Abstract
Patients with depressive episodes (PDE), such as unipolar disorder (UD) and bipolar disorder (BD), are often defined as distinct diagnostic categories, but increasing converging evidence indicated shared etiologies and pathophysiological characteristics across different clinical diagnoses. We explored whether these transdiagnostic deficits are caused by the common neural substrates across diseases or disease-sensitive mechanisms, or a combination of both. In this study, we utilized a Bayesian model to decompose the resting-state brain activity into multiple hyper- and hypo-activity patterns (refer to as “factors”), so as to explore the shared and disease-sensitive alteration patterns in PDE. The model was constructed over a total of 259 patients (131 UD and 128 BD) with 100 healthy controls as the reference. The other 32 initial depressive episode BD (IDE-BD) patients who had symptoms of mania or hypomania during follow-up were taken as an independent set to estimate the factor composition using the established model for further analysis. We revealed three transdiagnostic alteration factors in PDE. Based on the distribution of factors and the tendency of factor composition at the group level, these factors were defined as BD sensitive factor, UD sensitive factor and shared basic alteration factor. We further found that the factor composition and the ROIs-based alteration degree (mainly involving in orbitofrontal gyrus and part of parietal lobe) were associated with the bipolar index in IDE-BD patients. Our findings contributed to understanding the core transdiagnostic shared and disease-sensitive alterations in PDE and to predicting the risk of emotional state transition in IDE-BD patients.
Similar content being viewed by others
Introduction
Unipolar disorder (UD) and bipolar disorder (BD), both of which have depressive episodes symptoms, are defined as distinct diagnostic categories according to Diagnostic and Statistical Manual of Mental Disorders (DSM) Fifth Edition. However, the validity of psychiatric classification based on DSM classification is still questionable [1]. Studies combining genetics, epidemiology and neuroscience showed that groups of patients with depressive episodes (PDE) have no sharp or discrete neurobiological boundaries between diagnostic categories [2]. For example, there is evidence that UD and BD share a common genetic cause [3]. Furthermore, PDE show the same pattern of disrupted neural circuits and networks associated with cognition and adaptive emotional responses, regulation [4, 5]. Therefore, a promising alternative approach has been proposed as a transdiagnostic approach [6], which is expected to go beyond existing diagnostic categories and improve the way we classify and treat PDE [1].
Several studies have reported shared and specific functional or structural changes in PDE with different diagnoses. Meta-analysis has revealed that the gray matter volume reduction in prefrontal cortex, anterior cingulate cortex and insula was observed in both UD and BD, while the gray matter volume of right dorsolateral prefrontal cortex (DLPFC) and parietal regions decreased only in UD [7]. Moreover, several studies have found significant alterations in the amplitude of low-frequency fluctuation (ALFF) across both UD and BD compared to healthy controls (HC), most prominently in frontal, parietal and occipital regions [8,9,10], which was also found based on the regional homogeneity [11]; however, inconsistencies have also been reported and suggested further exploration [12, 13]. By identifying physiological characteristics through functional connectivity (FC), studies have found that both BD and UD show decreased FC between default mode network (DMN) and central control network [14] or limbic system network [15], and the interruption of topological properties within DMN and limbic [16, 17]. Patient groups could be differentiated by large-scale network alterations: significantly increased FC within DMN in UD, increased FC within frontoparietal network in BD, and increased FC within cingulo-opercular network in both two diseases [18]. Furthermore, a recent review suggested that functional and structural changes in the neural circuits involved in emotional and reward processing may be the common neurological characteristics for PDE [19].
The MRI studies above have found some transdiagnostic similarities and disease-specific changes between BD and UD. However, existing methods of analysis, such as standard FC analysis, tended to be limited to several key areas. First, the traditional case-control approach used in most researches, which independently identifies a single disease from HC, may lead to an illusion of group specificity [2]. To make a breakthrough in understanding how brain function changes to cause depressive episodes, a transdiagnostic neuroimaging study is urgently needed. Secondly, there is evidence that differences in individual behavior may be reflected in the variability of the whole brain, which can serve as a stable and reliable fingerprint [20, 21]. Namely, individual patients, whether UD or BD, are biologically highly heterogeneous [22,23,24], who may be clinically diffuse. Thus, the Latent Dirichlet Allocation (LDA), a data-driven Bayesian framework, was utilized in this study, which reconciles categorical and dimensional perspectives of individual heterogeneity. This mathematical framework has been successfully used to reveal latent atrophy factors in Alzheimer’s disease with structural MRI [25] and the novel subtype study of autism spectrum disorder [26]. The LDA model allows each individual to express multiple latent factors to varying degrees rather than categorizing them via a certain standard, which represents the unique characteristics of this patient’s disease.
