Abstract
The field of anatomic pathology has been evolving in the last few decades and the advancements have been largely fostered by innovative technology. Immunohistochemistry enabled a paradigm shift in discovery and diagnostic evaluation, followed by booming genomic advancements which allowed for submicroscopic pathologic characterization, and now the field of digital pathology coupled with machine learning and big data acquisition is paving the way to revolutionize the pathology medical domain. Whole slide imaging (WSI) is a disruptive technology where glass slides are digitized to produce on-screen whole slide images. Specifically, in the past decade, there have been significant advances in digital pathology systems that have allowed this technology to promote integration into clinical practice. Whole slide images (WSI), or digital slides, can be viewed and navigated comparable to glass slides on a microscope, as digital files. Whole slide imaging has increased in adoption among pathologists, pathology departments, and scientists for clinical, educational, and research initiatives. Integration of digital pathology systems requires a coordinated effort with numerous stakeholders, not only within the pathology department, but across the entire enterprise. Each pathology department has distinct needs, use cases and blueprints, however the framework components and variables for successful clinical integration can be generalized across any organization seeking to undergo a digital transformation at any scale. This article will review those components and considerations for integrating digital pathology systems into clinical practice.
Similar content being viewed by others
Technology adoption
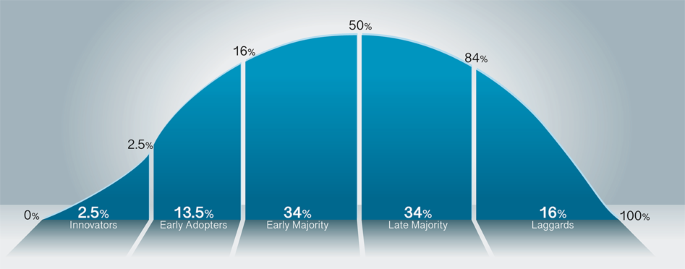
The adoption of digital pathology follows a cycle similar to other new disruptive technologies, known as the technology adoption lifecycle or diffusion of innovation1 (Fig. 1). This curve follows the route most often taken related to the adoption of novel technology. The innovators are the first to invent or adopt the new technology; they are venturesome and interested in realizing new ideas. The innovators are very willing to take risks and need no appeal to adopt the new technology. The subsequent group on the adoption curve includes the early adopters, who represent key opinion leaders. They embrace change opportunities and are already aware of the need to change, thus are very comfortable adopting new ideas. Early adopters do not need significant convincing to change and will eagerly adopt the new technology. Once the innovators and early adopters show value in the technology, the early majority is the next large group that will adopt the new technology. The early majority typically need to see evidence that the innovation works and adds value before they are willing to adopt it, such as success stories or visualizing the innovation’s effectiveness. Only when the technology has been mastered will the late majority adopt the new technology. The late majority are skeptical of change and will only adopt an innovation after it has been tried and proven by the early majority. The last and final group who may or may not adopt the new technology are termed laggards (e.g., skeptics). This group is bound by tradition and tend to be very conservative. They are skeptical of change and are the hardest group to convince that the new technology has value and should be adopted. They may only join after significant statistics, publications, appeals, and pressure from people in the prior adopter groups. There are various approaches to adopting new medical technologies in a clinical organization as these organizations have complex sets of processes as well as cohorts of individuals with varying degrees of risk averseness. Inquiry into and implementation of new technologies will depend on multiple factors that include the specific use cases and expertise available to determine which approach is best for the organization and for improved patient care. Regarding digital pathology systems, most early adopters implemented a phased approach as the technology matured over the years, in addition to evolving use cases becoming apparent. At Memorial Sloan Kettering Cancer Center, a large tertiary cancer center in NYC, the Department of Pathology has been using digital pathology for more than a decade, after the introduction of the first whole slide scanner in 2007. Various phases were involved in the implementation timeline: telepathology, frozen sections (e.g., intraoperative consultations), and recently, increased retrospective and prospective scanning for clinical diagnostics. As pathologists became increasingly familiar with using digital pathology for primary diagnostic use, we have witnessed an increase in pathologist buy-in and comfort using the technology. Two digital pathology validation studies conducted 2 years apart at our institution collected pathologists’ responses related to the comfortability to render a primary diagnosis digitally. In 2018, pathologists were 54% and 23% comfortable with rendering a digital primary diagnosis with or without access to glass slides, respectively. This is in comparison to 90% and 60% in 20202,3. One key proponent for this increase in primary diagnostic comfortability was the daily introduction of digital pathology in clinical workflows and the familiarity with WSI and digital pathology use at our institution. These numbers may continue to increase as more pathologists become comfortable and familiar using these digital systems and more trainees get exposed to digital pathology use during their training.
The adoption curve shows the innovators who develop the technology are the smallest percentage of adopters (2.5%), with the early adopters encompassing an additional 13.5%. Between the early adopters and early majority exists a gap, termed the chasm, where the critical market divide occurs. The early majority encompasses the first large (34%) group of interest in adopting the technology. Following suite, the equal in proportion, but late majority (34%) ensue. The last group in the technology adoption cycle is the laggards, or skeptics (16%).
Digital pathology developments
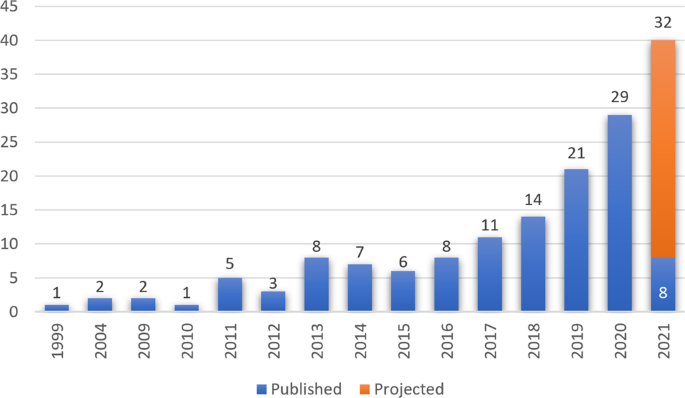
In general, the practice of medicine including pathology is undergoing a digital transformation and digital pathology is emerging as a potential new standard of care. Digital pathology systems were first initiated in the 1960s where telepathology systems were used in demonstrations as a way to practicing pathology at a distance4,5. The early systems were robotically controlled, motorized light microscopes. These were further developed into transmission of static images captured on camera-integrated microscopes. The first commercial whole slide scanning system was designed in the 1990s and has since been adapted to modern whole slide scanners incorporating state-of-the-art optics, robotics, and computers6. Clinical adoption and literature have increased as shown by the numerous publications over the last two decades, with an upward trend in the last 5 years (Fig. 2). The current digital pathology landscape provides myriads of hardware and software solutions that offer technology for most laboratory use cases, each with their own limitations and added value7.
