Abstract
HER2 is an established therapeutic biomarker in advanced or recurrent endometrial serous carcinoma. Current clinical guidelines recommend HER2 testing exclusively in this endometrial carcinoma (EC) subtype; however, the full spectrum of ECs harboring HER2 amplification remains ill-defined. The present study characterizes the clinicopathologic and molecular features of HER2-amplified ECs across all histologic subtypes. Retrospective analysis of our institutional cohort of 2,042 ECs subjected to targeted clinical massively parallel sequencing identified 77 (3.8%) cases with HER2 amplification, a group comprised of serous (n = 29), endometrioid (low-grade, n = 2, high-grade, n = 1) and clear cell (n = 4) carcinomas, carcinosarcomas (n = 18) and high-grade ECs with ambiguous features (HGEC, n = 23). A co-existing TP53 mutation was identified in 94% (72/77) of HER2-amplified ECs. Other recurrent genetic alterations included amplification of CCNE1 (22%) and ERBB3 (10%), FBXW7 mutations or deletions (13%), and mutations in PIK3CA (40%) and PPP2R1A (13%). The HER2 immunohistochemistry score was 2+ or 3+ for all evaluable cases (n = 61). Apart from carcinosarcomas, which often showed lower HER2 expression, particularly in the sarcomatous component, HER2 immunohistochemical staining pattern and intensity were similar across EC subtypes. Intratumor heterogeneity in HER2 expression was common and correlated with genetic heterogeneity as detected by fluorescence in-situ hybridization. These results demonstrate the frequent co-occurrence of HER2 amplification with TP53 mutation and high-grade histology, rather than being specific to serous carcinoma, per se. Overall, these findings suggest that HER2 targeted therapy may be more broadly applicable to all high-grade EC histotypes and consideration should be given to expanding therapeutic eligibility.
Similar content being viewed by others
Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy and is traditionally subclassified based on histomorphology. Endometrioid, serous, and clear cell carcinomas constitute the major epithelial subtypes. Carcinosarcoma is also considered a form of EC that has undergone sarcomatous transformation. It is not uncommon, particularly in high-grade tumors, to encounter overlapping histologic features between various subtypes, and considerable interobserver variability in subtype classification of high-grade ECs is well-documented1. Nevertheless, genetic analyses have identified associations between tumor histology and underlying somatic genetic alterations. Early studies, later confirmed by The Cancer Genome Atlas, have established endometrial serous carcinomas to be characterized by TP53 mutation and high frequency of HER2 amplification, in contrast to endometrioid carcinomas, which are often driven by PTEN loss2,3,4,5,6.
Human epidermal growth factor receptor 2 (HER2), a transmembrane tyrosine kinase receptor, is encoded by ERBB2 (HER2), located on 17q127. HER2 amplification leads to overexpression of the gene and is correlated with poor prognosis in several tumor types, including breast8, gastroesophageal9, and ECs10. HER2 targeted therapies, including the monoclonal antibody trastuzumab, have become the mainstay in the treatment of patients with HER2-positive breast and gastroesophageal tumors11. A randomized phase II study demonstrated progression-free and overall survival benefit in patients with advanced stage or recurrent HER2-overexpressing serous EC treated with carboplatin paclitaxel-trastuzumab compared to patients treated with chemotherapy alone, with the greatest benefit seen in stage III/IV disease12. Based on this pivotal work, trastuzumab has been incorporated into recent National Comprehensive Cancer Network (NCCN) guidelines for treatment of advanced or recurrent serous EC with HER2 overexpression/amplification13.
Given the clinical efficacy of anti-HER2 targeted therapy in HER2-positive serous carcinoma, and the paucity of effective treatment options currently available for high-grade EC, the potential of extending this therapy to other types of EC is of great interest. At our institution, the vast majority of ECs are subjected to clinical next-generation sequencing (NGS) using the MSK-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay14, presenting an unprecedented opportunity to interrogate specific genetic alterations in a large cohort of ECs encompassing all histologic subtypes. In this study, we conducted a retrospective analysis of ECs subjected to MSK-IMPACT to characterize the clinicopathologic and molecular features associated with HER2 amplification. Intratumor heterogeneity in HER2 expression and amplification were assessed by immunohistochemistry (IHC) and fluorescence in-situ hybridization (FISH), respectively.