In this study, we applied the LDA to decompose the resting-state ALFF patterns in transdiagnostic PDE into multiple latent hyper- and hypo-activity patterns (i.e., factors). We hypothesized that there are shared and disease-sensitive functional patterns in patients with different diagnostic categories even though all are in a depressive state. Our study was conducted along with the following three points: (1) the dominant patients without labels of UD or BD were put into the LDA model to estimate the transdiagnostic latent factors in the whole brain, enabling in-depth exploration of alteration patterns across PDE; (2) the compositions of these transdiagnostic alteration factors in patients were further deduced to find various expression tendency over patients with different diagnosis categories; (3) a particular group of patients with an early major depressive episode, whose nature was found to be BD during their following-up, were taken as an independent sample set to further validate the value of alteration factors suggested via LDA model in emotional state prediction.
Materials and methods
Participants
All participants were recruited from September 2011 to December 2018. Using the Mini-International Neuropsychiatric Interview (M.I.N.I, Chinese version), all patients were diagnosed by at least two psychiatric attending physicians based on the DSM-IV. Then, the 17-item Hamilton Depression Rating Scale (HAMD-17) was used to quantify the severity of depression in patients. The resulting sample comprised 259 PDE (131 UD and 128 BD) patients and 100 HC, for primary analyses (age, gender and education matched). An independent sample of 32 initial depressive episode BD (IDE-BD) patients, who were initially strictly diagnosed as UD during scanning and then convert to BD with the episodes of mania or hypomania during follow-up, was used for secondary analyses. As described in our previous study [27], the IDE-BD patients had never experienced a manic episode when seeking treatment.
The recruitment and exclusion criteria for patients and HC are detailed in Supplementary Material. All participants were 18–55 years old, native Han Chinese, right-handed and had normal intelligence (Table 1). All participants have signed a written informed consent after a full written and verbal explanation. This research was approved by the Research Ethics Review Board of Affiliated Brain Hospital of Nanjing Medical University.
MRI acquisition and preprocessing
Resting-state fMRI data in this study were obtained from the Department of Psychiatry of Affiliated Brain Hospital of Nanjing Medical University. The MRI scan parameters and preprocessed pipeline were detailed in Supplementary Methods. All data satisfied the criteria of maximum motion in translation <2 mm, rotation <2 degrees. Linear regression of multiple nuisance variables was applied, which consisted of head motion (Friston 24-parameter), white matter, cerebrospinal fluid and global signal. We chose to regress out global signal because of its effectiveness in removing motion-related and respiratory artifacts [28, 29].
Latent factors based on LDA model
The LDA [30], which is a hierarchical Bayesian model, was applied over those resting-state voxel-level ALFF to capture latent alteration factor for transdiagnostic PDE. The LDA model consists of a three-tier structure of voxels, latent factors and patients (as shown in Supplementary Fig. S1). Given the ALFF data for all patients and the predefined factor number K, the model can estimate K latent alteration factors. The brain activity of patients was estimated as approximations of latent factor Pr(Voxel | Factor) multiplied by personalized factor composition Pr(Factor | Patient). The following steps were followed to estimate the latent factors for 259 PDE.
Step one: feature extraction and dimension reduction
The ALFF reflects spontaneous fluctuations of a given voxel, which has higher test-retest reliability than other functional measures [31]. We computed the ALFF within the frequency band of 0.01–0.08 Hz as previous [32]. In this study, we chose the automated anatomical labeling atlas as a predefined mask. Specifically, only the cortical and subcortical regions of the brain were selected for subsequent analysis, while the cerebellum was excluded due to the incomplete MRI scan coverage for some of the subjects. Furthermore, the ALFF of each voxel was divided by the global mean value for standardization. Age, gender, education and head motion (mean frame-wise displace [33]) were regressed out from all participants’ ALFF via a general linear model. To identify principal ALFF alterations, we extracted the voxels with significant differences in ALFF after two-sample t-test (p < 0.05, no correction) between each group pairs in BD, UD and HE, respectively. The union of three voxel sets was retained as the mask for further analysis.
Step two: applying LDA to fMRI data
To map the resting-state fMRI data to the LDA model, the continuous ALFF images of PDE calculated in the previous section were discretized via z-normalized with respect to 100 HC participants, so that the greater alteration corresponded to the higher word frequency. A z-score greater (or less) than zero in patient indicates hyper-activity (or hypo-activity) relative to the activity (ALFF value) observed in HC. Finally, the z-scores were multiplied by 10 and rounded to the nearest integer. Although the discretization of the ALFF data might lead to some loss of information, we note that a sufficiently large multiplicative factor can minimize the loss of information [26]. It is worth noting that traditional LDA can only deal with positive input variables, such as the positive frequency of dictionary words. However, PDE are associated with both hyper- and hypo-activity of the brain (represented by ALFF), and the positive (or negative) values cannot simply be ignored or taken as absolute values here. Therefore, in this study, we used an improved LDA model, named polar LDA [34], to indicate the information of hyper-activity or hypo-activity for each voxel with an additional binary variable. Details about our LDA can be found in Supplementary Methods.
