Abstract
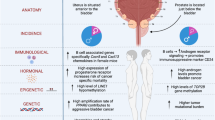
Biological sex, being female or male, broadly influences diverse immune phenotypes, including immune responses to diseases at mucosal surfaces. Sex hormones, sex chromosomes, sexual dimorphism, and gender differences all contribute to how an organism will respond to diseases of the urinary tract, such as bladder infection or cancer. Although the incidence of urinary tract infection is strongly sex biased, rates of infection change over a lifetime in women and men, suggesting that accompanying changes in the levels of sex hormones may play a role in the response to infection. Bladder cancer is also sex biased in that 75% of newly diagnosed patients are men. Bladder cancer development is shaped by contributions from both sex hormones and sex chromosomes, demonstrating that the influence of sex on disease can be complex. With a better understanding of how sex influences disease and immunity, we can envision sex-specific therapies to better treat diseases of the urinary tract and potentially diseases of other mucosal tissues.
Similar content being viewed by others
Introduction
The composition and function of the human immune system are highly variable among healthy individuals. Biological sex, being female or male, broadly influences diverse immune phenotypes. Sex is a biological variable that comprises differences in chromosomes, steroid hormone levels, reproductive organs, and sexually dimorphic features between female and male organisms. Sex is distinct from gender, which is determined by behaviors in society and cultural norms1. To fully understand differences in immunity between women and men, the combined effects of intrinsic (e.g., sex hormones, chromosomes), extrinsic (e.g., environment), and behavioral factors (e.g., gendered occupations) must be considered together2,3,4. Experimental approaches such as cell culture or preclinical animal models can be used to isolate and assess the impact of some of these factors, such as sex hormone levels. However, the cumulative impact of these factors on immunity is challenging to understand, and much remains to be discovered as to how sex and gender influence immunity.
Women and men experience different susceptibility to and severity of infectious and non-infectious diseases3. In general, women are at greater risk of developing autoimmune diseases, such as systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, or scleroderma5. By contrast, men are more susceptible to infectious diseases caused by viruses, bacteria, fungi, and parasites, including COVID-19, leptospirosis, aspergillosis, and schistosomiasis6,7. Generally, adult female organisms mount more robust systemic cell-mediated and humoral immune responses than adult males, which lead to more rapid pathogen clearance or stronger responses to vaccines8,9,10,11. In addition, the female immune system is characterized by greater antigen-presenting capability, more circulating CD4+ T cells, higher immunoglobulin levels after infection, and enhanced tumor and allograft rejection12,13,14,15. The male immune system, on the other hand, exhibits increased pro-inflammatory cytokine production and higher numbers of circulating leukocytes such as neutrophils or monocytes in homeostasis and disease3,16.
Similar sex-based differences in immunity are also apparent at mucosal surfaces, such as the lung or the gut, which provide the first line of defense against invading pathogens17. In lung infections, for example, women mount stronger immune responses to the H1N1 and the H5N1 influenza virus, but also exhibit greater morbidity and mortality than men of the same age18. The same holds true in animal models, in which influenza infection in female mice induces higher production of the pro-inflammatory cytokines TNF-α, IFN-γ, IL-6, and CCL2 in the lungs, and increased weight loss, hypothermia, and mortality compared to infected male mice, despite similar viral titers between the sexes19. Sex also influences immunity in the bladder, a less well-studied mucosal surface. Here, we focus on the impact of biological sex on urinary tract diseases. Importantly, when referring to factors that are exclusively biological differences, the terms female and male are used, whereas the terms women and men are used when gender may contribute to factors or phenotypes discussed1.
Urinary tract infection is strongly sex biased
Urinary tract infections (UTI) exhibit one of the most prominent sex disparities among infectious diseases, with premenopausal women being 20–40 times more likely to have a UTI than men of the same age20,21,22. One hypothesis to explain this disparity is that anatomical differences, such as the shorter distance between the anus and the urethral opening in females or a longer urethra in males, influence UTI prevalence in humans. The major challenge is that hypotheses based on anatomical differences lack supporting evidence because they are nearly impossible to test experimentally23,24. Arguing against anatomical differences as the primary reason underlying the sex disparity in UTI, this pronounced increase in susceptibility in women is most striking in adolescents and adults under the age of 50 (Fig. 1). By contrast, male infants under 6 months of age are approximately twice as likely to have an initial UTI than female infants, while recurrence risk in female and male infants less than 1 year of age who have experienced a UTI is equivalent (32% vs. 35% respectively)25,26. Similarly, in populations over 65 years old, the prevalence of bacteriuria (bacteria in the urine) and UTI increase substantially in men until they are nearly equivalent to that of elderly women27,28. The prevalence of UTI in the institutionalized elderly population is similar between the sexes. However, this may be influenced by the use of urinary catheters, which bring their own risk of UTI29,30. Importantly, the increase in UTI prevalence in an older male population cannot be explained by anatomy, as urethral length does not change over time in men31. Instead, changes in UTI incidence in men may be due to urodynamic changes (e.g., prostatic hypertrophy in older men), or potentially to biological or immunological changes that occur over time. Interestingly, the peak of UTI risk in male infants correlates with a postnatal testosterone surge, in which testosterone levels increase at 1 month of age and subside by 6 months of age32,33.
The prevalence of bacteriuria, indicated by the percentages shown, is highly variable between the sexes and during life. Infancy is the only period of life during which human males have a higher risk for bacteriuria and UTI than females. During adulthood, the prevalence of bacteriuria is lower in men than women and increases with age in men or during pregnancy for women. Differences in incidence between men and women decrease in the elderly, and both sexes face high rates of recurrent infection after 85 years of age. yo years old, *UTI prevalence is shown instead of bacteriuria. This figure was drawn summarizing data from refs. 20,25,26,27,28,29,30,32,38,39,43,149. Figures were generated with images from Servier Medical Art (www.servier.com), licensed under the Creative Commons Attribution 3.0 Unported License (http://creativecommons.org/license/by/3.0/).
Sex hormones and UTI incidence
Indeed, changes in susceptibility to and prevalence of UTI over time in women and men suggest that biological factors influence an individual’s risk for infection. Particularly during the reproductive years, most female vertebrate animals, including humans, exhibit increased levels of estrogens, whereas male organisms generally have higher levels of androgens, such as testosterone34,35,36,37. Sex differences in UTI susceptibility are the most apparent in post-pubescent adults under the age of 50, correlating with when estrogen and testosterone levels are highest in females and males, respectively (Fig. 2)38,39. Specifically in women, the incidence of UTI increases after puberty, when estrogen levels increase, while, when testosterone levels are at their highest and estrogens at their lowest, adult men are at their lowest risk of UTI37,38,39. However, men that experience infection are at increased risk of chronic UTI, and increased morbidity and mortality from complicated UTI39,40,41,42. Then, when women, after menopause, and men, after andropause, undergo a decrease in estrogen and testosterone levels, respectively, the incidence of UTI rises in both sexes, such that men face nearly the same risk as women at the same age (Figs. 1 and 2)20,37,38. Moreover, pregnancy, which is characterized by high levels of estrogen, is strongly correlated with high incidence of UTI and recurrent infection43. Overall, those striking correlations suggest that sex hormones contribute to the risk or response to infection.
The global percentage of sex hormone levels, as the maximum mean testosterone and the maximum mean estradiol level, in men (blue squares) and women (red circles) and bacteriuria prevalence in men (green triangles) and women (purple diamonds) is shown over time. In infancy, UTI prevalence is represented instead of bacteriuria. In the first 6 months of life, male infants have a higher prevalence of UTI than female infants, but this sex difference is reversed during childhood and remains over the lifetime of men and women. Figure adapted from ref. 37 with bacteriuria data from refs. 20,25,26,27,28,29,30,32,38,39,43,149.
