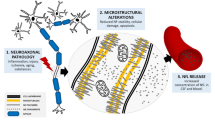
Abstract
Neurofilament proteins have been validated as specific body fluid biomarkers of neuro-axonal injury. The advent of highly sensitive analytical platforms that enable reliable quantification of neurofilaments in blood samples and simplify longitudinal follow-up has paved the way for the development of neurofilaments as a biomarker in clinical practice. Potential applications include assessment of disease activity, monitoring of treatment responses, and determining prognosis in many acute and chronic neurological disorders as well as their use as an outcome measure in trials of novel therapies. Progress has now moved the measurement of neurofilaments to the doorstep of routine clinical practice for the evaluation of individuals. In this Review, we first outline current knowledge on the structure and function of neurofilaments. We then discuss analytical and statistical approaches and challenges in determining neurofilament levels in different clinical contexts and assess the implications of neurofilament light chain (NfL) levels in normal ageing and the confounding factors that need to be considered when interpreting NfL measures. In addition, we summarize the current value and potential clinical applications of neurofilaments as a biomarker of neuro-axonal damage in a range of neurological disorders, including multiple sclerosis, Alzheimer disease, frontotemporal dementia, amyotrophic lateral sclerosis, stroke and cerebrovascular disease, traumatic brain injury, and Parkinson disease. We also consider the steps needed to complete the translation of neurofilaments from the laboratory to the management of neurological diseases in clinical practice.
Key points
-
Neurofilament proteins have emerged as one of the most important body fluid biomarkers of neuro-axonal injury in a wide range of neurological diseases.
-
High-sensitivity analytical platforms enable reliable quantification of neurofilament light chain (NfL) levels in blood samples, paving the way for their use in clinical practice.
-
Establishment of large reference databases of physiological blood levels of NfL adjusted for age and BMI was a major milestone towards the clinical use of NfL.
-
Neurofilament levels can often not be used to diagnose disease entities but are useful as a diagnostic type biomarker in the preclinical phases of neurodegenerative diseases and as markers of disease progression, prognosis, and treatment response.
-
Neurofilament levels are increasingly used as an outcome measure in clinical trials; FDA approval of tofersen was based on changes in blood NfL levels, marking a paradigm shift in the importance of biomarkers in regulatory approvals.
-
Standardization and cross-compatibility of neurofilament measures taken with current emerging analytic platforms are key to completing the translation of neurofilaments into clinical practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14, 577–589 (2018).
Petzold, A. The 2022 Lady Estelle Wolfson lectureship on neurofilaments. J. Neurochem. 163, 179–219 (2022).
Norgren, N., Karlsson, J.-E., Rosengren, L. & Stigbrand, T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid. Hybridomics 21, 53–59 (2002).
Disanto, G. et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870 (2017).
Gisslén, M. et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3, 135–140 (2016).
Spitzenberger, F. et al. Laboratory-developed tests: design of a regulatory strategy in compliance with the international state-of-the-art and the regulation (EU) 2017/746 (EU IVDR [In Vitro Diagnostic Medical Device Regulation]). Ther. Innov. Regul. Sci. 56, 47–64 (2022).
Hauser, S. L. et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 383, 546–557 (2020).
Tabrizi, S. J. et al. Targeting huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 380, 2307–2316 (2019).
Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Mullard, A. NfL makes regulatory debut as neurodegenerative disease biomarker. Nat. Rev. Drug Discov. 22, 431–434 (2023).
Biogen. FDA Grants Accelerated Approval for QALSODYTM (Tofersen) for SOD1-ALS, a Major Scientific Advancement as the First Treatment to Target a Genetic Cause of ALS https://investors.biogen.com/news-releases/news-release-details/fda-grants-accelerated-approval-qalsodytm-tofersen-sod1-als (2023).
Leptak, C. & Kozauer, N. Letter of Support to the International Progressive Multiple Sclerosis Alliance. U.S. Food & Drug Administration https://www.fda.gov/media/149608/download (2021).
Cooke, E. Letter of Support of Neurofilament Light in Childhood Neurological Diseases. European Medicines Agency https://www.ema.europa.eu/en/documents/other/letter-support-neurofilament-light-childhood-neurological-diseases_en.pdf (2022).
Koini, M. et al. Factors influencing serum neurofilament light chain levels in normal aging. Aging 13, 25729–25738 (2021).
Fitzgerald, K. C. et al. Contributors to serum NfL levels in people without neurologic disease. Ann. Neurol. 92, 688–698 (2022).
Benkert, P. et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 21, 246–257 (2022).
Shaw, G. et al. Uman-type neurofilament light antibodies are effective reagents for the imaging of neurodegeneration. Brain Commun. 5, fcad067 (2023).
Gafson, A. R. et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain 143, 1975–1998 (2020).
Andreasson, U. et al. Assessing the commutability of candidate reference materials for the harmonization of neurofilament light measurements in blood. Clin. Chem. Lab. Med. 61, 1245–1254 (2023).
Eldirany, S. A., Lomakin, I. B., Ho, M. & Bunick, C. G. Recent insight into intermediate filament structure. Curr. Opin. Cell Biol. 68, 132–143 (2021).
Ghosh, K., Huihui, J., Phillips, M. & Haider, A. Rules of physical mathematics govern intrinsically disordered proteins. Annu. Rev. Biophys. 51, 355–376 (2022).
Janmey, P. A., Leterrier, J.-F. & Herrmann, H. Assembly and structure of neurofilaments. Curr. Opin. Colloid Interface Sci. 8, 40–47 (2003).
Trimpin, S. et al. Identification of endogenous phosphorylation sites of bovine medium and low molecular weight neurofilament proteins by tandem mass spectrometry. Biochemistry 43, 2091–2105 (2004).
Petzold, A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 233, 183–198 (2005).
Rebelo, A. P. et al. Cryptic amyloidogenic elements in the 3’ UTRs of neurofilament genes trigger axonal neuropathy. Am. J. Hum. Genet. 98, 597–614 (2016).
Murray, K. A. et al. Identifying amyloid-related diseases by mapping mutations in low-complexity protein domains to pathologies. Nat. Struct. Mol. Biol. 29, 529–536 (2022).
Xiao, S., McLean, J. & Robertson, J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim. Biophys. Acta Mol. Basis Dis. 1762, 1001–1012 (2006).
Petzold, A. et al. Protein aggregate formation permits millennium-old brain preservation. J. R. Soc. Interface 17, 20190775 (2020).
Briot, J., Simon, M. & Méchin, M.-C. Deimination, intermediate filaments and associated proteins. Int. J. Mol. Sci. 21, 8746 (2020).
Cloos, P. A. C. & Christgau, S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology 5, 139–158 (2004).
Yuzwa, S. A. et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Chem. Biol. 8, 393–399 (2012).
Zucchi, E. et al. A motor neuron strategy to save time and energy in neurodegeneration: adaptive protein stoichiometry. J. Neurochem. 146, 631–641 (2018).
Jones, J. B. & Safinya, C. R. Interplay between liquid crystalline and isotropic gels in self-assembled neurofilament networks. Biophys. J. 95, 823–835 (2008).
Lasek, R. J., Phillips, L., Katz, M. J. & Autilio-Gambetti, L. Function and evolution of neurofilament proteins. Ann. N. Y. Acad. Sci. 455, 462–478 (1985).
Monaco, S., Autilio-Gambetti, L., Lasek, R. J., Katz, M. J. & Gambetti, P. Experimental increase of neurofilament transport rate: decreases in neurofilament number and in axon diameter. J. Neuropathol. Exp. Neurol. 48, 23–32 (1989).
Lasek, R. J., Oblinger, M. M. & Drake, P. F. Molecular biology of neuronal geometry: expression of neurofilament genes influences axonal diameter. Cold Spring Harb. Symp. Quant. Biol. 48, 731–744 (1983).
Balaratnasingam, C. et al. Axonal transport and cytoskeletal changes in the laminar regions after elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 48, 3632–3644 (2007).
