Abstract
Susceptibility or resilience to posttraumatic stress disorder (PTSD) depends on one’s ability to appropriately adjust synaptic plasticity for coping with the traumatic experience. Activity-regulated mRNA translation synthesizes plasticity-related proteins to support long-term synaptic changes and memory. Hence, cytoplasmic polyadenylation element-binding protein 3-knockout (CPEB3-KO) mice, showing dysregulated translation-associated synaptic rigidity, may be susceptible to PTSD-like behavior. Here, using a context-dependent auditory fear conditioning and extinction paradigm, we found that CPEB3-KO mice exhibited traumatic intensity-dependent PTSD-like fear memory. A genome-wide screen of CPEB3-bound transcripts revealed that Nr3c1, encoding glucocorticoid receptor (GR), was translationally suppressed by CPEB3. Thus, CPEB3-KO neurons with elevated GR expression exhibited increased corticosterone-induced calcium influx and decreased mRNA and protein levels of brain-derived neurotrophic factor (Bdnf). Moreover, the reduced expression of BDNF was associated with increased GR level during fear extinction in CPEB3-KO hippocampi. Intracerebroventricular delivery of BDNF before extinction training mitigated spontaneous fear intrusion in CPEB3-KO mice during extinction recall. Analysis of two GEO datasets revealed decreased transcriptomic expression of CPEB3 but not NR3C1 in peripheral blood mononuclear cells of humans with PTSD. Collectively, this study reveals that CPEB3, as a potential PTSD-risk gene, downregulates Nr3c1 translation to maintain proper GR-BDNF signaling for fear extinction.
Similar content being viewed by others
Introduction
Posttraumatic stress disorder (PTSD) is a psychiatric disorder after a traumatic experience, such as combat- or domestic violence-caused distress. The manifestation of PTSD depends on not only the intensity of trauma but also genetic risk factors [1, 2]; the latter may result in enhanced fear memory formation and/or impaired fear extinction after exposure to trauma reminders. A significant number of individuals with PTSD do not respond well to exposure therapy [3,4,5], which modifies fear memory through extinction learning. Therefore, the risk alleles that influence the development and treatment of PTSD must be identified.
However, the genetic predisposition to PTSD is difficult to determine [6] because an extensive trauma is necessary to trigger its development, and healthy controls can carry PTSD-risk alleles in the absence of over-threshold trauma exposure. Many candidate-driven studies have investigated genes involved in regulating stress and fear responses, including the hypothalamic-pituitary-adrenal axis (HPA-axis) and limbocortical circuit of fear memory. At the molecular level, glucocorticoid receptor (GR), a glucocorticoid-activated transcription factor, is the key mediator of the stress response by modulating the HPA-axis [7] and synaptic plasticity in the limbocortical circuit [8]; when dysregulated, GR affects the pathogenesis and therapy of stress-related emotional disorders [9, 10]. Additional molecular factors contributing to the magnitude of PTSD symptoms have been addressed, such as serotonin transporter (5-HTT), dopamine receptor, brain-derived neurotrophic factor (BDNF) and FK506-binding protein 5 (FKBP5). Some are GR-regulated genes or molecules controlling GR sensitivity [11]. Because PTSD is caused by an inability to appropriately adjust synaptic plasticity after severe trauma, plasticity-related proteins, such as BDNF and metabotropic glutamate receptor 5 (mGluR5), have also been reported as PTSD-risk factors [12, 13]. Recently, a large-scale meta-analysis of PTSD genome-wide association studies reported additional risk alleles under the consideration of sex and ethnicity. For example, single nucleotide polymorphisms in PARK2 are related to dopamine signaling, ZDHHC14 is involved in regulating β-adrenergic receptors, and several non-coding RNAs with unidentified relations are related to the etiology of PTSD [14]. Because non-coding RNAs can affect a wide-spectrum of gene expression [15, 16], they may shift the balance in gene regulatory networks to affect the HPA-axis and/or synaptic plasticity and consequently accentuate a vulnerability to trauma.
Generating PTSD animal models aims to uncover neural and molecular mechanisms underlying PTSD and then develop effective therapeutic strategies. However, multiple stressful events in combination are required to induce PTSD-like symptoms in mice and rats [17, 18]. To include the genetic risk factor, mutant mice such as FKBP5-knockout (KO), 5-HTT-KO, hippocampal KO of BDNF and BDNF-Val66Met have been used for studying aberrant fear responses [19,20,21,22,23]. Cytoplasmic polyadenylation element-binding protein 3 (CPEB3) is a sequence-specific RNA-binding protein that regulates the translation of several plasticity-related proteins, including the subunits of N-methyl-D-aspartate receptor (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) [24,25,26]. Our previous studies found that CPEB3 could inhibit translation via downregulating the GTPase activity of the translation elongation factor eEF2. NMDAR signaling triggers calpain 2-mediated proteolysis of CPEB3, thereby increasing translation of CPEB3-repressed RNAs [26,27,28]. The other study identified that monoubiquitination of CPEB3 by Neuralized1 switches CPEB3 from a repressor to an activator to promote polyadenylation-induced synthesis of AMPAR subunits [29]. Therefore, similar to its family members, CPEB1, CPEB2 and CPBE4, CPEB3 can regulate mRNA translation bidirectionally depending on the interacting partners and signaling [30]. Several lines of evidence indicate an increased synaptic rigidity in the CPEB3-KO hippocampus and cortex, including slow reversal learning in the Morris water maze and chemical long-term depression-induced synaptic remodeling as well as impaired hippocampal depotentiation [31, 32]. Moreover, CPEB3-KO mice with normal responses to electric shocks exhibited accelerated acquisition and retarded extinction in the contextual fear conditioning test, but their long-term consolidated memory appeared normal [31].