Sex hormones and the immune response to UTI
Sex hormones directly impact immunity and, therefore, are obvious candidates in the search for drivers of sex differences in prevention of and response to UTI. For example, pregnancy in humans is associated with a shift toward Th2 T cell differentiation, which would be less effective at fighting intracellular uropathogens44,45. Indeed, estrogen and progesterone, which are high during pregnancy, are reported to decrease secretion of IFNγ and IL-2 in peripheral blood mononuclear cells isolated from the blood of women who have experienced multiple spontaneous miscarriages, which would inhibit Th1 differentiation, and upregulate IL-6 expression, which is implicated in Th2 differentiation ex vivo46. Moreover, in vitro, testosterone inhibits Th1 T cell differentiation, which could explain a decreased male immune response to intracellular uropathogens, for example47. In addition, another striking example of an innate cell subset regulated by sex hormones is the innate lymphoid cell (ILC) 2 subset. Female mice and humans have higher numbers of ILC2s compared to males, in specific pathologies such as asthma, and ILC2 development is inhibited by androgen signaling at barrier surfaces in vivo48,49. Immune cells widely express sex hormone receptors, and many immunity-related genes carry promoters with hormone-responsive elements that bind estrogens and/or androgens3,34. Together, these findings suggest that sex hormone signaling may directly regulate distinct immune responses.
To understand how sex impacts the response to UTI in the bladder, our teams applied two different approaches to directly compare female and male mice. Using a mouse UTI model, in which uropathogenic Escherichia coli (UPEC) are intravesically instilled directly into the bladders of female and male C57BL/6 mice using a catheter placed in the urethra, we observed that female mice resolve infection without intervention, whereas male mice remain chronically infected for up to 1 month50,51 (Fig. 3). In infected C57BL/6 mice, the amplitude of early inflammatory responses in the female bladder is significantly greater than in male mice, with increased pro-inflammatory cytokine expression, inflammatory cell infiltration, and bacterial phagocytosis at 24 h post-infection, despite equivalent bacterial burden between the sexes50. These differences largely resolve by 48 h post-infection, suggesting that sex modulates the immediate innate response to UTI. Similarly, using a surgical method to instill UPEC directly into the bladders of C57BL/6 mice, bypassing the urethra entirely, bladder-associated bacterial titers are equal at 24 h post-infection between the sexes; however, female mice have higher pro-inflammatory cytokine expression in bladder tissue at this time point52.
The innate immune response to female mice (top) is characterized by robust cytokine expression and immune cell infiltration, which is notably absent in male mice (bottom, transparent immune cells and missing cytokines). The events depicted inside the dotted box have been observed in the context of UTI in female mice, however, it is unknown whether these events unfold in male mice with UTI. For example, both sexes have two subtypes of resident macrophages, however, whether they behave similarly in defense against infection is unknown. Notably, both sexes mount a non-sterilizing adaptive immune response to infection, although whether this response is mediated by similar mechanisms in female and male mice is unclear. Figure drawn in BioRender with data from ref. 50 and Rousseau et al., BioRxiv, https://www.biorxiv.org/content/10.1101/2021.10.28.466224v1.
Supporting a role for androgens in mediating differences in immunity, female C57BL/6 mice pretreated with testosterone or dihydrotestosterone (androgenized mice) prior to initiation of UTI fail to eliminate UPEC from the bladder for up to 1 month, compared to mock-treated female mice, which spontaneously resolve their infections within 4 weeks50,53. Androgenized female C57BL/6 mice infected via the surgical approach and treated with the androgen receptor (AR) inhibitor enzalutamide clear their infections at the same rate as unmanipulated female mice53. Of note, IL-17, a pro-inflammatory cytokine whose expression in the lung is also sex-discrepant54,55,56,57, is one of the only cytokines significantly higher in female mouse bladders compared to male mice at both 24 and 48 h post-infection. In female C57BL/6 mice infected intravesically, testosterone treatment reduces infiltration of CD4+ T cells, type 3 innate lymphoid cells (ILC3), and γδ T cells50, all of which are major producers of IL-1758. While this study identified a difference in the total number of ILCs in female mice compared to male mice, in which female mice have more bladder resident ILCs50, another study showed no differences in the percentages of ILC subsets among CD45+ cells in the naive urinary tract when comparing female and male mice59. Interestingly, female mice treated with an IL-17-neutralizing antibody fail to resolve their infection50. However, IL-17 supplementation is not sufficient to rescue the resolution defect in male mice50. Castrating C57BL/6 male mice prior to intravesical infection does not alter UTI resolution50.
Mouse host strain differences can be used to delineate the role of androgens and other factors in UTI outcomes. For example, male C3H/HeN mice naturally have three times the circulating testosterone of male C57BL/6 mice60. Compared with female C3H/HeN mice, surgically infected male C3H/HeN and androgen-treated female mice develop persistent high-titer chronic cystitis significantly more frequently at 2 weeks post-infection, with increased IL-1β, IL-6, and CXCL1 at 6 h post-infection52,53. Of note, CSF-3 and TNF-α are increased in female C3H/HeN mice compared to male mice at 24 h post-infection in this model52,53. Castration does ameliorate the severe UTI phenotype seen in male mice, as nearly all castrated males resolve UTI by 2 weeks post-infection. Androgen replacement in castrated male mice, by subcutaneous implantation of testosterone or dihydrotestosterone, leads to bacterial burdens similar to that observed in unmanipulated male animals52,53. Female C3H/HeN mice given testosterone or dihydrotestosterone are also more susceptible to chronic cystitis, whereas ovariectomy does not alter the infection52,53. Taken together, these data support that androgens contribute to high bacterial burdens in UTI and suggest that the increased testosterone in male hosts contributes to severity of infection.
Sex differences in upper-tract UTI in C3H/HeN mice are even more striking than those apparent in cystitis, potentially due to the vesicoureteral reflux observed in this mouse strain61. By 6 days after inoculation of the bladder, 90% of male C3H/HeN mice develop severe renal abscesses, with neutrophil infiltration and loss of normal tubular architecture53. By contrast, only 20–30% of C3H/HeN female mice develop chronic high-titer infections, with abscesses visible in ~5% of animals. Male TLR4-deficient C3H/HeJ mice develop high-titer pyelonephritis but no abscesses53. Instead, these mice fail to limit infection to the kidney parenchyma, exhibiting increased rates of bacterial dissemination and mortality53. In the non-refluxing C57BL/6 strain, female mice treated with testosterone also develop persistent kidney infection52,53,62,63. These androgenized C57BL/6 females have increased monocyte recruitment, increased M2-polarized macrophages in the kidney from 14 to 28 days post-infection, and increased kidney-associated M2 macrophage cytokines CXCL1 and CSF-3 from day 10 to 21 post-infection63. Finally, androgen exposure in female mice induces a burst of epithelial-derived TGFβ1 at 24 h post-infection, which polarizes macrophages toward an alternatively activated, pro-fibrotic phenotype, promoting the development of scars surrounded by pro-fibrotic M2a macrophages62,64. Whether testosterone plays a role in the severity of sequelae following pyelonephritis in humans remains to be investigated.
