Abstract
Diagnosis of Wilson disease (WD) can be difficult because of its protean clinical presentations, but early diagnosis is important because effective treatment is available and can prevent disease progression. Similarly, diagnosis of WD on liver biopsy specimens is difficult due to the wide range of histologic appearances. A stain that could help identify WD patients would be of great value. The goal of this study was to use mass spectrometry-based proteomics to identify potential proteins that are differentially expressed in WD compared to controls, and could serve as potential immunohistochemical markers for screening. Several proteins were differentially expressed in WD and immunohistochemical stains for two (metallothionein (MT) and cytochrome C oxidase copper chaperone (COX17)) were tested and compared to other methods of diagnosis in WD including copper staining and quantitative copper assays. We found diffuse metallothionein immunoreactivity in all liver specimens from patients with WD (n = 20); the intensity of the staining was moderate to strong. This staining pattern was distinct from that seen in specimens from the control groups (none of which showed strong, diffuse staining), which included diseases that may be in the clinical or histologic differential of WD (steatohepatitis (n = 51), chronic viral hepatitis (n = 40), autoimmune hepatitis (n = 50), chronic biliary tract disease (n = 42), and normal liver (n = 20)). COX17 immunostain showed no significant difference in expression between the WD and control groups. MT had higher sensitivity than rhodanine for diagnosis of WD. While the quantitative liver copper assays also had high sensitivity, they require more tissue, have a higher cost, longer turnaround time, and are less widely available than an immunohistochemical stain. We conclude that MT IHC is a sensitive immunohistochemical stain for the diagnosis of WD that could be widely deployed as a screening tool for liver biopsies in which WD is in the clinical or histologic differential diagnosis.
Similar content being viewed by others
Introduction
Wilson disease (WD) is an autosomal recessive disorder of cellular copper transport caused by mutations in the ATP7B gene, which codes for a copper transporting ATPase. Mutations in ATP7B prevent excretion of copper into bile, which results in accumulation of copper in the liver, brain, cornea, and other organs1. Diagnosis of WD can be difficult because of the protean clinical manifestations, as well as the lack of highly sensitive and specific diagnostic tests. Children often initially present with hepatic involvement, which can range from asymptomatic disease to mild biochemical derangement, chronic hepatitis, fulminant hepatic failure, or cirrhosis. Adolescent and adult patients can also have neurologic and/or psychiatric symptoms, which often occur in later stages of the disease. Diagnosis in the early stage of disease is important, because effective treatment is available and early therapy can prevent progression to severe liver injury or the development of neurologic disease.
In patients with clinical findings suggestive of WD, the recommended initial assessment includes serum ceruloplasmin and copper levels, ocular slit-lamp examination for Kayser-Fleischer rings (copper deposition in the periphery of the cornea), and 24-h urinary copper excretion2. The results of these tests can be sufficient for a diagnosis of WD in some patients, but each has low sensitivity and specificity, and the results are often not diagnostic.
Diagnosis of WD on liver biopsy specimens is difficult due to the wide range of histologic appearances, analogous to the wide range of clinical manifestations mentioned above. Liver biopsy can be particularly helpful in patients in whom there is a clinical suspicion of WD3,4. However, when liver biopsies are performed on patients without a high prior index of suspicion for WD, the diagnosis can easily be missed due to the nonspecificity of the histologic findings. The spectrum of morphologic findings in WD may include steatosis, nuclear glycogenation, Mallory bodies, portal and lobular mononuclear inflammation, advanced fibrosis, and hepatocyte necrosis5. In some cases, copper deposition can be identified using a rhodanine stain6, and Orcein stain can also be used to highlight sulfhydryl-rich copper-binding proteins. However, these histochemical stains are unreliable for diagnosis of WD due to poor sensitivity, and a negative histochemical stain cannot be used to exclude the diagnosis. Quantitative copper assays using mass spectrometry have higher sensitivity; using a cutoff of 250 mcg/g, the sensitivity and specificity of hepatic copper quantification for a diagnosis of WD are 83% and 99%, respectively7. However, these assays are not widely available, and have stringent specimen requirements including submitting almost all of the biopsy tissue or obtaining a separate core of liver for analysis.