Vial, J. D. The early changes in the axoplasm during Wallerian degeneration. J. Biophys. Biochem. Cytol. 4, 551–555 (1958).
Lasek, R. J. Bidirectional transport of radioactively labelled axoplasmic components. Nature 216, 1212–1214 (1967).
Nixon, R. A. & Logvinenko, K. B. Multiple fates of newly synthesized neurofilament proteins: evidence for a stationary neurofilament network distributed nonuniformly along axons of retinal ganglion cell neurons. J. Cell Biol. 102, 647–659 (1986).
Mutalik, S. P. & Ghose, A. Axonal cytomechanics in neuronal development. J. Biosci. 45, 64 (2020).
Gentil, B. J. et al. Normal role of the low-molecular-weight neurofilament protein in mitochondrial dynamics and disruption in Charcot-Marie-Tooth disease. FASEB J. 26, 1194–1203 (2012).
Wagner, O. I. et al. Mechanisms of mitochondria-neurofilament interactions. J. Neurosci. 23, 9046–9058 (2003).
Zhu, P.-P. et al. Transverse endoplasmic reticulum expansion in hereditary spastic paraplegia corticospinal axons. Hum. Mol. Genet. 31, 2779–2795 (2022).
Dashiell, S. M., Tanner, S. L., Pant, H. C. & Quarles, R. H. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J. Neurochem. 81, 1263–1272 (2002).
Petzold, A. et al. In vivo monitoring of neuronal loss in traumatic brain injury: a microdialysis study. Brain 134, 464–483 (2011).
Altmann, P. et al. Seven day pre-analytical stability of serum and plasma neurofilament light chain. Sci. Rep. 11, 11034 (2021).
Brureau, A. et al. NF-L in cerebrospinal fluid and serum is a biomarker of neuronal damage in an inducible mouse model of neurodegeneration. Neurobiol. Dis. 104, 73–84 (2017).
Geisler, N. & Weber, K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc. Natl Acad. Sci. USA 78, 4120–4123 (1981).
Budelier, M. M. et al. A map of neurofilament light chain species in brain and cerebrospinal fluid and alterations in Alzheimer’s disease. Brain Commun. 4, fcac045 (2022).
Woltsche, N. et al. Neurofilament light chain: a new marker for neuronal decay in the anterior chamber fluid of patients with glaucoma. Br. J. Ophthalmol. 107, 1432–1437 (2022).
Plog, B. A. et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 35, 518–526 (2015).
Engel, S. et al. Elevated neurofilament light chain CSF/serum ratio indicates impaired CSF outflow in idiopathic intracranial hypertension. Fluids Barriers CNS 20, 3 (2023).
Hier, D. B. et al. Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues. Biomark. Res. 9, 70 (2021).
Azizi, S. et al. A kinetic model for blood biomarker levels after mild traumatic brain injury. Front. Neurol. 12, 668606 (2021).
Thelin, E. P. et al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front. Neurol. 8, 300 (2017).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7, eabh2169 (2021).
Keddie, S. et al. Peripherin is a biomarker of axonal damage in peripheral nervous system disease. Brain 146, 4562–4573 (2023).
Yuan, A. et al. Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. J. Neurosci. 32, 8501–8508 (2012).
Petzold, A., Keir, G., Green, A. J. E., Giovannoni, G. & Thompson, E. J. A specific ELISA for measuring neurofilament heavy chain phosphoforms. J. Immunol. Methods 278, 179–190 (2003).
Gaiottino, J. et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 8, e75091 (2013).
Rissin, D. M. & Walt, D. R. Digital concentration readout of single enzyme molecules using femtoliter arrays and Poisson statistics. Nano Lett. 6, 520–523 (2006).
Wilson, D. H. et al. The simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J. Lab. Autom. 21, 533–547 (2016).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010).
Petzold, A. et al. Neurofilament ELISA validation. J. Immunol. Methods 352, 23–31 (2010).
Kuhle, J. et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 54, 1655–1661 (2016).
Brousse, M. et al. Serum neurofilament light chain cut-off definition for clinical diagnosis and prognosis of amyotrophic lateral sclerosis. Eur. J. Neurol. 30, 1919–1927 (2023).
Lee, S. et al. Development of a highly sensitive neurofilament light chain assay on an automated immunoassay platform. Front. Neurol. 13, 935382 (2022).
Yuan, A. & Nixon, R. A. Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front. Neurosci. 15, 689938 (2021).
Harp, C. et al. Development of an age-adjusted model for blood neurofilament light chain. Ann. Clin. Transl. Neurol. 9, 444–453 (2022).
Khalil, M. et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 11, 812 (2020).
Yilmaz, A. et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev. Mol. Diagn. 17, 761–770 (2017).
Bridel, C. et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 76, 1035–1048 (2019).
Idland, A.-V. et al. CSF neurofilament light levels predict hippocampal atrophy in cognitively healthy older adults. Neurobiol. Aging 49, 138–144 (2017).
Reiber, H. Flow rate of cerebrospinal fluid (CSF) — a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122, 189–203 (1994).
Mattsson, N., Andreasson, U., Zetterberg, H. & Blennow, K., Alzheimer’s Disease Neuroimaging Initiative.Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 74, 557–566 (2017).
Manouchehrinia, A. et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 7, 139–143 (2020).
Simrén, J. et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5–90 years. Brain Commun. 4, fcac174 (2022).
Tang, R. et al. Association of neurofilament light chain with renal function: mechanisms and clinical implications. Alzheimers Res. Ther. 14, 189 (2022).
Akamine, S. et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci. Rep. 10, 20350 (2020).
Kosa, P. et al. Enhancing the clinical value of serum neurofilament light chain measurement. JCI Insight 7, e161415 (2022).
Simonsen, A. H. et al. Neurofilament light chain levels in serum among a large mixed memory clinic cohort: confounders and diagnostic usefulness. Alzheimers Dement. 15, e12512 (2023).
Sjölin, K. et al. Serum neurofilament light chain in patients with atrial fibrillation. J. Am. Heart Assoc. 11, e025910 (2022).
Polymeris, A. A. et al. Serum neurofilament light in atrial fibrillation: clinical, neuroimaging and cognitive correlates. Brain Commun. 2, fcaa166 (2020).
Li, Y. et al. Neurofilament light chain is a promising biomarker in alcohol dependence. Front. Psychiatry 12, 754969 (2021).
Sareban, M. et al. Serum neurofilament level increases after ascent to 4559 m but is not related to acute mountain sickness. Eur. J. Neurol. 28, 1004–1008 (2021).
Sönksen, S.-E. et al. Brain structure and neurocognitive function in two professional mountaineers during 35 days of severe normobaric hypoxia. Eur. J. Neurol. 29, 3112–3116 (2022).
Isung, J. et al. Differential effects on blood and cerebrospinal fluid immune protein markers and kynurenine pathway metabolites from aerobic physical exercise in healthy subjects. Sci. Rep. 11, 1669 (2021).
Joisten, N. et al. Exercise diminishes plasma neurofilament light chain and reroutes the kynurenine pathway in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 8, e982 (2021).
Bazarian, J. J. et al. Effects of physical exertion on early changes in blood-based brain biomarkers: implications for the acute point of care diagnosis of concussion. J. Neurotrauma 40, 693–705 (2023).
Kuhle, J. et al. Sustained reduction of serum neurofilament light chain over 7 years by alemtuzumab in early relapsing-remitting MS. Mult. Scler. 28, 573–582 (2022).
Sormani, M. P. et al. Blood neurofilament light as a potential endpoint in phase 2 studies in MS. Ann. Clin. Transl. Neurol. 6, 1081–1089 (2019).
Leppert, D. et al. Blood neurofilament light in progressive multiple sclerosis: post hoc analysis of 2 randomized controlled trials. Neurology 98, e2120–e2131 (2022).
Vermunt, L. et al. Age‐ and disease‐specific reference values for neurofilament light presented in an online interactive support interface. Ann. Clin. Transl. Neurol. 9, 1832–1837 (2022).