Materials and methods
Case selection
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center and written informed consent was obtained from all patients. All ECs that underwent clinical FDA-authorized tumor-normal targeted massively parallel sequencing analysis of up to 468 cancer-related genes by MSK-IMPACT14, from 2014 to 2019 were evaluated (n = 2,042).
In addition to mutations, the MSK-IMPACT assay also provides an assessment of genome-wide copy number14. All ECs with HER2 amplification, defined by tumor/normal fold change (FC) ≥ 2.0 with P < 0.05, were identified (n = 77), based on a prior study establishing optimal amplification threshold criteria using MSK-IMPACT data from breast and gastroesophageal carcinomas15.
Morphologic review
Review of the original pathology reports and histomorphologic re-review of representative slides were performed on all cases with HER2 amplification identified from the MSK-IMPACT database to confirm the diagnosis. Slide review was performed by a subspecialty-trained gynecologic pathologist (MHC) and discrepancies with the original rendered diagnosis were confirmed with a second gynecologic pathologist (LHE). Classification of tumor subtype was rendered using WHO 2020 criteria16, and based primarily on histomorphology, and if applicable, immunohistochemical stains performed at the time of original diagnosis. For high-grade ECs, tumors with variable morphologic features overlapping between the major subtypes (namely, endometrioid, serous and/or clear cell carcinoma) were classified as high-grade EC with ambiguous features (HGEC). The designation of “mixed carcinoma” was reserved for ECs with spatially distinct components of different histotypes.
From pathology re-review, the tumor subtype was revised from original reported diagnosis for 6 cases: carcinosarcoma to serous (n = 1, due to lack of convincingsarcomatous component), endometrioid grade 2 to HGEC (n = 2; both cases showing overlapping features between serous and endometrioid carcinoma, and moderate-to-high grade nuclear atypia), and mixed serous/endometrioid carcinoma to HGEC (n = 3, due to lack of distinct spatial separation between morphologic components).
Ancillary testing for HER2 status
Of 77 ECs with HER2 amplification by MSK-IMPACT, HER2 IHC was performed for 61, FISH results were available for 47 (n = 23 performed specifically for this study; n = 24 previously reported for clinical management) and 43 were analyzed by both IHC and FISH. Where possible, the same tissue block used for NGS was used for IHC and/or FISH (see Supplementary Table S1 for details).
Immunohistochemistry
HER2 immunohistochemical stains were performed (4B5, Ventana, Tucson, AZ, USA) and HER2 IHC scores were assigned based on the percentage of positive cells, staining intensity and membranous staining pattern (i.e. incomplete, complete, basolateral/lateral), according to modified American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) 2007 HER2 testing guidelines for breast cancer17,18. In brief, tumors with intense complete or lateral/basolateral membranous HER2 immunostaining in more than 30% of tumor cells were assigned a 3+ score, and 2+ score was assigned when intense complete or lateral/basolateral membrane staining was seen in ≤ 30%, or weak to moderate staining in ≥ 10%, of tumor cells. These guidelines have been previously validated for the assessment of HER2 IHC in EC, with high interobserver reproducibility and demonstrated higher IHC-FISH concordance in EC compared to the 2018 ASCO/CAP breast and 2016 ASCO/CAP gastric criteria18,19,20. HER2 heterogeneity by IHC was defined by the presence of at least two-degree difference in staining intensity (0 vs 2+, 1+ vs 3+, or 0 vs 3+) involving at least 5% of tumor cells.
Immunohistochemical staining for p53 (DO-7, Ventana, AZ) was performed on tumors lacking somatic TP53 genetic alterations by MSK-IMPACT and the expression pattern was interpreted as wildtype or aberrant (diffuse overexpression or complete absence of expression), as previously described21.
HER2 FISH
HER2 FISH was performed on 23 tumors with available archival tissue using an FDA-approved HER2 dual-probe FISH assay [HER2 IQFISH pharmDx (Dako, Glostrup, Denmark) or PathVysion HER2 DNA Probe Kit (Vysis, Abbott Molecular, Des Plaines, IL, USA)]. HER2 (red) and chromosome enumeration probe 17 (CEP17; green) signals were enumerated in at least 20 tumor cell nuclei by two independent observers (KAD, DSR). HER2 amplification by FISH was defined as HER2/CEP17 ratio of ≥ 2.0. The entire section was assessed for heterogeneity both independently of and in conjunction with HER2 immunohistochemical staining pattern. Areas with low/absent HER2 immunohistochemical staining (0/1+) were scored separately from areas with moderate/high intensity staining (2+ /3+) for tumors in which these areas are spatially distinct. For cases in which spatial separation of cells with variable HER2 expression was not feasible, an overall FISH score was assigned.