Step three: estimation of latent alteration factors in PDE
The discrete z-scores of ALFF within the predefined mask above for 259 PDE were fed into the polar LDA model to estimate the latent alteration factors. We chose factor number K = 2–5 to estimate the reasonable setting. Solutions were calculated 100 times from 100 random initializations, where the variational expectation-maximization algorithm was applied to estimate Pr(Factor | Patient) and Pr(Voxel | Factor) under each setting of K. The detailed algorithm flow of model estimation was shown in the Supplementary Methods. Then, a bootstrapping procedure, which generated 100 new sample sets by randomly sampling 259 subjects from the original sample set, was used to estimate the confidence intervals for the factor-specific patterns (i.e., Pr(Voxel | Factor)). To further explore the statistically significant brain regions of each factor in more detail, the Human Brainnetome Atlas [35] based on both anatomical and functional connections was applied over the estimated latent alteration factors in subsequent analyses, which included refined 210 cortical and 36 subcortical subregions. The bootstrapped z-scores were averaged across 246 brain regions. The p values were converted from the z-scores that were calculated by dividing factor-specific patterns by the bootstrapping-estimated standard deviation. In addition, we applied separate general linear models (or logistic regression for binary variables) to the factor compositions and each clinical characteristic (age, gender, education, HAMD-17, etc.) of PDE (outside of diagnosis) to explore if there were any particular behavioral or clinical differences between individuals that loaded onto a particular factor (Supplementary Methods).
Result
Latent alteration factors in PDE
With the predefined mask (13,059 voxels remaining), the unsupervised polar LDA model was applied to the voxel-wise ALFF of 259 PDE. The solutions were robust for K = 2 and 3 factors but unstable for K = 4 and 5 factors (see Supplementary Fig. S2). As K increased, the stability of factor estimation decreased gradually. Therefore, more factors were not considered in our study. The Pr(Voxel | Factor) for each latent factor could be visualized as a probabilistic map overlaid brain (each row in Fig. 1), which represented a factor-specific hyper- and hypo-activity pattern in the brain regions. The pattern distribution of each alteration factor indicated that these estimated factors from K = 2–3 were presented in a hierarchical manner. Factor 1 in two-factor model was highly similar to factor 1 in three-factor model (with a correlation of 0.99), while another two factors in three-factor model seemed to be the inheritance and extension of factor 2 in two-factor model (Supplementary Fig. S3). Suggested via pattern distribution of each alteration factor, we focused on the three-factor model for the subsequential analysis. In addition, we performed control analyses without the global signal regression when preprocessing the fMRI data and the results were generally consistent (see Supplementary Results and Supplementary Fig. S7).
The Pr(Voxel | Factor) for each latent factor was visualized as a probabilistic alteration map overlaid on the Montreal Neurological Institute template (each row), which was a probability distribution over all the voxels in the defined mask. Bright colors indicated the higher probability of hyper- or hypo-activity at this spatial location for a specific alteration factor.
The hyper- and hypo-activity patterns across three factors
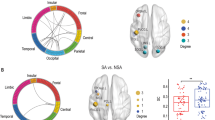
Statistically significant brain regions were obtained via a bootstrapping procedure for LDA model re-estimation and the probabilities were summed within them (Fig. 2A–C). Factor 1 was associated with hyper-activities within precentral gyrus (PrG), postcentral gyrus, superior parietal lobule (SPL), and occipital cortex (OC). By contrast, there were hypo-activities within DLPFC, orbital frontal gyrus (OFG), precuneus and posterior cingulate cortex (PCC). The patterns of hyper- and hypo- activities in factor 2 were almost opposite to those in factor 1 (with a correlation of −0.71), however with some slight deviations. For example, the parietal lobe and OFG were significant only in factor 1. Factor 3 was also associated with strong hyper-activity in OFG and hypo-activity in precuneus and PCC. Furthermore, we binarized these regions (ignoring directionality of abnormality) and summed them across the three factors (Supplementary Fig. S4) to explore the shared and specific hyper- and hypo-activity patterns among these three factors.
A–C Statistically significant patterns of hyper- and hypo-activity associated with each factor, summed within regions. Different colors represent different polarities (red, hyper-activity; blue, hypo-activity). D After mapping the six functional circuits associated with depressive disorder, the probability sum of hyper- or hypo-activity patterns for each factor was displayed on a radar chart. The number represented the scale of web and the dotted lines represented no impairment compared to healthy controls. DLPFC dorsolateral prefrontal cortex, OC occipital cortex, PCC posterior cingulate cortex, OFG orbital frontal gyrus.
In order to further analyze the functional pattern of factor-specific activity on large-scale neural circuits, six neural circuits associated with PDE [36] were selected to map to the latent alteration factors based on the Human Brainnetome Atlas, including default mode circuit, salience circuit, threat circuit, reward circuit, attention circuit and cognitive control circuit (see Supplementary Table S1 for details). As shown in Fig. 2D, except for the cognitive control circuit, the polarity of ALFF activity patterns between factor 1 and factor 2 was completely opposite, that is, the circuits exhibiting hypo-activity in factor 1 happened to be hyper-activity in factor 2. Secondly, factor 3 was similar to factor 1 on the default mode and attention circuits, while similar to factor 2 on the threat and salience circuits, suggesting that factor 3 might work as the impairment basis of PDE. Particularly, on the reward circuit, factor 3 showed a significantly higher degree of functional impairment than the other two factors.