Bornhorst, J. A. et al. Plasma neurofilament light chain (NfL) reference interval determination in an age-stratified cognitively unimpaired cohort. Clin. Chim. Acta 535, 153–156 (2022).
Borghi, E. et al. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat. Med. 25, 247–265 (2006).
Abdelhak, A. et al. Serum neurofilament light chain reference database for individual application in paediatric care: a retrospective modelling and validation study. Lancet Neurol. 22, 826–833 (2023).
Wilson, D. et al. Development and multi-center validation of a fully automated digital immunoassay for neurofilament light chain: toward a clinical blood test for neuronal injury. Clin. Chem. Lab. Med. 62, 322–331 (2023).
Janiaud, P. et al. Personalized treatment decision algorithms for the clinical implementation of serum neurofilament light chain in multiple sclerosis: a modified Delphi study. Mult. Scler. J. 29, 650–1044 (2023).
Lycke, J. N., Karlsson, J. E., Andersen, O. & Rosengren, L. E. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 64, 402–404 (1998).
Rosengren, L. E., Karlsson, J. E., Karlsson, J. O., Persson, L. I. & Wikkelsø, C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J. Neurochem. 67, 2013–2018 (1996).
Delcoigne, B. et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 94, e1201–e1212 (2020).
Kuhle, J. et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 92, e1007–e1015 (2019).
Bittner, S. et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: a longitudinal multicentre cohort study. EBioMedicine 56, 102807 (2020).
Piehl, F. et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult. Scler. 24, 1046–1054 (2018).
Ziemssen, T. et al. Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front. Immunol. 13, 852563 (2022).
Bar-Or, A. et al. Blood neurofilament light levels predict non-relapsing progression following anti-CD20 therapy in relapsing and primary progressive multiple sclerosis: findings from the ocrelizumab randomised, double-blind phase 3 clinical trials. EBioMedicine 93, 104662 (2023).
Kapoor, R. et al. Natalizumab reduces serum concentrations of neurofilament light chain in secondary progressive multiple sclerosis patients from the phase 3 ASCEND study (S12.008). Neurology 92, https://doi.org/10.1212/WNL.92.15_supplement.S12.008 (2019).
Kuhle, J. et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 88, 826–831 (2017).
Røsjø, E. et al. Natural variation of vitamin D and neurofilament light chain in relapsing-remitting multiple sclerosis. Front. Neurol. 11, 329 (2020).
Smolders, J. et al. Vitamin D 3 supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol. Scand. 141, 77–80 (2020).
Bridel, C. et al. Serum neurofilament light association with progression in natalizumab-treated patients with relapsing-remitting multiple sclerosis. Neurology 97, e1898–e1905 (2021).
Barro, C. et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 141, 2382–2391 (2018).
Chitnis, T. et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann. Clin. Transl. Neurol. 5, 1478–1491 (2018).
Cantó, E. et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 76, 1359 (2019).
Thebault, S. et al. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 10, 10381 (2020).
Thebault, S. et al. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol. Neuroimmunol. Neuroinflamm. 6, e598 (2019).
Buchmann, A. et al. High serum neurofilament light chain levels correlate with brain atrophy and physical disability in multiple sclerosis. Eur. J. Neurol. 30, 1389–1399 (2023).
Lie, I. A. et al. Serum neurofilament as a predictor of 10-year grey matter atrophy and clinical disability in multiple sclerosis: a longitudinal study. J. Neurol. Neurosurg. Psychiatry 93, 849–857 (2022).
Maggi, P. et al. Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology 97, e543–e553 (2021).
Abdelhak, A. et al. Neurofilament light chain elevation and disability progression in multiple sclerosis. JAMA Neurol. 80, 1317–1325 (2023).
Meier, S. et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol. 80, 287–297 (2023).
Voigt, I., Inojosa, H., Wenk, J., Akgün, K. & Ziemssen, T. Building a monitoring matrix for the management of multiple sclerosis. Autoimmun. Rev. 22, 103358 (2023).
Miyazawa, I. et al. High CSF neurofilament heavy chain levels in neuromyelitis optica. Neurology 68, 865–867 (2007).
Mariotto, S. et al. Neurofilament light chain serum levels reflect disease severity in MOG-Ab associated disorders. J. Neurol. Neurosurg. Psychiatry 90, 1293–1296 (2019).
Mariotto, S. et al. Serum neurofilament light chain in NMOSD and related disorders: comparison according to aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies status. Mult. Scler. J. Exp. Transl. Clin. 3, 205521731774309 (2017).
Watanabe, M. et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology 93, e1299–e1311 (2019).
Chang, X. et al. Serum neurofilament light and GFAP are associated with disease severity in inflammatory disorders with aquaporin-4 or myelin oligodendrocyte glycoprotein antibodies. Front. Immunol. 12, 647618 (2021).
Kim, H. et al. Longitudinal follow-up of serum biomarkers in patients with neuromyelitis optica spectrum disorder. Mult. Scler. 28, 512–521 (2022).
Kim, H. et al. Serum biomarkers in myelin oligodendrocyte glycoprotein antibody-associated disease. Neurol. Neuroimmunol. Neuroinflamm. 7, e708 (2020).
Lista, S. et al. Diagnostic accuracy of CSF neurofilament light chain protein in the biomarker-guided classification system for Alzheimer’s disease. Neurochem. Int. 108, 355–360 (2017).
Mattsson, N., Cullen, N. C., Andreasson, U., Zetterberg, H. & Blennow, K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 76, 791–799 (2019).
Ashton, N. J. et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 12, 3400 (2021).
Dhiman, K. et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimers Dement. 12, e12005 (2020).
Verberk, I. M. W. et al. Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res. Ther. 12, 118 (2020).
Moore, E. E. et al. Neurofilament relates to white matter microstructure in older adults. Neurobiol. Aging 70, 233–241 (2018).
Meeker, K. L. et al. Cerebrospinal fluid neurofilament light chain is a marker of aging and white matter damage. Neurobiol. Dis. 166, 105662 (2022).
Gaetani, L. et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881 (2019).
Benussi, A. et al. Diagnostic and prognostic value of serum NfL and p-Tau 181 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 91, 960–967 (2020).
Steinacker, P. et al. Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt-Jakob disease. Sci. Rep. 6, 38737 (2016).
Abu-Rumeileh, S. et al. The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res. Ther. 10, 3 (2018).
Preische, O. et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat. Med. 25, 277–283 (2019).
Lleó, A. et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimers Dement. 15, 742–753 (2019).
Zetterberg, H. et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 73, 60–67 (2016).
Fortea, J. et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 17, 860–869 (2018).
Aamodt, W. W. et al. Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov. Disord. 36, 2945–2950 (2021).
Bäckström, D. C. et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 72, 1175–1182 (2015).
Hansson, O. et al. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88, 930–937 (2017).
Koga, S., Sekiya, H., Kondru, N., Ross, O. A. & Dickson, D. W. Neuropathology and molecular diagnosis of synucleinopathies. Mol. Neurodegener. 16, 83 (2021).
Thijssen, E. H. et al. Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia. Alzheimers Dement. 14, e12285 (2022).
Baiardi, S. et al. Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimers Res. Ther. 14, 153 (2022).
The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J. Neurol. Neurosurg. Psychiatry 57, 416–418 (1994).
Grossman, M. et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Prim. 9, 40 (2023).
Forgrave, L. M., Ma, M., Best, J. R. & DeMarco, M. L. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Alzheimers Dement. 11, 730–743 (2019).
Davy, V., Dumurgier, J., Fayosse, A., Paquet, C. & Cognat, E. Neurofilaments as emerging biomarkers of neuroaxonal damage to differentiate behavioral frontotemporal dementia from primary psychiatric disorders: a systematic review. Diagnostics 11, 754 (2021).
Al Shweiki, M. R. et al. Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J. Psychiatr. Res. 113, 137–140 (2019).