Estrogens also influence the response to infection in the urinary tract. Post-menopausal and elderly women are at greater risk of recurrent infection38,65, which may be explained in part by changes in estrogen levels over time (Fig. 2). Decreased estrogen levels in post-menopausal women may limit production of antimicrobial peptides and contribute to decreased integrity of the urothelial lining, which would render the bladder more susceptible to bacterial colonization66,67. Compared with sham-operated mice, ovariectomized C57BL/6 female animals have greater bacterial burdens 24 h after intravesical instillation of UPEC and increased circulating IL-6 at 6 h post-infection66,68. Fourteen days after infection, ovariectomized C57BL/6 mice have more quiescent intracellular bacterial reservoirs, a phenotype which can be reversed if ovariectomized mice are treated with estradiol68.
However, estrogen can also increase uptake of bacteria by urothelial cells, while at the same time strengthening tight junctions between urothelial cells and stimulating antimicrobial peptide production, both of which would act to protect the urothelium from infection66,67. Following estradiol supplementation in post-menopausal women, exfoliated human urothelial cells show significantly increased expression of antimicrobial peptide genes such as hBD3 and the cell-cell contact-associated protein, zona occludens protein 1 (ZO-1)66. These observations were also apparent in vitro using 5637 cells, a human urothelial cell line, in which estradiol exposure enhances the expression of hBD1, hBD2, psoriasin, RNase 7, and cathelicidin66. In addition, ZO-1 and occludin mRNA levels are significantly up-regulated and E-cadherin is redistributed at cell-cell contact points66. Importantly, however, the ability to make direct comparisons of the impact of estrogen supplementation between this in vitro system and in vivo in female organisms is complicated by the fact that 5637 cells were derived from an elderly male patient with bladder cancer. In addition, one study showed that the composition and time course of thickening after infection of the glycosaminoglycan layer, a major component of mucosal surfaces that plays a critical role in passive defenses against UTI, was significantly influenced by estradiol level69. Finally, increasing estrogen levels, apparent at puberty, can elicit production of natural anti-enteropathogenic E coli (EPEC) antibodies, leading to faster clearance of blood-borne EPEC in female mice compared to male mice, thus providing a sex-biased protection against bacteremia and sepsis70. Whether these or other natural antibodies recognize UPEC and whether they could protect against UTI is unknown. It is interesting, however, to speculate they may play a role in immunity as antibodies present in the urine of previously infected women can prevent UPEC binding to human urothelial cells, potentially limiting infection71. Together, these findings support that sex hormones directly and indirectly impact immunity to UTI and suggest that their modulation may have potential in the treatment of acute, chronic, and recurrent UTI, but also highlight the need for optimized models to understand these interactions.
Bladder cancer is also sex biased
Bladder cancer is one of the ten most common cancers with more than 500,000 new cases each year, worldwide72, although it is less well studied than other cancers with similar incidence. In direct contrast to UTI, bladder cancer is more common in men. Interestingly, however, women tend to be in later stages at diagnosis and experience worse outcomes compared to men73. Seventy-five percent of new diagnoses are non-muscle-invasive bladder cancer, and when classified as high-risk, can be treated with intravesical instillation of Bacillus Calmette-Guérin (BCG), the vaccine for tuberculosis74. Although the reasons are not known, and contrary to earlier reports75, women are at greater risk of recurrence and progression after BCG therapy compared to men76. A more aggressive malignancy, muscle-invasive bladder cancer, can be treated with chemotherapy, radiation, checkpoint inhibitors, or a combination of these approaches. While differences in response to chemotherapy between the sexes have not been identified77, some studies suggest that women and men have divergent outcomes following checkpoint inhibition therapy for bladder cancer78,79. The reasons for differences in incidence, progression, and response to therapy between women and men are complex, and likely include lifestyle and gender-associated factors, but evidence supports that hormone signaling and sex chromosomes themselves contribute to the underlying mechanisms of tumor development.
Sex hormone signaling and bladder cancer
As early as the 1970s, preclinical research models demonstrated that sex hormone signaling contributes to the development of bladder cancer. Experimental bladder cancer is typically induced in mice and rats through administration of the carcinogen N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) in the drinking water. Using this model, tumor incidence can be inhibited through the administration of the estrogen receptor agonist diethylstilbestrol in male rats, whereas ovariectomy or testosterone treatment increases cancer incidence in female rats80,81. More recent studies, using genetically modified mice to dissect the role of sex hormone signaling, report that androgen signaling is generally pro-tumorigenic. Remarkably, AR-deficient C57BL/6-129Sv mosaic mice do not develop tumors following BBN exposure, whereas nearly all wild-type male and almost half of all wild-type female mice had bladder cancer after 40 weeks of BBN exposure82. Interestingly, C57BL/6-129Sv male mice with a conditional deletion of AR only in the urothelium also did not develop tumors following BBN exposure, indicating that androgen signaling in the bladder epithelium drives development of these tumors83. Reinforcing this conclusion, female and male C57BL/6 mice expressing human AR from a tamoxifen-inducible, urothelial-specific promoter have a higher incidence of more advanced tumors after BBN treatment compared to littermate controls. Furthermore, castration of these transgenic animals protects them from more severe disease84. Finally, in a model using MB49 bladder cancer cells injected in the flank, male mice accumulate greater numbers of CD8+ T cells with a progenitor exhausted phenotype in the tumor microenvironment compared to female mice. This phenotype was dependent upon AR signaling in T cells which regulated this T cell phenotype via the transcription factor Tcf7/TCF185. Inhibition of T cell maturation toward an effector cell phenotype and T cell exhaustion would be expected to negatively impact tumor immunity.
Evidence of androgen-driven tumor development exists in humans, as well. Perhaps the strongest evidence comes from studies reporting that androgen-deprivation therapy is protective in bladder cancer patients. In a study with a median follow-up of 5 years, bladder cancer patients treated with androgen-deprivation therapy for concurrent prostate cancer had a significantly longer interval of recurrence-free survival86. The same authors reported that AR expression in tumor tissue is an independent predictor of tumor recurrence in bladder cancer87. This finding confirms an earlier report analyzing samples from 148 men and 40 women, demonstrating that AR and estrogen receptors alpha (ERα) and beta (ERβ) are expressed in both benign urothelium and tumor samples, but that all three receptors are decreased in malignant tissue88.
While a majority of these bladder cancer studies focus on androgens, estrogen likely also plays a role in tumor development in the bladder. Estrogens exert their function by binding to two subtypes of intracellular receptors, ERα and ERβ89. Expression of ER subtypes varies among immune cell populations. T cells express higher levels of ERα, whereas B cells have greater ERβ expression90. Of the two ERs, ERβ is more likely to be found in bladder cancer samples, with a 60% detection rate in 224 bladder cancer samples, and a statistically significant increased presence in later-stage tumors (75–80%) compared to early-stage Ta/T1 tumors (53–56%)91. Assessments of recurrence-free or progression-free survival reveal that only ERβ expression is significantly associated with worse outcomes in patients with low-grade tumors88. Analysis of steroid sulfatase and aromatase, which activate estrogen in circulation, in 113 urothelial carcinoma samples, showed that steroid sulfatase labeling is lost with increasing tumor grade, suggesting estrogen levels and ERs may correlate in urothelial tumors92. Importantly, studies of hormone receptor expression and association with bladder cancer recurrence or survival do not always find positive correlations93. Thus, further studies with larger cohorts specifically powered to analyze hormone receptors in both women and men with bladder cancer are needed.