Factor composition of the PDE
The Pr(Factor | Patient) is the probability distribution based on the latent factors estimated by the polar LDA model, which represents the patients’ factor composition. After false discovery rate (FDR, q < 0.05) multiple comparisons correction, only age and gender showed significant differences across three factors (Supplementary Results and Supplementary Fig. S5). As shown in Fig. 3, most patients expressed multiple latent alteration factors rather than only one. Notably, the tendency of factor expression was different in these two diseases: BD tended to express factor 1, while UD tended to express factor 2. Thereafter, the averaged Pr(Factor | Patient) in different groups were calculated to explore whether patients with BD or UD were biased in terms of factor composition. The mean factors composition of BD groups was 43.98%, 30.98%, 25.04% (corresponding to factors 1–3), while 25.65%, 45.89%, 28.46% in UD groups.
Each patient corresponds to a point, with the location in barycentric coordinates representing the factor composition (i.e., Pr(Factor | Patients)). The angles of the triangle represent pure factors, and the point closer to the angle indicates the higher probability of the corresponding factor. Different colors represent different sets of patients with depressive episodes. The tendency of factor expression was different: BD patients tended to express factor 1, while UD patients tended to express factor 2. BD bipolar disorder, UD unipolar disorder.
Emotional state prediction based on the latent alteration factors
To validate the effect of the factors obtained from the polar LDA model on emotion prediction, we took the IDE-BD patients as an independent data set to infer their factor compositions from the model trained by the 259 PDE. By multiplying Pr(Voxel | Factor) and Pr(Factor | Patient), the alteration degree of each patient in voxel-level (i.e., D(Voxel | Patient)) could be calculated. Then, according to the spatial location, polarity and specificity of the factor distribution, all the above significant regions after the bootstrapping procedure for LDA model re-estimation were divided into five regional sets (i.e., five regions of interest, ROIs) (see Supplementary Fig. S6): OFG, PrG+SPL, SFG+middle frontal gyrus (MFG), precuneus+PCC+IPLr (inferior parietal lobule rostrodorsal area) and OC+fusiform gyrus (FuG)+IPLc (inferior parietal lobule caudal area). The alteration degree of patients in each ROI (i.e., D(ROI | Patient)) was obtained accordingly by averaging the D(Voxel | Patient) in this region.
Then, we used the bipolar index to explore the risk indicators for the transition from depression to mania in IDE-BD patients. The bipolar index is a clinical rating scale used to determine whether a patient is at risk for BD, a higher score indicating greater risk. The correlation between the expression on each factor and bipolar index was analyzed over these IDE-BD patients, with age, gender, education and head motion as covariates. We found that the expression of factor 1 was positively correlated with bipolar index in IDE-BD patients (r = 0.4271, p = 0.0148) (Fig. 4A). Then, we applied canonical correlation analysis to find an optimal linear combination of ROI alteration degrees D(ROI | Patient) in the whole brain between that maximally correlated with bipolar index. Age, gender, education and head motion were also regressed out before canonical correlation analysis. After 10,000 runs of permutations to test the statistical significance, the results showed that the alteration degrees in these five ROIs were positively correlated with bipolar index (r = 0.5935, p = 0.005) (Fig. 4B), and the regions with the highest absolute importance were mainly in OFG and PrG+SPL.
A The Pr(Factor | Patient) of factor 1 was positively associated with the bipolar index and in IDE-BD patients, suggesting that a higher factor 1 expression was associated with the higher risk of emotional state conversion. B The CCA between the loading of D(ROI | Patient) and the bipolar index. Positive correlation indicated that a higher loading on the alteration degree was associated with a greater risk of emotional state conversion. The scatter plots showed the relationship between CCA D(ROI | Patient) loading and CCA bipolar index loading, where each dot represented a patient with IDE-BD. The bar chart showed the importance of ROI in CCA. C The distributions of expression (i.e., Pr(Factor | Patient)) on each factor in the three groups were shown as the violin plot. The inner box-plot showed the quartile and the mean value (white dot). The group differences on the factor compositions of patients in each factor were examined using the one-way analysis of covariance. D The bar graph showed the mean ROI alteration degree of patients (i.e., D(ROI | Patient)) in different clinical diagnosis groups. Error bars denote standard error of mean. The Kruskal–Wallis test was utilized to test the group difference due to the heterogeneity of variance between groups. BD bipolar disorder, IDE-BD bipolar disorder with the initial depressive episode but manic or hypomanic symptoms during follow-up, UD unipolar disorder, ROI region of interest, CCA canonical correlation analyses, OFG orbital frontal gyrus, PrG precentral gyrus, SPL superior parietal lobule, SFG superior frontal gyrus, MFG middle frontal gyrus, PreC precuneus, PCC posterior cingulate cortex, IPLr inferior parietal lobule (rostrodorsal area), OC occipital cortex, FuG fusiform gyrus, IPLc inferior parietal lobule (caudal area).