In this study, we identified that CPEB3-KO mice showed enhanced fear intrusion during extinction recall in a traumatic intensity-dependent manner. Molecular characterizations identified that CPEB3-suppressed translation of GR mRNA (encoded by the Nr3c1 gene) and its deficiency rendered elevated GR signaling to impair Bdnf transcription and fear extinction. Intracranial supplementation of BDNF into CPEB3-KO mice before extinction training mitigated their abnormal fear responses. Together, CPEB3 may be a novel regulator of GR-mediated stress signaling for coping with traumatic experiences.
Materials and methods
Animals, genotyping and corticosterone (CORT) treatment
All animal experiments were approved and performed in accordance with the guidelines of the Institutional Animal Care and Utilization Committee in Academia Sinica. To obtain CPEB3-wild type (WT) and -KO mice, male littermates from heterozygous mating were genotyped and housed in a 12-h light-dark room with ad libitum access to food and water as described [31]. Male mice at age 2.5–4 months were used for behavioral assays or intraperitoneally (i.p.) injected with CORT (5 mg/kg) at 2 pm. To avoid circadian influences, all mice were euthanized between 2 and 4 pm for tissue collection.
Antibodies and chemicals
Antibodies used in the study were β-actin (A5441), α-tubulin (T5168) and pY816-TrkB (ABN1381) from Sigma-Aldrich; GAPDH (SC-25778), GR (SC-1004) and TrkB (SC-8316) from Santa Cruz Biotechnology; pS211-GR (#8765), CREB (#9197) and pS133-CREB (#9196) from Cell Signaling; myc-tag (9E10 clone) from Abcam; CaMK2α (05-532) and pT286-CaMK2α (05-533) from Millipore; BDNF (25699-1-AP) from Proteintech; mGluR5 (A9819) from ABclonal; Kalirin (PA5-18214) from Invitrogen; and CPEB3 monoclonal antibody generated in-house (Chao et al., 2012). AlexaFluor-conjugated secondary antibodies, ProLong Gold antifade and 4’,6-diamidino-2-phenylindole (DAPI) reagents and Fura-2 AM calcium indicator (F1221) were from Thermo Fisher. Avertin (T48402), papain (P4762) and CORT (27840) were from Sigma-Aldrich.
Context-dependent auditory fear acquisition and extinction
Adult male mice were tested by observers who were blinded to genotypes except for the BDNF-rescue experiment. In brief, the fear response was assessed by the freezing percentage during the tone-given period (FreezeScan, CleverSys Inc.). A mouse was first placed in the acquisition chamber (context A) cleaned with 70% ethanol for 200 s. For fear conditioning, the habituated mouse received a 2k-Hz tone for 20 s (conditioned stimulus [CS]) with a 0.5-mA foot shock during the last 2 s (unconditioned stimulus [US]), followed by a 100-s interval (total 2 min/trial). The CS-US paired conditioning was repeated for 4 or 6 trials before returning to the cage. After 24-h consolidation, extinction training was performed in an apparatus of different shape, texture and color (context B), which was cleaned with scented soap water. The fear-conditioned mouse was habituated for 100 s and then received a 2k-Hz tone for 20 s, followed by a 50-s post-trial interval (total 70 s/training). The extinction training was repeated 18 times before returning to the cage. One extinction trial was to analyze the freezing time in 3 consecutive tone periods and the extinction blocks #1 and #2 were spaced 30 min apart. A recall task, including a 100-s habituation and 4 repeats of 20-s 2k-Hz tone with a 50-s interval, was performed in context B to test spontaneous fear recovery and then in context A 30 min later to evaluate fear renewal. The mouse stayed in both contexts for 50 s more after the last 20-s tone before returning back to the cage.
Intracerebroventricular (ICV) Infusion of BDNF
Mice at age 8–10 weeks were anesthetized and implanted with a 25-gauge cannula to the lateral ventricle (AP −0.6 mm, ML −1.5 mm, DV −2.2 mm relative to Bregma) as described [33]. After recovery from the surgery for 2 weeks, the mice underwent CS-US conditioning. After 24 h, mice were briefly anesthetized with isoflurane and a 33-gauge micro-injector was connected to infuse 1 μl saline or BDNF (1 μg/μl) at 15 μl/h. The mouse recovered for 1 h in the cage was given extinction trainings.
RNA Immunoprecipitation (RIP) and RT-qPCR
A previous protocol was used with modifications [31]. A pair of mouse cortices was harvested for immunoprecipitation by using control or CPEB3 IgG-bound beads. About 10% beads was used for immunoblotting and the precipitated RNAs from the remaining beads were used for microarray analysis. For validating microarray-identified targets, the RNA samples isolated from independent RIPs were reverse-transcribed. Quantitative PCR involved using comparative threshold cycle and the Universal Probe Library system (Roche) with the primers listed in Supplementary Table S1.
Microarray and gene ontology analysis
A previous protocol was followed with modifications [34]. The precipitated RNAs from 2 independent RIPs were submitted to a microarray service (Agilent SurePrint G3 Mouse GE 8x60K, Welgene, Taiwan). The genes with average signal showing more than 1.5-fold enrichment in CPEB3 versus control precipitates were analyzed by the Gene Ontology enRIchment anaLysis and visual LizAtion tool (GORILLA) [35]. The term “neuron” was applied to all selected ontologies and the genes in “learning or memory” (Supplementary Table S2) with signal in the top 50% were further analyzed for the number of CPE (UUUUA1-2U).