The role of sex chromosomes in the development of bladder cancer
The X chromosome encodes ~1100 genes, in contrast to the 100 genes encoded by the Y chromosome34. Many X-linked genes play important immune modulatory functions. For example, the X chromosome encodes Toll-like receptors TLR7 and TLR8, the common gamma chain cytokine receptor subunit IL2RG, the IL-13 receptor subunit IL13RA2, the T regulatory cell master regulator FOXP3, the TNF superfamily protein CD40L, the NADPH oxidase catalytic subunit NOX2 (CYBB), and IL1 receptor-associated kinase 1 (IRAK1)6,94,95.
In humans, females carry two copies of the X chromosome (XX), whereas males are heterogametic (XY). To compensate for the double dose of X-linked genes in females, cells initiate X-chromosome inactivation. In this process, random silencing of one of the X chromosomes occurs in each female cell early in development, leading to mosaic expression of either the maternal or paternal X chromosome1,96. Approximately 15% of X-linked genes escape inactivation in a subset of cells in humans, leading to increased expression of certain genes in females compared to males97. For example, TLR7, expressed by plasmacytoid dendritic cells, B cells, monocytes, and macrophages, escapes X inactivation in about 30% of cells, which impacts recognition of viral and self RNA, enhanced proliferative capacity of CD27hi B cells, and increased expression of interferon regulatory factor IRF5 and IFNα in plasmacytoid dendritic cells in females98,99. In males, mutations in IL2RG, encoding a cytokine receptor subunit critical for the development and function of T cells, lead to X-linked severe combined immunodeficiency100,101.
Tools to study the contribution of sex chromosomes to bladder cancer development include the “four core genotypes” mouse. In this transgenic C57BL/6 mouse, in which the testis-determining gene Sry has been moved from the Y chromosome to an autosome, breeding gives rise to animals that are gonadally female or male and have either an XX or XY sex chromosome complement102,103. Using these mice in a BBN-induced model of bladder cancer demonstrated that both hormones and sex chromosomes contribute to increased susceptibility in male mice104. Specifically, XX female mice had the best overall survival, followed by XY female mice, XX male mice, and finally XY male animals. RNA-sequencing revealed that the X-linked gene Kdm6a, a lysine demethylase and tumor suppressor, escapes X inactivation in these mice, and its conditional deletion results in decreased survival in female, but not male animals. In humans, KDM6A expression is also negatively correlated with advancing tumor stages only in tumors from female patients in The Cancer Genome Atlas data set104. In early-stage non-muscle-invasive bladder cancers from women, sequencing of tumors showed that 20 of 27 samples had at least one mutation in KDM6A105. In mice, Kdm6a differentially regulates multiple immune response mechanisms including T cell proliferation, cytokine production, and the expression of genes such as CXCR3 and CCR5, which are involved in the regulation of Th1 and Th2 activation pathways106. Together, these findings support that sex chromosomes themselves, through escape of X-inactivation by tumor suppressor genes, influence the development of bladder cancer, at least in women.
Sex and patient care in bladder cancer
While the contributions of hormone signaling or the X chromosome can be dissected in the laboratory, and observed or manipulated to some extent in patient populations; determining how additional factors contribute to the sex disparity seen in bladder cancer diagnoses and outcomes is more difficult. Evaluation of more than 27,500 patients (of whom 27% were female) revealed that female patients have a significantly higher proportion of nonurothelial squamous carcinomas compared to male bladder cancer patients107. This finding was confirmed through the sequencing of 1000 muscle-invasive bladder tumors (24% from women), which demonstrated that basal and squamous tumors were significantly more represented among female patients108. This supports the hypothesis that sex-specific disease outcomes may be due, in part, to disease subtype or variant histology108.
Sex differences in patient care itself may also have an effect on disease outcomes. Contrary to studies asserting that women experience delays in treatment due to longer follow-up times, delayed or a lack of referral to urologists, or misdiagnoses (e.g., female patients presenting with hematuria are commonly diagnosed with UTI)73, one study found that women are actually significantly less likely to experience delayed treatment (odds ratio 0.89, 95% confidence interval (CI) 0.84–0.93, p < 0.001)107. Additionally, with the exception that women were less likely to be treated at high-volume hospitals, no differences in treatment were apparent between women and men with respect to receipt of definitive treatment, neoadjuvant treatment, or pelvic lymph node dissection. The lack of significance in these parameters led the authors to conclude that although women are more likely to die from bladder cancer compared to men, delay or differences in treatment may not explain this phenomenon107. Given that this conclusion is at odds with many previous studies uncovering delays and differences in treatment, additional studies are needed.
Sex and the urinary tract microbiome
Covered in greater detail in another article of this special issue, the urinary or bladder microbiome likely impacts susceptibility or response to UTI and the development and response to therapy for bladder cancer. How the urinary or bladder microbiome may be impacted by sex, though, is poorly understood.
Contrary to a long-held clinical belief, the urine of healthy individuals is not sterile. Using approaches similar to those applied to study the intestinal microbiome, analysis of bacterial 16S rDNA sequences amplified from clean catch, midstream urine from healthy subjects or from individuals at risk for infection, such as those with neuropathic bladders, demonstrate that healthy human urine has considerably variable bacterial 16S rDNA sequence richness among individuals, including bacteria associated with urogenital pathologies109,110. Moreover, urinary microbiomes differ according to physiological or pathological bladder function and are altered by infections and their duration, antibiotic administration, and method of urinary catheterization109,110,111,112. Furthermore, women carry a more heterogeneous mix of bacterial genera compared to men, with representative members of the Actinobacteria and Bacteroidetes phylas, absent in most male samples109,110,111,112. Lactobacillus is found exclusively in women, and its presence varies significantly among women depending upon their bladder function113. For example, reanalysis of previously acquired urine samples showed that healthy women have higher proportions of Lactobacillus and healthy men have higher proportions of Staphylococcus haemolyticus than women or men with neuropathic bladders113. In addition, regardless of sex, individuals with neuropathic bladders, who are at greater risk for UTI, have greater proportions of Enterococcus faecalis, Proteus mirabilis, and Klebsiella pneumoniae113,114. In women over the age of 70, genera such as Jonquetella, Parvimonas, Proteiniphilum, and Saccharofermentans appear exclusively, and the overall number of genera and bacterial abundance are reduced compared to those aged 20 to 49111. The number of genera greatly increases with age in men, including the appearance of Parvimonas111. Overall, sex differences in the total number of bacteria and genera within the urinary microbiome exist and change with age, supporting a potential link between sex hormone levels or other sex-specific factors and microbiome composition, warranting continued investigation111.
Interestingly, a recent study examined urine and vaginal microbiota compositions of healthy women or patients with recurrent UTI at pre- and post-menopausal ages. While menopausal status correlated with significant differences in both urinary and vaginal microbiota, including a decline in Lactobacillus in post-menopausal women115, no significant differences were found to correlate with recurrent UTI in the absence of chronic antibiotic use116. Estrogen can support Lactobacillus growth by increasing glycogen storage in human vaginal epithelial cells117. Therefore, it is possible that the decreased Lactobacillus carriage observed in post-menopausal women is due, at least in part, to the decline in estrogen levels67,118. Interestingly, while a second study also did not identify significant differences in the abundance of Lactobacillus among patients with recurrent UTI compared to age-matched healthy individuals119, at least two studies report that the use of intravaginal Lactobacillus reduces recurrence in women with recurrent UTI120,121. Together, these findings suggest that a complex relationship among microbiomes at different anatomical sites may influence the incidence of UTI but additional studies are needed122.