Ducharme, S. et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain 143, 1632–1650 (2020).
Willemse, E. A. J., Scheltens, P., Teunissen, C. E. & Vijverberg, E. G. B. A neurologist’s perspective on serum neurofilament light in the memory clinic: a prospective implementation study. Alzheimers Res. Ther. 13, 101 (2021).
Gendron, T. F. et al. Comprehensive cross-sectional and longitudinal analyses of plasma neurofilament light across FTD spectrum disorders. Cell Rep. Med. 3, 100607 (2022).
Staffaroni, A. M. et al. Temporal order of clinical and biomarker changes in familial frontotemporal dementia. Nat. Med. 28, 2194–2206 (2022).
Giannini, L. A. A. et al. Clinical value of longitudinal serum neurofilament light chain in prodromal genetic frontotemporal dementia. Neurology 101, e1069–e1082 (2023).
Brettschneider, J., Petzold, A., Süssmuth, S. D., Ludolph, A. C. & Tumani, H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66, 852–856 (2006).
Zetterberg, H., Jacobsson, J., Rosengren, L., Blennow, K. & Andersen, P. M. Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. Eur. J. Neurol. 14, 1329–1333 (2007).
Lu, C.-H. et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84, 2247–2257 (2015).
Menke, R. A. L. et al. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann. Clin. Transl. Neurol. 2, 748–755 (2015).
Steinacker, P. et al. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J. Neurol. Neurosurg. Psychiatry 87, 12–20 (2016).
Steinacker, P. et al. Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: data from the German MND-net. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 112–119 (2017).
De Schaepdryver, M. et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 89, 367–373 (2018).
Schreiber, S. et al. Significance of CSF NfL and tau in ALS. J. Neurol. 265, 2633–2645 (2018).
Abu-Rumeileh, S. et al. Diagnostic-prognostic value and electrophysiological correlates of CSF biomarkers of neurodegeneration and neuroinflammation in amyotrophic lateral sclerosis. J. Neurol. 267, 1699–1708 (2020).
Halbgebauer, S. et al. Comparison of CSF and serum neurofilament light and heavy chain as differential diagnostic biomarkers for ALS. J. Neurol. Neurosurg. Psychiatry 93, 68–74 (2022).
Verde, F. et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 157–164 (2019).
Meyer, T. et al. Performance of serum neurofilament light chain in a wide spectrum of clinical courses of amyotrophic lateral sclerosis-a cross-sectional multicenter study. Eur. J. Neurol. 30, 1600–1610 (2023).
Abu-Rumeileh, S. et al. Comparison between plasma and cerebrospinal fluid biomarkers for the early diagnosis and association with survival in prion disease. J. Neurol. Neurosurg. Psychiatry 91, 1181–1188 (2020).
Halbgebauer, S. et al. Blood β-synuclein and neurofilament light chain during the course of prion disease. Neurology 98, e1434–e1445 (2022).
Gille, B. et al. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 45, 291–304 (2019).
Poesen, K. et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88, 2302–2309 (2017).
Oeckl, P. et al. Proteomics in cerebrospinal fluid and spinal cord suggests UCHL1, MAP2 and GPNMB as biomarkers and underpins importance of transcriptional pathways in amyotrophic lateral sclerosis. Acta Neuropathol. 139, 119–134 (2020).
Benatar, M., Wuu, J., Andersen, P. M., Lombardi, V. & Malaspina, A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann. Neurol. 84, 130–139 (2018).
Benatar, M. et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 95, e59–e69 (2020).
Thompson, A. G. et al. Multicentre appraisal of amyotrophic lateral sclerosis biofluid biomarkers shows primacy of blood neurofilament light chain. Brain Commun. 4, fcac029 (2022).
Feneberg, E. et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90, e22–e30 (2018).
De Schaepdryver, M. et al. Serum neurofilament heavy chains as early marker of motor neuron degeneration. Ann. Clin. Transl. Neurol. 6, 1971–1979 (2019).
Bjornevik, K. et al. Prediagnostic neurofilament light chain levels in amyotrophic lateral sclerosis. Neurology 97, e1466–e1474 (2021).
Benatar, M. et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 538–548 (2019).
Weydt, P. et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann. Neurol. 79, 152–158 (2016).
Goutman, S. A. et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 21, 480–493 (2022).
Boylan, K. B. et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 84, 467–472 (2013).
Gendron, T. F. et al. Phosphorylated neurofilament heavy chain: a biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann. Neurol. 82, 139–146 (2017).
Su, W.-M. et al. Predictors of survival in patients with amyotrophic lateral sclerosis: a large meta-analysis. EBioMedicine 74, 103732 (2021).
Devos, D. et al. A ferroptosis-based panel of prognostic biomarkers for amyotrophic lateral sclerosis. Sci. Rep. 9, 2918 (2019).
Shefner, J. M. et al. Amyotrophic lateral sclerosis clinical trials and interpretation of functional end points and fluid biomarkers: a review. JAMA Neurol. 79, 1312–1318 (2022).
Witzel, S. et al. Neurofilament light and heterogeneity of disease progression in amyotrophic lateral sclerosis: development and validation of a prediction model to improve interventional trials. Transl. Neurodegener. 10, 31 (2021).
Miller, T. et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 383, 109–119 (2020).
Meyer, T. et al. Neurofilament light-chain response during therapy with antisense oligonucleotide tofersen in SOD1-related ALS: treatment experience in clinical practice. Muscle Nerve 67, 515–521 (2023).
Benatar, M. et al. Design of a randomized, placebo-controlled, phase 3 trial of tofersen initiated in clinically presymptomatic SOD1 variant carriers: the ATLAS study. Neurotherapeutics 19, 1248–1258 (2022).
Esselin, F. et al. Repeated neurofilament light chain measurements did not capture riluzole therapeutic effect in amyotrophic lateral sclerosis patients. CNS Neurosci. Ther. 28, 1532–1538 (2022).
Shefner, J. M. et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 131, 1975–1978 (2020).
The German Neurological Society (DGN). Guidelines motoneuron diseases. dgn.org, https://dgn.org/leitlinie/motoneuronerkrankungen [in German] (2021).
Gattringer, T. et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89, 2108–2114 (2017).
Tiedt, S. et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology 91, e1338–e1347 (2018).
Pedersen, A. et al. Circulating neurofilament light in ischemic stroke: temporal profile and outcome prediction. J. Neurol. 266, 2796–2806 (2019).
Gendron, T. F. et al. Plasma neurofilament light predicts mortality in patients with stroke. Sci. Transl. Med. 12, eaay1913 (2020).
Sanchez, J. D. et al. Temporal patterning of neurofilament light as a blood-based biomarker for stroke: a systematic review and meta-analysis. Front. Neurol. 13, 841898 (2022).
De Marchis, G. M. et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur. J. Neurol. 25, 562–568 (2018).
Onatsu, J. et al. Serum neurofilament light chain concentration correlates with infarct volume but not prognosis in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 28, 2242–2249 (2019).
Traenka, C. et al. Serum neurofilament light chain levels are associated with clinical characteristics and outcome in patients with cervical artery dissection. Cerebrovasc. Dis. 40, 222–227 (2015).
Pujol-Calderón, F. et al. Neurofilament changes in serum and cerebrospinal fluid after acute ischemic stroke. Neurosci. Lett. 698, 58–63 (2019).
Uphaus, T. et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke 50, 3077–3084 (2019).
Wang, P., Fan, J., Yuan, L., Nan, Y. & Nan, S. Serum neurofilament light predicts severity and prognosis in patients with ischemic stroke. Neurotox. Res. 37, 987–995 (2020).
Rahmig, J. et al. Serum neurofilament light chain levels are associated with stroke severity and functional outcome in patients undergoing endovascular therapy for large vessel occlusion. J. Neurol. Sci. 429, 118063 (2021).
Jacob, M. A. et al. Increased neurofilament light chain is associated with increased risk of long-term mortality in cerebral small vessel disease. J. Stroke 24, 296–299 (2022).