Results
Histologic subtypes of HER2-amplified ECs
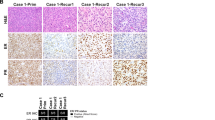
Interrogation of 2,042 ECs identified 77 with HER2 amplification by NGS using the MSK-IMPACT assay. For this subgroup, the median age was 68 at time of pathologic diagnosis (range 54–86) and stage distribution was as follows: I (n = 17, 22%), II (n = 4, 5%), III (n = 16, 21%), IV (n = 38, 49%), information not available (n = 2, 3%). Almost all HER2-amplified ECs were high-grade (75/77, 97%, Fig. 1A–H), comprised of serous (n = 29, 38%), clear cell (n = 4, 5%), endometrioid FIGO grade 3 (n = 1, 1%), HGEC (n = 20, 26%), carcinosarcomas (n = 18, 23%), and mixed carcinomas (n = 3, 4%, including 2 serous/endometrioid and 1 endometrioid/clear cell). The remaining 2 (3%) cases were low-grade endometrioid carcinomas (grade 1, n = 1; grade 2, n = 1). A serous or HGEC component was present in most of the HER2-amplified carcinosarcomas (16/18, 89%); the remaining 2 carcinosarcomas had yolk sac and clear cell carcinoma components, respectively.
Representative photomicrographs illustrating: (A, B) serous carcinoma, (A) with papillary and (B) solid architecture; (C) carcinosarcoma, with serous carcinoma component; (D) clear cell carcinoma; (E) low-grade endometrioid carcinoma; (F-H) high-grade endometrial carcinomas with ambiguous features (HGEC), (F) with serous and endometrioid features (note areas of squamous differentiation); (G) with serous and clear cell features; and (H) HGEC as a component of carcinosarcoma.
HER2 amplification was observed across serous, HGEC, carcinosarcoma and clear cell carcinomas at similar frequencies, ranging from 6–14% (Table 1), but was rare in endometrioid carcinomas (0.2%, all grades included) and absent in rare EC subtypes (0/13 undifferentiated/de-differentiated, mesonephric-like, and neuroendocrine carcinomas).
Molecular genetic landscape of HER2-amplified ECs
Somatic genetic alterations involving 468 cancer-related genes of the MSK-IMPACT assay were interrogated in the 77 HER2-amplified ECs (Fig. 2). The most striking finding was the high frequency of TP53 mutation in this cohort (n = 72, 94%), irrespective of histologic subtype. All of these were somatic mutations, except for one case, in which the TP53 truncating mutation was germline with loss-of-heterozygosity in tumor cells (HER2-27) and correlated with complete absence of p53 expression by IHC. Of the 5 tumors lacking TP53 genetic alterations, 2 harbored MDM2 amplification (HER-28 and HER-77), and 1 had low tumor purity and low sequencing reads (HER-29); only 2 of these (1 endometrioid and 1 clear cell carcinoma, out of 4 tumors with available tissue for IHC) showed a definitive wildtype p53 expression pattern (Supplementary Table S2). After accounting for these cases, there is evidence to support p53 dysfunction (TP53 mutation, aberrant p53 immunohistochemical staining pattern, and/or MDM2 amplification) in a total of 75 of 77 (97%) cases.
For clarity of presentation, only genes with pathogenic or likely pathogenic alterations occuring in at least 3 cases or previously reported to be recurrently altered in endometrial carcinoma are shown. CSRC carcinosarcoma, HGEC high-grade endometrial carcinoma with ambiguous features, IHC immunohistochemistry, FISH fluorescence in-situ hybridization.
