2002;164:121–37.
Griffin ML, Weiss RD, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–6.
Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abus Treat. 1993;10:63–66.
Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–9.
Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, et al. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–33.
Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5.
Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol. 1999;144:77–82.
Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, et al. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacol. 2005;182:245–52.
Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacol. 2000;148:196–200.
Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–7.
Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacol. 2011;216:53–62.
Kohtz AS, Aston-Jones G. Cocaine seeking during initial abstinence is driven by noradrenergic and serotonergic signaling in hippocampus in a sex-dependent manner. Neuropsychopharmacology. 2017;42:408–18.
Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci. 2014;111:11822–7.
Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacol. 2002;159:397–406.
Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharm Biochem Behav. 2002;72:431–5.
Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83.
Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–9.
Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacol. 1989;98:408–11.
Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–45.
Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharm Biochem Behav. 1997;57:571–99.
Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharm Biochem Behav. 2004;78:699–705.
Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–74.
Mendelson J. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303.
Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566:255–64.
Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharm Exp Ther. 2000;293:879–86.
Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behavioural Brain Res. 2007;184:174–84.
Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, et al. Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology. 2019;44:1189–97.
Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharm Biochem Behav. 2001;68:641–6.
Kohtz AS, Paris JJ, Frye CA. Low doses of cocaine decrease, and high doses increase, anxiety-like behavior and brain progestogen levels among intact rats. Hormones Behav. 2010;57:474–80.
Kohtz AS, Walf AA, Frye CA. Effects of non-contingent cocaine on 3 alpha-androstanediol. II. Disruption of lordosis of proestrous rats. Physiol Behav. 2019;203:113–9.
Budziszewska B, Jaworska-Feil L, Lason W. The effect of repeated amphetamine and cocaine administration on adrenal, gonadal and thyroid hormone levels in the rat plasma. Exp Clin Endocrinol Diabetes. 1996;104:334–8.
Mello NK, Mendelson JH, Kelly M, Bowen CA. The effects of cocaine on basal and human chorionic gonadotropin-stimulated ovarian steroid hormones in female rhesus monkeys. J Pharm Exp Ther. 2000;294:1137–45.
King TS, Schenken RS, Kang IS, Javors MA, Riehl RM. Cocaine disrupts estrous cyclicity and alters the reproductive neuroendocrine axis in the rat. Neuroendocrinology. 1990;51:15–22.
Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17.
Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 2008;36:375–84.
Kohtz AS, Lin B, Smith ME, Aston-Jones G. Attenuated cocaine-seeking after oxytocin administration in male and female rats. Psychopharmacol. 2018;235:2051–63.
Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–26.
Frye CA, Walf AA, Kohtz AS, Zhu Y. Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of rats. Horm Behav. 2013;64:539–45.
Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41:1149–56.
Quadagno DM, McCullough J, Langan R. The effect of varying amounts of exogenous estradiol benzoate on estrous behavior in the rat. Horm Behav. 1972;3:175–9.
Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–9.
Green R, Luttge WG, Whalen RE. Induction of receptivity in ovariectomized female rats by a single intravenous injection of estradiol-17. Physiol Behav. 1970;5:137–41.
Clark AS, Roy EJ. Behavioral and cellular responses to pulses of low doses of estradiol-17 beta. Physiol Behav. 1983;30:561–5.
Sodersten P, Eneroth P, Hansen S. Induction of sexual receptivity in ovariectomized rats by pulse administration of oestradiol-17 beta. J Endocrinol. 1981;89:55–62.
Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacol. 2013;226:113–25.
Porter-Stransky KA, Bentzley BS, Aston-Jones G. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 2015;22:303–17
Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98.
Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF. 5alpha-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav Immun. 2016;55:202–14.
Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–71.
Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–80.
Dluzen DE, Ramirez VD. Modulatory effects of progesterone upon dopamine release from the corpus striatum of ovariectomized estrogen-treated rats are stereo-specific. Brain Res. 1991;538:176–9.
Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990;517:117–22.
Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–72.
King TS, Canez MS, Gaskill S, Javors MA, Schenken RS. Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharm Exp Ther. 1993;264:29–34.
Potter D. Low-dose follicular-phase cocaine administration disrupts menstrual and ovarian cyclicity in rhesus monkeys. J Soc Gynecol Investig. 1999;6:88–94.
Potter DA, Moreno A, Luther MF, Eddy CA, Siler-Khodr TM, King TS, et al. Effects of follicular-phase cocaine administration on menstrual and ovarian cyclicity in rhesus monkeys. Am J Obstet Gynecol. 1998;178:118–25.
Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96.
Acknowledgements
The authors would like to thank Joshua Zhao and Dennis Kim for their technical assistance in the project.
Funding
This research was funded by PHS grants R01-DA006214, K99-DA045758, and ES007148-30.
Author information
Authors and Affiliations
Contributions
ASK and GAJ designed experiments. ASK, BL, HD, and MP performed experiments. ASK, BL and HD analyzed data. ASK and GAJ wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kohtz, A.S., Lin, B., Davies, H. et al. Hormonal milieu drives economic demand for cocaine in female rats. Neuropsychopharmacol. 47, 1484–1492 (2022). https://doi.org/10.1038/s41386-022-01304-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01304-6