Abstract
The human body harbors a diverse ecosystem of microorganisms, including bacteria, viruses, and fungi, collectively known as the microbiota. Current research is increasingly focusing on the potential association between the microbiota and various neuropsychiatric disorders. The microbiota resides in various parts of the body, such as the oral cavity, nasal passages, lungs, gut, skin, bladder, and vagina. The gut microbiota in the gastrointestinal tract has received particular attention due to its high abundance and its potential role in psychiatric and neurodegenerative disorders. However, the microbiota presents in other body tissues, though less abundant, also plays crucial role in immune system and human homeostasis, thus influencing the development and progression of neuropsychiatric disorders. For example, oral microbiota imbalance and associated periodontitis might increase the risk for neuropsychiatric disorders. Additionally, studies using the postmortem brain samples have detected the widespread presence of oral bacteria in the brains of patients with Alzheimer’s disease. This article provides an overview of the emerging role of the host microbiota in neuropsychiatric disorders and discusses future directions, such as underlying biological mechanisms, reliable biomarkers associated with the host microbiota, and microbiota-targeted interventions, for research in this field.
Similar content being viewed by others
Introduction
According to the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, neuropsychiatric disorders such as schizophrenia, autism spectrum disorder (ASD), major depressive disorder (MDD), bipolar disorder (BD), anxiety disorders, and substance use disorder, continue to rank among the top ten leading causes of global burden, with no evidence of a reduction since 1990 [1]. These disorders have profound impacts on individuals, their families, and communities, posing a significant public health concern worldwide. Additionally, the prevalence of MDD, anxiety disorders, and post-COVID-19 condition has increased during and following the COVID-19 pandemic [2,3,4,5,6,7,8,9]. Accumulating evidence indicates that both genetic and environmental factors contribute significantly to the development and manifestation of these neuropsychiatric disorders [10,11,12,13,14,15,16,17,18]. Environmental factors encompass early life experiences, social and cultural factors, traumatic events, chronic stress, substance abuse and addiction, as well as limited access to mental healthcare services. However, the precise biological mechanisms underlying the development and progression of neuropsychiatric disorders remain elusive.
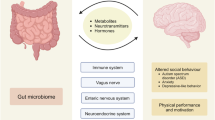
Brain–body crosstalk constitutes a bidirectional network that facilitates the regulation and maintenance of overall homeostasis in the body by enabling communication between the brain and peripheral organs [19,20,21,22,23,24,25,26,27,28,29,30]. This crosstalk involves various components, including the central nervous system (CNS), peripheral nervous system, neurotransmitters, chemical signaling, hormones, feedback loops and homeostasis, and mind–body connection associated with emotions. A comprehensive understanding of brain–body crosstalk is crucial for scientists to unravel the underlying mechanisms contributing to the pathogenesis of neuropsychiatric disorders.
The human body is known to harbor a diverse and abundant community of microorganisms called the microbiota. Host microbiota can be categorized into various types, including oral, nasal, lung, gut, skin, bladder, and vagina microbiota (Fig. 1) [31,32,33,34,35]. The gut microbiota, which resides in the gastrointestinal (GI) tract, has garnered increasing attention due to its role in brain–body crosstalk, known as the gut–brain axis [20, 28, 29, 36,37,38,39,40]. However, limited research has been conducted on the role of other host microbiota in neuropsychiatric disorders due to their lower abundance compared to the gut microbiota. It is essential to comprehensively understand the role of the predominant gut microbiota as well as other microbiota in neuropsychiatric disorders.
The human microbiota resides in the various tissues of the body, including the mouth, nose, gastrointestinal (GI) tract, lung, skin, bladder, and vagina. In the GI trats, the density of microbes in different locations, such as the stomach, duodenum, jejunum, ileum, and colon, has been shown [43]. Part of the figure was designed using resources from Biorender.com.
In this article, the author provides an overview of the host microbiota in humans and explores the emerging role of the host microbiota in neuropsychiatric disorders. Furthermore, the author proposes future research directions for investigating the role of the host microbiota in neuropsychiatric disorders.
Host microbiome in human
The role of the host microbiota in maintaining health and contributing to various diseases has gained considerable attention. The host-microbiota encompasses a range of microbial communications, including the oral, nasal, lung, gut, skin, bladder, and vaginal microbiota (Fig. 1) [31, 34, 35]. Notably, the oral and nasal microbiota serve as crucial entry points for potential pathogens that could spread to the CNS. Human microbial communities are complex and interconnected (Fig. 2). Microbiota in one organ have the potential to influence those in another. The gut, which houses the majority of the host’s microbiome, plays a central role in affecting overall health and influencing various diseases throughout the body [31, 34, 41, 42]. Although potential interactions among microbiota in different organs have been suggested, empirical evidence to support these claims remains limited (Fig. 2). Current research is focused on elucidating these microbial interactions across various organs.
The microbiota in one organ may potentially influence that in another organ. The gut microbiota, which constitutes the majority of the host’s microbiome, plays a central role in affecting health and disease throughout the body. Although there are proposed interactions between the microbiota of different organs, current evidence supporting these interactions remains limited. Dysbiosis in the microbiota across various different tissues may contribute to the incidence of organ-specific diseases. COPD: chronic obstructive pulmonary disease. Part of the figure was designed using resources from Biorender.com.
While numerous studies have focused on the gut microbiota in the GI tract, research on other microbiota has been limited due to their lower abundance compared to the gut microbiota [43]. In the next sections, the author provides a summary of the role of the host microbiota in the GI tract and the other different tissues.
Gut microbiota
The density (gram) of microbiota in the GI tract varies across different regions: stomach (1 × 101), duodenum (1 × 103), jejunum (1 × 104), ileum (1 × 107), and colon (1 × 1012) (Fig. 1) [43]. The composition and diversity of gut microbiota in these different regions may differ across these regions; however, studying the gut microbiota from fecal samples, which are closely related to the colon, is more straightforward. The term “gut microbiota–brain axis” refers to the bidirectional communications between the gut microbiota in the GI tract and the brain. Accumulating evidence strongly suggests that the gut microbiota plays a crucial role in regulating brain function and behavior through various mechanisms. These mechanisms include the production of various neurotransmitters, including serotonin, dopamine, γ-aminobutyric acid (GABA), kynurenic acid, as well as microbe-derived metabolites (e.g., short-chain fatty acids [SCFAs], bile acids, D-amino acids). With regard to D-amino acids, some studies have demonstrated reduced blood levels of D-glutamate in patients with Alzheimer’s disease (AD) compared to healthy controls [44, 45]. Moreover, it has been observed that plasma levels of D-glutamate were correlated with cognitive functions [44, 45]. Given that D-glutamate is a component of the peptidoglycan cell wall in bacteria, it is plausible that the gut microbiota contributes to its production [46]. The immune system also plays a role in this axis [20, 28, 29, 47]. Preclinical findings have highlighted the significance of the vagus nerve in the gut microbiota–brain axis [48,49,50,51,52,53,54,55,56,57]. Understanding the gut microbiota–brain axis via the vagus nerve has opened up new possibilities for the development of novel treatments (e.g., dietary interventions, prebiotics, probiotics, symbiotics [synergistic combination of prebiotics and probiotics], fecal microbiota transplantation, and vagus nerve stimulation) for neuropsychiatric disorders [9, 39, 40, 58].
Research on the gut microbiota–brain axis in neuropsychiatric disorders is a rapidly evolving field. Alterations in the composition and diversity of the gut microbiota have been linked to various psychiatric disorders, including schizophrenia, MDD, BD, ASD, anxiety, and even neurodegenerative disorders such as AD and Parkinson’s disease (PD) [59,60,61,62,63,64,65,66,67,68,69,70,71]. A meta-analysis identified a transdiagnostic pattern associating gut microbiota imbalances with schizophrenia, MDD, BD, and anxiety [72]. These imbalances were characterized by a reduction of certain anti-inflammatory butyrate-producing bacteria and an increase in pro-inflammatory bacteria [72]. Specifically, consistent reductions in the levels of Faecalibacterium and Coprococcus, along with elevated levels of Eggerthella, were observed across psychiatric disorders such as schizophrenia, MDD, BD, and anxiety [72]. A separate systematic review highlighted bacterial taxa frequently linked to psychiatric disorders (e.g., schizophrenia, MDD, BD) [64]. These findings include reduced levels of bacterial genera that produce SCFAs (e.g., butyrate), increased levels of lactic acid-producing bacteria, and a heightened presence of bacteria involved in the metabolism of neurotransmitters such as glutamate and GABA [64].
A meta-analysis, which included discovery and replication samples, confirmed that ten bacterial genera were significantly correlated with AD [73]. Among these, four genera were significantly associated with the APOE rs429358 risk allele, either as protective or risk factors for AD. Importantly, the pro-inflammatory genus Collinsella, identified as a risk factor for AD, exhibited a positive correlation with the APOE rs429358 risk allele across both sample sets. These findings indicate that the influence of host genetic factors on the abundance of these ten genera is significantly correlated with AD, suggesting that these genera could serve as potential biomarkers and therapeutic targets for the disease [73]. Another recent meta-analysis showed that, at the phylum level, the relative abundance of Firmicutes was significantly lower in AD patients compared to healthy controls [74]. Conversely, the relative abundance of Bacteroidetes was significantly higher in patients with MCI than in healthy controls [74]. Collectively, these findings highlight gut microbiota abnormalities associated with AD.
PD is a neurodegenerative disorder primarily characterized by motor symptoms, including tremors, rigidity, bradykinesia, and postural instability. Notably, a significant number of PD patients experience GI symptoms, such as constipation, well before the onset of motor symptoms. This suggests that alterations in gut motility could be linked to gut microbiota dysbiosis. A protein named α-synuclein accumulates in the brains of PD patients, forming aggregates termed Lewy bodies. Interestingly, these protein aggregates are also found in the enteric nervous system (the nerve of the gut). Current hypotheses suggest that pathologic α-synuclein may originate in the gut and subsequently migrate to the brain via the vagus nerve, thereby contributing to the pathology of PD [60, 71, 75,76,77,78].