Pre-analytic considerations
Scanning workflow
The breadth and volume of pathology glass slide scanning is dependent on the specific organizational volume of cases and resources. Cases can be considered for clinical prospective scanning or retrospective archival scanning. In determination of prospective clinical scanning workflows, assessment of laboratory volumes is critical to ensure adequate scanning throughput as to not delay turnaround time. Based on resources, departments may also deploy a hybrid digitization model where certain groups of slides (i.e., subspecialty, procedure type) are routinely scanned and the glass slides are also concurrently distributed. For glass slides that were not prospectively scanned, these can be marked for scanning and retrospectively scanned for archiving after manual microscopic review and case reporting. In such a scenario, guidelines implemented at our institution during early phases of digital scanning of glass slides were as follows: (1) for biopsies, 1 block from each specimen part is selected, (2) for resection cases, all frozen section slides and frozen section controls, glass slides with diagnostically relevant grading/staging information, diagnostic, predictive or prognostic immunohistochemistry, (3) for consultation cases, all diagnostic and prognostically relevant glass slides, and (4) relevant H&E slides for cases being sent for molecular analysis8. Archival scanning may also include deployment of additional whole slide scanners at off-site storage locations where glass slide pathology assets are stored for regulatory or warehousing purposes. Staff to retrieve, clean and prepare, load and unload, and quality control glass slides would need to be allocated for such an endeavor. The digital slides would be available for review in the laboratory information system (LIS) for clinical, research, or educational use. Anecdotally, the pathologists who were initially skeptical of image quality and potential relevant use of whole slide images, now expect the ready availability of WSI and have altered their initial perspectives on the value and use of digital pathology (Table 1).
Barcoding and tracking
The gateway of integrating digital pathology into a clinical practice is barcoding and tracking. Digital pathology prerequisites include having glass slides barcoded, likely with the other pathology assets as well. There are various glass slide barcoding printing technologies available such as printing barcodes on adhesive labels or directly printing on the glass slide. Some institutions use secondary labels for consultation services that piggyback atop or are placed adjacent to the originating institutions slide labels. The barcoding and tracking integration with the LIS will link digital slides to their respective database information within the LIS. Once the whole slide scanners decode barcodes captured in the glass slide label during the digitization process, healthcare standard messaging (e.g., HL7, XML) and network communication protocols allow seamless integration with digital pathology system databases as well as the LIS9. Benefits from using these integrated systems include automated slide aggregation, where cases are automatically collated and are digitally available. With digital pathology, glass slides do not need to be organized and matched with paperwork for distribution. Digital slides of cases are also immediately available within the LIS for review without reliance on courier transport services for glass slide delivery. These assurances however require additional appropriate slide label printer maintenance. At our institution, a quality assurance study found that proper slide label printer maintenance reduced barcode scanning errors by up to 20% (unpublished). In addition, for consultations, various pathology departments have different workflows for secondary labeling of glass slides received from their primary institutions. For laboratories that affix a second label below the primary label as to not obscure the outside laboratory label text, this may present multiple barcodes in the same field of view to the whole slide scanner slide label macrocamera. Our department initially used affixed label stickers (e.g., red dot stickers) for indication of glass slides to be scanned. These red dot label stickers were placed over outside institutional barcodes to hide them from the whole slide scanner camera, such that the camera would only recognize and decode our department’s internal barcode. However, as our operation scaled, these were too cumbersome to be used for placement atop the referring institution’s barcodes, as well as inefficient for removal after scanning before returning to the consulting institution. The department then instituted liquid chalk ink markers, which facilitated easier placement and removal of “erasable chalk ink” for scanning workflow purposes8. Additionally, through collaborating with a digital pathology system vendor, we were able to successfully develop software to automatically suppress outside institutional barcodes without the need of using stickers or chalk ink markers to cover the outside institutional barcodes. This minor modification enabled remarkable and efficient enhancements for consultation whole slide scanning workflows in our department.
Laboratory
Understanding the laboratory logistics is an integral part of establishing a digital pathology workflow. Monitoring laboratory volumes and statistics will help workflow planning and resource allocation. Each laboratory may display variations (multifactorial) in their respective workflows, however general considerations for data collection may be applicable across all clinical laboratories. Data analytics should be detailed hierarchically with daily counts of patient accessioned cases, block generation, and total stained slides including frozen sections. These numbers should include additional control slides for immunohistochemistry if applicable, and exclude any unstained slides, such as sections for molecular studies, as these slides should not be digitized. These data should then be aggregated for each operational day of the week, as certain trends in volume may be identified based on surgical and clinic operating schedules. Furthermore, the number of initial hematoxylin and eosin (H&E) stained slides may be differentiated from frozen section slides, immunohistochemistry, special stains, and subsequent H&E levels/recuts. Volume statistics including maximum and average volumes of the aforementioned stains based on procedure type (e.g., biopsy, resection) or consultation slides across a large time span will enable adequate resource planning. These volumes may also differ by pathology subspecialty or domain (e.g., surgical pathology, cytopathology, hematopathology, and molecular pathology), thus it is important to also quantify based on intended digital use cases of the laboratory.