Germline sequencing of ATP7B is available and is the current gold standard test for diagnosing WD. It is, however, limited by the very large number and diversity of pathogenic variants as well as a substantial number of variants of uncertain significance8. Indeed, variants of uncertain significance are a major challenge across clinical molecular genetics practice and are not easily resolved in WD or other hereditary disorders9,10. Approximately 80% of Wilson patients have two pathogenic mutations identified8. Consequently, results are ambiguous in a significant minority of cases, and thus patients may still lack a definite diagnosis despite molecular testing. The challenge is compounded by the fact that most molecular data on WD is from Eurocentric populations, with much less data available from Indian, Asian and Latin American patients, which contributes to reduced diagnostic sensitivity in these populations11. Further, the cost of germline sequencing has decreased substantially over the last decade, but it remains more costly than immunohistochemistry. All of these factors have led to a continued interest in newer testing methodologies for WD8,11, and a more widely available screening test with high sensitivity and less stringent tissue requirements that could be used on liver biopsies would be of great value for diagnosis of WD.
Mass spectrometry proteomics is a powerful tool for tissue-based peptide analysis. We hypothesized that this method might allow for a high sensitivity comparison between the proteins present in WD compared to normal liver and other liver diseases in the differential diagnosis with WD. Our goal was to use mass spectrometry-based quantitative proteomics to study liver FFPE tissues from patients with WD and control groups in order to identify potential proteins that were differentially expressed. We anticipated that such proteins could serve as potential immunohistochemical markers for WD in liver biopsies, and we aimed to evaluate their utility as screening tests.
Materials and methods
Patient Selection
After Institutional Review Board approval, the institutional pathology database was searched for liver specimens (biopsy and resection) with the diagnosis of WD, and additional cases of WD were obtained from other institutions. WD cases were included in the study if they had sufficient tissue available for additional immunohistochemistry, and had either a hepatic copper concentration of >250 mcg/g dry weight, urinary copper excretion of >100 mcg/24 h, and/or molecular genetic confirmation of WD. Clinicopathologic features were reviewed for each case including, age, sex, liver enzymes, serum ceruloplasmin, urine copper, histologic findings, stage of fibrosis (Batts–Ludwig), rhodanine stain results, quantitative hepatic copper result, and molecular testing results for WD. Control cases (biopsies and resections) with clinically established diagnoses were selected to represent a range of liver diseases, including steatohepatitis, chronic viral hepatitis C, autoimmune hepatitis, and chronic biliary tract disease, at various stages of disease.
Sample preparation for quantitative proteomics analysis
Archived liver FFPE sections from patients with WD (n = 8) and control individuals (n = 8) (three 10 µm sections each) were used for quantitative proteomics analysis. Deparaffinization was performed by heating the slides (65 °C for 10 min) followed by serial incubation in xylene, 99%, 95%, 70% ethanol and water. The tissue was scraped and resuspended in lysis buffer (50% 2,2,2- Triflouroethanol, 300 mM Tris-HCl pH 8.0). Proteins were de-crosslinked by heating (90 °C for 90 min) and further protein extraction was performed by subjecting to 90 cycles of high pressure 45,000 psi for 45 s and ambient pressure for 15 s in a Barocycler (Pressure Biosciences Inc., South Easton, MA, USA). Samples were then reduced, alkylated and digested using lys-C/trypsin. After C18 stage tip-based cleanup, peptide estimation was performed using Bicinchoninic acid (BCA) peptide estimation kit (Pierce, Waltham, MA, USA). Digested peptides were labeled with TMTpro reagents using manufacturer’s protocol. Labeled peptides were pooled and subjected to basic pH reverse phase fractionation on UltiMate 3000 HPLC system (Thermo Fisher Scientific, Waltham, MA, USA). For basic pH fractionation, labeled peptides were resuspended in 5 mM TEABC pH 8.5 buffer (solvent A) and separated on a C18 XBridge column (3 μm, 250 × 4.6 mm, Waters) using a gradient of 8% to 40% of 5 mM TEABC, pH 8.5, 90% acetonitrile (solvent B). Fractions collected in 96 well plate were subsequently pooled into 24 fractions.