Genetic alterations previously implicated in serous EC4,6,22, including amplification of CCNE1 (n = 17, 22%), FBXW7 mutations or deletions (n = 10, 13%), and PPP2R1A mutations (n = 10, 13%), were also frequent, and not restricted to serous subtype. Interestingly, RB1 truncating mutations (n = 3, 4%) were only present in carcinosarcomas. Mutations of genes associated with endometrioid carcinoma5,6, namely, PTEN (n = 2, 3%), ARID1A (n = 2, 3%) and CTNNB1 (0%), were rare in HER2-amplified ECs. Co-amplification of other 17q genes, CDK12 (located at 17q12, same band as HER2) and RARA (17q21.2), was present in 41 (53%) and 8 (10%) of cases, respectively. Other notable recurrent genetic alterations included PIK3CA activating mutations (n = 31, 40%) and ERBB3 amplification (n = 8, 10%), both of which have been reported to impact the efficacy of anti-HER2 therapy in other cancer types23,24.
HER2 IHC
Immunohistochemical analysis for HER2 expression was performed on 61 of 77 (79%) ECs with HER2 amplification detected by NGS (Table 2). Uniform, strong, diffuse membranous staining throughout the tumor section was only observed in 16 (26%) cases, whilst the majority exhibited heterogeneous HER2 expression (n = 45, 74%), with varying proportions of tumor cells showing negative/weak, moderate, or strong intensity staining (Fig. 3A-G). Applying the modified 2007 ASCO/CAP breast criteria to our cohort, 28 (46%) ECs had a HER2 IHC score of 2+ and 33 (54%) had a score of 3+. There was a highly significant association between the HER2 IHC score and the magnitude of gene amplification quantified by NGS (HER2 IHC 2+, mean FC = 3.6 versus HER2 IHC 3+, mean FC = 8.6; p = 0.0005, Welch’s t-test, two-tailed). Of note, applying a 10% threshold for assigning a score of 3+ (per 2018 ASCO/CAP breast criteria and 2016 ASCO/CAP gastric criteria), results in re-classification of 10 cases from 2+ to 3+ (Fig. 3G), and less separation in the degree of amplification between 2+ and 3+ cases (HER2 IHC 2+, mean FC = 4.2 versus HER2 IHC 3+, mean FC = 7.2; p = 0.04, Welch’s t-test, two-tailed).
Representative areas with (A) weak, incomplete membranous staining (1+); (B) moderate intensity basolateral/complete membranous staining (2+), juxtaposed with an area showing 1+ staining; (C) diffuse, strong complete membranous staining (3+). HER2 expression is seen in non-serous endometrial carcinoma subtypes, including (D) clear cell carcinoma, (E) carcinosarcoma, with loss of expression in the sarcomatous component, and (F) HGEC, including areas showing endometrioid/squamoid differentiation. (G) Bar graph showing semi-quantitative estimates of the proportion of tumor cells at different HER2 staining intensities for each evaluated case. Red asterisks mark cases with strong staining between 10–30% of tumor cells, which would be given a HER2 IHC score of 2+ if using a 30% cutoff (2007 ASCO/CAP breast criteria), and 3+ if using a 10% cut-off (2018 ASCO/CAP criteria). CSRC carcinosarcoma, HGEC high-grade endometrial carcinoma with ambiguous features.
Comparing the distribution of HER2 IHC scores across histologic subtypes, there were significantly more cases with 2+ staining in carcinosarcomas (carcinosarcomas: 13/17, 76% vs. other EC subtypes: 15/44, 34%, p = 0.004, Fisher exact test). This was due to both lower proportion of positive cells and intensity of staining (in both carcinoma and sarcoma components). HER2 expression tended to be restricted to the carcinoma component (Fig. 3E); of 12 cases with HER2 IHC evaluable on the sarcoma component, 6 were completely negative, and 6 showed weak to moderate expression, which was typically focal (Table 3). HER2 IHC was available on a few pure endometrioid (n = 1) and clear cell carcinomas (n = 4), and while statistical comparisons are not meaningful with such low numbers, there did not appear to be any differences in the HER2 expression in these tumor types compared to serous carcinomas (Fig. 3D). Furthermore, in morphologically heterogeneous tumors containing a combination of serous, endometrioid and/or clear cell carcinoma features (including HGEC, epithelial components of carcinosarcomas, and mixed ECs), there was no clear relationship between tumor morphology and the extent or intensity of HER2 expression (Fig. 3F).