Women with bacterial vaginosis, characterized by overrepresentation of a polymicrobial mixture of bacteria, including Gardnerella, in the vagina have a 13-fold higher UTI risk than women with Lactobacillus-dominated vaginal microbiotas123,124,125. In addition, in a study of 22 women with recurrent UTI and 17 healthy individuals, those with recurrent UTI had an abnormally high (>3) mean Nugent score (a microscopic measure of bacterial morphologies used to diagnose bacterial vaginosis) of 4.6 compared to 1.7 among the control subjects126. In a mouse model, inoculation of Gardnerella in bladders of mice previously infected with UPEC is sufficient to induce urothelial apoptosis and exfoliation, which would be expected to induce UPEC emergence from reservoirs, thereby inducing recurrent UTI127,128. Corroborating those observations, whole bladder RNA-sequencing after Gardnerella inoculation in previously UPEC-infected mice identified host pathways involved in Gardnerella-induced recurrent UTI such as epithelial turnover, apoptosis, and expression of inflammatory cytokines129. Gardnerella can be found in human urine collected directly from the bladder through suprapubic aspiration or catheterization and may mediate a similar mechanism of increased susceptibility to recurrent UTI, although it is thought these bacteria cannot directly colonize the bladder127,128,130,131. In vitro, co-aggregation of Gardnerella and UPEC leads to the formation of biofilms132. Interestingly, E. coli may be able to establish vaginal intracellular reservoirs in human and mice. Indeed, UPEC can invade a normal vaginal mucosal tissue cell line from a premenopausal woman, VK2 E6/E7, via a mechanism distinct from that observed in the bladder. In this same study, UPEC was found within cells from four vaginal swabs of women with a history of recurrent UTI133. Finally, E. coli formed reservoirs in vaginal epithelial cells from C3H/HeN mice up to 28 days post-inoculation133. In vivo, bacteria able to form reservoirs would be protected from antibiotics. However, how these bacteria might then induce UTI is not known. Interestingly, a recent longitudinal multi-omics analysis demonstrated that the gut microbiomes in women with recurrent UTI are distinct from those in women that have not experienced UTI, and that bacteria frequently transit the gut–bladder axis, with strain persistence noted in patients with recurrent UTI134. Overall this study suggests that recurrent UTI susceptibility is, at least in part, mediated by gut dysbiosis and differential immune responses to bacterial bladder colonization134. Taken together, these findings illuminate potential mechanisms underlying susceptibility to recurrent UTI in women.
Unfortunately, studies directly assessing correlative or causative relationships among the urinary, bladder, or genital microbiome and disease states in women and men are few and many are not sufficiently powered to make strong conclusions. Future application of transcriptomic, proteomic, and metabolomic analyses will enable discovery of the roles and relationships the microbiomes of the urinary tract play in protection or disease pathogenesis135. These findings may then lead to personalized approaches for diagnosis, prevention, prophylaxis, and treatment of urinary tract diseases based on key individual characteristics, including sex and age135.
Taking sex into account for therapeutic approaches
Considering the clear role of sex-based differences in urinary tract pathology, it is crucial to understand the mechanisms underlying these differences to improve diagnoses for immune-mediated and infectious diseases and to develop therapies that will benefit both sexes136. Targeting sex hormones or their downstream signaling pathways has clear clinical benefits in diseases such as breast or prostate cancer. In bladder cancer, in addition to reports that androgen-deprivation therapy improves recurrence-free survival in men86, AR antagonism may synergize with chemotherapy or radiotherapy since AR positivity in tumor samples negatively correlates with response to chemotherapy137. A recent report has shown that androgen-deprivation therapy, used in prostate cancer in this study, may also improve the response to checkpoint blockade, as AR signaling in T cells inhibits expression of IFNγ, necessary for tumor immunity138. However, whether these approaches may be of benefit more generally to bladder cancer patients remains to be tested.
Given the impact of sex, and particularly sex hormones, on disease susceptibility and immune function, a patient’s sex and hormonal state could be considered in greater depth in prevention and therapy approaches. Indeed, sex hormones can be readily antagonized or supplemented, which makes them attractive as adjunct therapeutic targets or modifiers in bladder diseases. For example, screening of serum hormone levels in recurrent UTI patients is feasible and might greatly improve disease outcome if targeted treatments are developed. As declining levels of estrogen after menopause lead to changes in the urogenital tract, including the loss of commensal Lactobacillus in the vaginal and urinary microbiome that increase the risk of UTI67,117,139, clinical studies have investigated the impact of estrogen administration to prevent UTI. Oral estrogen supplementation is mildly effective in reducing recurrent UTI but carries increased risks of coronary heart disease, stroke, venous thromboembolism, and breast cancer140,141. A small study testing topical vaginal estrogen against placebo found that estrogen exposure significantly reduces the number of UTI in the first 6 months of treatment compared to the number of UTI in patients using a placebo cream142. To counter the decline in Lactobacillus correlated with decreased estrogen levels in post-menopausal women, oral or vaginal supplementation with Lactobacillus strains has been tested140. While the results vary among studies, including several that found no differences in Lactobacillus-treated groups compared to placebo or antibiotic-treated individuals; intravaginal suppositories of Lactobacillus crispatus reduced UTI recurrence compared to the placebo group, and this reduction was correlated with a high level of vaginal colonization of Lactobacillus120. However, as many of these studies include only small subject numbers, larger-scale studies are needed to assess the efficacy of hormone-based treatments to prevent or treat UTI143.
To support human studies of hormonal therapeutics, additional laboratory work and model development are needed to dissect the mechanisms of sex-based differences in urinary tract diseases. Additionally, with the exception of hormone replacement therapy in post-menopausal women, published preclinical and clinical studies do not consider individuals taking hormone supplements for other and varied reasons, including gender-affirming hormone therapy. For example, there is a paucity of information regarding the impact of hormone supplementation on risk of infection or tumor development in the transgender population. However, there is likely an impact, as for example, individuals transitioning from men to women can be at risk for other serious health issues, such as cardiovascular disease, due in part to estrogen supplementation144. In mouse models of UTI, anti-androgen therapy prevents testosterone-dependent chronic cystitis and pyelonephritis53. Treatment of male C3H/HeN mice with a typical antibiotic (ceftriaxone) clears UPEC from the urinary tract, but mice still develop sterile abscesses and renal scarring145. Renal scarring, particularly in childhood, significantly increases the likelihood of hypertension and end-stage renal disease later in life146,147. Thus, AR antagonism may prove to be a valuable addition to traditional antibiotic therapy in acute UTI, particularly in male patients, whereas individuals using testosterone supplementation may be at increased risk of infection. In the case of bladder cancer, AR antagonism may have a different outcome. We applied the BBN model to transgenic URO-Ova mice expressing a model antigen from the urothelium to follow tumor-specific T cell responses during immunotherapy148. We found that tumor-specific T cells infiltrated the bladder more robustly in male tumor-bearing mice treated with BCG and PD-L1 immunotherapies compared to female tumor-bearing mice, which may be one reason why men have better bladder cancer outcomes than women148. Importantly, however, whether this difference is mediated by sex hormones, sex chromosomes, or another mechanism is unclear and would require preclinical studies modulating each of these factors before one could conclude that AR antagonism is a viable treatment approach.
Conclusion
Men and women experience different susceptibility to, and morbidity from, infectious and non-infectious diseases. These differences are driven by multiple factors, including genetics, environmental exposure, hormones, and access to care. Sex hormones greatly influence the onset, severity, and outcomes of UTI. Sex chromosomes and hormones independently modulate the development of bladder cancer, and the urinary microbiome itself is influenced by sex. Given that disease development, susceptibility, and immune responses are shaped by levels of circulating hormones in both sexes, hormone manipulation may represent a viable therapeutic target in diseases of the urinary tract. Therefore, it is crucial to better understand the impact of sex as a biological variable in health and disease to optimize future individualized therapies for both women and men.