The difference of factor compositions and ROI alteration degree among three groups
Using one-way analysis of covariance, the group differences, including BD, IDE-BD and UD, on the factor compositions of patients in each factor were examined (Fig. 4C). Significant group differences on the expression of factor 1 and factor 2 were observed (all p < 0.0001, FDR corrected), with a descending (ascending) order of BD, IDE-BD, and UD for mean values in factor 1 (factor 2), but not in factor 3. The post hoc analyses revealed that compared with BD, IDE-BD and UD significantly decreased on the expression of factor 1 (all p < 0.016), while significantly increased on the expression of factor 2 (all p < 0.012).
Due to the heterogeneity of variance between groups, statistical analysis utilizing the Kruskal–Wallis test revealed significant differences in the four ROIs except OFG (all p < 0.0001, FDR corrected). In Fig. 4D, the corrected pairwise comparison results indicated that both IDE-BD and UD had significant differences with BD in SFG+MFG and OC+FuG+IPLc (all p < 0.044), while only UD and BD had significant differences in PrG+SPL and precuneus+PCC+IPLr (all p < 0.0001). Interestingly, compared with HC, BD and UD showed the opposite polarity of alterations (hyper-activity vs hypo-activity) in SFG+MFG and OC+FuG+IPLc. In addition, although no significant results were found, BD, IDE-BD, and UD tended to increase (decrease) in OFG (PrG+SPL).
Discussion
In this study, the polar LDA was used in a resting-state fMRI cohort of PDE to reveal three transdiagnostic latent alteration factors, each of which was associated with the hyper- and hypo-activity pattern of ALFF compared to HC. The majority of participants expressed multiple latent alteration factors rather than a single one, thus retaining interindividual variability. These three factors could be correspondingly referred to as the BD sensitive factor, the UD sensitive factor and the shared factor that characterized the basis of functional impairment in PDE. Finally, using an independent set of IDE-BD patients who had never experienced a manic episode during the fMRI scan, we could predict the emotional state transition risk of these patients based on the expression of BD sensitive factor or the alteration degrees of five ROIs (including OFG, PrG+SPL, SFG+MFG, precuneus+PCC+IPLr and OC+FuG+IPLc).
As the typical diseases of depressive episode, UD and BD with different diagnoses clinically had a neurobiological alteration overlap obviously. The alteration factor 3 was suggested to be the basis of functional impairment over the PDE, mainly involving the increased frontal region (OFG, subgenual anterior cingulate cortex, basal ganglia) and decreased posterior region (precuneus, PCC, IPL) ALFF, which was consistent with previous findings [37, 38]. Furthermore, in the archetypal major psychiatric disorder group obtained by ALFF-based transdiagnostic clustering, ALFF was also significantly increased in prefrontal cortex, limbic, and striatum and significantly decreased in posterior primary cortices, compared to HC [39]. Altogether, these findings further supported that the alteration pattern of factor 3 reflected the impaired balance between regions conventionally known for emotional perception and processing and the visual cortex, which was prevalent in the PDE. Moreover, our study also found that factor 3 manifested as highly hyper-activity in the reward circuit. Several studies have shown that both UD and BD patients had reduced activation in the reward-related brain regions or circuits while performing reward-related tasks, and BD showed significantly decreased activity compared with UD [40, 41]. This is consistent with our results, demonstrating that reward processing abnormalities share similar substrates across different PDE, with varying degrees of impairment [42].
In the absence of diagnostic labels, the factor composition estimated by our polar LDA model showed a significant clinical diagnostic group-level bias over factor 1 and factor 2, suggesting that these two factors may be disease-sensitive alteration patterns. Interestingly, the polarity of activity patterns between factor 1 (BD sensitive) and factor 2 (UD sensitive) was opposite in SFG, MFG, precuneus, PCC, FuG and OC. According to the spatial distribution, SFG and MFG in our factors belong to the DLPFC, which is primarily associated with cognitive control of perception and decision making and plays an important role in the top-down regulation of emotional processing [43]. Regarding structural MRI, UD patients revealed significant reduced gray matter volume in DLPFC compared to BD [7], while BD showed thinner cortical thickness [44, 45]. Furthermore, previous studies had shown that DLPFC activation in UD patients significantly increased [46] or decreased [47] compared to BD when performing various cognition-related tasks. We speculated that compared to BD, there was greater frontal control to external stimuli in UD patients whose DLPFC may be preferentially active for perpetuating negative bias processing in the resting state [37].