For each laboratory’s workflow, there are common scheduled times for batch processing of tissue blocks, specific subspecialty requirements, or priority (e.g., rush) cases. For instance, laboratories may process all received biopsy specimens overnight such that they are available for review in the early morning. Detailed understanding of laboratory operations will facilitate resource allocation and quantification of slides generated each hour. The hourly slide generation can be defined as the total number of stained slides that will be sent for digitization in a whole slide scanner. This will impact the total hourly and daily throughputs for each laboratory, however many other factors will affect true scanning throughput. Assessment of hourly glass slide generation on each respective day of the week is appropriate, as well as throughput of slide scanning per scanner. Statistical maximum and median volumes should be used for calculating the number of high-throughput scanners needed to scan the intended laboratory glass slide output. Additional operational factors include space, staffing, and scanner related considerations.
Another factor in analyzing pre-analytic workflows is related to specimen processing and laboratory hardware for glass slide staining, coverslipping, and drying. Similar to conventional microscopy, good laboratory practices should be kept in place to maximize quality glass slide production and minimize poor staining, air bubbles, tissue folds, etc. A suitable glass slide will translate to a quality whole slide image. Best practices should be maintained in relation to appropriate tissue size placement in tissue block cassettes. Large pieces in a tissue block may be sectioned beyond the width of the glass slide or the length of the coverslip; neither will be evaluable in the whole slide image. Process improvements in embedding have also been shown to reduce file sizes and cost if tissues are embedded close together minimizing superfluous space between the tissue pieces in multiple tissue sections10. Sectioning of tissue on the glass slide should be centered in the middle of the slide to avoid tissue being present out of the coverslip area, which will not be scanned in focus. This will also prevent unnecessary increases in scan times and file sizes. Standardization of coverslipping to ensure coverslips are not overhanging past the width of the glass slide are critical to avoid errors in whole slide scanning, and possible glass slide damage/breakage. In recent years, there have been considerable progress toward semi-automation of the anatomic pathology laboratory where automation has hitherto been lacking. For instance, there now exist automated embedding and microtomy devices. In addition, there are commercially available combined glass slide stainers and coverslippers with built-in ovens for reducing drying time of mounting media. In order to minimize reracking of glass slides for transfer to a whole slide scanner, evaluating the slide rack compatibility across the existing laboratory equipment is critical. This can be a considerable bottleneck in overall throughput for high volume scanning. Certain whole slide scanners may only be compatible with a single vendor glass slide cartridge (i.e., rack), which would require transferring and reracking of the glass slides to an otherwise compatible vendor-specific whole slide scanner slide cartridge. If the glass slide cartridge for the stainer, coverslipper, and oven are all compatible with the whole slide scanner, then the slide transfer process can be seamless, without the need for the time consuming and additional step to re-rack glass slides between instruments. Also, it is recommended that all glass slides are completely dry after coverslipping before being placed in a whole slide scanner. If no drying step is included in the workflow, coverslips may be displaced or excess mounting media may dry causing the glass slide to be adherent to the slide cartridge. These scenarios may cause significant scanning errors, with potential for glass slide damage. This is especially problematic when glass slides are positioned vertically and are stagnant. Superfluous mounting media from coverslipping instruments may cause downstream hardware errors as glass slides will become adherent to racks. In those cases, excess mounting media may pool towards the bottom of the rack due to gravity, and cause adhesion of the glass slide to the cartridge, which may cause scanning errors or slide damage when handled by the whole slide scanner. Inspection of immunohistochemistry and special stain workflows should also be included in this evaluation since they use separate hardware with potentially different glass slide cartridge inputs than typical H&E stainers. Newer high throughput whole slide scanners also offer true continuous loading technology where the whole slide scanner does not need to be interrupted or paused to add new racks to be scanned, or remove racks that have completed the scanning steps successfully to help mitigate some of the above mentioned issues.
The five S’s for successful implementation of a digital pathology system
Sponsorship from leadership
Leadership support is arguably one of the most critical factors for implementation of a clinical digital pathology system. The vision of a pathology department and allocation of resources is heavily impacted by enterprise and departmental leadership. This authority is crucial for the implementation and sustainability of a novel clinical care delivery modality and one of the most highly cited barriers to digital pathology adoption is lack of executive leadership buy-in11,12,13. It is important for change management best practices to have a project sponsor actively engaged to provide visible leadership and support for the practice change to advocate for resources, align stakeholders, and help clear potential roadblocks. Without institutional support and active project sponsorship from leadership, additional forecasted challenges to the digital transformation will ensue. It is the responsibility of those championing the digital pathology implementation to provide data on the value the system will bring to the institution or organization based on their specific use cases. Careful step wise expansion is also suggested as a strategy to gain leadership support, as the adoption of digital pathology requires added resources and the champions need to be aware of the risks involved in rapid expansion.
Space
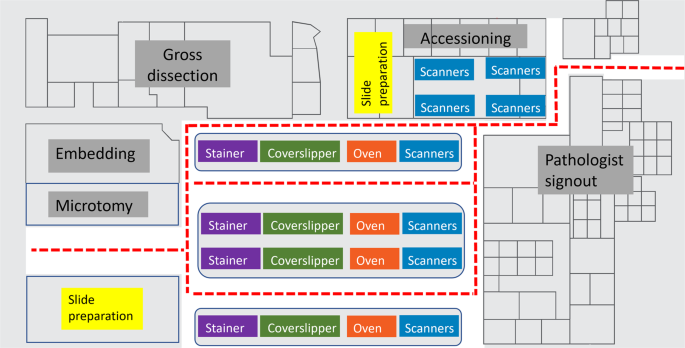
Space allocation in pathology operations has traditionally been challenging, especially in large metropolitan geographical areas where real estate is at a premium. With whole slide imaging (WSI), there may be multiple additional hardware with nontrivial device footprints to be included. It may be difficult to find optimal placement and location for whole slide scanners in an already existent laboratory. Ideally, the whole slide scanners are in a low-traffic, clean and chemical/spill free location with minimal floor vibration adjacent to the glass slide stainer(s), coverslipper(s), and drying oven(s). This allows the increased efficiency of the process from start to finish, maintains quality and turnaround time, and improves job satisfaction of the laboratory staff who are tasked with the digitization of the slides. A careful study of distance and timing in the creation of a Spaghetti diagram may prove helpful to track personnel across the laboratory in their various roles moving pathology assets from the point of accessioning to the point of slide distribution. An ideal digital laboratory will have stainers, coverslippers, ovens/drying stations, adjacent to whole slide scanners in a linear workflow (Fig. 3) In a “fully digital” laboratory, the space requirements could be reduced with significant efficiency gains from automated case assembly. This allows future optimization of the laboratory space to improve efficiency and quality operations.