HER2 FISH
To determine whether intratumor heterogeneity in HER2 expression was reflective of heterogeneity at the genetic level, HER2 FISH was performed on a subset of 23 tumors (Table 4). From this analysis, several common patterns of intratumor heterogeneity in HER2 amplification were observed (Fig. 4A): 1. Homogenous, 2. Near-homogenous (scattered non-amplified tumor cells present), 3. Heterogeneous—spatially distinct (previously termed “cluster” amplification25), 4. Heterogeneous—admixture of tumor cells with different degrees of amplification and non-amplified cells, 5. Heterogeneous—scattered amplified tumor cells in a background of non-amplified tumor cells (previously termed “mosaic” pattern25).
A Schematic depiction of common patterns of intratumor distribution of HER2-amplified tumor cells. B–I Representative images of cases with heterogeneous HER2 expression by immunohistochemistry and corresponding FISH of selected areas indicated by boxes (HER2—red signal; CEP17—green signal). B–D HER2-amplified/overexpressing serous carcinoma (HER2-61) with adjacent intraepithelial carcinoma component lacking amplification and exhibiting weak expression. E, F Small clusters of HER2-amplified/overexpressing tumor cells within a background of non-amplified/HER2-negative cells (HER2-30). G–I Spatially distinct HER2-amplified/HER2-positive areas and non-amplified/HER2-negative areas (HER2-06).
Diffuse, strong 3 + HER2 expression by IHC correlated with homogeneous HER2 amplification by FISH. However, subpopulations of non-amplified cells were observed in tumors with heterogeneous HER2 expression. For tumors with spatially distinct areas that showed high HER2 expression (HER2high: 2 + /3+ staining) and low HER2 expression (HER2low: 0/1 + staining), FISH demonstrated HER2 amplification, defined by HER2/CEP17 ratio of ≥ 2.0, to be restricted to the HER2high areas (Fig. 4B–I). Interestingly, in one case (HER2-61) of a Stage IA serous carcinoma with an adjacent “intraepithelial carcinoma” component, HER2 amplification and overexpression was restricted to the more florid papillary area, suggesting that HER2 may function as a driver of tumor progression, but not necessary for the initiation of early lesions (Fig. 4B–D).
Of 47 cases that underwent both NGS and FISH (n = 23 performed specifically for this study; n = 24 previously reported for clinical management), 44 were positive for HER2 amplification by both assays (Table 2). In 2 of the 3 discordant cases (HER2-30 and HER2-46), FISH demonstrated focal HER2-amplified cells, as single cells or small clusters (i.e., “mosaic” amplification, Fig. 4E-F), corresponding with overall HER2/CEP17 ratios of 1.4 and 1.8 (HER2 copy numbers of 3.4 and 4.3, respectively). The remaining case (HER2-36) had an HER2/CEP17 ratio of 1.7 and HER2 copy number of 3.8, as documented in the original diagnostic report. All 3 cases had HER2 IHC scores of 2+, with tumor cells showing weak to moderate staining only.
Discussion
The advent of clinical NGS has enabled a cost-effective means to evaluate multiple genetic biomarkers simultaneously and has generated a wealth of tumor profiling data in large patient cohorts. Detection of HER2 amplification by NGS has been shown to be highly concordant with IHC and FISH in several tumor types, including most recently, serous EC15,26. We identified HER2 amplification in 77 (3.8%) of 2,042 unselected ECs subjected to tumor genetic profiling by MSK-IMPACT.
Most of the studies on HER2 in EC were restricted to serous carcinomas, in which frequency of amplification ranged widely (less than 5% to over 40%), possibly owing, at least in part, to differences in composition of study cohorts and differences in testing methods10,18,26,27,28,29,30. Studies using IHC as the primary testing method followed by FISH testing of 2+ cases generally observed HER2 positivity in the range of 20–40% of serous carcinomas10,18,28,29. It is notable that the HER2 amplification frequency of 8% in serous carcinomas in our study is similar to the 9% reported by Robinson et al. who also used a targeted NGS approach26. Based on a prior study validating several cutoffs for defining HER2 amplification using the MSK-IMPACT assay, we chose a stringent FC ≥ 2.0 threshold in the present study to avoid capturing false positives. Samples with low tumor purity may have been missed, as high normal cell content would obscure signal from the tumor cell fraction. Another potential contributing factor to the relatively low frequency of HER2 amplification observed in NGS studies relates to intratumor heterogeneity. While IHC and FISH allow visualization of gene amplification at the single cell level, NGS provides an aggregate measurement over a larger tumor area and hence may not detect amplification when present only within a minor subpopulation.