However, it is important to note that the gut microbiota–brain axis is a complex and evolving field of research. Further studies are needed to elucidate the mechanisms and develop new effective therapies. Nonetheless, the emerging evidence underscores the significant role of the gut microbiota in psychiatric and neurodegenerative disorders, offering a new perspective on the treatment and management of these disorders. The author has not extensively explored the role of gut microbiota on neuropsychiatric disorders in this section, as numerous comprehensive review articles have already been published [64,65,66,67,68,69,70,71].
Oral microbiota
The oral microbiota refers to the collective microbial community inhabiting the human oral cavity. It represents the second-largest microbial community in the human body. Components of the oral microbiome are viruses, bacteria, archaea, fungi, and protozoa (Fig. 3) [34, 35, 79, 80]. The oral microbiota colonizes two distinct regions: the hard surfaces of the teeth, including dentures, and the soft tissues of the oral mucosa. Major phyla of the oral microbiota include Actionobacteria, Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria (Table 1) [34, 35]. Considering that the oral cavity serves as the primary entry point to the human body, disruptions in the oral microbiota may potentially contribute to the development and progression of psychiatric and neurodegenerative disorders, as well as autoimmune diseases (Fig. 3) [34, 35, 81,82,83]. In addition of the gut microbiota, research efforts have expanded to investigate the role of the oral microbiota in neuropsychiatric disorders [84,85,86,87,88,89].
The oral microbiota consists viruses, bacteria, archaea, fungi, and protozoa. In comparison to a healthy microbiome (eubiosis), patients with periodontitis exhibit an imbalanced microbiome (dysbiosis), which can contribute to the development of neuropsychiatric disorders. Studies utilizing postmortem brain samples have demonstrated the presence of the oral microbiota in the brains from patients with Alzheimer’s disease (AD) or Parkinson’s disease [105]. Part of the figure was designed using resources from Biorender.com.
Periodontal diseases in patients with psychiatric disorders
Patients with psychiatric disorders often exhibit poor oral hygiene and a compromised periodontal status [84]. A meta-analysis has indicated that all psychiatric disorders are associated with an increased risk of dental decay, as reflected by higher decayed, missing, and filled teeth scores, as well as greater tooth loss [90]. Dry mouth, a common side effect of medications, is prevalent among many individuals with neuropsychiatric disorders and serves as a significant risk factor for oral health issue [84]. Furthermore, the incidence of periodontitis in patients with psychiatric disorders is 1.45 times higher compared to those without psychiatric disorders [91]. Taken together, these findings suggest that periodontitis may partly contribute to the risk for developing neuropsychiatric disorders (Figs. 2 and 3). However, further detailed studies with larger sample sizes are needed.
In a case-control study involving BD patients (n = 176) and controls (n = 176), the prevalence of periodontitis was higher among BD patients, and BD patients with periodontitis exhibited elevated levels of Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis compared to controls [92]. Notably, periodontitis showed a strong association with both the total bacterial load and the depressive phase of BD [92]. These findings suggest that increased levels of these oral microbiota may contribute to periodontitis, potentially leading to depressive symptoms in BD patients. Additionally, the salivary microbiome in patients (n = 85) with drug-naïve first-episode schizophrenia was characterized by higher α-diversity (a measure of microbiome diversity) and lower β-diversity (a measure of the similarity or dissimilarity of microbial communities) heterogeneity than those of subjects (n = 43) with clinical high risk for psychosis and healthy controls (n = 80) [93]. Interestingly, hydrogen sulfide (H2S)-producing bacteria exhibited disease-stage-specific enrichment, and certain salivary microbiota exhibited disease-specific correlation patterns with symptom severity [93]. This supports previous findings highlighting the role of excess H2S in schizophrenia [94]. The same group reported changes in salivary metabolites in patients with drug-naïve first-episode schizophrenia compared to healthy controls, with these changes being closely associated with peripheral inflammatory markers and salivary microbiota [95]. These results suggest a connection between the disturbed oral microbiota, microbe-derived metabolites and the onset of schizophrenia, hinting at a possible role of the oral–brain connection in the initiation of this disease. However, further research involving larger sample sizes is necessary to confirm these findings.
A recent narrative review proposes a possible link between the oropharyngeal microbiome and schizophrenia, although additional research is needed to definitively establish this connection [96]. Another recent study showed that alcohol consumption influences the diurnal fluctuations in the oral microbiota of individuals with functional impairment due to alcohol dependence. This finding emphasizes the potential for interventions to mitigate the negative consequences of alcohol dependence [97].
Using a cohort of Israel veterans from the 1982 Lebanon war, Levert-Levitt et al. [98] found that the oral microbiota signature (specifically, reduced levels of bacteria sp_HMT_914, 332 and 871, as well as Noxia) correlated with the severity of post-traumatic stress disorder (PTSD). Conversely, the duration of education was associated with higher levels of sp_HMP_871 and decreased levels of Bacteroides and Firmicutes. Notably, air pollution positively correlated with PTSD symptoms, psychopathological symptoms, and changes in oral microbiota composition [98]. These findings suggest potential non-intrusive treatments for PTSD related to the oral microbiota pathway.
Neurodegenerative disorders and detection of bacteria in the brain
Mild cognitive impairment (MCI) represents an initial phase of memory decline or other cognitive functions, yet individuals with MCI retain the capability to conduct most daily activities. In subjects with MCI, higher levels of Pasteurellacae were observed compared to cognitively normal controls, whereas the abundance of Lautropia mirabilis was lower in individuals with MCI [99]. Furthermore, the abundance of Pasteurellacae was associated with inflammatory markers in the cerebrospinal fluid (CSF). These preliminary findings suggest that an altered composition of the oral microbiota may contribute to neuroinflammation, potentially leading to cognitive decline. However, further longitudinal studies involving elderly individuals are needed to better understand this relationship.
Both AD and PD are prominent neurodegenerative disorders, characterized by the accumulation of β-amyloid and α-synuclein in the brain, respectively. Patients with AD or PD often exhibit psychiatric symptoms, such as depression. A well-established association exists between poor oral health, specifically periodontitis, and an increased risk of developing AD or PD (Fig. 3) [100,101,102]. A cross-sectional study has revealed links between oral health-related stressors and neuropsychiatric symptoms in patients with AD [103]. Collectively, these findings highlight the importance of managing oral health in patients with neurodegenerative disorders.
Several microorganisms have been identified in the CSF and brains of individuals with AD or PD, suggesting a potential role in the progression of these diseases [104,105,106]. A recent study using the postmortem brain samples revealed the widespread presence of oral bacteria in regions associated with AD and PD pathology (Fig. 3). Interestingly, bacteria profiles in the brain were distinct from those in blood samples [107]. Additionally, a recent meta-analysis demonstrated a significant association between oral bacteria and AD, particularly when oral bacteria were detectable in the brain [108]. A recent study showed that the oral and gut microbiota of partners of AD patients resembled that of the AD patients themselves and differed from healthy controls [109], suggesting a potential transmission of microbiota. This observation could provide insight into why spouses of AD patients have an elevated risk of developing dementia [109]. Overall, it is plausible that periodontal microbiota could enter the brain, thereby contributing to the development of AD [110]. However, further cohort studies involving larger sample sizes are necessary to confirm the role of the oral microbiota in AD or PD. At present, it remains unknown whether oral bacteria are detected in the brains of patients with psychiatric disorders.
Increasing evidence highlights the role of oral microbiota in PD [111]. Notably, the oral microbiota in early-stage PD patients shows significant differences compared to healthy controls, with specific oral bacteria exhibiting associations with motor and non-motor functional measures [112]. Furthermore, significant disparities were observed in the composition of the oral and gut microbiome between PD patients and healthy controls [113]. Notably, the oral bacteria Lactobacillus demonstrated increased abundance in PD patients and was associated with opportunistic pathogens in the gut. Another study revealed significant differences in microbiota composition in the oral cavity and gut, but not the nasal cavity, between PD patients (n = 91) and healthy controls (n = 91) [114]. Interestingly, correlations between the genera in the oral cavity and the severity of depression and anxiety were observed in PD patients [114], suggesting a role of the oral microbiota in psychiatric symptoms of PD. These findings indicate a potential link between the oral and the gut microbiota in PD (Fig. 2), which could lead to functional changes within the microbiome of PD patients [113, 114]. Given the crucial role of oral microbiota in maintaining oral health, it is plausible that changes in the oral microbiota among the elderly population could contribute to the development and progression of various disorders such as AD, PD, and age-related systemic disorders [115, 116].
Nasal microbiota
The nasal cavity harbors a diverse community of microorganisms that contribute to the maintenance of the nasal mucosa health and overall immune system function [117,118,119]. Major phyla observed in the nasal microbiota include Actionobacteria, Firmicutes, and Proteobacteria (Table 1) [34, 35]. The composition and diversity of the nasal microbiota vary among individuals and are influenced by factors such as age, genetics, environmental exposures, and personal hygiene habits such as smoking [34, 117, 119]. Considering the role of the nasal microbiota in host immune responses, an imbalance in the nasal microbiota has been associated with various health conditions [34, 117, 119]. The role of the nasal microbiota in neuropsychiatric disorders is an emerging area of research that explores the potential link between the microbial communities in the nasal cavity and mental health conditions. Additionally, the nasal microbiota may influence the well-known gut microbiota–brain axis through various pathways, including direct contact with the olfactory system, immunes system modulation, and the production of neurotransmitters or metabolites capable of crossing the blood-brain barrier (Fig. 4). However, there are currently no reports available on the specific role of the nasal microbiota in patients with psychiatric disorders.
The nasal cavity can be divided into distinct regions, including the nasal vestibules, respiratory region, olfactory region, and nasopharyngeal region. The nasal microbiota colonizes these regions, and plays a crucial role in maintaining the health of the nasal mucosa and overall immune system function. There are three pathways from the mucus layer to the olfactory bulb: (a) the transcellular pathway, which involves passage through epithelial cells; (b) the paracellular pathway, which occurs between epithelial cells; and (c) the intracellular pathway, which occurs through the olfactory nerve. Considering the crucial role of the nasal microbiota within the nasal cavity, it is plausible that both the nasal microbiota and their metabolites may have a role in neuropsychiatric disorders. Part of the figure was designed using resources from Biorender.com.