LC-MS/MS analysis
LC-MS/MS analysis was carried out on an Orbitrap Eclipse mass spectrometer connected to UltiMate 3000 RSLC nano system (Thermo Scientific, San Jose, CA, USA). Online peptide separation was performed using a two-column strategy in which peptides were initially loaded on a trapping column (PepMap C18, 2 cm × 100 µm, 100 Å, Thermo Scientific, San Jose, CA, USA) using solvent A (0.1% formic acid) and separated on an analytical column (PepMap RSLC C18 2 µm, 75 µm × 50 cm, 100 Å, Thermo Scientific, San Jose, CA, USA) using a gradient of solvent B (100% acetonitrile, 0.1% formic acid). Total run time of the method was 150 min. During the peptide separation, an Orbitrap mass analyzer was operated in data-dependent mode in cycles of MS followed by MS/MS scans for 2 s. In the MS scan, precursor ions were acquired for a mass range of 350 – 1700 m/z using 120,000 resolution, AGC setting of 400,000 and ion injection time of 50 ms. Precursor ions were sequentially selected based on their abundance and filtered by quadrupole for MS/MS analysis using an isolation width of 0.7 Da. Additionally, precursor ions were filtered for a minimum intensity threshold of 20,000, charge state of 2–5 and excluded for repeated MS/MS analysis for 40 s. After fragmentation using high-energy collision-induced dissociation at 34% normalized collision energy, fragment ions were recorded in orbitrap using AGC setting of 100,000 and injection time of 100 ms.
Raw files were processed in Proteome Discoverer software version 2.5 (Thermo Scientific, San Jose, CA, USA). MS/MS spectra were searched against human UniProt protein database using Sequest search engines. The following parameters were specified during the database searching: full tryptic cleavage, 2 missed cleavages, precursor mass tolerance of 10 ppm, fragment ion tolerance of 0.02 Da, oxidation (methionine) and protein N-terminal acetylation as dynamic modifications and TMTpro at peptide N-terminus, lysine and carbamidomethylation (cysteine) as fixed modifications. False discovery rate was maintained to 1% at protein-level using Percolator. Peptide spectral matches with an average reporter ion S/N threshold of <10 and co-isolation threshold of >70 were not considered for quantitation.
Immunohistochemistry (IHC)
IHC staining was performed at the Pathology Research Core (Mayo Clinic, Rochester, MN) using the Leica Bond RX stainer (Leica, Buffalo Grove, IL, USA). FFPE tissues were sectioned at 5 microns and IHC staining was performed on-line. Slides for Metallothionein stain were retrieved for 20 min using Epitope Retrieval 1 (Citrate; Leica). The Metallothionein primary antibody (Abcam, Cambridge, MA, USA; Clone UC1MT) was diluted to 1/200 in Bkg Reducing Diluent (Dako; S3022) and incubated for 15 min.
The detection system used was Polymer Refine Detection System (Leica). This system includes the hydrogen peroxidase block, post primary and polymer reagent, DAB, and Hematoxylin. After the hydrogen peroxidase block a protein block (Dako; X0909) was applied for 5 min. Immunostaining visualization was achieved by incubating slides 10 min in DAB and DAB buffer (1:19 mixture) from the Bond Polymer Refine Detection System. To this point, slides were rinsed between steps with 1X Bond Wash Buffer (Leica). Slides were counterstained for 5 min using Schmidt hematoxylin and molecular biology grade water (1:1 mixture), followed by several rinses in 1X Bond wash buffer and distilled water, this is not the hematoxylin provided with the Refine kit. Once the immunochemistry process was completed, slides were removed from the stainer and rinsed in tap water for five minutes. Slides were dehydrated in increasing concentrations of ethyl alcohol and cleared in 3 changes of xylene prior to permanent coverslipping in xylene-based medium. A COX17 (Boster Biological Technology, Pleasanton, CA, USA; A04584, 1:750 dilution) immunostain was also performed on a subset of cases.
The immunostains were reviewed and the percentage of hepatocytes with cytoplasmic staining was recorded. The intensity of immunoreactivity was scored as 0, +1 (weak), +2 (moderate), or +3 (strong). We pre-determined that a qualitative threshold of at least 50% of hepatocytes showing at least 2+ staining was our threshold for positive. While we determined a qualitative threshold in advance, an H-score for MT immunostaining was calculated for each case to allow for a quantitative statistical analysis of the differences in staining in cases of WD and controls.
Statistical analysis
The Wilcox–Mann–Whitney test was used to compare H-scores. ROC curves were produced using SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Differential expression analysis was performed using 2 sample Student’s T test with permutation-based FDR correction. Principle component analysis and heat map visualization was performed in Perseus software platform12.