Immunohistochemistry, image acquisition and quantification
Mice i.p. injected with CORT at 2 pm were anesthetized 30 min later and perfused with 4% formaldehyde. Brains were isolated, post-fixed in 4% formaldehyde, embedded in paraffin and sectioned coronally. Solutions were prepared in PBS and all procedures were conducted at room temperature, unless otherwise specified. Antigen retrieval was performed in Tris-EDTA buffer (pH 9.0) in a pressure cooker for 5 min. Slices were permeabilized with 0.2% Triton X-100 for 30 min and blocked with 10% horse serum and 5% bovine serum albumin (BSA) for 2 h, followed by the incubation of primary antibodies in 1% BSA, 1% horse serum and 0.05% Triton X-100 solution at 4 °C for overnight. After 5 washes of PBS containing 0.2% Triton X-100, the slices were incubated with DAPI and fluorophore-conjugated secondary antibodies for 2 h and mounted in ProLong Gold antifade reagent. Images were captured with consistent parameters by LSM780 confocal microscopy and analyzed by Image J (NIH).
Neuronal culture, lentiviral infection and calcium imaging
Because of the infertility of CPEB3-KO females, two pregnant mice of heterozygous mating were used each time for culturing WT and KO neurons as described [31]. The preparation of lentiviruses and the infection of cultured neurons at 7 days in vitro (DIV) were as reported [24]. For calcium imaging, DIV17-18 neurons were washed with Hank’s balanced salt solution (HBSS) twice and then incubated with HBSS containing 2 µM Fura-2 AM, 1 mg/ml glucose and 0.1% BSA at 37 °C for 30 min. Neurons were recorded in a 37 °C chamber on Zeiss Axiovert 200 Inverted Fluorescent microscope and perfused with HBSS ± the indicated compounds at 4 ml/min. Regions of interest over the field of view were selected and the mean fluorescence intensity of each frame was measured.
Plasma corticosterone measurement and reporter assay
Mice completed the indicated behavior condition were sacrificed immediately to collect tissues and blood (1.5 mg EDTA/ml blood). After 10 min of 3000 x g centrifugation, plasma samples were collected to determine corticosterone concentration by using Corticosterone ELISA (KA0468, Abnova). The 3′-UTRs of hNR3C1 and mNr3c1 were amplified by using the primers listed in Supplementary Table S1 and cloned into XbaI-linearized pcDNA3.1-FLuc plasmid. HEK293T cells in a 12-well plate were transfected with a mixture of plasmids containing 0.2 µg firefly luciferase and 0.1 µg Renilla luciferase reporters along with 0.5 µg plasmid expressing enhanced green fluorescent protein (EGFP) or myc-CPEB3 by using Lipofectamine 2000. The reporter assay was performed 24 h later by using the Dual luciferase assay kit (Promega).
Statistical analysis
Excel and GraphPad Prism were used to produce the graphs and statistics. Single-factor comparisons were determined by nonparametric Mann–Whitney test. Multivariate data were analyzed by one-way or two-way ANOVA with Bonferroni post hoc comparisons except for behavioral data, which were analyzed with the Fisher LSD post hoc test.
Results
CPEB3-KO mice show PTSD-like behavior in response to intensified fear conditioning
To test whether CPEB3-KO mice are vulnerable to PTSD when facing increasing traumatic exposure, we used a context-dependent auditory fear conditioning and extinction paradigm to assess their fear responses (Fig. 1a). To reach a comparable freezing level after CS-US conditioning, CPEB3-WT and -KO mice underwent 6 and 4 acquisition trials, respectively (Fig. 1b). Both groups of mice explored the context A chamber with low freezing levels during habituation and then learned to pair the CS and US after acquisition training. CPEB3-KO mice learned faster than their WT littermates at the 2nd and 3rd trials but eventually reached a comparable freezing level at the last trial. On the next day, the extinction training successfully reduced the fear responses of all mice. We found no significant difference in freezing level between the last acquisition trial and first extinction trial, so the consolidation of fear memory appeared normal in both groups. During the recall tests, CS-induced freezing was significantly higher in context A (i.e., renewal) than context B (i.e., spontaneous recovery), but both WT and KO mice exhibited similar fear responses in both contexts.
a The protocol used to evaluate spontaneous fear recovery and fear renewal in adult male mice after CS-US paired conditioning in context A and CS-evoked extinction training in context B. CS (conditioned stimulus), 2k-Hz tone for 20 s; US (unconditioned stimulus), 0.5-mA foot shock given at the last 2 s of the tone for acquisition training. Each extinction trial consisted of 3 consecutive 20-s tones separated by a 50-s interval. b Freezing levels in CPEB3-WT and -KO mice during acquisition and extinction of cued fear conditioning. Consecutive extinctions (#1 and #2) were performed 30 min apart. The mice after extinction training were tested 6 days later for spontaneous recovery and renewal of fear in two different contexts. Acquisition: F(6,57) = 41.488, p < 0.001, extinction: F(1,18) = 5.364, p = 0.033, consolidation: F(1,18) = 3.635, p = 0.073, and recall: F(1,18) = 8.517, p = 0.009. c Similar to b except increasing acquisition trials in KO mice. Acquisition: F(6,84) = 75.044, p < 0.001, extinction: F(1,24) = 51.237, p < 0.001, consolidation: F(1,24) = 4.038, p = 0.056, and recall: F(1,24) = 6.798, p = 0.015. The number of mice used for each group is indicated in the parenthesis. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant, two-way ANOVA.
We then challenged CPEB3-KO mice with intensified traumatic exposure by increasing acquisition trials to 6 times (Fig. 1c). All mice were trained to associate CS with US during acquisition, but KO mice showed enhanced associated fear learning from the 2nd to 4th trial. Although both groups exhibited similar freezing levels at the end of acquisition, CPEB3-KO mice showed enhanced memory consolidation with increased freezing in the 1st extinction trial. Extinction training significantly diminished freezing in both groups to a similar extent. Notably, KO mice displayed comparable fear renewal with their WT littermates in context A, but their spontaneous recovery of fear in context B was markedly elevated (Fig. 1c). Therefore, CPEB3-KO mice showed PTSD-like fear intrusion after increasing trauma exposure (6 vs 4 acquisition trials), and their fear memory failed to be modified after extinction learning.