This diagram shows the visualization of slide flows through an ideal histology laboratory (not to scale) with whole slide scanners. Movements represented in this plot track the glass slides from the point of accession through the laboratory process including scanning on whole slide scanners in a linear, sequential fashion to optimize workflow. Instrument automation is important to minimize device footprints in the laboratory, automated all-in-one stainer, coverslipper, and ovens may improve efficiency and provide better workflows in minimizing device transfers. Whole slide scanners present in accessioning may be suitable for slides generated at other institutions received for consultation.
Staffing
Staff resources may need repurposing of existing personnel or hiring additional positions in the laboratory. Whole slide scanners used in a clinical workflow ideally should be located in the laboratory. To that end, histotechnologists or laboratory aides may be trained for loading glass slide racks into the whole slide scanners and removing scanned racks when completed. Additional staff for troubleshooting whole slide scanners and WSI quality assurance may be needed or trained as determined by the laboratory leadership. Adequate staffing for high volume digital scanning operation will be required to ensure appropriate throughput, as well as rescanning for slides that require remediation. Consultation workflows may also include point of scanning in the accessioning area such that stained glass slides received in consultation may be placed in racks and directly scanned prior to, or in place of, glass slide distribution. Glass slides from consultation cases may require additional cleaning steps to reduce digital scanning artifacts. Depending on specific use cases, institutions may need to allow staffing in additional shifts to maximize the use of the scanners and to maintain turnaround times for their operations14,15. Additional staffing needs include network and IT support for both operational needs and also for software tool development as needs arise. An ideal team will include data scientists, engineers and technicians who will be available for support of the clinical and research needs, and will work in collaboration with the stakeholders at the department.
Storage
Storing whole slide images can be costly dependent on the volume, cadence, location, and retention of files. For cloud storage services, this also includes the number of images reviewed and system users. Storage considerations are crucial when implementing a digital pathology system. The size of each generated WSI is dependent on tissue size (e.g., scan area), scanning magnification, μm per pixel (e.g., resolution), and multiple plane (e.g., z-stack) scanning. Conservative estimates for single plane, ×40 equivalent resolution scanning of formalin fixed paraffin embedded surgical pathology glass slides, shows average file sizes to be approximately 2 gigabytes per WSI. To estimate WSI storage, the laboratory can divide the number of stained glass slides intended to be scanned by 500 to get an estimate of terabytes (TB) required (i.e., 500,000 stained slides generated per year = 1000 TB of storage space per year). The overall cost of digital media storage decreases every year, however at scale can be a significant cost. Data can be saved on-premises or in the cloud. Cloud based data storage has allowed users to upload their files to internet-connected data server warehouses that store the files. For both storage methods, redundancy is key for disaster recovery. Additional costs may be incurred from cloud storage related to tiered storage (e.g., hot, cold) requirements and bandwidth usage. Regarding security, Health Insurance Portability and Accountability Act mandates backup and disaster managing plans for all medical images. The College of American Pathologists (CAP) Retention of Laboratory Records and Materials for glass slides is 10 or 20 years based on laboratory location and state requirements. The CAP has also added similar minimum retention requirements for digital images used for primary diagnosis if original glass slides are not available16. If digital slides storage is being retained, laboratories may consider moving glass slides off-site to less costly storage facilities in other locations. All local or cloud digital storage methods should allow for ease of use in viewing, navigating, and sharing the images. There are currently different available storage solutions that are based on practical and clinical needs. Digital storage should be monitored and as use cases change, each organization should determine the requirements and cost of their specific storage needs.
Scanners
Scanner considerations are dependent on the laboratory’s intended use cases, volumes, space and budgets. The selection of scanners will vary among institutions and will likely change over time with growing scanning needs of the users. Whole slide scanners are grouped into high throughput, low throughput, real-time hybrid/robotic, and integrated microscopy scanners17. Most medium to high volume laboratories will seek high-throughput scanners with true continuous scanning technology to suit their digitization workflows. Considerations for selection of scanner may also be dependent on the glass slide format compatibility. For a laboratory that performs routine whole mount slide sections, or other large glass slide format sizes, only certain scanners may accommodate scanning those larger formats, otherwise additional whole slide scanners will need to be acquired. The number of scanners will be dependent on the hourly slide generation from the lab, as well as scanning throughput of the whole slide scanner. This includes racking time (i.e., manual handling for noncompatible glass slide cartridges), scanner loading time (i.e., robotic handling), scan time over the glass slide (e.g., glass slide digitization), and network transfer time for the slide to be available in the LIS. There are four main clinically applicable categories of digital pathology hardware available in the vendor market: high throughput whole slide scanners, low throughput whole slide scanners, dynamic real-time scanners, and integrated microscopes.
High throughput
This category of whole slide scanners is purposed for high volume scanning. High-throughput scanners are typically used by large volume laboratories that either prospectively scan clinical cases or digitally archive glass slides. These whole slide scanners range in slide input capacities between 100 and 1000 slides. However, newer scanner technologies also offer continuous loading capabilities such that the scanners support a “load and walk away” workflow. Glass slide scanning will not be interrupted when loading new or unloading completed slide cartridges. All high-throughput scanners support conventional glass slides, 1 × 3 inches (25 × 75 mm) × 1 mm thick; and some offer larger, 2 × 3 inch slide scanning or up to 8 × 6 inch (200 × 150 mm). Most scanners in this category offer image resolution at ×40 equivalent magnification: ~0.25 μm per pixel or ×20 equivalent magnification ranging from ~0.5 μm per pixel. Whole slide scanners are available with higher pixel resolutions, such as equivalent ×63 (~0.16 μm per pixel, or ×83 magnification (~0.12 μm per pixel). Slide scanning speeds are dependent on the engineering and robotics of the hardware. Total scanning time factors include, but are not limited to: imaging acquisition process, sensor size, tissue scan area, objective lens/equivalent magnification, automation, motorized stage and robotics speed, focusing method, number of z-planes to image, and network connectivity.