Neurodegenerative disorders
Age significantly influences olfactory dysfunction, making it a potential early indicator of neurodegenerative disorders [119]. Anosmia (complete loss of olfactory function) and hyposmia (decreased olfactory function) are commonly observed in patients with neurodegenerative disorders. Although there is currently no direct evidence supporting the association between the inflammatory response of the nasal microbiota and neurodegenerative disorders, several reports explore the role of the nasal microbiota in AD and PD [119]. These studies suggest a potential link between microbial communities in the nasal cavity and the development or progression of these neurological disorders. Additionally, a potential association between PD and nasal microbiota has been proposed. Dysbiosis of the nasopharyngeal microbiota could trigger inflammatory responses to α-synuclein, contributing to the pathological changes seen in PD [119].
Nasal microbiota in the olfactory function
Accumulating evidence suggests the involvement of olfactory dysfunction in neuropsychiatric disorders (Fig. 2). Specific alterations in various components of the sense of smell have been observed in patients with neuropsychiatric disorders such as schizophrenia [120,121,122,123,124,125,126]. However, there are currently no reports available on the relationship between the nasal microbiota and olfactory functions in patients with neuropsychiatric disorders. Notably, patients with substance use disorder often use inhalation of drugs of abuse. It is known that the respiratory system, including the nasal cavity and lungs, can be exposed to various drugs through inhalation, resulting in dysbiosis of nasal and lung microbiota. Given the crucial role of the nasal microbiota in olfactory functions, it is of great interest to investigate whether the nasal microbiota is altered in patients with neuropsychiatric disorders.
Lung microbiota
The human respiratory tract was traditionally believed to be a sterile environment; however, emerging research using advanced techniques has revealed the presence of a diverse community of microorganisms known as the lung microbiota. Major phyla observed in the lung microbiota include Bacteroidetes, Firmicutes, and Proteobacteria (Table 1) [34, 35]. Various factors, including environmental exposers, host genetics, immune function, and lifestyle, influence the composition of the lung microbiota, highlighting its potential role in maintaining lung health and immune function [127,128,129]. Notably, alterations in the lung microbiota have been associated with respiratory diseases such as asthma, chronic obstructive pulmonary disease, pneumonia, cystic fibrosis, and lung cancer (Fig. 2) [127,128,129,130,131]. Interestingly, a gut–lung axis has been described, indicating crosstalk between the microbiomes of the gut and lungs (Fig. 2) [132, 133].
Currently, there is a limited body of research specifically focusing on the role of the lung microbiota in neuropsychiatric disorders. The lung–brain axis remains underexplored, although three potential mechanisms have been proposed [133]. First, microorganisms or their by-products might directly translocate across the capillary barrier into the bloodstream, eventually reaching the brain. Second, the lung microbiome could influence systemic humoral factors, given its role in local pulmonary immune homeostasis. Third, the lung microbiome may affect systemic cell-mediated immunity, which could subsequently impact brain function [133]. A recent prospective randomized study demonstrated that traumatic brain injury patients who developed ventilator-associated pneumonia during their ICU stay exhibited distinct structures of bronchoalveolar lavage microbiota both at admission and at seven days post-ICU admission [134]. This finding suggests the potential utility of lung microbiota management as a strategy for preventing infections in critically ill patients [134].
Additionally, a recent preclinical study demonstrated that antibiotic-induced disruption of the lung microbiome significantly increased the susceptibility of rats to developing autoimmune diseases in the CNS, suggesting the potential role of the lung microbiome on brain immune responses via the lung–brain axis [135]. Furthermore, antibiotic-induced microbiome depletion could reduce acute lung injury after lipopolysaccharide administration [136]. Another recent study demonstrated that small intestinal γδ T-cell migration into the lung and brain plays a role in stroke-associated pneumonia in mice [56]. Given the emerging recognition of the lung–brain axis [135, 137, 138], it becomes increasingly compelling to explore whether the lung microbiota plays a role in the development and progression of neuropsychiatric disorders.
Skin microbiota
Human skin is home to millions of bacteria, fungi and viruses that compose the skin microbiota. As the largest organ of the human body, the skin microbiota plays essential roles in the protection against the invasion of pathogens (Fig. 1) [139, 140]. The composition of the skin microbiota varies across different body regions (e.g., glabella, sebaceous, antecubital fossa, volar forearm, toe web space) and among individuals, influenced by the factors such as age, genetics, hygiene practices, and environmental exposures [139,140,141,142]. Major phyla of the skin microbiota include Actionobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (Table 1) [34, 35]. Given the important role of the skin microbiota in maintaining skin health and function, it is likely that the skin microbiota contributes to the regulation of the skin’s immune response, influencing inflammation and defense mechanisms. Disruptions in the skin microbiota can lead to various skin conditions and diseases. For example, an overgrowth of certain bacteria, such as Staphylococcus aures, has been associated with skin disorders such as acne, atopic dermatitis, and wound infections (Fig. 2) [139, 140].
The skin microbiota primarily influences the skin health; however, emerging research suggests a potential role of the skin microbiota in neuropsychiatric disorders due to its potential to influence the gut microbiota (Fig. 2) [34, 143, 144]. Therefore, it is possible that the skin microbiota may indirectly impact the gut–brain axis, leading to the development of neuropsychiatric disorders. Currently, there is limited research reporting alterations in the skin microbiota in patients with neuropsychiatric disorders. To the best of our knowledge, there is only one report that has reported alterations in the skin microbiota in patients with anorexia nervosa. Hermes et al. [145] identified significant correlations between Shannon diversity, the highly abundant skin antimicrobial peptide psoriasin, and bacterial load. Additionally, psoriasin was associated with Abiotrophia, an indicator for the healthy-weight control group. A significant correlation was observed between an individual’s body mass index and Lactobacillus, which serves as a microbial indicator of health. Further investigations examining the relationship between caloric and nutritional intake and the skin microbiota in patients with eating disorders are required to clarify the association between dietary factors and skin physiology [145]. In a recent study, Arikan et al. [146] demonstrated an association between axillary microbiota and cognitive impairment in PD patients (n = 103), suggesting a possible role of skin microbiota in cognitive impairments. Nonetheless, further studies with larger sample sizes are necessary to validate these findings.
Depression is a common psychiatric symptom in patients with skin diseases, such as psoriasis [147, 148]. Various studies have proposed the existence of a skin–microbiota–brain axis in the comorbidity of depression in patients with chronic wound [149,150,151]. Furthermore, alterations in both the skin and gut microbiota are believed to contribute to the pathogenesis of psoriasis through inflammatory and immune mechanisms [152]. A preclinical study using imiquimod-treated mice (a model of psoriasis) demonstrated correlations between the skin microbiota and the gut microbiota, suggesting bidirectional communication between the two [153]. Given the interaction between the skin microbiota and the gut microbiota in the immune system, it is intriguing to investigate whether skin microbiota is altered in patients of neuropsychiatric disorders.
Bladder microbiota
Bladder (or urinary) microbiota has been identified in the human urinary tract. The abundance and diversity of the urinary microbiota are distinct from the microbiota of other body sites such as the gut or the skin. Major phylum of the bladder microbiota is Firmicutes (Table 1) [34, 35]. Traditionally, the urinary environment was considered sterile; however, recent research has demonstrated the presence of a diverse microbial population in both healthy and diseases [154, 155]. In 2011, Siddiqui et al. [156] reported that the urinary microbiota in healthy women was predominantly composed to Lactobacillus species, similar to the vaginal microbiota, while women with urinary incontinence had a more diverse and less stable microbial community. Furthermore, Lewis et al. [157] identified a distinct microbial signature in men with symptomatic urinary tract infection. Additionally, Thomas-White et al. [158] reported that women with recurrent urinary tract infections had a higher prevalence of certain bacteria, such as Escherichia coli, compared to healthy controls, and that the bladder microbiota of women with recurrent urinary tract infections was less diverse and less stable over time. A recent cross-sectional study demonstrated that alterations in the urinary microbiota are correlated with the severity of overactive bladder symptom in patients with overactive bladder, suggesting that urinary dysbiosis may play a role in the deteriorations of functional bladder diseases [159]. Collectively, it is worth noting that the urinary microbiota may play a role in various urinary tract conditions, including urinary tract infections, intestinal cystitis, urinary incontinence, and kidney stones (Fig. 2) [160, 161].
To the best of our knowledge, there are currently no articles reporting alterations in bladder microbiota in patients with neuropsychiatric disorders. However, a study by Wu et al. [162] demonstrated negative correlations between the severity of depression and both richness (Chao1) and diversity (Shannon index) of urinary microbiota in patients with overactive bladder. Furthermore, Ren et al. [163] found that compared with healthy group, patients with BD exhibited significantly higher levels of betaine, glycerol, hippuric acid, indole sulfate, trimethylamine oxide, and urea in their urine samples, while the level of inositol was significantly lower. Given the role of microbiota in the production of these compounds [164,165,166], it is possible that alternations in bladder microbiota may contribute to the observed changes in the urine sample composition [167,168,169]. It is important to note that further research is needed to investigate the potential link between the bladder microbiota, neuropsychiatric disorders, and urine sample composition. Understanding these relationships could provide valuable insights into the role of the bladder microbiota in the context of neuropsychiatric disorders and urinary metabolites.
Notably, there is evidence suggesting a link between overactive bladder and psychiatric disorders such as depression and anxiety [170, 171]. A national cohort study conducted in Taiwan demonstrated significantly higher risk of depression and anxiety in patients with overactive bladder compared to those without overactive bladder [172]. Additionally, a study involving older Korean women (n = 3000) revealed a higher prevalence of depression, stress, and low self-esteem in women with urinary incontinence [173]. Moreover, a recent prospective UK cohort study found associations between mental health problems, stressful life evens, and new-onset urinary incontinence in primary school-age children [174]. These findings collectively suggest that disturbances in bladder function may contribute to mental health problems across different age groups, from children to elderly individuals. Given the emerging understanding of the role of bladder microbiota in bladder function [154, 155, 175], it is of great interest to investigate whether the bladder microbiota plays a role in both bladder function and psychiatric symptoms in patients with neuropsychiatric disorders. Further research in this area may provide valuable insights into the complex relationship between bladder function, neuropsychiatric disorders, and bladder microbiota.