CPEB3 represses Nr3c1 mRNA translation in neurons
We used RNA immunoprecipitation (RIP) to identify CPEB3-bound mRNAs (Fig. 2a) and classified them by Gene Ontology analysis [35] (Fig. 2b). Some known CPEB3-targeted mRNAs, such as Grin1 (with average fold enrichment of 1.19), Grin2a (0.92), Grin2b (1.48), Gria1 (1.61) and Gria2 (2.83), were not identified from this genome-wide approach or were clustered in the GO: glutamate receptor signaling pathway. We next examined transcripts in GO: learning or memory (Fig. 2c and Supplementary Table S2) and analyzed the number of cytoplasmic polyadenylation element (CPE, UUUUA1-2U) sequences in the 3’-untranslated region (3’-UTR) (Fig. 2d). Among the top 3 candidates, Grm5, Kalrn and Nr3c1, only the latter 2 could be validated from independent RIPs (Fig. 2e). Kalrn encodes Kalirin, a guanine nucleotide exchange factor for BDNF-stimulated neurite outgrowth [36]. Grm5 encodes mGluR5, which is upregulated in PTSD individuals [12, 37]. Only Nr3c1-encoded GR expression was upregulated in CPEB3-KO corticohippocampal tissue (Fig. 2f). The increased GR level was unlikely caused by a dysregulated HPA-axis because corticosterone (CORT) concentrations were comparable between WT and KO mice (Fig. 2g).
a Flow chart identifying CPEB3-bound transcripts by RNA-immunoprecipitation (RIP). b Gene ontology analysis (GORILLA) of enrichment clusters with more than 1.5-fold enrichment in the substance precipitated by CPEB3 (CP3) IgG relative to control (CTL) IgG. The statistical significance of each cluster is expressed as -log10 P in the X-axis. c Duplicate RIP signals of the identified genes in GO: learning or memory. The normalized RIP signals (RIPCP3 or RIPCTL/0.5 x [RIPCTL1 + RIPCTL2]) were ranked from smallest (min: 0.74) to largest (max: 9.99) and then color-coded. The median of the normalized RIP signals is 1.25. Nr3c1 is marked in red. d Genes with RIP signal intensity in the top 50% of all genes listed in c were analyzed for the consensus cytoplasmic polyadenylation elements (CPEs, UUUUAU and UUUUAAU) in their 3’-UTR and ranked according to number of CPEs. e Corticohippocampal lysates from adult male mice were used for RIP, followed by RT-qPCR to determine the relative levels of Nr3c1, Grm5 and Kalrn (n = 4 independent experiments). f Immunoblots of denoted proteins in WT and KO corticohippocampal tissues expressed as relative ratio to GAPDH expression. g Plasma corticosterone levels in mice with or without foot shock (6 mice per group). Data are mean ± SEM. *p < 0.05, n.s. not significant, nonparametric Mann–Whitney test.
The limbic regions, including the hippocampus, amygdala and prefrontal cortex, are involved in context-dependent auditory fear memory presentations [38, 39]. Only the protein (Fig. 3a) but not mRNA (Fig. 3b) level of GR elevated in these brain regions of CPEB3-KO mice. The phosphorylation of GR at S211 is enhanced upon ligand binding and important for nuclear translocation and transcriptional activation of GR [40]. Increased GR and pS211-GR levels were also detected in cultured KO neurons, which could be restored by ectopically expressing myc-tagged CPEB3 (Fig. 3c). Because aberrant GR signaling is highly relevant to PTSD, we examined whether CPEB3 may bind to and suppress the translation of human NR3C1 mRNA containing multiple CPEs (Fig. 3d). CPEB3 inhibited the expression of firefly luciferase reporter appended with the 3’-UTR of not only mouse Nr3c1 but also human NR3C1 (Fig. 3e). Thus, CPEB3 negatively regulates GR synthesis to confine GR signaling.
a Immunoblotting of hippocampal (Hippo), amygdala (Amyg) and prefrontal cortical (PF-Ctx) lysates from adult male mice. The protein level of GR was normalized to that of β-actin. b RT-qPCR of Nr3c1 and Gapdh mRNA levels expressed as relative ratio in various WT and KO brain tissues. c WT and KO neurons cultured at DIV7 were infected with lentiviruses expressing EGFP or myc-CPEB3 and harvested at DIV18 for immunoblotting. d The 9 and 12 CPEs in the 3’-UTR of mouse Nr3c1 and human NR3C1, respectively. e Dual luciferase reporter assay. The reporter plasmids, firefly luciferase (FLuc) with or without denoted 3’-UTR and Renilla luciferase (RLuc) were co-transfected with the plasmid expressing myc-tag or myc-CPEB3 into HEK293T cells. FLuc and RLuc activities were analyzed and expressed as relative ratios (3 independent experiments). Data are mean ± SEM. *p < 0.05, ***p < 0.001, n.s. not significant, nonparametric Mann–Whitney test.
Increased CORT-induced calcium influx in CPEB3-KO neurons
GR activation enhances Ca2+ conductance and its downstream signaling in the hippocampus and amygdala [41,42,43,44]. As expected, KO neurons treated with ≥200 ng/ml CORT showed increased calcium influx [Ca2+]i (Supplementary Fig. S1a). GR activation also sustains NMDAR-mediated [Ca2+]i [45], so NMDA-induced [Ca2+]i was potentiated by the presence of ≥200 ng/ml CORT (Supplementary Fig. S1b). Of note, we previously identified increased NMDAR expression in CPEB3-KO neurons [31], so NMDA alone caused elevated [Ca2+]i in KO neurons (Supplementary Fig. S1c). CORT-potentiated [Ca2+]i via NMDAR was augmented in KO neurons due to the elevated levels of both GR and NMDAR (Supplementary Fig. S1b). Ectopic expression of myc-CPEB3 sufficiently rescued CORT- and NMDA-induced abnormal [Ca2+]i in KO neurons (Supplementary Fig. S1a–c).