Low throughput
Whole slide scanners in this category are commonly used for low to medium volume scanning. Other uses of low throughput scanners may be for special niche needs that high-throughput scanners otherwise do not offer satisfactory solutions, such as fluorescence, scanning with oil, or intraoperative consultations. They are also good intermediate choices for labs that are taking initial steps in the area of digital pathology that have space and budget restrictions. Lower throughput scanners typically take up less laboratory space. The resolution, mechanics, and engineering are similar to their higher throughput counterparts; however also include higher resolution objectives such as ×100 equivalent resolution (0.1 μm per pixel). These instrument costs are generally lower and provide an affordable solution depending on slide volume when compared to the higher throughput whole slide scanners.
Dynamic-robotic imaging devices
Real-time dynamic-robotic imaging devices are increasingly being used for telepathology (i.e., intraoperative diagnosis). These scanners are low throughput, ranging from 1 to 5 slides per slide input bay. Pathologists can connect to the scanner workstation over the internet and operate the imaging device to view the glass slides in real time (i.e., pan, zoom, and focus). Pathologists have predominantly used these technologies for remote intraoperative consultations and rapid on-site cytologic evaluation18,19,20,21,22,23,24,25,26. The objective lenses and resolution of these scanners are similar to other scanners, however these scanners allow the pathologist to select from a range of objectives (e.g., 2×, 4×, 10×, 20×, 40×) to view the slides in real time. Imaging software allows for simultaneous viewing of different fields of view of the same, or multiple slides. Z-plane viewing can be more effective, while live-viewing the glass slide, since the pathologist operating the scanner can focus different z-planes efficiently in real time. Some of the scanners in this category have full scanning capabilities, but lack high-throughput capabilities.
Integrated microscopes
Integrated microscopes are digital cameras mounted on traditional brightfield microscopes. These systems are widely distributed in pathology departments today, initially available to capture static images from the objective lens of the microscope. They are limited to a single field of view per image captured. Through software enhancements, glass slides can be manually moved on the microscope stage where consecutive images can be captured and stitched together to generate a whole slide image27. These digital images are stored and can be shared through mechanisms as simple as email and also through dedicated web-based portals. Smartphones or other mobile devices can leverage their camera sensors via microscope attachment adapters to capture microscopy through the microscope’s eyepiece28. Cameras for integrated microscopes can be used for live streaming, whole slide image creation, or static image capture. The quality of the images generated with this methodology are operator dependent (i.e., field of view, magnification, blur). However, these modalities offer flexibility in multiple z-plane and field of view digitization, and generate smaller file sizes27,29,30,31. Integrated microscopes offer the lowest throughput yet are economical and can offer an affordable option in limited resource settings, especially for sharing images. Since these devices rely on the traditional microscopes that pathologists are familiar with, they can be seen as an intermediate step for digital imaging transformation of pathology operations.
Analytic (scanning) considerations
Clinical digital pathology adoption has considerably grown over the past decade with a range of vendor hardware and software to consider. Interoperability of a whole slide scanner file format, WSI viewer, and laboratory-hardware compatibility are key. Based on use case, imaging modality and the need for brightfield and/or fluorescence digital microscopy should be considered for appropriate scanner selection. Whole slide scanners also vary in optics and ultimately image resolution where scanners may contain one or several lens objectives, ranging in magnification from 1.25× to 100× oil. Evaluation of scanner specifications and footprint for placement in the designated areas are important for facilities management as well as network connectivity. Throughput (e.g., scan speeds) and continuous load considerations are also crucial to managing high volume scanning. Many vendors quote scanning speeds based on a 15 × 15 mm (225 mm2) industry standard. Actual clinical glass slides at a large tertiary cancer center have been shown to measure over 600 m2 scan area2,3. Scan times are also dependent on how many regions the scanner digitizes. Certain scanners capture a single area of interest including all background deadspace (e.g., scanned slide area with no tissue) on a given slide, versus other scanners that scan multiple areas of interest based on tissue detection. Contrasting scanners, one study showed 114 s average scan times with 290 mm2 scan area, while using a different scanner had an average scan time of 90 s, with 612 mm2 tissue area3,14. These vendor quoted scanning times naturally do not include operator slide handling times or robotic handling of slides and should be always evaluated at the deployment site for proper throughput assurance. Rescan rates are also important to document and audit for quality assurance purposes. These rates can be used for QA purposes and for guiding recommendations from scanner evaluations as well as providing further improvements to internal workflows or vendor feedback. Another consideration is scanning magnification, equivalent resolution and magnification supplied by hardware scanner vendors include familiar “20×” or “40×” labels, however unfortunately these terms at a high level are misnomers. Different vendor scanners have varied setups in terms of the native objective magnification, numerical aperture (e.g., NA), lenses, optical doublers, camera sensors, condensers, and software processing (i.e., image compression) of the acquired images. One scanner’s WSI with “40×” equivalent resolution is not necessarily equivalent to another WSI from a different vendor scanner. The higher the scan resolution (e.g., smaller microns/pixel ratio), the better the image resolution and ultimately better detection of microscopic detail (i.e., rare event detection, such as mitoses or microorganisms). From a regulatory clearance perspective, currently there are two whole slide scanners cleared for primary diagnosis using digital pathology, that include both ×40 and ×20 equivalent resolution scanning. Regardless of regulatory clearance, laboratories are still required to validate systems as per accreditation body guidelines and should validate the resolution(s) intended for primary diagnosis as would be clinically implemented. Similarly, there is still a lack of vendor interoperability between file formats, and laboratories requiring multiple whole slide scanners from multiple hardware vendors need to consider a vendor-agnostic whole slide image viewer as well as managing multiple vendor databases. However, efforts are underway to allow for increased vendor interoperability32. There is currently no single commercially available whole slide scanner that can accomplish all the various uses cases of clinical scanning (i.e., whole mount glass slide scanning, high-throughput with continuous load, high resolution, brightfield and fluorescent, etc). A multi-vendor operation may be a high consideration based on implementation uses cases, but may add additional integration requirements for the whole slide image viewer and LIS. It is suggested that laboratories work with vendors who allow the evaluation and customization of scanners that will best fit their needs, and also support the integration of those scanners into their existing IT solutions.