Vagina microbiota
The vaginal microbiota is a dynamic ecosystem that plays a role in women’s health [176,177,178]. The dominant bacteria in the vaginal microbiota are species of Lactobacillus (Table 1) [34, 35]. Lactobacillus species play several important roles in vaginal health, including the production of lactic acid, which helps maintain an acidic pH in the vagina (typically around 3.5–4.5), creating an inhospitable environment for pathogens [179, 180]. In women with a dominant microbiota Lactobacillus spp., the concentration of lactic acid is approximately 110 mM, acidifying the vagina to a pH of ~3.5 [178,179,180]. Two enantiomers of lactic acid exist. L-lactic acid is a common compound of human metabolism; however, both D- and L-lactic acid can be produced by certain strains of the microbiota or through unknown metabolic pathways (Fig. 5). D-lactate dehydrogenase is an enzyme that converts D-lactic acid to pyruvate [178, 181]. Currently, it remains unclear how Lactobacillus species in the vagina produce both enantiomers of lactic acid. Further studies on the quantification of two enantiomers of lactic acid in the vagina are needed.
The vaginal microbiota, particularly, the Lactobacillus species, plays several significant roles in maintaining vaginal health. There is an association between the composition of the vaginal microbiota and reproductive health, including the risk for spontaneous preterm birth. Maternal immune activation (MIA), which can result from a range of factors such as infections, immune challenges, stress, environmental exposures, has been linked with an increased risk of neuropsychiatric disorders in offspring. Dysbiosis of the vaginal microbiota due to MIA, along with subsequent disrupted immune responses, may foster the development of neuropsychiatric disorders in offspring. This imbalance in the vaginal microbiota might impact the synthesis of D- and L-lactic acid and other metabolites, leading to changes in pH and the immune system in the vagina. Furthermore, an imbalance in the vaginal microbiota may influence mental health issues in women throughout their lives. Part of the figure was designed using resources from Biorender.com.
The composition of the vaginal microbiota can be influenced by various factors, including different stages of a woman’s life (e.g., puberty, menstruation, pregnancy, menopause), hormonal status, sexual activity, hygiene practices, and underlying health conditions [176, 178]. There is a reported link between the profile of the vaginal microbiota and the incidence and prevalence of human papillomavirus [182]. It’s well established that women can encounter a variety of mental health issues, including depression, anxiety, postpartum depression, eating disorders, premenstrual dysphoric disorder, and perimenopausal depression. Although the direct relationship between the vaginal microbiota and neuropsychiatric disorders is not yet well understood, there are several potential ways in which the vaginal microbiota could influence these disorders. The vaginal microbiota plays a crucial role in maintaining a healthy immune system in the female reproductive tract. An imbalance in the vaginal microbiota can lead to inflammation and immune dysfunction, as well as changes in hormones and microbiota-derived metabolites, contributing to the development and progression of neuropsychiatric disorders (Fig. 5) [176, 178].
The successful application of fecal microbiota transplantation (FMT) has opened new avenues for the development of vaginal microbiota transplantation (VMT) [183,184,185,186,187]. VMT is an emerging clinical procedure designed to reestablish a balanced vaginal microbiota by transferring it from a healthy donor to a patient with vaginal microbiota dysbiosis [188, 189]. In 2019, Lev-Sagie et al. [190] reported the therapeutic effectiveness of VMT in women suffering from persistent and recurrent bacterial vaginosis, following pretreatment with antibiotics to eliminate pathogens. A recent case study demonstrated a successful VMT procedure, where donor strain engraftment was verified. This was followed by a successful pregnancy and childbirth after a series of previous late pregnancy losses or stillbirths [191]. If an imbalanced vaginal microbiota is found to be associated with mental health issues in women, VMT could emerge as a potential therapeutic option.
Emerging evidence suggests that maternal immune activation can increase the risk of neuropsychiatric disorders in offspring, including ASD, schizophrenia, and other neurodevelopmental and neuropsychiatric disorders [192, 193]. Various factors, such as infections, immune challenges, stress, and environmental exposures during pregnancy, can trigger an immune response in the mother (Fig. 5). There are significant concerns regarding the potential impact of maternal infection of COVID-19 on the development of neuropsychiatric disorders in offspring [194,195,196,197]. It remains unclear whether maternal immune activation can affect the vaginal microbiota in pregnant women. However, there is plausible that maternal immune activation could impact the vaginal microbiota, potentially leading to the development of neuropsychiatric disorders in offspring (Fig. 5). A recent meta-analysis using rodent studies showed that perinatal maternal microbiota disturbance is transmitted to the offspring, negatively impacting behavioral parameters related to neuropsychiatric disorders [198]. Understanding the relationship between maternal immune activation and the vaginal microbiota in the context of neuropsychiatric disorders in offspring is crucial for early detection, prevention, and intervention strategies. Further research is needed to uncover the underlying biological mechanisms, identify potential biomarkers (e.g., vaginal bacteria, and metabolites), and develop effective interventions (e.g., VMT) [189] to mitigate the impact of maternal immune activation on neurodevelopment and reduce the risk of neuropsychiatric disorders in the offspring of mothers who experience immune activation during pregnancy.
Conclusion remarks and future directions
As outlined earlier, multiple lines of evidence indicate that dysbiosis in the gut microbiota could contribute to the onset and progression of various psychiatric and neurodegenerative disorders. However, research on the host microbiota in other organs, such as mouth, nose, lung, skin, bladder, and vagina, remains limited. Investigating the microbiota in these other organs is essential, as they can interact with the gut microbiota in the GI tract through inflammatory and immune system pathways (Fig. 2) [31, 34, 41, 42]. Future comprehensive studies aim to uncover the biological mechanisms by which alterations in host-microbiota contribute to the development and progression of neuropsychiatric disorders. Another key goal is to identify reliable biomarkers linked to host microbiota that could facilitate early detection, diagnosis, and monitoring of these conditions.
Moreover, there is significant potential for microbiota-targeted interventions (e.g., plant-based diet, probiotics, prebiotics, symbiotics, microbiome-derived metabolites, and microbiota transplantation) in the treatment and prevention of neuropsychiatric disorders. A recent double-blind, placebo-controlled study demonstrated that adjunctive treatment of multiple probiotics significantly reduced depression scores in MDD patients, without causing serious adverse effects [199]. While the use of FMT in treating neuropsychiatric disorders is on the rise [185,186,187], microbiota transplantation in other organs, such as VMT, could also offer therapeutic possibilities. Longitudinal studies that monitor changes in host microbiota and their correlation with neuropsychiatric disorders will be essential. Ultimately, translating microbiota-based interventions into clinical practice will be a critical advancement in the field [200].
The human microbiota, consisting of trillions of microorganisms residing both within and on our bodies, interacts intricately with approximately 20,000–25,000 genes in each individual. This complex interplay between the host microbiota and human genes plays a crucial role in our health and disease susceptibility. In conclusion, the future research focusing on the role of the host microbiota in neuropsychiatric disorders offers significant promise for elucidating the complex relationships between the microbiota in various tissues and the brain. This research has the potential to open new avenues for diagnostic methodologies and innovative therapeutic strategies for these disorders.
References
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psych. 2022;9:137–50. https://doi.org/10.1016/S2215-0366(21)00395-3.
COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–12. https://doi.org/10.1016/S0140-6736(21)02143-7.
Yuan K, Zheng YB, Wang YJ, Sun YK, Gong YM, Huang YT, et al. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: a call to action. Mol Psych. 2022;27:3214–22. https://doi.org/10.1038/s41380-022-01638-z.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendation. Nat Rev Microbiol. 2023;21:133–46. https://doi.org/10.1038/s41579-022-00846-2.
Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psych. 2023;28:564–78. https://doi.org/10.1038/s41380-022-01836-9.
Zeng N, Zhao YM, Yan W, Li C, Lu QD, Liu L, et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psych. 2023;28:423–33. https://doi.org/10.1038/s41380-022-01614-7.
Zhao Y, Shi L, Jiang Z, Zeng N, Mei H, Lu Y, et al. The phenotype and prediction of long-term physical, mental and cognitive COVID-19 sequelae 20 months after recovery, a community-based cohort study in China. Mol Psych. 2023;28:1793–801. https://doi.org/10.1038/s41380-023-01951-1.
Hashimoto K. Overview of the potential use of fluvoxamine for COVID-19 and long COVID. Discov Ment Health. 2023;3:9 https://doi.org/10.1007/s44192-023-00036-3.
Hashimoto K Detrimental effects of COVID-19 in the brain and therapeutic options for long COVID: The role of Epstein-Barr virus and the gut-brain axis. Mol Psych.(2023) 4. https://doi.org/10.1038/s41380-023-02161-5.
Chen LP, Murad MH, Paras ML, Colbenson KM, Sattler AL, Goranson EN, et al. Sexual abuse and lifetime diagnosis of psychiatric disorders: systematic review and meta-analysis. Mayo Clin Proc. 2010;85:618–29. https://doi.org/10.4065/mcp.2009.0583.
Kendler KS, Zachar P, Craver C. What kinds of things are psychiatric disorders? Psychol Med. 2011;41:1143–50. https://doi.org/10.1017/S0033291710001844.
Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–51. https://doi.org/10.1038/nrg3240.
Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014;8:9 https://doi.org/10.3389/fnins.2014.00019.
Klengel T, Binder EB. Epigenetics of stress-related psychiatric disorders and gene X environment interactions. Neuron. 2015;86:1434–1357. https://doi.org/10.1016/j.neuron.2015.05.036.
Smigielski L, Jagannath V, Rössler W, Walitza S, Grünblatt E. Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. Mol Psych. 2020;25:1718–48. https://doi.org/10.1038/s41380-019-0601-3.
Nisar S, Bhat AA, Masoodi T, Hashem S, Akhtar S, Ali TA, et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol Psychiatry. 2022;27:2380–92. https://doi.org/10.1038/s41380-022-01506-w.
Teicher MH, Gordon JB, Nemeroff CB. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol Psych. 2022;27:1331–8. https://doi.org/10.1038/s41380-021-01367-9.
Taylor J, de Vries YA, van Loo HM, Kendler KS. Clinical characteristics indexing genetic differences in schizophrenia: a systematic review. Mol Psych. 2023;28:883–90. https://doi.org/10.1038/s41380-022-01850-x.