References
Mauvais-Jarvis, F. et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 396, 565–582 (2020).
Ortona, E., Pierdominici, M. & Rider, V. Editorial: sex hormones and gender differences in immune responses. Front. Immunol. 10, 1076 (2019).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Neyrolles, O. & Quintana-Murci, L. Sexual Inequality in Tuberculosis. PLoS Med. 6, e1000199 (2009).
Whitacre, C. C. Sex differences in autoimmune disease. Nat. Immunol. 2, 777–780 (2001).
Markle, J. G. & Fish, E. N. SeXX matters in immunity. Trends Immunol. 35, 97–104 (2014).
Ursin, R. L., Shapiro, J. R. & Klein, S. L. Sex-biased immune responses following SARS-CoV-2 infection. Trends Microbiol. 28, 952–954 (2020).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. USA 111, 869–874 (2014).
Fink, A. L., Engle, K., Ursin, R. L., Tang, W.-Y. & Klein, S. L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl Acad. Sci. USA 115, 12477–12482 (2018).
Engler, R. J. M. et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch. Intern. Med. 168, 2405–2414 (2008).
Angele, M. K., Pratschke, S., Hubbard, W. J. & Chaudry, I. H. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5, 12–19 (2014).
Hewagama, A., Patel, D., Yarlagadda, S., Strickland, F. M. & Richardson, B. C. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 10, 509–516 (2009).
Jacobsen, H. & Klein, S. L. Sex differences in immunity to viral infections. Front. Immunol. 12, 720952 (2021).
Lepeytre, F. et al. Association of sex with risk of kidney graft failure differs by age. J. Am. Soc. Nephrol. 28, 3014–3023 (2017).
Zhu, Y., Shao, X., Wang, X., Liu, L. & Liang, H. Sex disparities in cancer. Cancer Lett. 466, 35–38 (2019).
Gubbels Bupp, M. R., Potluri, T., Fink, A. L. & Klein, S. L. The confluence of sex hormones and aging on immunity. Front. Immunol. 9, 1269 (2018).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Klein, S. L., Hodgson, A. & Robinson, D. P. Mechanisms of sex disparities in influenza pathogenesis. J. Leukoc. Biol. 92, 67–73 (2012).
Robinson, D. P., Lorenzo, M. E., Jian, W. & Klein, S. L. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 7, e1002149 (2011).
Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7, 653–660 (2010).
Lipsky, B. A. Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis, and treatment. Ann. Intern. Med. 110, 138–150 (1989).
Ingersoll, M. A. Sex differences shape the response to infectious diseases. PLoS Pathog. 13, e1006688 (2017).
Hooton, T. M. et al. Perineal anatomy and urine-voiding characteristics of young women with and without recurrent urinary tract infections. Clin. Infect. Dis. 29, 1600–1601 (1999).
Lacerda Mariano, L. & Ingersoll, M. A. The immune response to infection in the bladder. Nat. Rev. Urol. 17, 439–458 (2020).
Shaikh, N., Morone, N. E., Bost, J. E. & Farrell, M. H. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr. Infect. Dis. J. 27, 302–308 (2008).
Nuutinen, M. & Uhari, M. Recurrence and follow-up after urinary tract infection under the age of 1 year. Pediatr. Nephrol. Berl. Ger. 16, 69–72 (2001).
Ruben, F. L. et al. Clinical infections in the noninstitutionalized geriatric age group: methods utilized and incidence of infections. The Pittsburgh Good Health Study. Am. J. Epidemiol. 141, 145–157 (1995).
Harper, M. & Fowlis, G. 3. Management of urinary tract infections in men. Trends Urol. Gynaecol. Sex. Health 12, 30–35 (2007).
Kaye, D. Urinary tract infections in the elderly. Bull. N. Y. Acad. Med. 56, 209–220 (1980).
Yoshikawa, T. T. Unique aspects of urinary tract infection in the geriatric population. Gerontology 30, 339–344 (1984).
Kohler, T. S., Yadven, M., Manvar, A., Liu, N. & Monga, M. The length of the male urethra. Int. Braz. J. Urol. 34, 451–454 (2008).
Albracht, C. D., Hreha, T. N. & Hunstad, D. A. Sex effects in pyelonephritis. Pediatr. Nephrol. Berl. Ger. 36, 507–515 (2021).
Lamminmäki, A. et al. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm. Behav. 61, 611–616 (2012).
Fish, E. N. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 8, 737–744 (2008).
Olsen, N. J. & Kovacs, W. J. Gonadal steroids and immunity. Endocr. Rev. 17, 369–384 (1996).
Amenyogbe, E. et al. A review on sex steroid hormone estrogen receptors in mammals and fish. Int. J. Endocrinol. 2020, 5386193 (2020).
Ober, C., Loisel, D. A. & Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 9, 911–922 (2008).
Johnson, C. C. Definitions, classification, and clinical presentation of urinary tract infections. Med. Clin. North Am. 75, 241–252 (1991).
Foxman, B. & Brown, P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. North Am. 17, 227–241 (2003).
Efstathiou, S. P. et al. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch. Intern. Med. 163, 1206–1212 (2003).
Nicolle, L. E., Friesen, D., Harding, G. K. & Roos, L. L. Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin. Infect. Dis. 22, 1051–1056 (1996).
Ki, M., Park, T., Choi, B. & Foxman, B. The epidemiology of acute pyelonephritis in South Korea, 1997-1999. Am. J. Epidemiol. 160, 985–993 (2004).
Gilstrap, L. C. & Ramin, S. M. Urinary tract infections during pregnancy. Obstet. Gynecol. Clin. North Am. 28, 581–591 (2001).
Reinhard, G., Noll, A., Schlebusch, H., Mallmann, P. & Ruecker, A. V. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochem. Biophys. Res. Commun. 245, 933–938 (1998).
Sykes, L., MacIntyre, D. A., Yap, X. J., Teoh, T. G. & Bennett, P. R. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm. 2012, 967629 (2012).
AbdulHussain, G., Azizieh, F., Makhseed, M. & Raghupathy, R. Effects of progesterone, dydrogesterone and estrogen on the production of Th1/Th2/Th17 cytokines by lymphocytes from women with recurrent spontaneous miscarriage. J. Reprod. Immunol. 140, 103132 (2020).
Kissick, H. T. et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl Acad. Sci. USA 111, 9887–9892 (2014).
Laffont, S. et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 214, 1581–1592 (2017).
Cephus, J.-Y. et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 21, 2487–2499 (2017).
Zychlinsky Scharff, A. et al. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight 5, 122998 (2019).
Zychlinsky Scharff, A., Albert, M. L. & Ingersoll, M. A. Urinary tract infection in a small animal model: transurethral catheterization of male and female mice. J. Vis. Exp. https://doi.org/10.3791/54432 (2017).
Olson, P. D., Hruska, K. A. & Hunstad, D. A. Androgens enhance male urinary tract infection severity in a new model. J. Am. Soc. Nephrol. 27, 1625–1634 (2016).
Olson, P. D. et al. Androgen exposure potentiates formation of intratubular communities and renal abscesses by Escherichia coli. Kidney Int. 94, 502–513 (2018).
Gautam, Y., Afanador, Y., Abebe, T., López, J. E. & Mersha, T. B. Genome-wide analysis revealed sex-specific gene expression in asthmatics. Hum. Mol. Genet. 28, 2600–2614 (2019).