Duering, M. et al. Serum neurofilament light chain levels are related to small vessel disease burden. J. Stroke 20, 228–238 (2018).
Peters, N. et al. Serum neurofilament light chain is associated with incident lacunes in progressive cerebral small vessel disease. J. Stroke 22, 369–376 (2020).
Peters, N. Neurofilament light chain as a biomarker in cerebral small-vessel disease. Mol. Diagn. Ther. 26, 1–6 (2022).
Gravesteijn, G. et al. Serum neurofilament light correlates with CADASIL disease severity and survival. Ann. Clin. Transl. Neurol. 6, 46–56 (2019).
Chen, C.-H., Cheng, Y.-W., Chen, Y.-F., Tang, S.-C. & Jeng, J.-S. Plasma neurofilament light chain and glial fibrillary acidic protein predict stroke in CADASIL. J. Neuroinflammation 17, 124 (2020).
Zhang, X., Wang, H., Li, L., Deng, X. & Bo, L. Neurofilament light chain: a candidate biomarker of perioperative stroke. Front. Aging Neurosci. 14, 921809 (2022).
Taylor, J. et al. Perioperative ischaemic brain injury and plasma neurofilament light: a secondary analysis of two prospective cohort studies. Br. J. Anaesth. 130, e361–e369 (2023).
Pinter, D. et al. Longitudinal MRI dynamics of recent small subcortical infarcts and possible predictors. J. Cereb. Blood Flow. Metab. 39, 1669–1677 (2019).
Egle, M. et al. Neurofilament light chain predicts future dementia risk in cerebral small vessel disease. J. Neurol. Neurosurg. Psychiatry 92, 582–589 (2021).
Heshmatollah, A. et al. Plasma β-amyloid, total-tau, and neurofilament light chain levels and the risk of stroke: a prospective population-based study. Neurology 98, e1729–e1737 (2022).
Korley, F. K. et al. Serum NfL (neurofilament light chain) levels and incident stroke in adults with diabetes mellitus. Stroke 50, 1669–1675 (2019).
Polymeris, A. A. et al. Renal function and body mass index contribute to serum neurofilament light chain levels in elderly patients with atrial fibrillation. Front. Neurosci. 16, 819010 (2022).
Lota, K. S. et al. Rotational head acceleration and traumatic brain injury in combat sports: a systematic review. Br. Med. Bull. 141, 33–46 (2022).
Bergman, J. et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol. Neuroimmunol. Neuroinflamm. 3, e271 (2016).
Shahim, P. et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6, 36791 (2016).
Gill, J. et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 91, e1385–e1389 (2018).
Iverson, G. L. et al. Serum neurofilament light is elevated differentially in older adults with uncomplicated mild traumatic brain injuries. J. Neurotrauma 36, 2400–2406 (2019).
Hossain, I. et al. Early levels of glial fibrillary acidic protein and neurofilament light protein in predicting the outcome of mild traumatic brain injury. J. Neurotrauma 36, 1551–1560 (2019).
Shahim, P. et al. Neurofilament light as a biomarker in traumatic brain injury. Neurology 95, e610–e622 (2020).
Shahim, P., Tegner, Y., Marklund, N., Blennow, K. & Zetterberg, H. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology 90, e1780–e1788 (2018).
Shahim, P., Zetterberg, H., Tegner, Y. & Blennow, K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 88, 1788–1794 (2017).
Farragher, C. D., Ku, Y. & Powers, J. E. The potential role of neurofilament light in mild traumatic brain injury diagnosis: a systematic review. Cureus 14, e31301 (2022).
Thelin, E. et al. A serum protein biomarker panel improves outcome prediction in human traumatic brain injury. J. Neurotrauma 36, 2850–2862 (2019).
Barro, C., Chitnis, T. & Weiner, H. L. Blood neurofilament light: a critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 7, 2508–2523 (2020).
Rosén, H., Karlsson, J.-E. & Rosengren, L. CSF levels of neurofilament is a valuable predictor of long-term outcome after cardiac arrest. J. Neurological Sci. 221, 19–24 (2004).
Rana, O. R. et al. Neurofilament light chain as an early and sensitive predictor of long-term neurological outcome in patients after cardiac arrest. Int. J. Cardiol. 168, 1322–1327 (2013).
Rosén, C. et al. Cerebrospinal fluid biomarkers in cardiac arrest survivors. Resuscitation 85, 227–232 (2014).
Rundgren, M., Friberg, H., Cronberg, T., Romner, B. & Petzold, A. Serial soluble neurofilament heavy chain in plasma as a marker of brain injury after cardiac arrest. Crit. Care 16, R45 (2012).
Abu-Rumeileh, S. et al. The multifaceted role of neurofilament light chain protein in non-primary neurological diseases. Brain 146, 421–427 (2022).
Moseby-Knappe, M. et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 76, 64 (2019).
Disanto, G. et al. Serum neurofilament light chain as a prognostic marker in postanoxic encephalopathy. Epilepsy Behav. 101, 106432 (2019).
Fisse, A. L. et al. Serum neurofilament light chain as outcome marker for intensive care unit patients. J. Neurol. 268, 1323–1329 (2021).
Wihersaari, L. et al. Neurofilament light as an outcome predictor after cardiac arrest: a post hoc analysis of the COMACARE trial. Intensive Care Med. 47, 39–48 (2021).
Wihersaari, L. et al. Neurofilament light compared to neuron-specific enolase as a predictor of unfavourable outcome after out-of-hospital cardiac arrest. Resuscitation 174, 1–8 (2022).
Hunziker, S. et al. Serum neurofilament measurement improves clinical risk scores for outcome prediction after cardiac arrest: results of a prospective study. Crit. Care 25, 32 (2021).
Andersson, P. et al. Predicting neurological outcome after out-of-hospital cardiac arrest with cumulative information; development and internal validation of an artificial neural network algorithm. Crit. Care 25, 83 (2021).
Blennow Nordström, E. et al. Serum neurofilament light levels are correlated to long-term neurocognitive outcome measures after cardiac arrest. Brain Inj. 36, 800–809 (2022).
Pouplet, C. et al. The accuracy of various neuro-prognostication algorithms and the added value of neurofilament light chain dosage for patients resuscitated from shockable cardiac arrest: an ancillary analysis of the ISOCRATE study. Resuscitation 171, 1–7 (2022).
Levin, H. et al. Plasma neurofilament light is a predictor of neurological outcome 12 h after cardiac arrest. Crit. Care 27, 74 (2023).
Adler, C. et al. Absolute serum neurofilament light chain levels and its early kinetics predict brain injury after out-of-hospital cardiac arrest. J. Neurol. 269, 1530–1537 (2022).
Kirschen, M. P. et al. Circulating neurofilament light chain is associated with survival after pediatric cardiac arrest*. Pediatr. Crit. Care Med. 21, 656–661 (2020).
Fink, E. L. et al. Association of blood-based brain injury biomarker concentrations with outcomes after pediatric cardiac arrest. JAMA Netw. Open 5, e2230518 (2022).
Hoiland, R. L. et al. Neurologic prognostication after cardiac arrest using brain biomarkers: a systematic review and meta-analysis. JAMA Neurol. 79, 390–398 (2022).
Fu, Y. et al. Neuroprognostication value of serum neurofilament light chain for out-of-hospital cardiac arrest: a systematic review and meta-analysis. PLoS ONE 18, e0290619 (2023).
Ashton, N. J. et al. Alzheimer disease blood biomarkers in patients with out-of-hospital cardiac arrest. JAMA Neurol. 80, 388–396 (2023).
Wang, S. L., Li, N., Feng, S. Y. & Li, Y. Serum neurofilament light chain as a predictive marker of neurologic outcome after cardiac arrest: a meta-analysis. BMC Cardiovasc. Disord. 23, 193 (2023).
Nolan, J. P. et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med 47, 369–421 (2021).
Panchal, A. R. et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 142, S366–S468 (2020).