The activation of calcium/calmodulin-dependent protein kinase 2α (CaMK2α) through NMDAR signaling as determined by autophosphorylation at T286 was elevated in CPEB3-KO neurons [32]. WT and KO neurons treated with 200 ng/ml CORT or in combination with 3-min NMDA stimulation were analyzed. The elevated GR and pT286-CaMK2α signal in KO neurons could be restored by ectopic myc-CPEB3 expression (Supplementary Fig. S1d). However, a slight increase of CORT-induced [Ca2+]i (Supplementary Fig. S1a) did not aggravate CaMK2α activation in KO neurons (Supplementary Fig. S1d). By contrast, in the presence of CORT with a brief NMDA stimulation, the level of pT286-CaMK2α was more obviously elevated in KO neurons (Supplementary Fig. S1e, conditions 3 and 4 vs 2).
Because CPEB3-KO neurons showed abnormal calcium signaling in response to CORT and NMDA (Supplementary Fig. S1), we analyzed whether CaMK2α is aberrantly activated in CPEB3-KO hippocampus and amygdala during the behavior test. Although hippocampal pT286-CaMK2α level was higher in habituated KO than WT mice (condition 1), it showed opposite changes with an increase in level in WT and a decrease in level in KO hippocampi after fear conditioning (condition 2). Such a phenomenon was not consistently found in amygdala (Supplementary Fig. S2). Moreover, the p-CaMK2α signal was downregulated to a similar level in WT and KO hippocampi after 24-h consolidation (condition 3). When spontaneous fear recovery was recalled in context B, KO hippocampi showed a slight increase in p-CaMK2α signal (condition 4). Because the activation of CaMK2α is necessary for fear consolidation and extinction [46, 47], the elevated p-CaMK2α signal in naïve KO mice may contribute to their accelerated fear acquisition.
Elevated GR signaling and reduced BDNF level in CPEB3-KO hippocampus
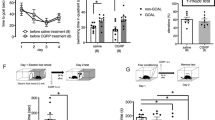
The data presented so far suggested that the loss of CPEB3-suppressed Nr3c1 translation causes elevated GR expression, thereby leading to PTSD-like behavior in fear-conditioned KO mice. To examine whether CPEB3 downregulation could be associated with PTSD, we analyzed 2 GEO DataSets, which contain the transcriptomes obtained from peripheral blood mononuclear cells (PBMCs) of humans. In the GSE860 dataset, we found reduced mRNA level of CPEB3 but not NR3C1 in PTSD individuals as compared with controls, both groups being trauma survivors [48]. The levels of CPEB3 and NR3C1 in individuals were not correlated (Fig. 4a). The GSE81761 dataset contains the transcriptomes from military members [49], which showed reduced expression of CPEB3 but not NR3C1 in the PTSD group (Supplementary Fig. S3).
a The microarray dataset (GSE860) containing transcriptomes of peripheral blood mononuclear cells collected from control (Ctrl) and PTSD individuals. The mRNA levels of CPEB3 and NR3C1 in these samples were retrieved. Pearson’s correlation coefficient revealed no strong correlation between levels of CPEB3 and NR3C1. b WT and KO mice (4 per group) treated with or without CORT were harvested for immunodetection of nuclear p-GR (Ser211) in the hippocampal CA1 region. Scales, 50 µm. c The hippocampal tissues isolated from WT and KO mice (6 per group) were processed for total RNA isolation and RT-qPCR to determine the normalized levels of various Bdnf transcripts relative to Actb. d Hippocampi isolated from mice at 0.5, 1.5 and 4 h after 5 mg/kg CORT injection or PBS injection (i.e., time 0) were used for immunoblotting of denoted proteins. Mice injected with PBS were used as 0-min control. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant, nonparametric Mann–Whitney test.
The integrated evidence indicates that elevated GR signaling attenuates Bdnf transcription to affect stress-induced emotional disorder and memory formation [50,51,52,53]. The nuclear pS211-GR signal was significantly higher in the hippocampal CA1 of KO than WT mice with or without intraperitoneal injection of CORT (Fig. 4b). We analyzed all Bdnf isoforms and found Bdnf I, III and IV more abundant than other isoforms in both genotypes (Fig. 4c), which is consistent with the previous study [54]. The most dominant Bdnf I transcript showed a consistent downregulation in the KO hippocampus (Fig. 4c), amygdala and prefrontal cortex (Supplementary Fig. S4). Moreover, the CPEB3-KO hippocampi showed elevated GR signaling (pS211-GR) in mice injected with PBS (0 min) or CORT (30 min) and reduced BDNF level, which lasted for 4 h after CORT injection (Fig. 4d).