Quality control and communication
Quality control for digitization of glass slides to whole slide images includes all of the pre-analytic workflow steps that are necessary to ensuring a high-quality glass slide, as well as additional steps that are introduced. Laboratories typically have performed manual quality control on the quality of the slide, however there are programmatic tools starting to emerge33,34. Guaranteeing a high-fidelity whole slide image requires proper specimen accessioning, barcoding and tracking (e.g., labeling of pathology assets), histology processes, and digital scanning workflow. The histology processes are well characterized and will not be further discussed other than what have been mentioned above in the Laboratory pre-analytics section. The additional quality control related to glass slide digitization includes four new quality control (QC) checkpoints: review of glass slide (macroscopic) prior to scanning, real-time QC during scanning, QC directly after scanning, and QC at the point of diagnostic review.
Pre-scanning QC
The pre-scanning QC process involves careful macroevaluation of pre-analytic slide artifacts to be resolved before scanning whenever possible. Glass slides may have laboratory histology artifacts such as air bubbles, ink markings on the coverslip (especially for glass slides received in consultation), overhanging slide labels and coverslips. Prior to inserting glass slides in the scanners, the slides must be visually examined and prepped such that glass slides are stained and dry, with no visible breaks or cracks, and slides are clean (slides with fingerprints, ink markings, etc are wiped clean with gauze and alcohol or linen cloth). If the tissue section extends underneath the edge of the slide label or to the edges of glass slide, the tissue present past those areas will not be scanned in focus. Coverslips are checked such that there are no overhanging edges beyond the edges of the glass slide or air bubbles. The slide label with the patient information should be legible and on the appropriate up-facing surface, firmly adherent to the glass slide without extending past the slide edges. The barcode on the label should be legible (not cutoff or smeared). This QC step is especially important for glass slides received in consultation. For sites with secondary (or tertiary) labels from consultation slides, multiple barcodes on the glass slides may cause barcode errors on detection and cause the whole slide image not to be linked or available within the LIS. Digital imaging assistants may check slide labels with available handheld barcode scanners to ensure readability of the barcode prior to placing on the whole slide scanner. Including a barcoding and tracking station to scan each glass slide either in the laboratory or in a separate area ensures each barcode is readable by handheld barcode readers and can also be configured to track the glass slide in the LIS as scanned or marked for scanning. Glass slides without barcodes, or barcodes that failed reading by the handheld barcode reader, should have new patient labels with barcodes printed and affixed on the glass slide. Training of accessioning staff or imaging assistants to apply slide labels with barcode in appropriate areas of the slide to keep essential data (patient identifiers, outside accession number, stain name etc) visible on WSI may be needed.
Real-time QC during scanning
For some whole slide scanners, there are real-time QC algorithms that run during slide scanning and can facilitate procedures of identifying slides of poor quality. Prior to unloading the scanned glass slide racks from the scanners, thumbnail images of the digitized glass slides on the user interface display of the whole slide scanner to ensure successful scans. There are multiple slide scanning error codes that the scanner can provide, such as barcode detection failure or if no tissue was identified on the slide (i.e., usually scant tissue or faint immunohistochemical slides), or image quality errors. Feedback and remediation of the glass slide scan can be provided in real time to the scanning operator who can address scanning issues immediately and rescan a slide, if necessary.
Post-scanning QC
A second QC workflow that may be aided by a digital imaging assistant or supervisor is performed to ensure the WSI itself, is of adequate quality and is present within the LIS. For continuous scanning whole slide scanners, thumbnail visualization on the scanner user interface monitor is essential for timely QC to ensure all tissue on the glass slide is within the scanned area. This second QC process may also involve investigation of the digital pathology system vendor’s database (i.e., image management systems) or within the LIS to review quality evaluation for typical WSI artifacts (i.e., tissue detection, out of focus, horizontal striping, color quality, etc). Any slide requiring a rescan is included in the subsequent batch of slides or can be manually scanned, if such a device is available. This QC step requires an alignment of the availability of quality digital images and the pathologist’s sign out schedules, and may require an adjustment of the digital scan associates’ schedules to avoid delays in image availability to the pathologists. All errors and troubleshooting should be recorded and monitored to maintain quality metrics and identify training operations to the staff. Automated software may be used in the future to allow for efficiency improvements to notify staff of slides scanned out of focus, or with other artifacts33.
QC at diagnostic review
Similar to glass slide QC, it is the responsibility of the laboratory staff and scanning team to ensure quality scanning operations and minimize WSI defects prior to reaching the pathologist for diagnostic review. In an ideal setting, all digital slides get reviewed by digital scan associates prior to pathologist’s review. When viewing a digital slide in the whole slide image viewer software, pathologists may identify digital slides with pre-analytic or WSI artifacts, and request prompt rescanning of the glass slide or defer to review the glass slide. At our institution, an additional QC tool has been implemented in the WSI viewer whereby the reviewer (e.g., pathologist, technologist) can provide direct feedback to the laboratory and digital scanning personnel on image quality, artifacts, or network performance (Fig. 4). All requested slides are then located and rescanned, and once the case is finalized, all glass slides proceed to be filed in the department slide library archive.