Next, the precuneus and PCC are the core regions of the DMN, which may be of great significance for self-consciousness and self-related mental representations during rest [48], which had been shown to be involved in the major pathogenesis of MDD and to play an important role in the successful retrieval of autobiographical and self-related information [49]. The decreased resting-state activity of precuneus both in BD and UD had been commonly demonstrated [9, 50, 51]. Consistent with our results, a meta-analysis showed that the ALFF in precuneus significantly increased in UD patients relative to BD patients, but both decreased compared to HC [52]. Furthermore, studies had shown that compared with HC, resting-state activity of PCC was increased in UD patients, associated with promoting ruminant self-awareness [53], while decreased in BD patients, associated with impaired integration of emotion and memory [54]. A transdiagnostic study across several mental illnesses and symptoms also revealed that the DMN may underlie a range of cognitive processes which were impaired in a variety of diseases [2]. Our previous studies had also confirmed that the dynamic connection of the DMN contributed to the identification between UD and IDE-BD [27].
Although no statistical differences were observed between the groups, the alteration degree of BD, IDE-BD, and UD on the OFG showed a gradual upward trend clearly, which explained why it had the highest absolute importance in the canonical correlation analysis. The OFC is involved in learning, prediction, and decision making for reward-related and emotional behaviors [55]. Alterations to OFC may abolish the top-down control of the amygdala and deeper limbic structures, which may account for the emotional and cognitive disinhibition as the typical characteristics of PDE [56]. Compared with HC, the reduced gray matter volume [56] and increased ReHo [11] or degree centrality [57] of patients with BD and UD on OFC have been reported. It has also been found that the activation of OFC in both BD and UD was related to the severity of depressive state [58, 59]. Our result further found that changes in OFC activity were also associated with the transformation of emotional state. The manic episodes in BD patients may cause a decrease in OFC activity, resulting in lower or higher than HC, which may be the reason for the inconsistency of previous studies [60, 61]. In conclusion, the OFC was not only a common transdiagnosis alteration region in PDE, but also a latent predictive risk factor for emotional state transition from unipolar to bipolar in our study.
Although covariate removal and inter-group matching were done, the significant differences for age and gender across factors were found in our LDA model, which were similar to a previous study [26]. In our study, with increasing age, patients tended to express factors 1, 2 or 3, respectively, which might reflect a neurodevelopmental stage. Furthermore, factor 2 was more frequently expressed in females, which might be related to the higher prevalence of depression among female participants. Across the lifespan, depression is almost twice as common in women as in men [62]. Studies have shown gender differences in the neuronal circuitry underlying depression [63, 64], which may be of relevance to sex differences in the clinical presentation of depression [65].
Compared with other current transdiagnostic studies [2, 3, 66], our study focused primarily on PDE, allowing us to better understand the association between these two disorders with similar clinical symptoms. Secondly, previous transdiagnostic studies mostly used clustering to divide each patient into a single subtype [39], which ignored the individual heterogeneity among patients. Our approach allows each individual to express multiple latent factors to varying degrees rather than a single factor. Finally, although no patient’s diagnostic category was given at the time of input, our results found that each factor has different sensitivity for different categories of patients.
Limitations
There are several limitations in our study. First, our conclusions need to be validated by additional clinical features or behavioral characteristics, which can simultaneously characterize UD and BD. We can use multiple modal data such as gene expression and structural neuroimaging to further explore the spectra of transdiagnostic PDE symptom profiles. Another limitation is the number of participants that needs to be improved and multicenter data may be more convincing. Moreover, the patients in this study were all in a major depressive episode state, and the inclusion of BD patients with manic episodes could better contribute to understand the shared and disease-sensitive impairment pattern between these two diseases. Finally, longitudinal data and medications are needed to explore the effects of medication status.
Conclusions
Using the LDA, our study revealed three transdiagnostic alteration factors associated with hyper- and hypo-activity patterns in PDE, each patient displaying a unique composition of these factors. The factors could be correspondingly divided into the BD sensitive factor, the UD sensitive factor and the shared basic alteration factor. Furthermore, our approach also contributed to the prediction of emotional state in IDE-BD patients, where the major brain regions involved include OFG and PrG+SPL. Understanding these core alterations in PDE was critical for mapping the major neural pathways resulting in psychopathology and the intersections in which distinct paths lead to varying clinical phenomenology within and across diagnoses in the future.
References
Fusar-Poli P, Solmi M, Brondino N, Davies C, Chae C, Politi P, et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry. 2019;18:192–207.
Baker JT, Dillon DG, Patrick LM, Roffman JL, Brady RO Jr, Pizzagalli DA, et al. Functional connectomics of affective and psychotic pathology. Proc Natl Acad Sci USA. 2019;116:9050–59.
Wang T, Zhang X, Li A, Zhu M, Liu S, Qin W, et al. Polygenic risk for five psychiatric disorders and cross-disorder and disorder-specific neural connectivity in two independent populations. Neuroimage Clin. 2017;14:441–49.
McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–85.
McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry. 2020;177:411–21.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51.
Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–63.
Yang Y, Liu S, Jiang X, Yu H, Ding S, Lu Y, et al. Common and specific functional activity features in schizophrenia, major depressive disorder, and bipolar disorder. Front Psychiatry. 2019;10:52.