Results
A total of 223 liver specimens were evaluated, including 20 specimens from patients with WD (12 resection specimens and 8 biopsy specimens), 50 with autoimmune hepatitis, 42 with chronic biliary tract disease, 51 with steatohepatitis, 40 with chronic hepatitis C, and 20 normal liver biopsies. All specimens from patients with WD had established diagnoses: 16 of the 20 patients had markedly elevated quantitative hepatic copper levels (>250 mcg/g dry weight), one had a confirmed diagnosis by molecular testing, and three had markedly elevated urinary copper excretion (>100 mcg/24 h) and low ceruloplasmin levels (Table 1).
Quantitative proteomics of FFPE liver tissue from Wilson disease and control groups
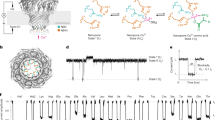
The methodology employed for the proteomics analysis is outlined in Fig. 1A. Quantitative proteomics analysis resulted in the identification of 7,491 proteins at 1% protein-level FDR. Principle component analysis showed striking separation of Wilson’s disease cases (n = 8) and control individuals (n = 8) indicating alterations at the proteomic-level in Wilson’s disease (Fig. 1B). Proteins differentially expressed in disease cases were identified using Student’s T test with multiple hypothesis correction. Overall, 588 proteins were over-expressed and 243 proteins were under-expressed with 1.5-fold difference (adjusted p value <0.05) in disease cases compared to controls (Fig. 1C). We did not identify the ATP7B protein in this study. We identified significant downregulation of COX17 (0.6-fold) which transports intracellular copper to cytochrome C oxidase complex. Despite COX17, we did not identify dysregulation of other copper chaperons such as ATOX1 and CCS. COMMD1, a protein involved in copper ion homeostasis, also remained unchanged in Wilson’s disease cases compared to control individuals. In order to understand the adaptive responses of liver cells upon copper accumulation, we performed functional characterization of the differentially expressed proteins using gene ontology enrichment analysis. Overexpressed proteins were significantly involved in several biological process such as interferon-γ-mediated signaling pathway, antigen processing and presentation, extracellular matrix reorganization, cell adhesion (Fig. 2A). Proteins involved in the immune response are shown as a protein-protein interaction network in Fig. 2B. Under-expressed proteins were significantly enriched in several metabolic processes such as oxidation-reduction processes, metabolism of xenobiotics, and steroid and lipid metabolic processes (Fig. 2C). The proteins involved in the redox reactions include CYP2C8, CYP2A6, CYP2C19, CYP3A4, HSD17B13, GFER, RDH16, VKORC1, SOD1 (Fig. 2D). Metallothionein-1M, was overexpressed by 1.7-fold in Wilson’s disease cases compared to control individuals (Fig. 3A). In addition to MT1M, MT1G was also overexpressed (1.5-fold; adj p value < 0.1) in WD cases (Fig. 3B). MS/MS spectrum of a representative peptide identified from MT1M is shown in Fig. 3C.
A Experimental strategy for quantitative proteomics analysis. B 2-dimensional principal component analysis of protein expression profiles across all the samples clearly segregating Wilson disease cases from control cases. C Volcano plot showing differentially expressed proteins in Wilson disease compared to controls (±1.5-fold; adj. p value < 0.05). Proteins overexpressed are highlighted in red and under-expressed proteins were highlighted in blue.
A Biological processes enriched among the overexpressed proteins in Wilson disease (FDR value < 0.05). B Protein-protein interaction network of the overexpressed proteins involved in immune response C Biological processes enriched among the under-expressed proteins in Wilson disease (FDR value < 0.05). D Protein-protein interaction network of the under-expressed proteins involved in oxidation-reduction process. Protein-protein interaction networks were generated using String database and analyzed in Cytoscape. Node color gradient is based on the fold-change of the over-expressed and under-expressed proteins. The width of the edge indicates the confidence of the interaction. The confidence ranges from 0.4 (low confidence) to 0.9 (high confidence) given by the String database.
Metallothionein immunohistochemistry
All cases of WD showed diffuse (>50%), moderate to strong cytoplasmic MT immunoreactivity within hepatocytes (Table 2; Fig. 4A, B). In WD cases with cirrhosis (11 of 20) the staining intensity varied among the cirrhotic nodules, but all cases contained some hepatocyte nodules with strong, diffuse MT1M immunoreactivity. Several other patterns of expression were identified in a subset of cirrhotic nodules, including a heterogeneous pattern of expression and a periseptal/zone 1 pattern of expression (Fig. 4C, D).
