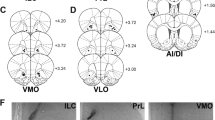
Abstract
Mu-opioid receptor (μ-OR) signaling in forebrain sites including nucleus accumbens (Acb) and ventromedial prefrontal cortex (vmPFC) modulates reward-driven feeding and may play a role in the pathophysiology of disordered eating. In preclinical models, intra-Acb or intra-vmPFC μ-OR stimulation causes overeating and vigorous responding for food rewards. These effects have been studied mainly in male animals, despite demonstrated sex differences and estrogen modulation of central reward systems. Hence, the present study investigated sex differences and estrogen modulation of intra-Acb and intra-vmPFC μ-OR-driven feeding behaviors. First, the dose-related effects of intra-Acb and intra-vmPFC infusions of the μ-OR-selective agonist, DAMGO, were compared among intact female, ovariectomized (OVX) female, and intact male rats. The DAMGO feeding dose-effect function was flattened in intact females relative to the robust, dose-dependent effects observed in OVX females and intact males. Thus, in intact females, intra-Acb DAMGO failed to elevate food intake relative to vehicle, while intra-vmPFC DAMGO elevated food intake, but to a smaller degree compared to males and OVX females. Next, to explore the possible role of estrogen in mediating the diminished DAMGO response observed in intact females, OVX rats were given intra-Acb or intra-vmPFC infusions of DAMGO either immediately after a subcutaneous injection of 17-beta-estradiol 3-benzoate (EB; 5 μg/0.1 mL) or 24 h after EB injection. Intra-Acb DAMGO effects were not changed at the immediate post-EB time point. At the delayed post-EB timepoint, significant lordosis was noted and the duration of intra-Acb DAMGO-driven feeding bouts was significantly reduced, with no change in the number of bouts initiated, locomotor hyperactivity, or Fos immunoreactivity in hypothalamic feeding and arousal systems. Similarly, EB failed to alter the motor-activational effects of intra-vmPFC DAMGO while reducing feeding. These findings indicate that delayed, presumably genomically mediated estrogen actions modulate the μ-OR-generated motivational state by reducing consummatory activity while sparing goal-approach and general arousal/activity. The results additionally suggest that EB regulation of consummatory activity occurs outside of forebrain-μ-OR control of hypothalamic systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E. Reciprocal opioid–opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating μ agonist-induced feeding in rats. Peptides. 2005;26:621–9.
Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and Fos expression. Neuroscience. 2000;99:267–77.
Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do -opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86.
Stratford TR, Wirtshafter D. Effects of muscimol, amphetamine, and DAMGO injected into the nucleus accumbens shell on food-reinforced lever pressing by undeprived rats. Pharm Biochem Behav. 2012;101:499–503.
Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–11.
Castro DC, Berridge KC. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci. 2017;114:E9125–34.
Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–60.
Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, et al. Endogenous opioid signaling in the medial prefrontal cortex is required for the expression of hunger-induced impulsive action. Neuropsychopharmacology. 2015;40:2464.
Tuulari JJ, Tuominen L, Boer FE, de, Hirvonen J, Helin S, Nuutila P, et al. Feeding releases endogenous opioids in humans. J Neurosci. 2017;37:8284–91.
Nummenmaa L, Saanijoki T, Tuominen L, Hirvonen J, Tuulari JJ, Nuutila P, et al. μ-opioid receptor system mediates reward processing in humans. Nat Commun. 2018;9:1500.
Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–51.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65.
Serdarevic M, Striley CW, Cottler LB. Sex differences in prescription opioid use. Curr Opin Psychiatr. 2017;30:238–46.
Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–72.
Barbosa-Leiker C, McPherson S, Layton ME, Burduli E, Roll JM, Ling W. Sex differences in opioid use and medical issues during buprenorphine/naloxone treatment. Am J Drug Alcohol Abus. 2018;44:488–96.
Kennedy AP, Epstein DH, Phillips KA, Preston KL. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 2013;132:29–37.
Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesthesia Analg. 2008;107:83–95.
Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem. 2014;14:83–89.
Yoest KE, Cummings JA, Becker JB. Oestradiol influences on dopamine release from the nucleus accumbens shell: sex differences and the role of selective oestradiol receptor subtypes. Br J Pharm. 2019;176:4136–48.
Yoest KE, Quigley JA, Becker JB. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav. 2018;104:119–29.
Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33.
Becker JB. Direct effect of 17β‐estradiol on striatum: sex differences in dopamine release. Synapse 1990;5:157–64.
Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118:169–71.
Peart DR, Andrade AK, Logan CN, Knackstedt LA, Murray JE. Regulation of cocaine-related behaviours by estrogen and progesterone. Neurosci Biobehav Rev. 2022;135:104584.
Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology. 2004;172:241–7.
Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci. 1989;103:36–45.
Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: Mu, Delta, and Kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34:4239–50.
Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90.
Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–59.
Newman S, Pascal L, Sadeghian K, Baldo BA. Sweetened-fat intake sensitizes gamma-aminobutyric acid—mediated feeding responses elicited from the nucleus accumbens shell. Biol Psychiatry. 2013;73:843–50.
Balthazart J, Choleris E, Remage-Healey L. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm Behav. 2018;99:1–8.
Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocr. 2008;29:238–57.
Taxier LR, Gross KS, Frick KM. Oestradiol as a neuromodulator of learning and memory. Nat Rev Neurosci. 2020;21:535–50.
Xiao L, Becker JB. Effects of estrogen agonists on amphetamine‐stimulated striatal dopamine release. Synapse. 1998;29:379–91.
Bryant DN, Bosch MA, Rønnekleiv OK, Dorsa DM. 17-β Estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–52.
Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc B Biol Sci. 2006;361:1251–63.
Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud H-R. Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol-Regul Integr Comp Physiol. 2003;284:R1436–44.
Zheng H, Patterson LM, Berthoud H-R. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82.
Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens μ-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–8.
Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 2013;33:18540–52.
Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–38.
Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-? and -? mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25.
Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA‐containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95.
Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41.
Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–59.
Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42.
Saux ML, Morissette M, Paolo TD. ERβ mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–7.
Bakshi V, Kelley A. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology. 1993;111:207–14.
Giacomini JL, Geiduschek E, Selleck RA, Sadeghian K, Baldo BA. Dissociable control of μ-opioid-driven hyperphagia vs. food impulsivity across subregions of medial prefrontal, orbitofrontal, and insular cortex. Neuropsychopharmacology. 2021;46:1981–9.
Giacomini JL, Sadeghian K, Baldo BA. Eating driven by the gustatory insula: contrasting regulation by infralimbic vs. prelimbic cortices. Neuropsychopharmacology. 2022;47:1358–66.
Chang L, Kigar SL, Ho JH, Cuarenta A, Gunderson HC, Baldo BA, et al. Early life stress alters opioid receptor mRNA levels within the nucleus accumbens in a sex-dependent manner. Brain Res. 2019;1710:102–8.
Gugusheff JR, Bae SE, Rao A, Clarke IJ, Poston L, Taylor PD, et al. Sex and age-dependent effects of a maternal junk food diet on the mu-opioid receptor in rat offspring. Behav Brain Res. 2016;301:124–31.
Smith CJW, Ratnaseelan AM, Veenema AH. Robust age, but limited sex, differences in mu-opioid receptors in the rat brain: relevance for reward and drug-seeking behaviors in juveniles. Brain Struct Funct. 2018;223:475–88.
Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming. Pharm Biochem Behav. 1999;64:53–57.
Corona R, Camacho FJ, García-Horsman P, Guerrero A, Ogando A, Paredes RG. Different doses of estradiol benzoate induce conditioned place preference after paced mating. Horm Behav. 2011;60:264–8.
Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–58.
Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–9.
Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38.
Sandberg D, David S, Stewart J. Effects of estradiol benzoate on the pattern of eating and ethanol consumption. Physiol Behav. 1982;29:61–65.