Moseby-Knappe, M. et al. Serum markers of brain injury can predict good neurological outcome after out-of-hospital cardiac arrest. Intensive Care Med. 47, 984–994 (2021).
Lybeck, A. et al. Postanoxic electrographic status epilepticus and serum biomarkers of brain injury. Resuscitation 158, 253–257 (2021).
Grindegård, L. et al. Association between EEG patterns and serum neurofilament light after cardiac arrest: a post hoc analysis of the TTM trial. Neurology 98, e2487–e2498 (2022).
Lagebrant, A. et al. Brain injury markers in blood predict signs of hypoxic ischaemic encephalopathy on head computed tomography after cardiac arrest. Resuscitation 184, 109668 (2023).
Tolosa, E., Garrido, A., Scholz, S. W. & Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397 (2021).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s progression markers initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Quadalti, C. et al. Neurofilament light chain and α-synuclein RT-QuIC as differential diagnostic biomarkers in parkinsonisms and related syndromes. NPJ Parkinsons Dis. 7, 93 (2021).
Holmberg, B., Rosengren, L., Karlsson, J. E. & Johnels, B. Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple-system atrophy compared with Parkinson’s disease. Mov. Disord. 13, 70–77 (1998).
Brettschneider, J. et al. Neurofilament heavy-chain NfH(SMI35) in cerebrospinal fluid supports the differential diagnosis of Parkinsonian syndromes. Mov. Disord. 21, 2224–2227 (2006).
Hall, S. et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452 (2012).
Magdalinou, N. K. et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 86, 1240–1247 (2015).
Mollenhauer, B. et al. Validation of serum neurofilament light chain as a biomarker of Parkinson’s disease progression. Mov. Disord. 35, 1999–2008 (2020).
Marques, T. M. et al. Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92, e1479–e1486 (2019).
Peng, L. et al. Diagnostic and prognostic performance of plasma neurofilament light chain in multiple system atrophy: a cross-sectional and longitudinal study. J. Neurol. 270, 4248–4261 (2023).
Angelopoulou, E. et al. CSF and circulating NfL as biomarkers for the discrimination of Parkinson disease from atypical parkinsonian syndromes: meta-analysis. Neurol. Clin. Pract. 11, e867–e875 (2021).
Martinez-Valbuena, I. et al. Combining skin α-synuclein real-time quaking-induced conversion and circulating neurofilament light chain to distinguish multiple system atrophy and Parkinson’s disease. Mov. Disord. 37, 648–650 (2022).
Bäckström, D. et al. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 95, e827–e838 (2020).
Rojas, J. C. et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 90, e273–e281 (2018).
Batzu, L. et al. Plasma p-tau181, neurofilament light chain and association with cognition in Parkinson’s disease. NPJ Parkinsons Dis. 8, 154 (2022).
Chelban, V. et al. Neurofilament light levels predict clinical progression and death in multiple system atrophy. Brain 145, 4398–4408 (2022).
Vijiaratnam, N. et al. Combining biomarkers for prognostic modelling of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 93, 707–715 (2022).
Ygland Rödström, E., Mattsson-Carlgren, N., Janelidze, S., Hansson, O. & Puschmann, A. Serum neurofilament light chain as a marker of progression in Parkinson’s disease: long-term observation and implications of clinical subtypes. J. Parkinsons Dis. 12, 571–584 (2022).
Donker Kaat, L. et al. Serum neurofilament light chain in progressive supranuclear palsy. Parkinsonism Relat. Disord. 56, 98–101 (2018).
Oosterveld, L. P. et al. CSF biomarkers reflecting protein pathology and axonal degeneration are associated with memory, attentional, and executive functioning in early-stage Parkinson’s disease. Int. J. Mol. Sci. 21, 8519 (2020).
Barba, L. et al. CSF synaptic biomarkers in AT(N)-based subgroups of Lewy body disease. Neurology 101, e50–e62 (2023).
Ye, R. et al. Serum NFL levels predict progression of motor impairment and reduction in putamen dopamine transporter binding ratios in de novo Parkinson’s disease: an 8-year longitudinal study. Parkinsonism Relat. Disord. 85, 11–16 (2021).
Yang, D. et al. Neurofilament light chain as a mediator between LRRK2 mutation and dementia in Parkinson’s disease. NPJ Parkinsons Dis. 9, 132 (2023).
Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Ann. Neurol. 89, 1212–1220 (2021).
Park, D. G. et al. Neurofilament light chain and cardiac MIBG uptake as predictors for phenoconversion in isolated REM sleep behavior disorder. J. Neurol. 270, 4393–4402 (2023).
Zhang, X. et al. Neurofilament light protein predicts disease progression in idiopathic REM sleep behavior disorder. J. Parkinsons Dis. 13, 485–499 (2023).
Byrne, L. M. et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol. 16, 601–609 (2017).
Byrne, L. M. et al. Neurofilament light protein as a potential blood biomarker for Huntington’s disease in children. Mov. Disord. 37, 1526–1531 (2022).
Gregory, S. et al. Characterizing white matter in Huntington’s disease. Mov. Disord. Clin. Pract. 7, 52–60 (2020).
Scahill, R. I. et al. Biological and clinical characteristics of gene carriers far from predicted onset in the Huntington’s disease Young Adult Study (HD-YAS): a cross-sectional analysis. Lancet Neurol. 19, 502–512 (2020).
Parkin, G. M., Corey-Bloom, J., Snell, C., Castleton, J. & Thomas, E. A. Plasma neurofilament light in Huntington’s disease: a marker for disease onset, but not symptom progression. Parkinsonism Relat. Disord. 87, 32–38 (2021).
Parkin, G. M. et al. Associations between prognostic index scores and plasma neurofilament light in Huntington’s disease. Parkinsonism Relat. Disord. 97, 25–28 (2022).
Byrne, L. M. et al. Evaluation of mutant huntingtin and neurofilament proteins as potential markers in Huntington’s disease. Sci. Transl. Med. 10, eaat7108 (2018).
Rodrigues, F. B. et al. Mutant huntingtin and neurofilament light have distinct longitudinal dynamics in Huntington’s disease. Sci. Transl. Med. 12, eabc2888 (2020).
Feasby, T. E. et al. An acute axonal form of Guillain-Barré polyneuropathy. Brain 109, 1115–1126 (1986).
Petzold, A. et al. CSF neurofilament levels: a potential prognostic marker in Guillain-Barré syndrome. Neurology 67, 1071–1073 (2006).
Axelsson, M. et al. Neurofilament light protein levels in cerebrospinal fluid predict long-term disability of Guillain-Barré syndrome: a pilot study. Acta Neurol. Scand. 138, 143–150 (2018).
Petzold, A. et al. CSF protein biomarkers for proximal axonal damage improve prognostic accuracy in the acute phase of Guillain-Barré syndrome. Muscle Nerve 40, 42–49 (2009).
Altmann, P. et al. Increased serum neurofilament light chain concentration indicates poor outcome in Guillain-Barré syndrome. J. Neuroinflammation 17, 86 (2020).
Körtvelyessy, P. et al. Ratio and index of Neurofilament light chain indicate its origin in Guillain-Barré syndrome. Ann. Clin. Transl. Neurol. 7, 2213–2220 (2020).
Martín-Aguilar, L. et al. Serum neurofilament light chain predicts long-term prognosis in Guillain-Barré syndrome patients. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2020-323899 (2020).
Dujmovic, I., Lunn, M. P., Reilly, M. M. & Petzold, A. Serial cerebrospinal fluid neurofilament heavy chain levels in severe Guillain-Barré syndrome. Muscle Nerve 48, 132–134 (2013).
Jin, M. et al. Cerebrospinal fluid neurofilament light chain predicts short-term prognosis in pediatric Guillain-Barré syndrome. Front. Neurol. 13, 972367 (2022).
Sandelius, Å. et al. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90, e518–e524 (2018).
Bomont, P. et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat. Genet. 26, 370–374 (2000).