The mean MT IHC H-score for WD cases was 261.0 ± 36.4 versus 13.6 ± 29.8 for all control group cases (p < 0.0001), highlighting the marked difference in extent and intensity of staining between WD and the control groups. Among the control groups, the chronic biliary tract disease group had the highest MT IHC H-score (28.0 ± 42.6) which was still significantly lower than the H-score of WD cases (p < 0.0001) (Table 3). In order to determine the optimal quantitative threshold for MT IHC, we performed a cutoff analysis using a Receiver Operator Characteristic (ROC) curve for MT IHC H-score which revealed that an H-score threshold of 165 had 100% sensitivity and 100% specificity for diagnosis of WD. Similarly, our pre-determined threshold of at least moderate intensity staining in >50% of hepatocytes had a 100% sensitivity and 100% specificity for a diagnosis of WD confirming that this practical and easy to implement threshold would yield excellent results (Tables 4, 5). There was no correlation between H-score and patient age or stage of fibrosis.
A rhodanine stain was available for 11 cases of WD. The rhodanine stain was positive for copper in 55% (6 of 11) of WD cases. In 4 of these 6 cases the staining was focal and mild. The sensitivity of rhodanine was therefore approximately half that of MT IHC and showed a much lower signal in most cases where it was positive.
In the control groups, 63% (128 of 203) of cases were completely negative for MT immunoreactivity (Fig. 5A, B) and 37% of cases (75 of 203) showed some degree of MT immunoreactivity (Supplementary Table 1). Several patterns of MT expression were identified including very focal (<5% of hepatocytes) strong expression (Fig. 5C), diffuse staining of weak intensity (8.4% of control cases showed MT expression in more than 20% of hepatocytes) (Fig. 5D), rare cases showed more extensive staining with more than weak intensity, but the staining never exceeded 50% of hepatocytes (Fig. 5E) – indeed it was always less than or equal to 20% of the hepatocytes. Several cases of cirrhosis showed a narrow rim of strong staining in zone 1 hepatocytes (Fig. 5F). None of the staining in control cases mimicked that seen in cases of WD.
A Negative MT staining in a case of chronic biliary tract disease. B Negative MT staining in a case of steatohepatitis. C Focal strong staining in ~10%v of the hepatocytes in a control case with minimal histologic change. D Mild diffuse staining in a case of steatohepatitis with cirrhosis. E Chronic hepatitis C case with diffuse mild to moderate staining (this case had an H-score of 150, which is the highest H-score in a control case), but could be readily distinguished from all WD cases given the weaker staining. F Narrow rim of zone 1 staining in a case of AIH.
COX17 immunohistochemistry
COX17 was found to be downregulated in WD patients by the mass spectrometry analysis. Therefore, a COX17 immunostain was performed and showed diffuse weak cytoplasmic immunoreactivity in hepatocytes of 6 cases of WD, 5 cases of autoimmune hepatitis, and 7 cases with minimal histologic change. There was no significant difference in staining pattern between the groups.
Discussion
To our knowledge, this is the first study that used mass spectrometry to identify proteins that are differentially expressed in WD to determine if any were suitable targets for IHC. As noted above, the quantitative proteomics analysis identified several potential protein targets including MTs and COX17. Metallothionein-1G (MT1G) is a low molecular mass cysteine-rich protein that binds to heavy metals, and acts as a scavenger of free radicals and is involved in metal detoxification and homeostasis13. MT expression in WD is thought to be induced by copper, and MT functions to bind copper within the cytoplasm and reduce its toxicity, although the molecular mechanism of the increased MT expression is not well understood14,15. Cytochrome c oxidase copper chaperone (COX17) is involved in the recruitment of mitochondrial copper16. Elevated levels of liver MTs have previously been identified in patients with WD and in some patients with primary biliary cirrhosis (PBC) using radioimmunoassays and chromatography17,18. Also, in 1987, Nartey, et al. reported positive MT immunoreactivity in the cytoplasm of hepatocytes of in a single patient with WD19. Given this previous work and the biological properties of the protein targets, we anticipated that WD would lead to dysregulation of any of these proteins, and thus they were attractive candidates for potential immunohistochemical assays.