The retrospective analysis of a large cohort of ECs in the present study enabled an unbiased interrogation of the frequency of HER2 amplification across tumor subtypes. While serous carcinomas made up the single largest group of HER2-amplified ECs (38.5%), our results are consistent with previous studies showing that this genetic alteration can also be found in other EC subtypes10,28,31,32,33. Importantly, almost all HER2-amplified ECs were of high-grade histology. It is not uncommon for high-grade ECs to exhibit ambiguous morphologic features overlapping between serous and endometrioid or clear cell carcinomas. Close to 30% of HER2-amplified cases were within this HGEC category, whereas only a handful were pure endometrioid or clear cell carcinomas. In HGEC tumors (and carcinosarcomas with a HGEC epithelial component), areas with endometrioid or clear cell features exhibited similar HER2 expression patterns as areas with more classic serous-like morphology, suggesting that cell type is the not primary determinant of HER2 status.
Regardless of histologic subtype classification, somatic genetic alterations were similar across HER2-amplified ECs and resembled the typical molecular profile of serous EC. Most striking was the co-existing TP53 mutation in nearly all cases. In a previous study from our institution, 17% of TP53-mutated ECs harbored HER2 amplification across histologic types31. Hence, while only a subset of TP53-mutated ECs acquire HER2 amplification, it appears that HER2 amplification generally only occurs in the setting of p53 dysregulation and is presumably a later event in tumor progression. Amplification of MDM2, a negative regulator of p53, in 2 of the rare HER2-amplified ECs that lacked TP53 mutation further support this contention.
From a treatment perspective, our molecular profiling analyses uncovered a high frequency of genetic alterations, which may potentially impact response to anti-HER2 targeted therapy. PIK3CA activating mutations were frequent (40% of HER2-amplified ECs) and are correlated with lower response rates to anti-HER2 therapy in breast cancer23 and trastuzumab resistance in serous EC cell lines34. Co-amplification of ERBB3, encoding HER3, was observed in 10% of cases. The formation of HER2/HER3 heterodimers have been implicated in trastuzumab resistance, and inhibitors of heterodimerization, such as pertuzumab, may represent an attractive therapeutic strategy in this setting24. The impact of concurrent PIK3CA mutation or ERBB3 amplification on treatment response to anti-HER2 therapy in HER2-amplified EC warrants further investigation.
As histotyping of high-grade EC is hampered by suboptimal interobserver reproducibility, our results are consistent with the notion that current clinical guidelines recommending HER2 testing limited to serous carcinomas may be too restrictive. To address the inherent shortcomings of histomorphology-based subtyping, a molecular subclassification of EC has been proposed. The Cancer Genome Atlas (TCGA) study of EC identified 4 distinct molecular subgroups: (1) POLE, ultramutated; (2) microsatellite instability-high (MSI), hypermutated; (3) copy number-high (serous-like); and (4) copy number-low (endometrioid)6. To implement this classification scheme into clinical practice, an algorithm for assigning ECs to each subgroup has been proposed, based on immunohistochemical analysis of p53 and MMR proteins and DNA sequencing of the POLE exonuclease domain35. This algorithm has demonstrated prognostic value in stratification of ECs36, and using this algorithm, Vermij et al showed that HER2-positive ECs were highly enriched in the p53-aberrant (corresponding to the copy number-high) subgroup28. Our MSK-IMPACT data showing HER2 amplification to be almost exclusive to TP53-mutated ECs provide additional support for the proposal to triage p53-aberrant ECs, identified by IHC, for HER2 testing.
All HER2-amplified ECs detected by MSK-IMPACT had complete or basolateral membranous HER2 staining at moderate intensity in ≥ 10% of cells, which corresponded to HER2 scores of 2+ or 3+. Consistent with prior studies, intratumor heterogeneity in HER2 expression was common in EC and HER2 staining correlated with genetic heterogeneity by FISH25.