Post-analytic considerations
Pathologist workstation
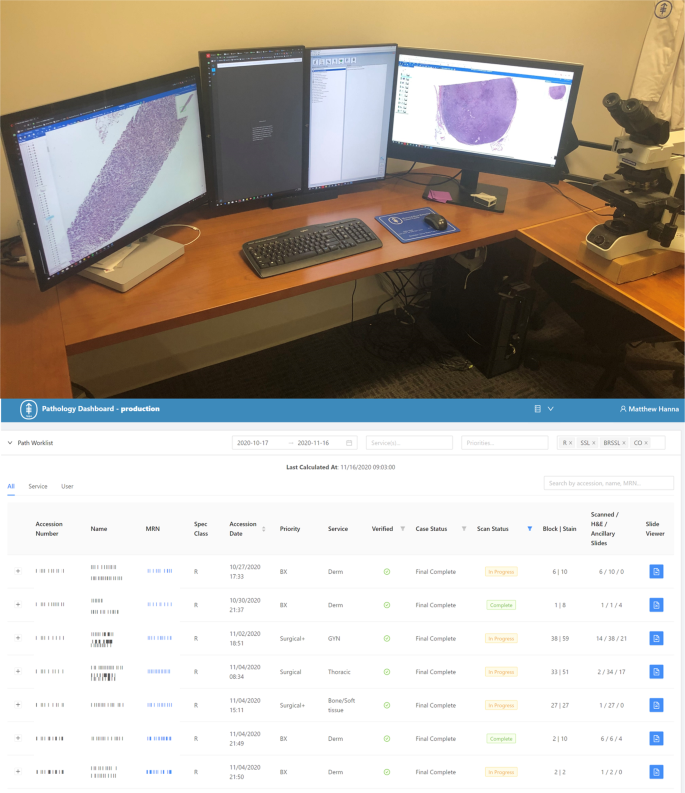
Digital transformation of a pathologist workstation has yet to be seamlessly realized. The pathologist digital workstation should integrate routine pathology workflows including multiple data typically found in the LIS such as patient clinical information, specimen data, digital image viewing, and integrated diagnostic reporting. In general, a pathologist workstation may have two or more computer monitors, ideally with one high-resolution display devoted entirely to the viewing of the whole slide images (Fig. 5) Most pathologists use a conventional computer mouse to view and navigate digital slides, however this may be time inefficient and several other input devices (e.g., 3Dmouse, trackpad, trackball, joystick, and touchscreen) have been evaluated35,36.
(Top) Pathologist workstation with multiple monitors can be used for navigating whole slide images on higher resolution monitors and incorporating the laboratory information system and electronic medical record or radiology systems in other screens, if available. Ideally, at least a dual screen monitor configuration should be used. (Bottom) Digital worklist software is critical to driving clinical workflows in a complete digital pathology operation as pathologists will need to know which cases have completed scanning and have been assigned.
LIS versus PACS
Pathology departments typically have a LIS-centric workflow. In contrast to this, radiology workflows are almost entirely Picture Archive and Communication System (PACS)-based, where all processes are centered around the patient imaging data. There are subtle, yet important differences in these two approaches, and while both medical domains are imaging based, clinical reporting workflows are vastly different between them. For instance, radiology clinical worklists are dynamic and are shared amongst various radiologists based on times of a given service day, modality, or specific organ system. All interpretative findings are reported for each radiology study with any further additional imaging having its own report and potentially being reported by a different radiologist. Pathologists are similarly assigned to cases based on scheduling, subspecialty, or procedure. However, pathologist workloads are also based on timing of multiple pre-analytic procedures, laboratory schedules, or shifts. Also, the initially assigned pathologist on a given case may order ancillary studies and will aggregate all findings of those tests into a single report. A dynamic worklist mentality would be a paradigm shift in pathology workflows and is currently impractical. Additionally, there have been a relative few “fully digital” laboratories to date, in addition to laboratories having adopted a hybrid approach. In the hybrid model, certain cases are digitally available, however glass slide and brightfield microscopes are also needed for case review either due to insufficient scanning throughput, challenging specimen or tissue types, or other needs. For those reasons, pathology has mostly maintained a LIS-centric adoption.
As the LIS is the center of pathology clinical workflows, digital pathology system integration into the LIS is highly recommended, and in most instances required. Negotiations with digital pathology system vendors and LIS vendors may require considerable planning and devotion of resources. Early introductions and discussions between vendors and the local deployment site should occur. LIS integration serves tremendous value to maintain workflows and processes where all of the other pathology metadata can be readily found. The LIS also serves as the primary site where the patient report is generated and authorized. The level of LIS integration with the digital pathology system requires file system locations in vendor databases with correlative reference identification of each slide and case in the LIS. This allows the LIS to offer flexibilities to the pathologist to identify which glass slides have digital slides available and inclusion of their respective metadata (e.g., part, block, stain, and scan resolution) in whole slide image viewers. Unique LIS interfaces are required for each vendor platform, which can individually launch WSI into separate vendor viewing platforms or a single vendor-agnostic WSI viewer. Each LIS vendor system varies in the capabilities and digital pathology features, thus it is critical to understand the technical opportunities and limitations available at the respective organization. Whole slide image viewing software may be implemented as a standalone viewer integrated with the LIS, however case management systems are also available for implementation. These software allow searching and manipulation of cases outside of the LIS, where through deeper LIS integration, other relevant data from the LIS are visible with the case management system. Additional features of such systems include enhanced user interfaces and intelligent workflows, case/metadata searching, workflow prioritization, tagging, education and conferencing tools, as well as potential for integrated clinical decision support tools. A third deployment strategy includes the use of an enterprise imaging system, whereby all imaging data from an institution are centrally stored and various dedicated applications are called upon based on file type or use case. The LIS centric or case management solutions are not mutually exclusive to having an enterprise imaging system. Challenges remain as certain digital pathology system vendors have proprietary file formats that may not work interchangeably with varying LIS or image viewers. Therefore, an enterprise imaging system may be insightful for organizations using various digital pathology system vendors to integrate with vendor-agnostic or vendor-specific viewers.