McEwen BS. Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Front Neuroendocrinol. 2018;49:8–30. https://doi.org/10.1016/j.yfrne.2017.11.001.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. https://doi.org/10.1152/physrev.00018.2018.
McEwen BS. Hormones and behavior and the integration of brain-body science. Horm Behav. 2020;119:104619 https://doi.org/10.1016/j.yhbeh.2019.104619.
Sui SX, Pasco JA. Obesity and brain function: the brain–body crosstalk. Med (Kaunas). 2020;56:499 https://doi.org/10.3390/medicina56100499.
Berthoud HR, Albaugh VL, Neuhuber WL. Gut-brain communication and obesity: understanding functions of the vagus nerve. J Clin Invest. 2021;131:e143770 https://doi.org/10.1172/JCI143770.
Gonçalves RA, De Felice FG. The crosstalk between brain and periphery: Implications for brain health and disease. Neuropharmacology. 2021;197:108728 https://doi.org/10.1016/j.neuropharm.2021.108728.
Matsubara Y, Kiyohara H, Teratani T, Mikami Y, Kanai T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology. 2022;205:108915 https://doi.org/10.1016/j.neuropharm.2021.108915.
Medawar E, Witte AV. Impact of obesity and diet on brain structure and function: a gut-brain-body crosstalk. Proc Nutr Soc. 2022;81:306–16. https://doi.org/10.1017/S0029665122002786.
Hashimoto K, Yang C. Special issue on “Brain-body communication in health and diseases”. Brain Res Bull. 2022;186:47–49. https://doi.org/10.1016/j.brainresbull.2022.05.014.
Chang L, Wei Y, Hashimoto K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res Bull. 2022;182:44–56. https://doi.org/10.1016/j.brainresbull.2022.02.004.
Wei Y, Wang T, Liao L, Fan X, Chang L, Hashimoto K. Brain-spleen axis in health and diseases: A review and future perspective. Brain Res Bull. 2022;182:130–40. https://doi.org/10.1016/j.brainresbull.2022.02.008.
Zhang X, Liu H, Hashimoto K, Yuan S, Zhang J. The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential. Crit Care. 2022;26:213 https://doi.org/10.1186/s13054-022-04090-1.
Santoro A, Zhao J, Wu L, Carru C, Biagi E, Franceschi C. Microbiomes other than the gut: inflammaging and age-related diseases. Semin Immunopathol. 2020;42:589–605. https://doi.org/10.1007/s00281-020-00814-z.
Ruff WE, Greiling TM, Kriegel MA. Host-microbiota interactions in immune-mediated diseases. Nat Rev Microbiol. 2020;18:521–38. https://doi.org/10.1038/s41579-020-0367-2.
Daniel N, Lécuyer E, Chassaing B. Host/microbiota interactions in health and diseases–Time for mucosal microbiology! Mucosal Immunol. 2021;14:1006–16. https://doi.org/10.1038/s41385-021-00383-w.
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and disease. Signal Transduct Target Ther. 2022;7:135 https://doi.org/10.1038/s41392-022-00974-4.
Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, Chang MW. Microbiome and human health: current understanding, engineering, and enabling technologies. Chem Rev. 2023;123:31–72. https://doi.org/10.1021/acs.chemrev.2c00431.
Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psych Clin Neurosci. 2019;73:613–27. https://doi.org/10.1111/pcn.12902.
Hashimoto K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem Pharm. 2020;177:113935 https://doi.org/10.1016/j.bcp.2020.113935.
Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psych. 2022;27:559–73. https://doi.org/10.1038/s41380-021-01121-1.
Hashimoto K. Neuroinflammation through the vagus nerve-dependent gut-microbiota-brain axis in treatment-resistant depression. Prog Brain Res. 2023;278:61–77. https://doi.org/10.1016/bs.pbr.2023.01.003.
Hashimoto K. Arketamine for cognitive impairment in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2023;273:1513–25. https://doi.org/10.1007/s00406-023-01570-5.
Martínez JE, Vargas A, Pérez-Sánchez T, Encío IJ, Cabello-Olmo M, Barajas M. Human microbiota network: unveiling potential crosstalk between the different microbiota ecosystems and their role in health and disease. Nutrients. 2021;13:2905 https://doi.org/10.3390/nu13092905.
Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 2022;28:1875–901. https://doi.org/10.3748/wjg.v28.i18.1875.
Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F, et al. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7:143 https://doi.org/10.1038/s41392-022-00986-0.
Lin CH, Yang HT, Chiu CC, Lane HY. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci Rep. 2017;7:14849 https://doi.org/10.1038/s41598-017-13951-7.
Lin CH, Yang HT, Lane HY. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharm Biochem Behav. 2019;185:172760 https://doi.org/10.1016/j.pbb.2019.172760.
Chang CH, Lin CH, Lane HY. D-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci. 2020;21:2676 https://doi.org/10.3390/ijms21082676.
Hashimoto K. Gut–microbiota–brain axis by bile acids in depression. Psychiatry Clin Neurosci. 2022;76:281 https://doi.org/10.1111/pcn.13370.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5. https://doi.org/10.1073/pnas.
Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psych. 2020;10:186 https://doi.org/10.1038/s41398-020-00878-3.
Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflam. 2020;17:241 https://doi.org/10.1186/s12974-020-01916-z.
Wang S, Ishima T, Qu Y, Shan J, Chang L, Wei Y, et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J Affect Disord. 2021;292:565–73. https://doi.org/10.1016/j.jad.2021.06.006.
Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. 2021;94:318–26. https://doi.org/10.1016/j.bbi.2020.12.032.
Wu Y, Zhang Y, Xie B, Abdelgawad A, Chen X, Han M, et al. RhANP attenuates endotoxin-derived cognitive dysfunction through subdiaphragmatic vagus nerve-mediated gut microbiota-brain axis. J Neuroinflammation. 2021;18:300 https://doi.org/10.1186/s12974-021-02356-z.
Yang Y, Eguchi A, Wan X, Chang L, Wang X, Qu Y, et al. A role of gut-microbiota-brain axis via subdiaphragmatic vagus nerve in depression-like phenotypes in Chrna7 knock-out mice. Prog Neuropsychopharmacol Biol Psych. 2023;120:110652 https://doi.org/10.1016/j.pnpbp.2022.110652.
Wang X, Eguchi A, Yang Y, Chang L, Wan X, Shan J, et al. Key role of the gut-microbiota-brain axis via the subdiaphragmatic vagus nerve in demyelination of the cuprizone-treated mouse brain. Neurobiol Dis. 2023;176:105951 https://doi.org/10.1016/j.nbd.2022.105951.
Xie B, Zhang Y, Han M, Wang M, Yu Y, Chen X, et al. Reversal of the detrimental effects of social isolation on ischemic cerebral injury and stroke-associated pneumonia by inhibiting small intestinal γδ T-cell migration into the brain and lung. J Cereb Blood Flow Metab. 2023;43:1267–84. https://doi.org/10.1177/0271678X231167946.
Siopi E, Galerne M, Rivagorda M, Saha S, Moigneu C, Moriceau S, et al. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol Psych.(2023) 2. https://doi.org/10.1038/s41380-023-02071-6.
Wang X, Yang J, Hashimoto K. (R)-ketamine as prophylactic and therapeutic drug for neurological disorders: beyond depression. Neurosci Biobehav Rev. 2022;139:104762 https://doi.org/10.1016/j.neubiorev.2022.104762.
Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psych. 2020;25:2905–18. https://doi.org/10.1038/s41380-019-0475-4.
Dong S, Sun M, He C, Cheng H. Brain-gut-microbiota axis in Parkinson’s disease: A historical review and future perspective. Brain Res Bull. 2022;183:84–93. https://doi.org/10.1016/j.brainresbull.2022.02.015.
Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. 2022;23:11245 https://doi.org/10.3390/ijms231911245.
Li Z, Lai J, Zhang P, Ding J, Jiang J, Liu C, et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol Psych. 2022;27:4123–35. https://doi.org/10.1038/s41380-022-01569-9.
Shoubridge AP, Choo JM, Martin AM, Keating DJ, Wong ML, Licinio J, et al. The gut microbiome and mental health: advances in research and emerging priorities. Mol Psych. 2022;27:1908–19. https://doi.org/10.1038/s41380-022-01479-w.
McGuinness AJ, Davis JA, Dawson SL, Loughman A, Collier F, O’Hely M, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psych. 2022;27:1920–35. https://doi.org/10.1038/s41380-022-01456-3.
Huang Y, Wu J, Zhang H, Li Y, Wen L, Tan X, et al. The gut microbiome modulates the transformation of microglial subtypes. Mol Psych. 2023;28:1611–21. https://doi.org/10.1038/s41380-023-02017-y.
Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527 https://doi.org/10.1016/j.ebiom.2023.104527.
Ortega MA, Álvarez-Mon MA, García-Montero C, Fraile-Martínez Ó, Monserrat J, Martinez-Rozas L, et al. Microbiota-gut-brain axis mechanisms in the complex network of bipolar disorders: potential clinical implications and translational opportunities. Mol Psych. (2023) 27. https://doi.org/10.1038/s41380-023-01964-w.
Jeong S, Chokkalla AK, Davis CK, Vemuganti R Post-stroke depression: epigenetic and epitranscriptomic modifications and their interplay with gut microbiota. Mol Psych. (2023) May 15. https://doi.org/10.1038/s41380-023-02099-8.
Zou B, Li J, Ma RX, Cheng XY, Ma RY, Zhou TY, et al. Gut microbiota is an impact factor based on the brain-gut axis to Alzheimer’s disease: a systematic review. Aging Dis. 2023;14:964–1678. https://doi.org/10.14336/AD.2022.1127.
Ferreiro AL, Choi J, Ryou J, Newcomer EP, Thompson R, Bollinger RM, et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci Transl Med. 2023;15:eabo2984 https://doi.org/10.1126/scitranslmed.abo2984.
Kleine Bardenhorst S, Cereda E, Severgnini M, Barichella M, Pezzoli G, Keshavarzian A, et al. Gut microbiota dysbiosis in Parkinson disease: A systematic review and pooled analysis. Eur J Neurol. (2023) 2. https://doi.org/10.1111/ene.15671.
Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psych. 2021;78:1343–54. https://doi.org/10.1001/jamapsychiatry.2021.2573.