Mariotto, S. et al. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst. 23, 174–177 (2018).
van Lieverloo, G. G. A. et al. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J. Peripher. Nerv. Syst. 24, 187–194 (2019).
Karam, C. Chronic inflammatory demyelinating polyradiculoneuropathy: five new things. Neurol. Clin. Pract. 12, 258–262 (2022).
Kapoor, M. et al. Association of plasma neurofilament light chain with disease activity in chronic inflammatory demyelinating polyradiculoneuropathy. Eur. J. Neurol. 29, 3347–3357 (2022).
Kmezic, I. et al. Neurofilament light chain and total tau in the differential diagnosis and prognostic evaluation of acute and chronic inflammatory polyneuropathies. Eur. J. Neurol. 29, 2810–2822 (2022).
Kim, S.-H. et al. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci. Rep. 10, 7995 (2020).
Huehnchen, P. et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 7, e154395 (2022).
Bischof, A. et al. Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann. Rheum. Dis. 77, 1093–1094 (2018).
Rossor, A. M. et al. A longitudinal and cross-sectional study of plasma neurofilament light chain concentration in Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 27, 50–57 (2022).
Millere, E. et al. Plasma neurofilament light chain as a potential biomarker in Charcot-Marie-Tooth disease. Eur. J. Neurol. 28, 974–981 (2021).
Rossor, A. M. et al. Plasma neurofilament heavy chain is not a useful biomarker in Charcot-Marie-Tooth disease. Muscle Nerve 53, 972–975 (2016).
Kapoor, M. et al. Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J. Peripher. Nerv. Syst. 24, 314–319 (2019).
Ticau, S. et al. Neurofilament light chain as a biomarker of hereditary transthyretin-mediated amyloidosis. Neurology 96, e412–e422 (2021).
Maia, L. F. et al. Plasma neurofilament light chain: an early biomarker for hereditary ATTR amyloid polyneuropathy. Amyloid 27, 97–102 (2020).
Carroll, A. S. et al. Serum neurofilament light chain in hereditary transthyretin amyloidosis: validation in real-life practice. Amyloid https://doi.org/10.1080/13506129.2024.2313218 (2024).
Faravelli, I. et al. Nusinersen treatment and cerebrospinal fluid neurofilaments: an explorative study on spinal muscular atrophy type 3 patients. J. Cell Mol. Med. 24, 3034–3039 (2020).
Kong, L. et al. Impaired prenatal motor axon development necessitates early therapeutic intervention in severe SMA. Sci. Transl. Med. 13, eabb6871 (2021).
Darras, B. T. et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann. Clin. Transl. Neurol. 6, 932–944 (2019).
Nitz, E. et al. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann. Clin. Transl. Neurol. 8, 2013–2024 (2021).
Jin, J. et al. Plasma neurofilament light chain in Chinese children with later-onset spinal muscular atrophy. Clin. Chem. Lab. Med. 60, e237–e239 (2022).
Reilly, A. et al. Central and peripheral delivered AAV9-SMN are both efficient but target different pathomechanisms in a mouse model of spinal muscular atrophy. Gene Ther. 29, 544–554 (2022).
Ru, Y. et al. Neurofilament light is a treatment-responsive biomarker in CLN2 disease. Ann. Clin. Transl. Neurol. 6, 2437–2447 (2019).
Wurster, C. D. et al. Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther. Adv. Neurol. Disord. 12, 1756286419846058 (2019).
Finkel, R. S. et al. Scientific rationale for a higher dose of nusinersen. Ann. Clin. Transl. Neurol. 9, 819–829 (2022).
De Wel, B., De Schaepdryver, M., Poesen, K. & Claeys, K. G. Biochemical and clinical biomarkers in adult SMA 3-4 patients treated with nusinersen for 22 months. Ann. Clin. Transl. Neurol. 9, 1241–1251 (2022).
Wilke, C. et al. Neurofilaments in spinocerebellar ataxia type 3: blood biomarkers at the preataxic and ataxic stage in humans and mice. EMBO Mol. Med. 12, e11803 (2020).
Peng, Y. et al. Association of serum neurofilament light and disease severity in patients with spinocerebellar ataxia type 3. Neurology 95, e2977–e2987 (2020).
Peng, L. et al. Blood neurofilament light chain in genetic ataxia: a meta-analysis. Mov. Disord. 37, 171–181 (2022).
Oender, D. et al. Evolution of clinical outcome measures and biomarkers in sporadic adult-onset degenerative ataxia. Mov. Disord. 38, 654–664 (2023).
Agrawal, N. et al. Neurofilament light chain in cerebrospinal fluid as a novel biomarker in evaluating both clinical severity and therapeutic response in Niemann-Pick disease type C1. Genet. Med. 25, 100349 (2023).
Nass, R. D. et al. Serum biomarkers of cerebral cellular stress after self-limiting tonic clonic seizures: an exploratory study. Seizure 85, 1–5 (2021).
Giovannini, G. et al. Serum neurofilament light as biomarker of seizure-related neuronal injury in status epilepticus. Epilepsia 63, e23–e29 (2022).
Ouédraogo, O. et al. Increased frequency of proinflammatory CD4 T cells and pathological levels of serum neurofilament light chain in adult drug-resistant epilepsy. Epilepsia 62, 176–189 (2021).
Nissen, M. S. et al. CSF-neurofilament light chain levels in NMDAR and LGI1 encephalitis: a national cohort study. Front. Immunol. 12, 719432 (2021).
Lardeux, P. et al. Core cerebrospinal fluid biomarker profile in anti-LGI1 encephalitis. J. Neurol. 269, 377–388 (2022).
Guasp, M. et al. Neurofilament light chain levels in anti-NMDAR encephalitis and primary psychiatric psychosis. Neurology 98, e1489–e1498 (2022).
Ziemssen, T. et al. Serum neurofilament light chain as a biomarker of brain injury in Wilson’s disease: clinical and neuroradiological correlations. Mov. Disord. 37, 1074–1079 (2022).
Hermann, P. et al. Plasma neurofilament light chain as a biomarker for fatal familial insomnia. Eur. J. Neurol. 29, 1841–1846 (2022).
Alagaratnam, J. et al. Correlation between cerebrospinal fluid and plasma neurofilament light protein in treated HIV infection: results from the COBRA study. J. Neurovirol. 28, 54–63 (2022).
Smeele, P. J. et al. Neurofilament light increases over time in severe COVID-19 and is associated with delirium. Brain Commun. 4, fcac195 (2022).
Cooper, J. et al. Quantification of neurological blood-based biomarkers in critically ill patients with coronavirus disease 2019. Crit. Care Explor. 2, e0238 (2020).
Masvekar, R. R. et al. Prognostic value of serum/plasma neurofilament light chain for COVID-19-associated mortality. Ann. Clin. Transl. Neurol. 9, 622–632 (2022).
Abdelhak, A. et al. Prognostic performance of blood neurofilament light chain protein in hospitalized COVID-19 patients without major central nervous system manifestations: an individual participant data meta-analysis. J. Neurol. 270, 3315–3328 (2023).
Syrjanen, J. A. et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 18, 1128–1140 (2022).
Bittner, S., Oh, J., Havrdová, E. K., Tintoré, M. & Zipp, F. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain 144, 2954–2963 (2021).
UniProt. P07197 HFM_Human https://www.uniprot.org/uniprotkb/P07197/entry (2024).
UniProt. P12036 NFH_Human https://www.uniprot.org/uniprotkb/P12036/entry (2024).
UniProt. P07196. NFL_Human https://www.uniprot.org/uniprotkb/P07196/entry (2024).
UniProt. Q16352. AINX_Human https://www.uniprot.org/uniprotkb/Q16352/entry (2024).
UniProt. P41219. PERI_Human https://www.uniprot.org/uniprotkb/P41219/entry (2024).