Sinopoli KJ, Floresco SB, Galea LAM. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem. 2006;86:293–304.
Wide JK, Hanratty K, Ting J, Galea LAM. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155:45–53.
Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 1971;2:287–97.
Laessig SA, Auger AP, McCarthy MM, Silbergeld EK. Effects of prenatal chlordecone on sexually differentiated behavior in adult rats. Neurotoxicol Teratol. 2007;29:255–63.
Hudson AE. Genetic reporters of neuronal activity: c-Fos and G-CaMP6. Methods Enzymol. 2018:197–220.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. sixth London: Academic Press; 2007.
Friard O, Gamba M. BORIS: a free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods Ecol Evol. 2016;7:1325–30.
Baldo BA, Gual‐Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin‐containing hypothalamic neurons by GABAA receptor‐mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–86.
Maldonado-Irizarry C, Swanson C, Kelley A. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–88.
Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–71.
Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67:141–7.
Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol Behav. 1971;7:847–52.
Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol. 1973;72:551–68.
Ferreira JA, Foley AM, Brown M. Sex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male rats. Eur J Appl Physiol. 2012;112:3007–18.
McNaughton N. Statistics for behavioral neuroscience. In: Saghal A, editor. Behavioral neuroscience: a practical approach. Oxford: Oxford University Press; 1993. p. 169–88.
Parker KE, McCabe MP, Johns HW, Lund DK, Odu F, Sharma R, et al. Neural activation patterns underlying basolateral amygdala influence on intra-accumbens opioid-driven consummatory versus appetitive high-fat feeding behaviors in the rat. Behav Neurosci. 2015;129:812–21.
Kelley A, Bakshi V, Fleming S, Holahan M. A pharmacological analysis of the substrates underlying conditioned feeding induced by repeated opioid stimulation of the nucleus accumbens. Neuropsychopharmacology. 2000;23:455–67.
Bakshi VP, Kelley AE. Sensitization and conditioning of feeding following multiple morphine microinjections into the nucleus accumbens. Brain Res. 1994;648:342–6.
Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharm Rev. 2016;68:242–63.
Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M. Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev. 2013;37:1985–98.
Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–82.
Ragnauth A, Znamensky V, Moroz M, Bodnar RJ. Analysis of dopamine receptor antagonism upon feeding elicited by mu and delta opioid agonists in the shell region of the nucleus accumbens. Brain Res. 2000;877:65–72.
Quiñones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW. Estrogen regulation of μ-opioid receptor mRNA in the forebrain of female rats. Mol Brain Res. 1997;47:134–8.
Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol-Regul Integr Comp Physiol. 2013;305:R1215–67.
Geary N. Estradiol, CCK and satiation. Peptides 2001;22:1251–63.
Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol-Regul Integr Comp Physiol. 2002;283:R1378–85.
Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–58.
Acknowledgements
The authors thank Juliana Giacomini and Alexius Lampkin for assistance with general laboratory tasks, and Joyce Borde for excellent care of the rat colony.
Funding
This work was supported by NIH grant MH074723 from the National Institute for Mental Health (PI: BAB). JCD was supported by the Science and Medicine Research Scholars Advanced Opportunity Fellowship of the University of Wisconsin-Madison. A subset of these data was presented in abstract form at the annual meeting of the Society for Neuroscience, 2018.
Author information
Authors and Affiliations
Contributions
JD: Writing original draft, Investigation, Formal analysis, Data curation, Visualization. KD: Investigation, Data curation. CZ: Investigation. ES: Investigation. KS: Investigation. AA: Conceptualization, Resources. BAB: Conceptualization, Supervision & project administration, Writing review & editing, Formal analysis, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diaz, J.C., Dunaway, K., Zuniga, C. et al. Delayed estrogen actions diminish food consumption without changing food approach, motor activity, or hypothalamic activation elicited by corticostriatal µ-opioid signaling. Neuropsychopharmacol. 48, 1952–1962 (2023). https://doi.org/10.1038/s41386-023-01711-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01711-3