There is some controversy over the appropriate proportion of cells with strong staining needed for separating a HER2 IHC score of 2+ from 3+ in EC. Compared to the 10% cut-off, our data demonstrate that using a 30% cut-off results in a stronger correlation between HER2 IHC score and the magnitude of HER2 amplification, in line with prior studies showing higher concordance between IHC and FISH using the more stringent cutoff18,19,20. Intuitively, the 30% cut-off restricts the 3+ category to tumors with relatively homogenous, high levels of amplification/overexpression, whereas a 10% cutoff allows cases with more intratumor heterogeneity (i.e. overall lower level of amplification) to be classified as 3+. Given the frequent intratumor heterogeneity in EC, we advocate applying a low threshold for performing FISH to confirm HER2 amplification, and therefore a higher cut-off for a score of 3 + (which bypasses the requirement for FISH) is recommended in the clinical setting.
Intratumor heterogeneity in HER2 expression/amplification was particularly marked in carcinosarcomas. Amongst HER2-amplified ECs identified by MSK-IMPACT, the proportion of cases with a HER2 score of 2+ was highest in carcinosarcomas and all 3 cases that were negative for amplification by FISH were carcinosarcomas. Consistent with previous reports37,38, we observed that HER2 expression was often less in sarcoma compared to carcinoma components. However, further work on a larger series of carcinosarcomas would be necessary to fully delineate the relationships between morphology, HER2 expression and amplification status in this tumor type.
The present study has several limitations. First, we did not evaluate different thresholds for calling HER2 amplification by MSK-IMPACT to establish optimal concordance with FISH. Some ECs with tumor/normal FC between 1.5 and 2.0 may potentially be amplified by FISH and would have been missed. As previously mentioned, detection of gene amplification is particularly problematic for samples with low tumor purity. Our reported frequency of HER2 amplification in EC should therefore be considered a conservative estimate. We were also unable to perform ancillary IHC and FISH testing on cases with inaccessible archival tissues, which were usually from patients whose surgeries were performed at other institutions. Of 46 cases with FISH results, there were 3 discordances (i.e. met criteria for HER2 amplification by MSK-IMPACT, but considered non-amplified by FISH, despite showing foci of HER2-amplified cells). Further optimization and standardization of criteria for NGS-based detection of HER2 amplification will be needed to improve concordance with FISH. Nevertheless, from a clinical standpoint, IHC remains the preferred primary HER2 testing modality, as it enables visual assessment of intratumor heterogeneity and if needed, can be performed on multiple tissue blocks at relatively low cost.
In conclusion, through an unbiased interrogation of a large cohort of ECs subjected to clinical sequencing, this study provides frequency estimates of HER2 amplification across EC subtypes, confirming prior observations that this genetic alteration is not restricted to serous carcinoma, but includes other TP53-mutated high-grade ECs. These findings support a co-operative pathogenic role between p53 dysregulation and HER2 amplification in driving high-grade EC progression. Frequent intratumor heterogeneity of HER2 expression/amplification and concurrent genetic alterations in PIK3CA and ERBB3 were found, and future work will correlate these molecular features with clinical response to anti-HER2 therapy.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Murali, R. et al. High-grade endometrial carcinomas: morphologic and immunohistochemical features, diagnostic challenges and recommendations. Int. J. Gynecol. Pathol. 38, S40–S63 (2019).
Tashiro, H. et al. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am. J. Pathol. 150, 177–185 (1997).
Prat, J., Oliva, E., Lerma, E., Vaquero, M. & Matías-Guiu, X. Uterine papillary serous adenocarcinoma. A 10-case study of p53 and c-erbB-2 expression and DNA content. Cancer 74, 1778–1783 (1994).
Kuhn, E. et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. JNCI 104, 1503–1513 (2012).
Tashiro, H. et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 57, 3935–3940 (1997).
Levine, D. A. et al. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013).
Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487 (2007).
Seshadri, R. et al. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J. Clin. Oncol. 11, 1936–1942 (1993).
Hechtman, J. F. & Polydorides, A. D. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Arch. Pathol. Lab. Med. 136, 691–697 (2012).
Morrison, C. et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 24, 2376–2385 (2006).
Oh, D. Y. & Bang, Y. J. HER2-targeted therapies—a role beyond breast cancer. Nat. Rev. Clin. Oncol. 17, 33–48 (2020).
Fader, A. N. et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 36, 2044–2051 (2018).
National Comprehensive Cancer Network. Uterine neoplasms (Version 1.2020)
Cheng, D. T. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Ross, D. S. et al. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J. Mol. Diagn. 19, 244–254 (2017).
WHO. Classification of Tumours 5th Edition - Female Genital Tumours. (IARC Press, Lyon, France, 2020).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 131, 18–43 (2007).