Cammann D, Lu Y, Cummings MJ, Zhang ML, Cue JM, Do J, et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci Rep. 2023;13:5258 https://doi.org/10.1038/s41598-023-31730-5.
Chen G, Zhou X, Zhu Y, Shi W, Kong L Gut microbiome characteristics in subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Eur J Neurol (2023) 3. https://doi.org/10.1111/ene.15961.
Tan AH, Lim SY, Lang AE. The microbiome-gut-brain axis in Parkinson disease—from basic research to the clinic. Nat Rev Neurol. 2022;18:476–95. https://doi.org/10.1038/s41582-022-00681-2.
Pant A, Bisht KS, Aggarwal S, Maiti TK. Human gut microbiota and Parkinson’s disease. Prog Mol Biol Transl Sci. 2022;192:281–307. https://doi.org/10.1016/bs.pmbts.2022.08.004.
Kumari S, Taliyan R, Dubey SK. Comprehensive review on potential signaling pathways involving the transfer of α-synuclein from the gut to the brain that leads to Parkinson’s disease. ACS Chem Neurosci. 2023;14:590–602. https://doi.org/10.1021/acschemneuro.2c00730.
Claudino Dos Santos JC, Oliveira LF, Noleto FM, Gusmão CTP, Brito GAC, Viana GSB. Gut-microbiome-brain axis: the crosstalk between the vagus nerve, alpha-synuclein and the brain in Parkinson’s disease. Neural Regen Res. 2023;18:2611–4. https://doi.org/10.4103/1673-5374.373673.
Deo PN, Deshmukh R. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23:122–8. https://doi.org/10.4103/jomfp.JOMFP_304_18.
Caselli E, Fabbri C, D’Accolti M, Soffritti I, Bassi C, Mazzacane S, et al. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 2020;20:120 https://doi.org/10.1186/s12866-020-01801-y.
Peng X, Cheng L, You Y, Tang C, Ren B, Li Y, et al. Oral microbiota in human systematic diseases. Int J Oral Sci. 2022;14:14 https://doi.org/10.1038/s41368-022-00163-7.
Huang X, Huang X, Huang Y, Zheng J, Lu Y, Mai Z, et al. The oral microbiome in autoimmune disease: friend or foe? J Transl Med. 2023;21:211 https://doi.org/10.1186/s12967-023-03995-x.
Kozak M, Pawlik A. The role of the oral microbiome in the development of diseases. Int J Mol Sci. 2023;24:531 https://doi.org/10.3390/ijms24065231.
Martínez M, Postolache TT, García-Bueno B, Leza JC, Figuero E, Lowry CA, et al. The role of the oral microbiota related to periodontal diseases in anxiety, mood and trauma- and stress-related disorders. Front Psych. 2022;12:814177 https://doi.org/10.3389/fpsyt.2021.814177.
Jungbauer G, Stähli A, Zhu X, Auber Alberi L, Sculean A, Eick S. Periodontal microorganisms and Alzheimer’s disease–A causative relationship? Periodontol 2000. 2022;89:59–82. https://doi.org/10.1111/prd.12429.
Bowland GB, Weyrich LS. The oral-microbiome-brain axis and neuropsychiatric disorders: an anthropological perspective. Front Psych. 2022;13:810008 https://doi.org/10.3389/fpsyt.2022.810008.
Malan-Müller S, Postolache TT. Editorial: The oral microbiota in mental health. Front Psych. 2022;13:1048179 https://doi.org/10.3389/fpsyt.2022.1048179.
Skallevold HE, Rokaya N, Wongsirichat N, Rokaya D. Importance of oral health in mental health disorders: An updated review. J Oral Biol Craniofac Res. 2023;13:544–52. https://doi.org/10.1016/j.jobcr.2023.06.003.
Richardson BN, Noh HI, Webster CI, Zhang W, Kim S, Yang I, et al. Oral microbiome, mental health, and sleep outcomes during the COVID-19 pandemic: An observational study in Chinese and Korean American immigrants. OMICS. 2023;27:180–90. https://doi.org/10.1089/omi.2022.0182.
Kisely S, Sawyer E, Siskind D, Lalloo R. The oral health of people with anxiety and depressive disorders-a systematic review and meta-analysis. J Affect Disord. 2016;200:119–32. https://doi.org/10.1016/j.jad.2016.04.040.
Coelho JMF, Miranda SS, da Cruz SS, Dos Santos DN, Trindade SC, Cerqueira EMM, et al. Common mental disorder is associated with periodontitis. J Periodontal Res. 2020;55:221–8. https://doi.org/10.1111/jre.12705.
Cunha FA, Cota LOM, Cortelli SC, Miranda TB, Neves FS, Cortelli JR, et al. Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: A case-control study. J Periodontal Res. 2019;54:63–72. https://doi.org/10.1111/jre.12605.
Qing Y, Xu L, Cui G, Sun L, Hu X, Yang X, et al. Salivary microbiome profiling reveals a dysbiotic schizophrenia-associated microbiota. NPJ Schizophr. 2021;7:51 https://doi.org/10.1038/s41537-021-00180-1.
Ide M, Ohnishi T, Toyoshima M, Balan S, Maekawa M, Shimamoto-Mitsuyama C, et al. Excess hydrogen sulfide and polysulfides production underlie a schizophrenia pathophysiology. EMBO Mol Med. 2019;11:e10695 https://doi.org/10.15252/emmm.201910695.
Cui G, Qing Y, Li M, Sun L, Zhang J, Feng L, et al. Salivary metabolomics reveals that metabolic alterations precede the onset of schizophrenia. J Proteome Res. 2021;20:5010–23. https://doi.org/10.1021/acs.jproteome.1c00504.
Martin S, Foulon A, El Hage W, Dufour-Rainfray D, Denis F. Is there a link between oropharyngeal microbiome and schizophrenia? A narrative review. Int J Mol Sci. 2022;23:846 https://doi.org/10.3390/ijms23020846.
Li X, Zhao K, Chen J, Ni Z, Yu Z, Hu L, et al. Diurnal changes of the oral microbiome in patients with alcohol dependence. Front Cell Infect Microbiol. 2022;12:1068908 https://doi.org/10.3389/fcimb.2022.1068908.
Levert-Levitt E, Shapira G, Sragovich S, Shomron N, Lam JCK, Li VOK, et al. Oral microbiota signature in post-traumatic stress disorder (PTSD) veterans. Mol Psych. 2022;27:4590–8. https://doi.org/10.1038/s41380-022-01704-6.
Yang I, Arthur RA, Zhao L, Clark J, Hu Y, Corwin EJ, et al. The oral microbiome and inflammation in mild cognitive impairment. Exp Gerontol. 2021;147:111273 https://doi.org/10.1016/j.exger.2021.111273.
Hamza SA, Asif S, Bokhan SKH. Oral health of individuals with dementia and Alzheimer’s disease: A review. J Indian Soc Peiodontol. 2021;25:96–101. https://doi.org/10.4103/jisp.jisp_287_20.
Mao S, Huang CP, Lan H, Lau HG, Chaing CP, Chen YW. Association of periodontitis and oral microbiomes with Alzheimer’s disease: A narrative systematic review. J Dent Sci. 2022;17:1762–79. https://doi.org/10.1016/j.jds.2022.07.001.
Verhoeff MC, Eikenboom D, Koutris M, de Vries R, Berendse HW, van Dijk KD, et al. Parkinson’s disease and oral health: A systematic review. Arch Oral Biol. 2023;151:105712 https://doi.org/10.1016/j.archoralbio.2023.105712.
Yang B, Tao B, Yin Q, Chai Z, Xu L, Zhao Q, et al. Associations between oral health status, perceived stress, and neuropsychiatric symptoms among community individuals with Alzheimer’s disease: a mediation analysis. Front Aging Neurosci. 2022;13:801209 https://doi.org/10.3389/fnagi.2021.801209.
Emery DC, Shoemark DK, Batstone TE, Waterfall CM, Coghill JA, Cerajewska TL, et al. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front Aging Neurosci. 2017;9:195 https://doi.org/10.3389/fnagi.2017.00195.
Siddiqui H, Rribe ERK, Singhrao SK, Olsen I. High throughput sequencing detects gingivitis and periodontal oral bacteria in Alzheimer’s disease autopsy brains. Neuro Res. 2019;1:3 https://doi.org/10.35702/nrj.10003.
Weber C, Dilthey A, Finzer P. The role of microbiome–host interactions in the development of Alzheimer’s disease. Front Cell Infect Microbiol. 2023;13:1151021 https://doi.org/10.3389/fcimb.2023.1151021.
Emery DC, Davies M, Cerajewska TL, Taylor J, Hazell M, Paterson A, et al. High resolution 16S rRNA gene Next Generation Sequencing study of brain areas associated with Alzheimer’s and Parkinson’s disease. Front Aging Neurosci. 2022;14:1026260 https://doi.org/10.3389/fnagi.2022.1026260.
Liu S, Dashper SG, Zhao R. Associations between oral bacteria and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2023;91:129–50. https://doi.org/10.3233/JAD-220627.
Zhang Y, Shen Y, Liufu N, Liu L, Li W, Shi Z, et al. Transmission of Alzheimer’s disease-associated microbiota dysbiosis and its impact on cognitive function: evidence from mice and patients. Mol Psych. (2023) 21. https://doi.org/10.1038/s41380-023-02216-7.
Pisani F, Pisani V, Arcangeli F, Harding A, Singhrao SK. The mechanistic pathways of periodontal pathogens entering the brain: the potential role of Treponema denticola in tracing Alzheimer’s disease pathology. Int J Environ Res Public Health. 2022;19:9386 https://doi.org/10.3390/ijerph19159386.
Berthouzoz E, Lazarevic V, Zekeridou A, Castro M, Debove I, Aybek S, et al. Oral and intestinal dysbiosis in Parkinson’s disease. Rev Neurol (Paris) (2023) Mar 16:S0035-3787(23)00876-7. https://doi.org/10.1016/j.neurol.2022.12.010.
Mihaila D, Donegan J, Barns S, LaRocca D, Du Q, Zheng D, et al. The oral microbiome of early-stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS One. 2019;14:e0218252 https://doi.org/10.1371/journal.pone.0218252.