Qiu M, Zhang H, Mellor D, Shi J, Wu C, Huang Y, et al. Aberrant neural activity in patients with bipolar depressive disorder distinguishing to the unipolar depressive disorder: a resting-state functional magnetic resonance imaging study. Front Psychiatry. 2018;9:238.
Wang X, Zhao N, Shi J, Wu Y, Liu J, Xiao Q, et al. Discussion on patients with bipolar disorder and depressive episode by ratio low frequency amplitude combined with grey matter volume analysis. J Med Syst. 2019;43:117.
Yao X, Yin Z, Liu F, Wei S, Zhou Y, Jiang X, et al. Shared and distinct regional homogeneity changes in bipolar and unipolar depression. Neurosci Lett. 2018;673:28–32.
Jiang X, Dai X, Kale Edmiston E, Zhou Q, Xu K, Zhou Y, et al. Alteration of cortico-limbic-striatal neural system in major depressive disorder and bipolar disorder. J Affect Disord. 2017;221:297–303.
Zhang K, Liu Z, Cao X, Yang C, Xu Y, Xu T, et al. Amplitude of low-frequency fluctuations in first-episode, drug-naive depressive patients: a 5-year retrospective study. PLoS One. 2017;12:e0174564.
Wang J, Wang Y, Huang H, Jia Y, Zheng S, Zhong S, et al. Abnormal dynamic functional network connectivity in unmedicated bipolar and major depressive disorders based on the triple-network model. Psychol Med. 2020;50:465–74.
Liu C, Pu W, Wu G, Zhao J, Xue Z. Abnormal resting-state cerebral-limbic functional connectivity in bipolar depression and unipolar depression. BMC Neurosci. 2019;20:30.
Wang Y, Wang J, Jia Y, Zhong S, Zhong M, Sun Y, et al. Topologically convergent and divergent functional connectivity patterns in unmedicated unipolar depression and bipolar disorder. Transl Psychiatry. 2017;7:e1165.
Wang Y, Wang J, Jia Y, Zhong S, Niu M, Sun Y, et al. Shared and specific intrinsic functional connectivity patterns in unmedicated bipolar disorder and major depressive disorder. Sci Rep. 2017;7:3570.
Goya-Maldonado R, Brodmann K, Keil M, Trost S, Dechent P, Gruber O. Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum Brain Mapp. 2016;37:808–18.
Han KM, De Berardis D, Fornaro M, Kim YK. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog Neuro-Psychoph. 2019;91:20–27.
Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–71.
Kong R, Li J, Orban C, Sabuncu MR, Liu H, Schaefer A, et al. Spatial topography of individual-specific cortical networks predicts human cognition, personality, and emotion. Cereb Cortex. 2019;29:2533–51.
Buch AM, Liston C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology. 2021;46:156–75.
Voineskos AN, Jacobs GR, Ameis SH. Neuroimaging heterogeneity in psychosis: neurobiological underpinnings and opportunities for prognostic and therapeutic innovation. Biol Psychiatry. 2020;88:95–102.
Marquand AF, Rezek I, Buitelaar J, Beckmann CF. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 2016;80:552–61.
Zhang X, Mormino EC, Sun N, Sperling RA, Sabuncu MR, Yeo BT, et al. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc Natl Acad Sci USA. 2016;113:E6535–E44.
Tang S, Sun N, Floris DL, Zhang X, Di Martino A, Yeo BTT. Reconciling dimensional and categorical models of autism heterogeneity: a brain connectomics and behavioral study. Biol Psychiatry. 2020;87:1071–82.
Shao J, Dai Z, Zhu R, Wang X, Tao S, Bi K, et al. Early identification of bipolar from unipolar depression before manic episode: evidence from dynamic rfMRI. Bipolar Disord. 2019;21:774–84.
Power JD, Plitt M, Gotts SJ, Kundu P, Voon V, Bandettini PA, et al. Ridding fMRI data of motion-related influences: removal of signals with distinct spatial and physical bases in multiecho data. Proc Natl Acad Sci USA. 2018;115:E2105–E14.
Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–87.
Blei DM, Ng AY, Jordan MI. Latent Dirichlet allocation. J Mach Learn Res. 2003;3:993–1022.
Zuo XN, Xing XX. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci Biobehav Rev. 2014;45:100–18.
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54.
Tang S, Sun N, Floris DL, Zhang X, Di Martino A, Yeo BTT. Reconciling dimensional and categorical models of autism heterogeneity: a brain connectomics and behavioral study. Biol Psychiatry. 2020;87:1071–82.
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26.
Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34:9–24.
Liu J, Ren L, Womer FY, Wang J, Fan G, Jiang W, et al. Alterations in amplitude of low frequency fluctuation in treatment-naive major depressive disorder measured with resting-state fMRI. Hum Brain Mapp. 2014;35:4979–88.
Xu K, Liu H, Li H, Tang Y, Womer F, Jiang X, et al. Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. J Affect Disord. 2014;152–154:237–42.
Chang M, Womer FY, Gong X, Chen X, Tang L, Feng R, et al. Identifying and validating subtypes within major psychiatric disorders based on frontal-posterior functional imbalance via deep learning. Mol Psychiatry. 2021;26:2991–3002.
Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–54.
Redlich R, Dohm K, Grotegerd D, Opel N, Zwitserlood P, Heindel W, et al. Reward processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology. 2015;40:2623–31.
Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12.
Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77.
Niu M, Wang Y, Jia Y, Wang J, Zhong S, Lin J, et al. Common and specific abnormalities in cortical thickness in patients with major depressive and bipolar disorders. EBioMedicine. 2017;16:162–71.
Lan MJ, Chhetry BT, Oquendo MA, Sublette ME, Sullivan G, Mann JJ, et al. Cortical thickness differences between bipolar depression and major depressive disorder. Bipolar Disord. 2014;16:378–88.
Young KD, Bodurka J, Drevets WC. Differential neural correlates of autobiographical memory recall in bipolar and unipolar depression. Bipolar Disord. 2016;18:571–82.
Rive MM, Koeter MW, Veltman DJ, Schene AH, Ruhe HG. Visuospatial planning in unmedicated major depressive disorder and bipolar disorder: distinct and common neural correlates. Psychol Med. 2016;46:2313–28.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83.
Zhong X, Pu W, Yao S. Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naive patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J Affect Disord. 2016;206:280–86.
Liu CH, Ma X, Yuan Z, Song LP, Jing B, Lu HY, et al. Decreased resting-state activity in the precuneus is associated with depressive episodes in recurrent depression. J Clin Psychiatry. 2017;78:e372–e82.
Han S, Cui Q, Wang X, Li L, Li D, He Z, et al. Resting state functional network switching rate is differently altered in bipolar disorder and major depressive disorder. Hum Brain Mapp. 2020;41:3295–304.
Gong J, Wang J, Qiu S, Chen P, Luo Z, Wang J, et al. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 2020;10:353.
Li W, Chen Z, Wu M, Zhu H, Gu L, Zhao Y, et al. Characterization of brain blood flow and the amplitude of low-frequency fluctuations in major depressive disorder: a multimodal meta-analysis. J Affect Disord. 2017;210:303–11.
Zhong SM, Chen GM, Zhao LP, Jia YB, Chen F, Qi ZZ, et al. Correlation between intrinsic brain activity and thyroid-stimulating hormone level in unmedicated bipolar II depression. Neuroendocrinology. 2019;108:232–43.
Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702.
Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771.
Li M, Das T, Deng W, Wang Q, Li Y, Zhao L, et al. Clinical utility of a short resting-state MRI scan in differentiating bipolar from unipolar depression. Acta Psychiatr Scand. 2017;136:288–99.
Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord. 2012;14:432–41.
Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527.
Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–17.
Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, et al. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry. 2012;169:316–25.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
Epp JR, Beasley CL, Galea LAM. Increased hippocampal neurogenesis and p21 expression in depression: dependent on antidepressants, sex, age, and antipsychotic exposure. Neuropsychopharmacology. 2013;38:2297–306.
Kong LT, Chen KY, Womer F, Jiang WY, Luo XG, Driesen N, et al. Sex differences of gray matter morphology in cortico-limbic-striatal neural system in major depressive disorder. J Psychiatr Res. 2013;47:733–39.
Eid RS, Gobinath AR, Galea LAM. Sex differences in depression: insights from clinical and preclinical studies. Prog Neurobiol. 2019;176:86–102.
Huang CC, Luo Q, Palaniyappan L, Yang AC, Hung CC, Chou KH, et al. Transdiagnostic and illness-specific functional dysconnectivity across schizophrenia, bipolar disorder, and major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:542–53.
Acknowledgements
The authors thank the patients who volunteered to participate in this study and the support of the Department of Psychiatry of Affiliated Brain Hospital of Nanjing Medical University.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81871066); Jiangsu Provincial Medical Innovation Team of the Project of Invigorating Health Care through Science, Technology and Education (Grant No. CXTDC2016004); Jiangsu Provincial Key Research and Development Program (Grant No. BE2018609 and BE2019675); Key Project supported by Medical Science and Technology Development Foundation, Jiangsu Commission of Health (Grant No. K2019011).
Author information
Authors and Affiliations
Contributions
JS and QL contributed to the conception and design of the study. JS, YZ and LX performed the analyses and JS drafted the manuscript. XW and HW participated in discussion of imaging analyses and statistical analyses. JS, YZ, LX XW, HW and RZ recruited participants and collected the data. JS, QL and ZY reviewed and revised the manuscript. ZY and QL obtained grant funding. All authors read and approved the final version of the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shao, J., Zhang, Y., Xue, L. et al. Shared and disease-sensitive dysfunction across bipolar and unipolar disorder during depressive episodes: a transdiagnostic study. Neuropsychopharmacol. 47, 1922–1930 (2022). https://doi.org/10.1038/s41386-022-01290-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01290-9
This article is cited by
-
Classification of bipolar disorders using the multilayer modularity in dynamic minimum spanning tree from resting state fMRI
Cognitive Neurodynamics (2023)