We found diffuse moderate or strong MT immunoreactivity in all liver specimens from patients with WD. This staining extent and intensity were substantially greater than that seen in specimens from the control groups, which included diseases that may be in the clinical or histologic differential of WD (steatohepatitis, chronic viral hepatitis, autoimmune hepatitis, and normal liver). We also included patients with chronic biliary tract disease as a control group because of the increased tissue copper levels frequently seen in these patients. Even in patients with chronic biliary tract disease, the staining was approximately an order of magnitude less than that seen in WD by H-score. The degree of difference was even greater for other control cases (see Figs. 4, 5 and Tables 3, 4)
The mean MT IHC H-scores were significantly higher in the WD group compared with each of the control groups (Table 3). A MT IHC cutoff of at least moderate intensity staining in >50% of hepatocytes had a 100% sensitivity and 100% specificity for a diagnosis of WD. No control group cases showed at least moderate expression in greater than 50% of hepatocytes (Supplementary Table 1). In the WD patients, we found no correlation between MT IHC H-score and patient age or stage of fibrosis. This suggests that MT IHC can detect even early-stage cases of WD before the development of cirrhosis.
In comparison to rhodanine for the diagnosis of WD on liver specimens, MT IHC has a far higher sensitivity, at 100%. vs 55%. This is likely because rhodanine only detects copper that is concentrated within lysosomes, and does not stain cytoplasmic copper; in early cases of WD, copper is often only cytoplasmic, without significant lysosomal deposition of copper20. Multiple studies have highlighted that rhodanine is suboptimal in diagnosis of WD6,21,22. In the largest of these studies, which included 74 patients with WD, rhodanine had a sensitivity ranging from 13.2–36.6% across the various morphologic patterns of WD, and the authors concluded that rhodanine had only minor diagnostic value in this situation21.
While quantitative liver copper assays are sensitive for WD diagnosis (in this study 15 of 16 cases had a quantitative hepatic copper level greater than 250 mcg/g dry tissue), they require more tissue, are more expensive, have a much longer turnaround time, and are less widely available than IHC. Our study included one case of WD with a hepatic copper level less than 250 mcg/g dry tissue, and this case showed diffuse positivity for MT. Similarly, all cases of WD with hepatic copper levels above 250 mcg/g dry tissue also had strong, diffuse positivity for MT IHC.
The Leipzig criteria are often used by clinicians to support a diagnosis of WD. The components include KF rings, neurologic symptoms, serum ceruloplasmin, hemolytic anemia, liver copper, urinary copper and mutation analysis. The sensitivity and specificity of several of these parameters are quite limited. For example, the sensitivities and specificities are as follows: KF rings (50%; not available), serum ceruloplasmin (21–56%;63–84% depending on method), liver copper (83.3%;98.6% using 250 mcg/g dry weight cutoff) and urinary copper (81.8%;89.8% using 70 mcg cutoff)4,7,23. These data further support the utility of the MT IHC assay with its much higher sensitivity and specificity. This assay is also particularly useful for the practicing pathologist, who may receive specimens in consultation or have limited clinical information and need to provide helpful information in a cost-effective, tissue-preserving manner.
In summary, we demonstrated that MT IHC was positive in all cases of WD diagnosed by other methods in this study (100% sensitivity), including those that had negative rhodanine stains and a single case with non-diagnostic quantitative hepatic copper levels. MT IHC is a sensitive, potentially widely available, inexpensive immunohistochemical stain for the diagnosis of WD that could be widely deployed as a screening tool for liver biopsies in which WD is in the clinical or histologic differential diagnosis. For patients with a positive MT IHC result, additional confirmatory testing would still be recommended, and in patients with a negative result, the delays and costs associated with referral for additional testing could be avoided. In conclusion, this study is the first to describe MT IHC as a highly sensitive and specific, inexpensive screening test for the diagnosis of WD on liver biopsy. Further studies involving larger numbers of liver biopsies are anticipated to confirm that this immunohistochemical test will be a valuable tool in the armamentarium of tests available for the diagnosis of WD.
References
Poujois, A. & Woimant, F. Wilson’s disease: a 2017 update. Clin. Res. Hepatol. Gastroenterol. 42, 512–520 (2018).
Ferenci, P. et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 23, 139–142 (2003).
Steindl, P. et al. Wilson’s disease in patients presenting with liver disease: a diagnostic challenge. Gastroenterology 113, 212–218 (1997).
Korman, J. D. et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 48, 1167–1174 (2008).