Bini, E. I. et al. The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PLoS ONE 9, e93831 (2014).
Yung, J. A., Fuseini, H. & Newcomb, D. C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 120, 488–494 (2018).
Fuseini, H. & Newcomb, D. C. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma Rep. 17, 19 (2017).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity 50, 892–906 (2019).
Huang, J. et al. Group 3 innate lymphoid cells protect the host from the uropathogenic Escherichia coli infection in the bladder. Adv. Sci. 9, 2103303 (2022).
Brouillette, J., Rivard, K., Lizotte, E. & Fiset, C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc. Res. 65, 148–157 (2005).
McLellan, L. K., Daugherty, A. L. & Hunstad, D. A. Sex differences in population dynamics during formation of kidney bacterial communities by uropathogenic Escherichia coli. Infect. Immun. 89, e00716–20 (2021).
Hreha, T. N. et al. TGFβ1 orchestrates renal fibrosis following Escherichia coli pyelonephritis. Physiol. Rep. 8, e14401 (2020).
Hreha, T. N. et al. Androgen-influenced polarization of activin A-producing macrophages accompanies post-pyelonephritic renal scarring. Front. Immunol. 11, 1641 (2020).
Gong, D. et al. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 13, 31 (2012).
Dwyer, P. L. & O’Reilly, M. Recurrent urinary tract infection in the female. Curr. Opin. Obstet. Gynecol. 14, 537–543 (2002).
Lüthje, P. et al. Estrogen supports urothelial defense mechanisms. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.3005574 (2013).
Lüthje, P., Hirschberg, A. L. & Brauner, A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas 77, 32–36 (2014).
Wang, C., Symington, J. W., Ma, E., Cao, B. & Mysorekar, I. U. Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect. Immun. 81, 733–739 (2013).
Anand, M. et al. Estrogen affects the glycosaminoglycan layer of the murine bladder. Female Pelvic Med. Reconstr. Surg. 18, 148–152 (2012).
Zeng, Z. et al. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat. Immunol. 19, 1100–1111 (2018).
Svanborg-Edén, C. & Svennerholm, A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect. Immun. 22, 790–797 (1978).
Saginala, K. et al. Epidemiology of bladder cancer. Med. Sci. 8, 15 (2020).
Dobruch, J. et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur. Urol. 69, 300–310 (2016).
Pettenati, C. & Ingersoll, M. A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 15, 615–625 (2018).
Boorjian, S. A., Zhu, F. & Herr, H. W. The effect of gender on response to bacillus Calmette-Guérin therapy for patients with non-muscle-invasive urothelial carcinoma of the bladder. BJU Int. 106, 357–361 (2010).
Uhlig, A. et al. Gender-specific differences in recurrence of non-muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur. Urol. Focus 4, 924–936 (2018).
D’Andrea, D. et al. Impact of sex on response to neoadjuvant chemotherapy in patients with upper-tract urothelial cancer. Eur. Urol. Open Sci. 19, 16–19 (2020).
Ye, Y. et al. Sex-associated molecular differences for cancer immunotherapy. Nat. Commun. 11, 1779 (2020).
Irelli, A., Sirufo, M. M., D’Ugo, C., Ginaldi, L. & De Martinis, M. Sex and gender influences on cancer immunotherapy response. Biomedicines 8, 232 (2020).
Okajima, E., Hiramatsu, T., Iriya, K., Ijuin, M. & Matsushima, S. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol. Res. 3, 73–79 (1975).
Tanahashi, N. K., Suzawa, N. & Azuma, C. Effects of sex hormones on oncogenesis in rat urinary bladder by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Int. J. Clin. Pharmacol. Biopharm. 15, 101–105 (1977).
Miyamoto, H. et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl Cancer Inst. 99, 558–568 (2007).
Hsu, J.-W. et al. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am. J. Pathol. 182, 1811–1820 (2013).
Johnson, D. T. et al. Conditional expression of the androgen receptor increases susceptibility of bladder cancer in mice. PLoS ONE 11, e0148851 (2016).
Kwon, H. et al. Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 0, eabq2630 (2022).
Izumi, K. et al. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget 5, 12665–12674 (2014).
Izumi, K. et al. Expression of androgen receptor in non-muscle-invasive bladder cancer predicts the preventive effect of androgen deprivation therapy on tumor recurrence. Oncotarget 7, 14153–14160 (2016).
Miyamoto, H. et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 109, 1716–1726 (2012).
Dechering, K., Boersma, C. & Mosselman, S. Estrogen receptors alpha and beta: two receptors of a kind? Curr. Med. Chem. 7, 561–576 (2000).
Phiel, K. L., Henderson, R. A., Adelman, S. J. & Elloso, M. M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 97, 107–113 (2005).
Shen, S. et al. Expression of estrogen receptors-α and -β in bladder cancer cell lines and human bladder tumor tissue. Cancer 106, 2610–2616 (2006).
Sato, N. et al. Clinicopathological significance of estrogen receptor β and estrogen synthesizing/metabolizing enzymes in urothelial carcinoma of urinary bladder. Pathol. Oncol. Res. 27, 589649 (2021).
Mir, C. et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU Int. 108, 24–30 (2011).
Bianchi, I., Lleo, A., Gershwin, M. E. & Invernizzi, P. The X chromosome and immune associated genes. J. Autoimmun. 38, J187–192 (2012).
Youness, A., Miquel, C.-H. & Guéry, J.-C. Escape from X chromosome inactivation and the female predominance in autoimmune diseases. Int. J. Mol. Sci. 22, 1114 (2021).
Migeon, B. R. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA 295, 1428–1433 (2006).
Berletch, J. B., Yang, F., Xu, J., Carrel, L. & Disteche, C. M. Genes that escape from X inactivation. Hum. Genet. 130, 237–245 (2011).
Souyris, M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018).
Griesbeck, M. et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J. Immunol. Author Choice 195, 5327–5336 (2015).
Schmalstieg, F. C. & Goldman, A. S. Immune consequences of mutations in the human common gamma-chain gene. Mol. Genet. Metab. 76, 163–171 (2002).
Brooks, E. G. et al. A novel X-linked combined immunodeficiency disease. J. Clin. Invest. 86, 1623–1631 (1990).
Lovell-Badge, R. & Robertson, E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109, 635–646 (1990).
Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121 (1991).
Kaneko, S. & Li, X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv. 4, eaar5598 (2018).
Hurst, C. D. et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell 32, 701–715.e7 (2017).
Itoh, Y. et al. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J. Clin. Invest. 129, 3852–3863 (2019).
Krimphove, M. J. et al. Sex-specific differences in the quality of treatment of muscle-invasive bladder cancer do not explain the overall survival discrepancy. Eur. Urol. Focus 7, 124–131 (2021).
de Jong, J. J. et al. Distribution of molecular subtypes in muscle-invasive bladder cancer is driven by sex-specific differences. Eur. Urol. Oncol. 3, 420–423 (2020).
Siddiqui, H., Nederbragt, A. J., Lagesen, K., Jeansson, S. L. & Jakobsen, K. S. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol 11, 244 (2011).
Fouts, D. E. et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 10, 174 (2012).
Lewis, D. A. et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 3, 41 (2013).
Gottschick, C. et al. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 5, 99 (2017).
Groah, S. L. et al. Redefining healthy urine: a cross-sectional exploratory metagenomic study of people with and without bladder dysfunction. J. Urol. 196, 579–587 (2016).
Jahromi, M. S., Mure, A. & Gomez, C. S. UTIs in patients with neurogenic bladder. Curr. Urol. Rep. 15, 433 (2014).