Acknowledgements
C.E.T.’s research is supported by the European Commission (Marie Curie International Training Network, grant agreement No. 860197 (MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant No. 831434), European Platform for Neurodegenerative Diseases (IMI 2 Joint Undertaking (JU), grant No. 101034344) and European Union Joint Programme – Neurodegenerative Disease Research (JPND; bPRIDE), National MS Society (Progressive MS alliance), Alzheimer Association, Health Holland, the Dutch Research Council (ZonMw), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, and Alzheimer Netherlands. C.T.E. is a recipient of ABOARD, which is a public–private partnership receiving funding from ZonMw (#73305095007) and Health Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). C.E.T. is recipient of TAP-dementia, a ZonMw-funded project (#10510032120003) in the context of the Dutch National Dementia Strategy. S.L. is supported by the Fondation pour la Recherche Médicale (FRM), the Claude Pompidou Foundation, H2020 Marie Skłodowska-Curie Actions MIRIADE project, and the European Metrology Programme for Innovation and Research Neuromet2 project. M.O. was supported by the JPND networks Genfi-Prox (01ED2008A) and bPRIDE (01ED2001), the German Federal Ministry of Education and Research (FTLDc 01GI1007A, Moodmarker 01EW200), the EU (MIRIADE 860197, FAIR-PARK II 633190), the German Research Foundation/DFG (SFB1279), the Foundation of the State Baden-Württemberg (D.3830), Boehringer Ingelheim Ulm University BioCenter (D.5009) and the Thierry Latran Foundation. F.P. is funded by the Swedish MRC (grant no. 2020-02700), the Knut and Alice Wallenberg Foundation, and the Swedish Brain Foundation. S.B. is supported by the Deutsche Forschungsgemeinschaft (DFG, SFB CRC-TR-128 and CRC-TR-355) and the Hermann and Lilly Schilling Foundation. T.G. acknowledges research support from the Austrian Science Fund and the Austrian Neurological Society. S.A.-R. received support from the Medical Faculty of Martin-Luther-University Halle-Wittenberg (Clinician Scientist-Programm No. CS22/06). S.T. is supported by the MS Canada for Postdoctoral Fellowship. H.T. acknowledges research support by DMSG (German Multiple Sclerosis Society), MWK-BW (Ministry of Science, Research and Arts of the State Baden-Württemberg), University of Ulm, and Chemische Fabrik Karl Bucher. A.P. acknowledges support from the National Institute of Health and Care Research Biomedical Research Centre at Moorfields Eye Hospital and the UK Department of Health. K.B. is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF agreement (#ALFGBG-715986 and #ALFGBG-965240), the JPND (JPND2019-466-236), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), and the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC). H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No. 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 860197 (MIRIADE), the JPND (JPND2021-00694), the National Institute for Health and Care Research UCL Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). D.L. is supported by the International Progressive MS Alliance (grant PA-2002-36227). J.K. is supported by the Swiss MS Society, the Swiss National Research Foundation (320030_189140/1 and 212534/1), the University of Basel and the International Progressive MS Alliance. Parts of the manuscript have been generated by members of the BioMS-eu consortium.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.K. has received funding for travel and speaker honoraria from Bayer, Biogen Idec, Merck, Novartis, and Teva Pharmaceutical Industries and serves on scientific advisory boards for Biogen Idec, Merck Serono, Novartis, and Roche outside the submitted work. C.E.T. has collaboration contracts with ADx Neurosciences, Eli Lilly, and Quanterix and has performed contract research or received grants from AC Immune, Axon Neurosciences, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, PeopleBio, Roche, Siemens, Toyama, and Vivoryon. She is editor of Alzheimer Research and Therapy, and serves on the editorial boards of Medidact Neurologie/Springer and Neurology: Neuroimmunology & Neuroinflammation. S.L. has served as a consultant or on advisory boards for Biogen, Fujirebio-Europe, Lilly, Roche Diagnostics and Shimadzu. M.O. has given scientific advice to AXON, AviadoBio, Biogen, Fujirebio and Roche. F.P. has received research grants from Janssen, Merck KGaA and UCB, has received fees for serving on Data Monitoring Committees (DMC) in clinical trials with Chugai, Lundbeck and Roche, and has prepared an expert witness statement for Novartis. T.Z. has contributed to scientific advisory boards and/or has consulted for Biogen, Celgene, Novartis, Merck and Roche, has received compensation for serving on speakers bureaus for Biogen, Celgene, Merck, Novartis, Roche and Sanofi, and has received research support from Biogen, Merck, Novartis, and Sanofi. S.B. has received honoraria from Biogen Idec, Bristol Meyer Squibbs, Hexal, Merck Healthcare, Novartis, Roche, Sanofi-Genzyme and Teva. M.P.S. has received consulting fees from Alexion, Biogen, Immunic, Merck, Novartis, Roche and Sanofi. T.G. has received travel grants and speakers’ honoraria from Amgen, Bayer, Boehringer Ingelheim, Novartis and Pfizer outside the submitted work. A.A. has received research grants from Denali Therapeutics and Roche, outside the submitted work. A.G. has received research grants from Denali Therapeutics and F. Hoffmann-La Roche. A.G. has received personal fees from JAMA Neurology, Neurona and Pipeline Pharmaceuticals outside the submitted work. A.G. also has a patent pending for a small-molecule drug for remyelination. The institutes of L.K. (University Hospital Basel and the Research Center for Neuroimmunology and Neuroscience Basel) have received the following exclusively for research support: steering committee, advisory board and consultancy fees from Bayer, Biogen, BMS, Janssen (J&J), Merck, Novartis, Roche, Sanofi, Santhera and TG Therapeutics; speaker fees from Bayer, Biogen, Merck, Novartis, Roche and Sanofi; support of educational activities from Merck, Novartis, Roche and Sanofi; license fees for Neurostatus products; and grants from Bayer, Biogen, the European Union, Innosuisse, Merck, Novartis, Roche, the Swiss MS Society and the Swiss National Research Foundation. M.C. has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Biogen Idec, Bristol Myers Squibb, Genzyme, Merk Serono, Novartis, Teva Pharmaceuticals and Sanofi-Aventis. H.T. has participated in meetings sponsored by or received honoraria for acting as an advisor or speaker for Alexion, Bayer, Biogen, Bristol Myers Squibb, Celgene, Diamed, Fresenius, Fujirebio, GlaxoSmithKline, Horizon, Janssen-Cilag, Merck, Novartis, Roche, Sanofi-Genzyme, Siemens and Teva. M.S.F. has received research or educational grants from Sanofi-Genzyme Canada, has received honoraria or consultation fees from Alexion/Astra Zeneca, BiogenIdec, EMD Inc./EMD Serono/Merck Serono, Find Therapeutics, Hoffman-La Roche, Novartis, Quanterix, Sanofi-Genzyme, and Teva Canada Innovation, is a member of company advisory boards, boards of directors or similar for Alexion/Astra Zeneca, Atara Biotherapeutics, Bayer Healthcare, Celestra Health, EMD Inc./Merck Serono, Find Therapeutics, Hoffman-La Roche, Actelion/Janssen (J&J), Novartis, Sanofi-Genzyme, and Setpoint Medical, and has participated in a company-sponsored speaker’s bureau for EMD Serono and Sanofi-Genzyme. A.P. has received grant support for remyelination trials in multiple sclerosis to the Amsterdam University Medical Centre. K.B. has served as a consultant and on advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Lilly, Moleac, Novartis, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programmes for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work in this paper. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work in this paper. D.L. is Chief Medical Officer of GeNeuro. J.K. has received speaker fees, research support, travel support, and/or served on advisory boards from Alnylam, Bayer, Biogen, Bristol Myers Squibb, Celgene, Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Quanterix, Roche, Sanofi and Stata DX. S.A.-R., S.T. and P.B. declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks T. Cronberg, G. Shaw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BioMS-eu consortium: https://bioms-eu.com
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalil, M., Teunissen, C.E., Lehmann, S. et al. Neurofilaments as biomarkers in neurological disorders — towards clinical application. Nat Rev Neurol (2024). https://doi.org/10.1038/s41582-024-00955-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41582-024-00955-x