Jo S, Kang W, Hwang YS, Lee SH, Park KW, Kim MS, et al. Oral and gut dysbiosis leads to functional alterations in Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:87 https://doi.org/10.1038/s41531-022-00351-6.
Li Z, Lu G, Luo E, Wu B, Li Z, Guo J, et al. Oral, nasal, and gut microbiota in Parkinson’s disease. Neuroscience. 2022;480:65–78. https://doi.org/10.1016/j.neuroscience.2021.10.011.
Mosaddad SA, Mahootchi P, Safari S, Rahimi H, Aghili SS. Interactions between systemic diseases and oral microbiota shifts in the aging community: A narrative review. J Basic Microbiol. 2023;63:831–54. https://doi.org/10.1002/jobm.202300141.
Huang X, Huang X, Huang Y, Zhang J, Lu Y, Mai Z, et al. The oral microbiome in autoimmune diseases: friend or foe? J Transl Med. 2023;21:211 https://doi.org/10.1186/s12967-023-03995-x.
Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019;17:87 https://doi.org/10.1186/s12915-019-0703-z.
Tai J, Han MS, Kwak J, Kim TH. Association between microbiota and nasal mucosal diseases in terms of immunity. Int J Mol Sci. 2021;22:4744 https://doi.org/10.3390/ijms22094744.
Thangaleela S, Sivamaruthi BS, Kesika P, Bharathi M, Chaiyasut C. Nasal microbiota, olfactory health, neurological disorders and aging—a review. Microorganisms. 2022;10:1405 https://doi.org/10.3390/microorganisms10071405.
Athanassi A, Dorado Doncel R, Bath KG, Mandairon N. Relationship between depression and olfactory sensory function: a review. Chem Senses. 2021;46:bjab044 https://doi.org/10.1093/chemse/bjab044.
Hasegawa Y, Ma M, Sawa A, Lane AP, Kamiya A. Olfactory impairment in psychiatric disorders: Does nasal inflammation impact disease psychophysiology? Transl Psych. 2022;12:314 https://doi.org/10.1038/s41398-022-02081-y.
Kulason S, Ratnanather JT, Miller MI, Kamath V, Hua J, Yang K, et al. A comparative neuroimaging perspective of olfaction and higher-order olfactory processing: on health and disease. Semin Cell Dev Biol. 2022;129:22–30. https://doi.org/10.1016/j.semcdb.2021.08.009.
Cothren TC, Evonko CJ, MacQueen DA. Olfactory dysfunction in schizophrenia: evaluating olfactory abilities across species. Curr Top Behav Neurosci. 2023;63:363–92. https://doi.org/10.1007/7854_2022_390.
Etyemez S, Narita Z, Mihaljevic M, Coughlin JM, Nestadt G, Nucifora FCJ, et al. Brain regions associated with olfactory dysfunction in first episode psychosis patients. World J Biol Psychiatry. 2023;24:178–86. https://doi.org/10.1080/15622975.2022.2082526.
Marin C, Alobid I, Fuentes M, López-Chacón M, Mullol J. Olfactory dysfunction in mental illness. Curr Allergy Asthma Rep. 2023;23:153–64. https://doi.org/10.1007/s11882-023-01068-z.
Wang Q, Ren H, Li Z, Li J, Dai L, Dong M, et al. Differences in olfactory dysfunction and its relationship with cognitive function in schizophrenia patients with and without auditory verbal hallucinations. Eur Arch Psychiatry Clin Neurosci. (2023) 22. https://doi.org/10.1007/s00406-023-01589-8.
Whiteside SA, McGinniss JE, Collman RG. The lung microbiome: progress and promise. J Clin Invest. 2021;131:e150473 https://doi.org/10.1172/JCI150473.
Li C, Chen W, Lin F, Li W, Wang P, Liao G, et al. Functional two-way crosstalk between brain and lung: the brain–lung axis. Cell Mol Neurobiol. 2023;43:991–1003. https://doi.org/10.1007/s10571-022-01238-z.
Natalini JG, Singh S, Segal LN. The dynamic lung microbiome in health and disease. Nat Rev Microbiol. 2023;21:222–35. https://doi.org/10.1038/s41579-022-00821-x.
Di Simone SK, Rudloff I, Nold-Petry CA, Forster SC, Nold MF. Understanding respiratory microbiome-immune system interactions in health and disease. Sci Transl Med. 2023;15:eabq5126 https://doi.org/10.1126/scitranslmed.abq5126.
O’Shaughnessy M, Sheils O, Baird AM. The lung microbiome in COPD and lung cancer: Exploring the potential of metal-based drugs. Int J Mol Sci. 2023;24:12296 https://doi.org/10.3390/ijms241512296.
Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol. 2017;15:55–63. https://doi.org/10.1038/nrmicro.2016.142.
Bell JS, Spencer JI, Yates RL, Yee SA, Jacobs BM, DeLuca GC. Invited review: From nose to gut–the role of the microbiome in neurological disease. Neurophathol Appl Neurobiol. 2019;45:195–215. https://doi.org/10.1111/nan.12520.
Cotoia A, Paradiso R, Ferrara G, Borriello G, Santoro F, Spina I, et al. Modifications of lung microbiota structure in traumatic brain injury ventilated patients according to time and enteral feeding formulas: a prospective randomized study. Crit Care. 2023;27:244 https://doi.org/10.1186/s13054-023-04531-5.
Hosang L, Canals RC, van der Flier FJ, Hollensteiner J, Daniel R, Flügel A, et al. The lung microbiome regulates brain autoimmunity. Nature. 2022;603:138–44. https://doi.org/10.1038/s41586-022-04427-4.
Hashimoto Y, Eguchi A, Wei Y, Shinno-Hashimoto H, Fujita Y, Ishima T, et al. Antibiotic-induced microbiome depletion improves LPS-induced acute lung injury via gut–lung axis. Life Sci. 2022;307:120885 https://doi.org/10.1016/j.lfs.2022.120885.
Azzoni R, Marsland BJ. The lung-brain axis: A new frontier in host-microbe interactions. Immunity. 2022;55:589–91. https://doi.org/10.1016/j.immuni.2022.03.015.
Yang L, Feng S, Wu C, Yang L. The lung microbiome: A potential target in regulating autoimmune inflammation of the brain. Neurosci Bull. 2022;38:1435–7. https://doi.org/10.1007/s12264-022-00912-y.
Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16:143–55. https://doi.org/10.1038/nrmicro.2017.157.
Chen YE, Fischbach MA, Belkaid Y. Skin microbiota-host interactions. Nature. 2018;553:427–36. https://doi.org/10.1038/nature25177.
Flowers L, Grice EA. The skin microbiome: balancing risk and reward. Cell Host Microbe. 2020;28:190–200. https://doi.org/10.1016/j.chom.2020.06.017.
Harris-Tryon TA, Grice EA. Microbiota and maintenance of skin barrier function. Science. 2022;376:940–5. https://doi.org/10.1126/science.abo0693.
Goswami A, Wendt FR, Pathak GA, Tylee DS, De Angelis F, De Lillo A, et al. Role of microbes in the pathogenesis of neuropsychiatric disorders. Front Neuroendocrinol. 2021;21:100917 https://doi.org/10.1016/j.yfrne.2021.100917.
Ferraretto A, Donetti E, García-Mena J, Pacheco-López G. Editorial: The gut-skin-brain axis in human health and disease. Front Nutr. 2023;10:1155614 https://doi.org/10.3389/fnut.2023.1155614.
Hermes BM, Rademacher F, Chung C, Tiegs G, Bendix MC, de Zwaan M, et al. Skin microbiota analysis in patients with anorexia nervosa and healthy-weight controls reveals microbial indicators of healthy weight and associations with the antimicrobial peptide psoriasin. Sci Rep. 2022;12:15515 https://doi.org/10.1038/s41598-022-19676-6.
Arikan M, Yildiz Z, Kahraman Demir T, Yilmaz NH, Sen A, et al. Axillary microbiota is associated with cognitive impairment in Parkinson’s disease patients. Microbiol Spectr. 2022;10:e0235821 https://doi.org/10.1128/spectrum.02358-21.
Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–60. https://doi.org/10.1001/jama.2020.4006.
Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301–15. https://doi.org/10.1016/S0140-6736(20)32549-6.
Hadian Y, Fregoso D, Nguyen C, Bagood MD, Dahle SE, Gareau MG, et al. Microbiome-skin-brain axis: A novel paradigm for cutaneous wounds. Wound Repair Regen. 2020;28:282–92. https://doi.org/10.1111/wrr.12800.
Woo YR, Han YJ, Kim HS, Cho SH, Lee JD. Updates on the risk of neuropsychiatric and gastrointestinal comorbidities in rosacea and its possible relationship with the gut-brain-skin axis. Int J Mol Sci. 2020;21:8427 https://doi.org/10.3390/ijms21228427.
Chen G, Chen ZM, Fan XY, Jin YL, Li X, Wu SR, et al. Gut-brain-skin axis in psoriasis: a review. Dermatol Ther (Heidelb). 2021;11:25–38. https://doi.org/10.1007/s13555-020-00466-9.
Wang X, Li Y, Wu L, Xiao S, Ji Y, Tan Y, et al. Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed Pharmacother. 2021;137:111065 https://doi.org/10.1016/j.biopha.2020.111065.
Shinno-Hashimoto H, Hashimoto Y, Wei Y, Chang L, Fujita Y, Ishima T, et al. Abnormal composition of microbiota in the gut and skin of imiquimod-treated mice. Sci Rep. 2021;11:112665 https://doi.org/10.1038/s41598-021-90480-4.
Brubaker L, Wolfe A. The urinary microbiota: a paradigm shift for bladder disorders? Curr Opin Obstet Gynecol. 2016;28:407–12. https://doi.org/10.1097/GCO.0000000000000298.
Hrbacek J, Tlaskal V, Cermak P, Hanacek V, Zachoval R. Bladder microbiota are associated with clinical conditions that extend beyond the urinary tract. Microorganisms. 2022;10:874 https://doi.org/10.3390/microorganisms10050874.
Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011;11:244 https://doi.org/10.1186/1471-2180-11-244.
Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41 https://doi.org/10.3389/fcimb.2013.00041.