Stromeyer, F. W. & Ishak, K. G. Histology of the liver in Wilson’s disease: a study of 34 cases. Am. J. Clin. Pathol. 73, 12–24 (1980).
Mounajjed, T., Oxentenko, A. S., Qureshi, H. & Smyrk, T. C. Revisiting the topic of histochemically detectable copper in various liver diseases with special focus on venous outflow impairment. Am. J. Clin. Pathol. 139, 79–86 (2013).
Ferenci, P. et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson’s Disease. Clin. Gastroenterol. Hepatol. 3, 811–818 (2005).
Chang, I. J. & Hahn, S. H. The genetics of Wilson disease. Handb. Clin. Neurol. 142, 19–34 (2017).
Fatkin, D. & Johnson, R. Variants of uncertain significance and “missing pathogenicity”. J. Am. Heart Assoc. 9, e015588 (2020).
Hoffman-Andrews, L. The known unknown: the challenges of genetic variants of uncertain significance in clinical practice. J. Law Biosci. 22, 648–657 (2018).
Collins, C. J. et al. Direct measurement of ATP7B peptides is highly effective in the diagnosis of Wilson Disease. Gastroenterology 160, 2367–2382 (2021).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2018).
Carpene, E., Andreani, G. & Isani, G. Metallothionein functions and structural characteristics. J. Trace Elem. Med. Biol. 21, 35–39 (2007). Suppl 1.
Zhang, C. C., Volkmann, M., Tuma, S., Stremmel, W. & Merle, U. Metallothionein is elevated in liver and duodenum of Atp7b((-/-)) mice. Biometals 31, 617–625 (2018).
Salazar, G., Huang, J., Feresin, R. G., Zhao, Y. & Griendling, K. K. Zinc regulates Nox1 expression through a NF-kappa B and mitochondrial ROS dependent mechanism to induce senescence of vascular smooth muscle cells. Free Radic. Bio. Med. 108, 225–235 (2017).
Amaravadi, R., Glerum, D. M. & Tzagoloff, A. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum. Genet. 99, 329–333 (1997).
Mulder, T. P., Janssens, A. R., Verspaget, H. W., van Hattum, J. & Lamers, C. B. Metallothionein concentration in the liver of patients with Wilson’s disease, primary biliary cirrhosis, and liver metastasis of colorectal cancer. J. Hepatol. 16, 346–350 (1992).
Hunziker, P. E. & Sternlieb, I. Copper metallothionein in patients with hepatic copper overload. Eur. J. Clin. Invest. 21, 466–471 (1991).
Nartey, N. O., Frei, J. V. & Cherian, M. G. Hepatic copper and metallothionein distribution in Wilson’s disease (hepatolenticular degeneration). Lab. Invest. 57, 397–401 (1987).
Goldfischer, S. & Sternlieb, I. Changes in the distribution of hepatic copper in relation to the progression of Wilson’s disease (hepatolenticular degeneration). Am. J. Pathol. 53, 883–901 (1968).
Pilloni, L. et al. Value of histochemical stains for copper in the diagnosis of Wilson’s disease. Histopathology 33, 28–33 (1998).
Goldfischer, S., Popper, H. & Sternlieb, I. The significance of variations in the distribution of copper in liver disease. Am. J. Pathol. 99, 715–730 (1980).
Aksu, A. U., Sari, S., Gurkan, O. E. & Dalgic, B. Urinary 24-hour copper excretion at the time of diagnosis in children with Wilson’s disease. Acta Gastroenterol. Belg. 81, 410–414 (2018).
Author information
Authors and Affiliations
Contributions
D.J.R. performed study design, wrote and revised the manuscript and performed data collection and interpretation. K.K.M., S.S, and A.P. wrote portions of the manuscript, performed study design, and performed data collection and interpretation. R.K.M. and T.M. performed study concepts and designs and reviewed the manuscript. L.L., M.W., J.C., A.M.B., and D.S.A. contributed cases and reviewed the manuscript. R.P.G. performed study concept and design and reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Mayo Clinic Institutional Review Board (IRB).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rowan, D.J., Mangalaparthi, K.K., Singh, S. et al. Metallothionein immunohistochemistry has high sensitivity and specificity for detection of Wilson disease. Mod Pathol 35, 946–955 (2022). https://doi.org/10.1038/s41379-021-01001-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-01001-7