GR-mediated BDNF downregulation contributes to PTSD-like behavior in CPEB3-KO mice
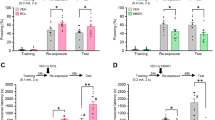
To assess whether the PTSD-like phenotype in CPEB3-KO mice is caused by enhanced GR signaling to suppress BDNF expression and impair extinction, we collected hippocampal and plasma samples during the behavioral assay (Fig. 5a). Plasma CORT concentration was correlated with the stress condition of mice, with an increase right after acquisition and a decrease during extinction, but showed no changes in KO mice (Fig. 5b). Despite comparable Nr3c1 mRNA levels between WT and KO hippocampi (Fig. 5c), KO hippocampi at the basal condition (habituation) and at extinction showed elevated GR level accompanied by decreased BDNF level (Fig. 5e, f). Because BDNF signaling is important for fear extinction [23, 55, 56], we speculated that the increased fear intrusion in CPEB3-KO mice may be caused by insufficient BDNF to impair extinction. If so, the supply of BDNF should facilitate extinction to reduce the intrusive fear recovery in KO mice. Thus, we first examined the temporal activation profiles of tyrosine receptor kinase B (TrkB) and its downstream c-AMP-responsive element-binding protein (CREB) signaling in the amygdala, hippocampus and prefrontal cortex of mice after intracranial delivery of BDNF. A slight increase in the level of BDNF was found in the hippocampus and prefrontal cortex within 1 h after the delivery. Moreover, BDNF-activated Y816-phosphorylation of TrkB and subsequent Ser133-phophorylation of CREB could last for 1–2 h in these brain regions (Supplementary Fig. S5). Thus, we infused mice with saline or BDNF 1 h before the extinction procedure (Fig. 5g). All groups of mice learned to pair the CS and US and exhibited normal memory consolidation and effective extinction. The BDNF treatment significantly rescued PTSD-like responses in KO mice: their freezing was reduced in both spontaneous recovery and renewal tasks to a similar extent as CPEB3-WT mice. Therefore, insufficient BDNF signaling in CPEB3-KO mice may account for their risk of PTSD-like behavior.
a Mice were trained for fear conditioning behavior, and their hippocampi and blood were collected at the illustrated times. Three mice per genotype were collected at each time and processed for (b-f) experiments. b Level of plasma corticosterone in mice during behavioral training. c The left hippocampus of each mouse was processed for total RNA isolation and RT-qPCR to determine the normalized Nr3c1 mRNA level relative to Gapdh. d The right hippocampus of each mouse was processed for western blot analysis of denoted proteins. e, f The immunostained signals of GR and BDNF relative to β-actin. g WT and KO mice implanted with cannula were used for behavioral training. Single dose of BDNF or saline was infused to the lateral ventricle 1 h before extinction training. Acquisition: F(6,105) = 97.374, p < 0.001, extinction: F(1,30) = 4.320, p = 0.046, consolidation: F(1,30) = 0.118, p = 0.734, and recall: F(1,30) = 20.987, p < 0.001. The number of mice in each group is in parentheses. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant, nonparametric Mann–Whitney test (for b, e, f), two-way ANOVA (for c, g).
Discussion
This study identified a novel regulatory mechanism via CPEB3 that constrains GR synthesis, which is critical for BDNF-mediated extinction of fear memory and resilience to PTSD-like behavior after traumatic exposure.
Stress-mediated activation of CORT-GR signaling can have central (HPA-axis) and peripheral (immune system) effects [9, 10, 57]. Hence, the number and affinity of GR to its ligands are instrumental in regulating stress-induced physiological changes. The increased GR number as determined by 3H-dexamethasone binding in PBMCs was found in deployed soldiers with co-morbid depressive symptoms [58] and correlated with the risk of developing PTSD and the severity of PTSD symptoms after deployment [59]. GR-overexpressing mice showed altered cognitive behaviors and emotional lability similar to PTSD symptoms [60]. Because over-activation of GR signaling is conceptualized for PTSD, understanding how the expression and activation of GR could be dysregulated in PTSD individuals should help with therapeutic strategies. A previous study identified that the single nucleotide polymorphism rs11186856 in the intron of human CPEB3 is associated with episodic memory [61]. The reduced CPEB3 level in PBMCs (Fig. 4a and Supplementary Fig. S3) likely increases GR number, but whether it can mirror the scenario in neurons of PTSD individuals to cause elevated NR3C1 translation requires further investigation.
GR is associated with a chaperone complex in the cytoplasm and translocated into the nucleus upon ligand binding, so its function as a transcription factor is tightly influenced by its interaction between the chaperone complex and glucocorticoids. Aberrant epigenetic modifications and genetic mutations in a co-chaperone of this complex, FKBP5, are related to PTSD [19, 49, 62]. Moreover, hyper- or hypo-methylation of CpG sites in the NR3C1 promoter affect NR3C1 transcription and were reported to be associated with PTSD, depression and early-life stress experiences [63]. While Nr3c1 transcription is well characterized [64, 65], much less is known about how Nr3c1 is posttranscriptionally regulated. The AU-rich elements in Nr3c1 3′-UTR can mediate Nr3c1 decay in COS cells [66] and several adrenal and brain microRNAs target Nr3c1 3′-UTR to downregulate GR expression [67,68,69]. Together with CPEB3-controlled translation, GR expression is subjected to multi-layered regulation, and any maladaptive changes could lead to altered sensitivity to cortisol and stress responses.
CPEB3 regulates the translation of multiple plasticity-related proteins, including those subunits constituting AMPAR and NMDAR [25, 26, 31, 70]. Although the elevated NMDAR expression and NMDAR-mediated CaMK2α activation in CPEB3-deficient neurons could explain enhanced consolidation of long-term spatial memory in KO mice [31], why fear extinction is defective in CPEB3-KO mice is not intuitively obvious because the activation of AMPAR, NMDAR and CaMK2α is also required for fear extinction [46, 71,72,73,74]. In this study, we found that CORT/NMDA-induced calcium signaling was moderately elevated in KO neurons (Supplementary Fig. S1). However, the activation of CaMK2α was higher in naïve CPEB3-KO than WT mice but was reversed in 1 h after fear-associative learning and then returned to the same level after fear consolidation (Supplementary Fig. S2). Moreover, a partial reduction of CaMK2 activation impaired fear extinction [46], so we reasoned that elevated p-CaMK2 level in naïve CPEB3-KO mice may facilitate fear acquisition but not extinction. By contrast, the reduced BDNF expression was associated with elevated GR level in naïve and fear-conditioned CPEB3-KO mice (Fig. 5d–f). BDNF signaling is important for both fear acquisition and extinction [23, 55, 56]. Because intracranial delivery of BDNF before extinction dampened fear recall in KO mice (Fig. 5g), the loss of CPEB3-regulated GR/BDNF signaling may be the key reason underlying defective fear extinction. A recent study identified that CPEB3 in cerebellar Bergmann glia suppressed GluA1 expression in response to a predator odor-induced acute stress. Such a suppression is important to shorten the lateral processes and reduce AMPAR current in Bergmann glia for stress adaptation [70]. Thus, CPEB3-controlled translation appears to fine-tune stress responses in neurons and also other types of cells.