Buza, N., English, D. P., Santin, A. D. & Hui, P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod. Pathol 26, 1605–1612 (2013).
Buza, N. et al. Reproducibility of scoring criteria for HER2 immunohistochemistry in endometrial serous carcinoma: a multi-institutional interobserver agreement study. Mod. Pathol 34, 1194–02 (2021).
Buza, N. HER2 testing in endometrial serous carcinoma: time for standardized pathology practice to meet the clinical demand. Arch. Pathol. Lab. Med. 145, 687–691 (2021).
Chui, M. H. et al. Somatic intronic TP53 c.375+5G mutations are a recurrent but under-recognized mode of TP53 inactivation. J. Pathol. Clin. Res. https://doi.org/10.1002/cjp2.242 (2021).
Zhao, S. et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 110, 2916–2921 (2013).
Loibl, S. et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann. Oncol. 27, 1519–1525 (2016).
Lee-Hoeflich, S. T. et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 68, 5878–5887 (2008).
Buza, N. & Hui, P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes Chromosomes Cancer 52, 1178–1186 (2013).
Robinson, C. L. et al. Detection of ERBB2 amplification in uterine serous carcinoma by next-generation sequencing: an approach highly concordant with standard assays. Mod. Pathol. 34, 603–612 (2021).
Odicino, F. E. et al. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int. J. Gynecol. Cancer 18, 14–21 (2008).
Vermij L. et al. HER2 status in high-risk endometrial cancers (PORTEC-3): relationship with histotype, molecular classification, and clinical outcomes. Cancers (Basel) 13, https://doi.org/10.3390/cancers13010044 (2020).
Slomovitz, B. M. et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 22, 3126–3132 (2004).
Santin, A. D. et al. Determination of HER2/neu status in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gyn. Oncol. 98, 24–30 (2005).
Momeni-Boroujeni, A. et al. Clinicopathologic and genomic analysis of TP53-mutated endometrial carcinomas. Clin. Cancer Res. 27, 2613–2623 (2021).
Cagaanan, A. et al. HER2 Expression in endometrial cancers diagnosed as clear cell carcinoma. Int. J. Gynecol. Pathol. https://doi.org/10.1097/PGP.0000000000000783 (2021).
Da Cruz Paula, A. et al. Genetic and molecular subtype heterogeneity in newly diagnosed early- and advanced-stage endometrial cancer. Gyn. Oncol. 161, 535–544 (2021).
Black, J. D. et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br. J. Cancer 113, 1020–1026 (2015).
Talhouk, A. et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 113, 299–310 (2015).
Talhouk, A. et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 123, 802–813 (2017).
Raspollini, M. R. et al. COX-2, c-KIT and HER-2/neu expression in uterine carcinosarcomas: prognostic factors or potential markers for targeted therapies? Gyn. Oncol. 96, 159–167 (2005).
Rottmann, D. et al. HER2 testing of gynecologic carcinosarcomas: tumor stratification for potential targeted therapy. Mod. Pathol 33, 118–127 (2020).
Funding
Research reported in this publication was supported by a Cancer Center Support Grant of the NIH/NCI (Grant No. P30CA008748) and internal funding from the Department of Pathology, Memorial Sloan Kettering Cancer Center. B.W. is funded in part by NIH/NCI P50 CA247749 01, Breast Cancer Research Foundation and Cycle for Survival grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
V.M. received study support (all funding to institution)/consultancy/advisory board membership from AstraZeneca, Clovis, Eisai, Faeth, Genentech, GSK, iTEOS, Karyopharm, Moreo, MSD, Takeda, and Zymeworks, unrelated to this work. B.W. reports ad hoc membership of the scientific advisory board of REPARE Therapeutics, unrelated to this work. M.H.C. has served on an ovarian cancer medical advisory board for Roche Diagnostics, unrelated to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ross, D.S., Devereaux, K.A., Jin, C. et al. Histopathologic features and molecular genetic landscape of HER2-amplified endometrial carcinomas. Mod Pathol 35, 962–971 (2022). https://doi.org/10.1038/s41379-021-00997-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00997-2
This article is cited by
-
Molekulare Klassifikation beim Endometriumkarzinom
Die Gynäkologie (2023)
-
HER2-amplified endometrial carcinoma and AFP-producing endometrial carcinoma
Modern Pathology (2022)