Stapleton, A. E. The vaginal microbiota and urinary tract infection. Microbiol. Spectr. 4 https://doi.org/10.1128/microbiolspec.UTI-0025-2016 (2016).
Hugenholtz, F. et al. Urine and vaginal microbiota compositions of postmenopausal and premenopausal women differ regardless of recurrent urinary tract infection and renal transplant status. Sci. Rep. 12, 2698 (2022).
Baldassarre, M. et al. Effects of long-term high dose testosterone administration on vaginal epithelium structure and estrogen receptor-α and -β expression of young women. Int. J. Impot. Res. 25, 172–177 (2013).
Heinemann, C. & Reid, G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can. J. Microbiol. 51, 777–781 (2005).
Vaughan, M. H. et al. The urinary microbiome in postmenopausal women with recurrent urinary tract infections. J. Urol. 206, 1222–1231 (2021).
Stapleton, A. E. et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 52, 1212–1217 (2011).
Sadahira, T. et al. Efficacy of Lactobacillus vaginal suppositories for the prevention of recurrent cystitis: a phase II clinical trial. Int. J. Urol. 28, 1026–1031 (2021).
Reid, G. & Bruce, A. W. Probiotics to prevent urinary tract infections: the rationale and evidence. World J. Urol. 24, 28–32 (2006).
Sumati, A. H. & Saritha, N. K. Association of urinary tract infection in women with bacterial vaginosis. J. Glob. Infect. Dis. 1, 151–152 (2009).
Sharami, S. H., Afrakhteh, M. & Shakiba, M. Urinary tract infections in pregnant women with bacterial vaginosis. J. Obstet. Gynaecol. 27, 252–254 (2007).
Lewis, A. L. & Gilbert, N. M. Roles of the vagina and the vaginal microbiota in urinary tract infection: evidence from clinical correlations and experimental models. GMS Infect. Dis. 8, Doc02 (2020).
Kirjavainen, P. V. et al. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clin. Vaccin. Immunol. 16, 29–36 (2009).
Gilbert, N. M., O’Brien, V. P. & Lewis, A. L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 13, e1006238 (2017).
O’Brien, V. P., Joens, M. S., Lewis, A. L. & Gilbert, N. M. Recurrent Escherichia coli urinary tract infection triggered by gardnerella vaginalis bladder exposure in mice. J. Vis. Exp. 10, 3791/61967 (2020).
O’Brien, V. P., Lewis, A. L. & Gilbert, N. M. Bladder exposure to gardnerella activates host pathways necessary for Escherichia coli recurrent UTI. Front. Cell. Infect. Microbiol. 11, 788229 (2021).
Wolfe, A. J. et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 50, 1376–1383 (2012).
Thomas-White, K. J. et al. Incontinence medication response relates to the female urinary microbiota. Int. Urogynecol. J. 27, 723–733 (2016).
Castro, J., Machado, D. & Cerca, N. Escherichia coli and Enterococcus faecalis are able to incorporate and enhance a pre-formed Gardnerella vaginalis biofilm. Pathog. Dis. 74, ftw007 (2016).
Brannon, J. R. et al. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat. Commun. 11, 2803 (2020).
Worby, C. J. et al. Longitudinal multi-omics analyses link gut microbiome dysbiosis with recurrent urinary tract infections in women. Nat. Microbiol. 7, 630–639 (2022).
Mouraviev, V. & McDonald, M. An implementation of next generation sequencing for prevention and diagnosis of urinary tract infection in urology. Can. J. Urol. 25, 9349–9356 (2018).
Brodin, P. & Davis, M. M. Human immune system variation. Nat. Rev. Immunol. 17, 21–29 (2017).
Ide, H. & Miyamoto, H. Sex hormone receptor signaling in bladder cancer: a potential target for enhancing the efficacy of conventional non-surgical therapy. Cells 10, 1169 (2021).
Guan, X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 1–6 https://doi.org/10.1038/s41586-022-04522-6 (2022).
Sihra, N., Goodman, A., Zakri, R., Sahai, A. & Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 15, 750–776 (2018).
Beerepoot, M. & Geerlings, S. Non-antibiotic prophylaxis for urinary tract infections. Pathogens 5, 36 (2016).
Beerepoot, Ma. J., Geerlings, S. E., van Haarst, E. P., van Charante, N. M. & ter Riet, G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J. Urol. 190, 1981–1989 (2013).
Ferrante, K. L., Wasenda, E. J., Jung, C. E., Adams-Piper, E. R. & Lukacz, E. S. Vaginal estrogen for the prevention of recurrent urinary tract infection in postmenopausal women: a randomized clinical trial. Female Pelvic Med. Reconstr. Surg. 27, 112–117 (2021).
Fox, K. A., Lokken, E. M., Reed, S. D. & Rahn, D. D. Evaluation of systemic estrogen for preventing urinary tract infections in postmenopausal women. Menopause 28, 836–844 (2021).
Getahun, D. et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann. Intern. Med. 169, 205–213 (2018).
Olson, P. D. et al. Renal scar formation and kidney function following antibiotic-treated murine pyelonephritis. Dis. Model. Mech. 10, 1371–1379 (2017).
Shaikh, N. et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr. 168, 893–900 (2014).
Calderon-Margalit, R. et al. History of childhood kidney disease and risk of adult end-stage renal disease. N. Engl. J. Med. 378, 428–438 (2018).
Rousseau, M. et al. Identification of sex differences in tumor-specific T cell infiltration in bladder tumor-bearing mice treated with BCG immunotherapy. Bladder Cancer 6, 507–524 (2020).
Rowe, T. A. & Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 9 https://doi.org/10.2217/ahe.13.38 (2013).
Acknowledgements
The authors would like to acknowledge critical reading by Rixa-Mareike Köhn. We also gratefully acknowledge our finding sources. L.D. and L.L.M. have received support from the Labex Milieu Intérieur (ANR-10-LABX-69-01). L.L.M. was part of the Pasteur-Paris University (PPU) International PhD Program, which received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 665807. Work cited herein is supported by the US National Institutes of Health (D.A.H.: R01-DK111541, R01-DK126697, R01-AI158418) and the Agence Nationale de la Recherche (M.A.I.: ANR-19-CE15-0015-01, ANR-21-CE15-0006-02).
Author information
Authors and Affiliations
Contributions
All authors contributed to evaluating relevant literature, drafting, and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.A.H. serves on the Board of Directors of BioVersys AG, Basel, Switzerland and has received research funding from BioAge Labs, Richmond, CA, USA. All other authors have no conflicts to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deltourbe, L., Lacerda Mariano, L., Hreha, T.N. et al. The impact of biological sex on diseases of the urinary tract. Mucosal Immunol 15, 857–866 (2022). https://doi.org/10.1038/s41385-022-00549-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-022-00549-0
This article is cited by
-
Welche Rolle spielt das Geschlecht bei der Nierentransplantation?
Die Nephrologie (2024)
-
Does immune cell exhaustion lie at the heart of BCG-unresponsive disease?
Nature Reviews Urology (2023)
-
Correlation of procalcitonin and c-reactive protein levels with pathogen distribution and infection localization in urinary tract infections
Scientific Reports (2023)
-
Correlation between androgen and estrogen receptor expression and clinicopathologic features in carcinoma urinary bladder
Journal of Cancer Research and Clinical Oncology (2023)
-
Procedure Matters in Gender-Associated Outcomes following Metabolic-Bariatric Surgery: Five Year North American Matched Cohort Analysis
Obesity Surgery (2023)