Bi-directional control of synaptic efficacy is instrumental for dynamic and adaptive changes of memory and behaviors. On the basis of previously identified behavioral and electrophysiological abnormalities, we predicted that CPEB3-KO mice may develop PTSD-like responses [31, 32]. Importantly, molecular changes identified in naïve KO mice did not persist through the entire fear behavioral test (Fig. 5 and Supplementary Fig. S2), which suggests that the target mRNAs translationally regulated by CPEB3 undergo context-dependent changes. Such a scenario was found in CPEB4, which activates c-Fos translation in olfactory granule cells only at the early postnatal stage despite the presence of CPEB4 and c-Fos continuously in granule cells [75]. Thus, it is difficult and limited to explain aberrant behaviors simply based on the altered gene expression profile in naïve mouse brain or human PBMCs. For example, the regulatory axis of CPEB3-GR-BDNF is more important in fear extinction than acquisition, so CPEB3-KO mice showed enhanced spontaneous recovery of fear, which could be rescued by supplementing BDNF right before extinction training. Because PTSD is precipitated by the failure to appropriately adjust synaptic plasticity after severe trauma, KO or transgenic mice showing enhanced memory consolidation and/or impaired extinction should be more prone to PTSD-like behavior with increased intensity of the traumatic experience. We proposed that such mouse models could simulate the contribution of genetic risk factors to PTSD and resilience in response to exposure therapy and thus facilitate the molecular underpinnings of PTSD.
Funding and disclosure
This work was supported by the Ministry of Science and Technology of Taiwan [MoST108-2320-B-001-020-MY3], National Health Research Institutes [NHRI-EX109-10719SI] and Academia Sinica in Taiwan. The authors report no biomedical financial interests or potential conflicts of interest.
Data availability
Microarray data can be viewed in GEO accession GSE159731.
References
Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2016;41:297–319.
Afifi TO, Asmundson GJ, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin Psychol Rev. 2010;30:101–12.
Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167–81.
Mello PG, Silva GR, Donat JC, Kristensen CH. An update on the efficacy of cognitive-behavioral therapy, cognitive therapy, and exposure therapy for posttraumatic stress disorder. Int J Psychiatry Med. 2013;46:339–57.
Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337–46.
Logue MW, Amstadter AB, Baker DG, Duncan L, Koenen KC, Liberzon I, et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology. 2015;40:2287–97.
Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57:561–70.
Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014;35:180–96.
Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35.
Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–65.
Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35.
Holmes SE, Girgenti MJ, Davis MT, Pietrzak RH, DellaGioia N, Nabulsi N, et al. Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc Natl Acad Sci USA. 2017;114:8390–95.
Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–74.
Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558.
Gil N, Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet. 2020;21:102–17.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402.
Deslauriers J, Toth M, Der-Avakian A, Risbrough VB. Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol Psychiatry. 2018;83:895–907.
Richter-Levin G, Stork O, Schmidt MV. Animal models of PTSD: a challenge to be met. Mol Psychiatry. 2019;24:1135–56.
Criado-Marrero M, Morales Silva RJ, Velazquez B, Hernandez A, Colon M, Cruz E, et al. Dynamic expression of FKBP5 in the medial prefrontal cortex regulates resiliency to conditioned fear. Learn Mem. 2017;24:145–52.
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70.
Sabbagh JJ, O’Leary JC 3rd, Blair LJ, Klengel T, Nordhues BA, Fontaine SN, et al. Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PLoS One. 2014;9:e107241.
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–91.
Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–64.
Chao HW, Lai YT, Lu YL, Lin CL, Mai W, Huang YS. NMDAR signaling facilitates the IPO5-mediated nuclear import of CPEB3. Nucleic Acids Res. 2012;40:8484–98.
Fioriti L, Myers C, Huang YY, Li X, Stephan JS, Trifilieff P, et al. The persistence of hippocampal-based memory requires protein synthesis mediated by the prion-like protein CPEB3. Neuron 2015;86:1433–48.
Huang YS, Kan MC, Lin CL, Richter JD. CPEB3 and CPEB4 in neurons: analysis of RNA-binding specificity and translational control of AMPA receptor GluR2 mRNA. EMBO J. 2006;25:4865–76.
Chen PJ, Huang YS. CPEB2-eEF2 interaction impedes HIF-1alpha RNA translation. EMBO J. 2012;31:959–71.
Wang CF, Huang YS. Calpain 2 activated through N-methyl-D-aspartic acid receptor signaling cleaves CPEB3 and abrogates CPEB3-repressed translation in neurons. Mol Cell Biol. 2012;32:3321–32.
Pavlopoulos E, Trifilieff P, Chevaleyre V, Fioriti L, Zairis S, Pagano A, et al. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell. 2011;147:1369–83.
Ivshina M, Lasko P, Richter JD. Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu Rev Cell Dev Biol. 2014;30:393-415.
Chao HW, Tsai LY, Lu YL, Lin PY, Huang WH, Chou HJ, et al. Deletion of CPEB3 enhances hippocampus-dependent memory via increasing expressions of PSD95 and NMDA receptors. J Neurosci. 2013;33:17008–22.
Huang WH, Chao HW, Tsai LY, Chung MH, Huang YS. Elevated activation of CaMKIIalpha in the CPEB3-knockout hippocampus impairs a specific form of NMDAR-dependent synaptic depotentiation. Front Cell Neurosci. 2014;8:367.