Thomas-White KJ, Kliethermes S, Rickey L, Lukacz ES, Richter HE, Moalli P, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol. 2017;216:55.e1–e16. https://doi.org/10.1016/j.ajog.2016.07.049.
Li K, Chen C, Zeng J, Wen Y, Chen W, Zhao J, et al. Interplay between bladder microbiota and overactive bladder symptom severity: a cross-sectional study. BMC Urol. 2022;22:39 https://doi.org/10.1186/s12894-022-00990-0.
Grobeisen-Duque O, Mora-Vargas CD, Aguilera-Arreola MG, Helguera-Repetto AC. Cycle biodynamics of women’s microbiome in the urinary and reproductive systems. J Clin Med. 2023;12:4003 https://doi.org/10.3390/jcm12124003.
Kim DS, Lee JW. Urinary tract infection and microbiome. Diagnostics (Basel). 2023;13:1921 https://doi.org/10.3390/diagnostics13111921.
Wu P, Chen Y, Zhao J, Zhang G, Chen J, Wang J, et al. Urinary microbiome and psychological factors in women with overactive bladder. Front Cell Infect Microbiol. 2017;7:488 https://doi.org/10.3389/fcimb.2017.00488.
Ren Y, Chen ZZ, Sun XL, Duan HJ, Tian JS, Wang JY, et al. Metabolomic analysis to detect urinary molecular changes associated with bipolar depression. Neurosci Lett. 2021;742:135515 https://doi.org/10.1016/j.neulet.2020.135515.
Vernocchi P, Del Chierico F, Putignani L. Gut microbiota profiling: metabolomics based approach to unravel compounds affecting human health. Front Microbiol. 2016;7:1144 https://doi.org/10.3389/fmicb.2016.01144.
Popkov VA, Zharikova AA, Demchenko EA, Andrianova NV, Zorov DB, Plotnikov EY. Gut microbiota as a source of uremic toxins. Int J Mol Sci. 2022;23:483 https://doi.org/10.3390/ijms23010483.
Haddad EN, Nel NH, Petrick LM, Kerver JM, Comstock SS. Associations between the gut microbiota, urinary metabolites, and diet in women during the third trimester of pregnancy. Curr Dev Nutr. 2022;7:100025 https://doi.org/10.1016/j.cdnut.2022.100025.
Yang Y, Ma C, Li S, Cai W, Dai W, Zhang X, et al. Urinary microbiota and serum metabolite analysis in patients with diabetic kidney disease. Heliyon. 2023;9:e17040 https://doi.org/10.1016/j.heliyon.2023.e17040.
Chao YT, Lin YK, Chen LK, Huang P, Hsu YC. Role of the gut microbiota and their metabolites in hemodialysis patients. Int J Med Sci. 2023;20:725–36. https://doi.org/10.7150/ijms.82667.
Chen X, Cheng Y, Tian X, Li J, Ying X, Zhao Q, et al. Urinary microbiota and metabolic signatures associated with inorganic arsenic-induced early bladder lesions. Ecotoxicol Environ Saf. 2023;259:115010 https://doi.org/10.1016/j.ecoenv.2023.115010.
Rogowski A, Krowicka-Wasyl M, Chotkowska E, Kluz T, Wróbel A, Berent D, et al. Psychiatry history and overactive bladder symptom severity in ambulatory urogynecological patients. J Clin Med. 2021;10:3988 https://doi.org/10.3390/jcm10173988.
Hughes FM Jr, Odom MR, Cervantes A, Livingston AJ, Purves JT. Why are some people with lower urinary tract symptoms (LUTS) depressed? New evidence that peripheral inflammation in the bladder causes central inflammation and mood disorders. Int J Mol Sci. 2023;24:2821 https://doi.org/10.3390/ijms24032821.
Tzeng NS, Chang HA, Chung CH, Kao YC, Yeh HW, Yeh CB, et al. Risk of psychiatric disorders in overactive bladder syndrome: a nationwide cohort study in Taiwan. J Investig Med. 2019;67:312–8. https://doi.org/10.1136/jim-2018-000835.
Lee HY, Rhee Y, Choi KS. Urinary incontinence and the association with depression, stress, and self-esteem in older Korean Women. Sci Rep. 2021;11:9054 https://doi.org/10.1038/s41598-021-88740-4.
Warne N, Heron J, von Gontard A, Joinson C Mental health problems, stressful life events and new-onset urinary incontinence in primary school-age children: a prospective cohort study. Eur Child Adolesc Psych. (2023) Apr 24. https://doi.org/10.1007/s00787-023-02211-x.
Yacouba A, Tidjani Alou M, Lagier JC, Dubourg G, Raoult D. Urinary microbiota and bladder cancer: A systematic review and a focus on uropathogens. Semin Cancer Biol. 2022;86:875–84. https://doi.org/10.1016/j.semcancer.2021.12.010.
Gupta S, Kakkar V, Bhushan I. Crosstalk between vaginal microbiome and female health: a review. Micro Pathog. 2019;136:103696 https://doi.org/10.1016/j.micpath.2019.103696.
Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Micro Cell Fact. 2020;19:203 https://doi.org/10.1186/s12934-020-01464-4.
France M, Alizadeh M, Brown S, Ma B, Ravel J. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol. 2022;7:367–78. https://doi.org/10.1038/s41564-022-01083-2.
Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164 https://doi.org/10.3389/fphys.2015.00164.
Amabebe E, Anumba DOC. The vaginal microenvironment: the physiological role of Lactobacilli. Front Med. 2018;5:181 https://doi.org/10.3389/fmed.2018.00181.
Pohanka M. D-lactic acid as a metabolite: toxicology, diagnosis, and detection. Biomed Res Int. 2020;2020:3419034 https://doi.org/10.1155/2020/3419034.
Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG. 2020;127:171–80. https://doi.org/10.1111/1471-0528.15854.
Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9:88–96. https://doi.org/10.1038/nrgastro.2011.244.
Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: Review and update. J Formos Med Assoc. 2019;118:S23–31. https://doi.org/10.1016/j.jfma.2018.08.011.
Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98 https://doi.org/10.3389/fcimb.2020.00098.
Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psych. 2020;20:299 https://doi.org/10.1186/s12888-020-02654-5.
Matheson JT, Holsinger RMD. The role of fecal microbiota transplantation in the treatment of neurodegenerative diseases: A review. Int J Mol Sci. 2023;24:1001 https://doi.org/10.3390/ijms24021001.
Ma D, Chen Y, Chen T. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol Lett. 2019;366:fnz025 https://doi.org/10.1093/femsle/fnz025.
Tuniyazi M, Zhang N. Possible therapeutic mechanisms and future perspectives of vaginal microbiota transplantation. Microorganisms. 2023;11:1427 https://doi.org/10.3390/microorganisms11061427.
Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25:1500–4. https://doi.org/10.1038/s41591-019-0600-6.
Wrønding T, Vomstein K, Bosma EF, Mortensen B, Westh H, Heintz JE, et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: a proof of concept case study. EClinicalMedicine. 2023;61:102070 https://doi.org/10.1016/j.eclinm.2023.102070.
Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–7. https://doi.org/10.1126/science.aag3194.
Brown AS, Meyer U. maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psych. 2018;175:1073–83. https://doi.org/10.1176/appi.ajp.2018.17121311.
Hashimoto K.Molecular mechanisms of the rapid-acting and long-lasting691 antidepressant actions of (R)-ketamine.Biochem Pharmacol.2020;177:113935.
Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psych. 2022;27:1898–907. https://doi.org/10.1038/s41380-021-01432-3.
Wang R, Wu Z, Huang C, Hashimoto K, Yang L, Yang C. Deleterious effects of nervous system in the offspring following maternal SARS-CoV-2 infection during the COVID-19 pandemic. Transl Psychiatry. 2022;12:232. https://doi.org/10.1038/s41398-022-01985-z.
Falahi S, Abdoli A, Kenarkoohi A. Maternal COVID-19 infection and the fetus: Immunological and neurological perspectives. N Microbes N Infect. 2023;53:101135. https://doi.org/10.1016/j.nmni.2023.101135.
Hassib L, de Oliveira CL, Rouvier GA, Kanashiro A, Guimarães FS, Ferreira FR. Maternal microbiome disturbance induces deficits in the offspring’s behaviors: a systematic review and meta-analysis. Gut Microbes. 2023;15:2226282. https://doi.org/10.1080/19490976.2023.2226282.
Nikolova VL, Cleare AJ, Young AH, Stone JM. Acceptability, tolerability, and estimates of putative treatment effects of probiotics as adjunctive treatment in patients with depression: A randomized clinical trial. JAMA Psych. 2023;80:842–7. https://doi.org/10.1001/jamapsychiatry.2023.1817.
Vasilliu O. Is fecal microbiota transplantation a useful therapeutic intervention for psychiatric disorders? A narrative review of clinical and preclinical evidence. Curr Med Res Opin. 2023;39:161–77. https://doi.org/10.1080/03007995.2022.2124071.
Acknowledgements
The author would like to thank our collaborators who are listed as the co-authors of our papers in the reference list. This study was supported by the grant from Japan Society for the Promotion of Science (to KH, 21H02846 and 23K17634).
Author information
Authors and Affiliations
Contributions
KH is the sole author that conceived, drafted and approved the final version of this work. The author used ChatGPT-4 to edit the draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
KH is the inventor of filed patent applications on “The use of R-Ketamine in the treatment of psychiatric diseases”, “(S)-norketamine and salt thereof as pharmaceutical”, “R-Ketamine and derivative thereof as prophylactic or therapeutic agent for neurodegeneration disease or recognition function disorder”, “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases or bone diseases”, and “R-Ketamine and its derivatives as a preventive or therapeutic agent for a neurodevelopmental disorder” by the Chiba University. KH has also received speakers’ honoraria, consultant fee, or research support from Abbott, Boehringer-Ingelheim, Daiichi-Sankyo, Meiji Seika Pharma, Seikagaku Corporation, Dainippon-Sumitomo, Taisho, Otsuka, Murakami Farm and Perception Neuroscience.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashimoto, K. Emerging role of the host microbiome in neuropsychiatric disorders: overview and future directions. Mol Psychiatry 28, 3625–3637 (2023). https://doi.org/10.1038/s41380-023-02287-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02287-6