Lu WH, Yeh NH, Huang YS. CPEB2 activates GRASP1 mRNA translation and promotes AMPA receptor surface expression, long-term potentiation, and memory. Cell Rep. 2017;21:1783–94.
Lee YL, Kung FC, Lin CH, Huang YS. CMTR1-catalyzed 2’-o-ribose methylation controls neuronal development by regulating Camk2alpha expression independent of RIG-I signaling. Cell Rep. 2020;33:108269.
Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinforma. 2009;10:48.
Yan Y, Eipper BA, Mains RE. Kalirin is required for BDNF-TrkB stimulated neurite outgrowth and branching. Neuropharmacology. 2016;107:227–38.
Davis MT, Hillmer A, Holmes SE, Pietrzak RH, DellaGioia N, Nabulsi N, et al. In vivo evidence for dysregulation of mGluR5 as a biomarker of suicidal ideation. Proc Natl Acad Sci USA. 2019;116:11490–95.
Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–54.
Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717–23.
Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–80.
Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–49.
Chameau P, Qin Y, Spijker S, Smit AB, Joels M. Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J Neurophysiol. 2007;97:5–14.
Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, et al. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur J Neurosci. 2002;16:1083–9.
Karst H, Wadman WJ, Joels M. Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons. Brain Res. 1994;649:234–42.
Takahashi T, Kimoto T, Tanabe N, Hattori TA, Yasumatsu N, Kawato S. Corticosterone acutely prolonged N-methyl-d-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. J Neurochem. 2002;83:1441–51.
Kimura R, Silva AJ, Ohno M. Autophosphorylation of alphaCaMKII is differentially involved in new learning and unlearning mechanisms of memory extinction. Learn Mem. 2008;15:837–43.
Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–37.
Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:500–13. 425
Rusch HL, Robinson J, Yun S, Osier ND, Martin C, Brewin CR, et al. Gene expression differences in PTSD are uniquely related to the intrusion symptom cluster: a transcriptome-wide analysis in military service members. Brain Behav Immun. 2019;80:904–08.
Chen H, Lombes M, Le Menuet D. Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol Brain. 2017;10:12.
Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–29.
Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J Neuroendocrinol. 2006;18:104–14.
Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213.
Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–35.
Liu IY, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24:7958–63.
Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–90.
de Kloet CS, Vermetten E, Bikker A, Meulman E, Geuze E, Kavelaars A, et al. Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Mol Psychiatry. 2007;12:443–53.
van Zuiden M, Geuze E, Maas M, Vermetten E, Heijnen CJ, Kavelaars A. Deployment-related severe fatigue with depressive symptoms is associated with increased glucocorticoid binding to peripheral blood mononuclear cells. Brain Behav Immun. 2009;23:1132–9.
van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, et al. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 2011;168:89–96.
Chourbaji S, Vogt MA, Gass P. Mice that under- or overexpress glucocorticoid receptors as models for depression or posttraumatic stress disorder. Prog Brain Res. 2008;167:65–77.
Vogler C, Spalek K, Aerni A, Demougin P, Muller A, Huynh KD, et al. CPEB3 is associated with human episodic memory. Front Behav Neurosci. 2009;3:4.
de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
Palma-Gudiel H, Cordova-Palomera A, Leza JC, Fananas L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: a critical review. Neurosci Biobehav Rev. 2015;55:520–35.
Ramamoorthy S, Cidlowski JA. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol Cell Biol. 2013;33:1711–22.
Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev. 2014;35:671–93.
Schaaf MJ, Cidlowski JA. AUUUA motifs in the 3’UTR of human glucocorticoid receptor alpha and beta mRNA destabilize mRNA and decrease receptor protein expression. Steroids. 2002;67:627–36.
Riester A, Issler O, Spyroglou A, Rodrig SH, Chen A, Beuschlein F. ACTH-dependent regulation of microRNA as endogenous modulators of glucocorticoid receptor expression in the adrenal gland. Endocrinology. 2012;153:212–22.
Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–61.
Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–8.
Bender CL, Sun X, Farooq M, Yang Q, Davison C, Maroteaux M, et al. Emotional stress induces structural plasticity in bergmann glial cells via an AC5-CPEB3-GluA1 pathway. J Neurosci. 2020;40:3374–84.
Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24.
Radulovic J, Ren LY, Gao C. N-Methyl D-aspartate receptor subunit signaling in fear extinction. Psychopharmacology. 2019;236:239–50.
Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–8.
Trent S, Barnes P, Hall J, Thomas KL. AMPA receptors control fear extinction through an Arc-dependent mechanism. Learn Mem. 2017;24:375–80.
Tseng CS, Chao HW, Huang HS, Huang YS. Olfactory-experience- and developmental-stage-dependent control of CPEB4 regulates c-Fos mRNA translation for granule cell survival. Cell Rep. 2017;21:2264–76.
Acknowledgements
The authors thank Huei-Fang Wu in NPAS Neuro-Electrophysiology Core for calcium imaging, the institutional pathology core for brain tissue sectioning, and DNA sequencing support under the Academia Sinica Core Facility and Innovative Instrument Project (AS-CFII-108-115).
Author information
Authors and Affiliations
Contributions
W.-H.L. H.-W.C. designed and conducted the experiments, analyzed data and wrote the manuscript. P-Y Lin identified PTSD-like phenotype, S-H Lin, H-W Chen and T-H Liu helped with some experiments and analyses. Y.-S.H. designed and supervised the study and co-wrote the manuscript with H.-W.C. and W.-H.L. and is responsible for its content.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, WH., Chao, HW., Lin, PY. et al. CPEB3-dowregulated Nr3c1 mRNA translation confers resilience to developing posttraumatic stress disorder-like behavior in fear-conditioned mice. Neuropsychopharmacol. 46, 1669–1679 (2021). https://doi.org/10.1038/s41386-021-01017-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01017-2