Technical considerations
Digital pathology systems are relatively new to enterprise networks, and implementation of these systems will benefit from inclusion and discussions with all relevant enterprise stakeholders (e.g., network, security, storage, information technology, informatics, clinical leads, etc). Involvement from each team will ensure a seamless integration and allow various participants’ inputs. For instance, enterprise network systems may not be familiar with whole slide image file extensions (e.g., SVS, NDPI, MRXS, and ISYNTAX). At our institution this resulted in WSI viewing that was initially throttled as files were being continuously scanned for viruses and other malware by security software as the file formats were not the typical file extensions that security teams were used to monitoring. These negatively affected WSI viewing performance and worsened pathologist’s experience using digital pathology until discussions ensued to resolve the throttling of such file extensions. Furthermore, data management is also critical to maintaining a successful digital pathology system operation. Data transfer speeds should be considered as most high-throughput scanners recommend 1 gigabit/s network connectivity from the whole slide scanner to the database server and 10 gigabit/s connectivity to the image storage location. Access and administration of digital pathology system vendor databases can facilitate data capture and monitoring of the digital pathology repository and associated metadata. Research and education use cases required robust de-identification systems for internal and external collaboration, especially for data gathering to train, test, and validate machine learning models. The whole slide image viewer is another critical software component that requires compatibility with the whole slide images being generated. For a multi-vendor digital pathology system, a single vendor-agnostic WSI viewer would provide the best training and user experience. The alternative is having each vendor’s WSI viewer launch to view slides from each vendor scanner system. This would require pathologists to learn various viewer applications, user interfaces, and functions and would also split viewing of clinical cases in multiple viewers for cases where slides were scanned on multiple vendor scanners, potentially adding delays and QC issues. Additionally, monitor display considerations for clinical review of digital pathology are becoming of increasing interest37,38,39. Variables affecting the clinical perspective include display resolution and screen size. High power field quantification (e.g., mitosis) in the digital workflow needs to be considered and evaluating comparing to clinical diagnostic criteria37. Another goal for clinical implementation should be to assess user experience to ensure appropriate pathologist buy-in, and improve workflows based of the collected feedback.
Use cases
Digital pathology implementation will differ based on the needs of individual laboratories. The type as well as number of use cases will vary based on enterprise initiatives, organizational leadership, specimen volumes, geographical location, available resources and budget. Use cases in digital pathology include, but are not limited to: primary diagnosis, second opinion (e.g., consultation), telepathology, quality assurance, archiving and sharing, education, conferencing, image analysis/machine learning, research/publications, marketing and business, tracking, and tissue procurement, amongst others. Several publications highlighting the use cases and advantages of digital pathology are available6,11,40,41,42,43,44,45,46,47. Appraisal of each use case is out of scope for this clinical implementation review, however use cases are defined in Table 2. Each use case should be properly scoped and vetted for achieving goals within the organization to fulfill their vision and mission statements.
Business case
Developing a business case for implementation of digital pathology systems is essential and dependent on the organizational use cases and vision for innovation. Cost analysis and return on investment should be properly scoped to include required personnel, hardware, software, service agreements, information technology infrastructure and interfaces, digital storage (in comparison to glass slide storage), and off-site vendor services, if applicable. The return on investment from digital pathology has largely been shown as either patient safety, quality improvements, efficiency (e.g., time), or related cost savings48,49. A large distributed health network analyzed their potential productivity and laboratory consolidation, as well as avoidance in treatment costs due to increased access to subspecialty expertise. Their projected costs savings with a full scale enterprise wide digital pathology adoption after 5 years was ~$18 million50. A pilot study from the University Health Network, Toronto also showed their digital pathology implementation enabled the ready access of whole slide images to be available for pathologist review between 1 and 4 days sooner than glass slides. This demonstrated a 2-day improvement in turnaround time for final reporting of clinical cases. Their calculated cost savings per year were $CA 131,000 in courier costs, travel, and accommodations51. Data from our own institution showed a decrease in turnaround time for reported cases with digital images by 1 day. In addition, a $1.3 million USD 5-year cost savings from digital pathology implementation in comparison to routine glass slide workflows was projected8. Centers in The Netherlands with well-established digital pathology adoption assessed various laboratory roles and their tasks in a typical workday and found more than 19 h collectively saved per day using digital pathology52. Several institutions in California utilizing a telepathology service currently validated at five centers similarly showed telepathology provided shortened turnaround time and significant financial savings53.
Replicating the brightfield microscope purely for digital review of whole slide images as a singular use case may not derive significant benefit, as data suggests pathologists unassisted digital review time of a whole slide image is less efficient when compared to glass slide review time2,54,55. However, with the advent of machine learning and other clinical decision support tools, additional value and efficiency has been shown for certain detection and quantification tasks. Raciti et al. showed that by using clinical decision support tools, there is an average increase of pathologist sensitivity in detection of prostate adenocarcinoma in core needle biopsies by 16%56. Similarly, automated detection of prostate adenocarcinoma showed estimated reduction in diagnostic review time by 65.5%57. Artificial intelligence (AI) related to detection of mitosis in breast cancer has shown an overall time savings of 27.8% with increased accuracy58. Introduction of AI automation of acid fast bacilli had average overall increase in sensitivity by ~15% with a 2.5% increase in overall accuracy, however with significant increases in efficiency59.
The initial investment in digital pathology systems and potential business cases vary and projected value in digital pathology may be realized based on needed use cases and workflows. Centers with hybrid (e.g., digital and analog) systems will likely incur the most cost as they are required to maintain duplicative, parallel workflows with the same resources. Hybrid workflows require additional staffing, space, storage, and other support, and several benefits of a fully digital pathology laboratory may not be realized with such a design. However, mandating complete digital transformation without appropriate buy-in and comfort level would be disruptive to clinical practice, and most institutions will rely on a step wise approach to complete digital transformation. Determination of success after digital pathology implementation may be derived from experience surveys, analysis of utilization, turnaround time assessments, or medium to long term cost analyses.
Implementation and validation
Institutions contemplating their digital transformations have options for their implementation roadmap. Most undergo a phased approach remediating challenges as they arise, however other laboratories with dedicated resources or new initiatives, may be able to achieve success with an end to end implementation for all intended uses case. The digital pathology systems are composed of hardware (e.g., whole slide scanner, monitor) and software (e.g., whole slide image viewer, decision support tools) components. The quantity and variety of each component will be based on the laboratories intended use cases.
To date, no whole slide scanner can encompass all pathology needs (including cytology and other cellular specimens) such as traditional and whole mount glass slide formats, continuous loading capabilities, high-resolution scanning, with rapid scan speeds. For this reason, institutions with high complexity anatomic pathology testing may opt to acquire multiple digital pathology systems to use the best technology for each specified intended use case. While this enables image acquisition of all use cases, it requires maintenance and expense of multiple vendor service contracts, hosting of varied technological infrastructure, added training to staff, and lack of interoperability. Whole slide image viewer performance should also be evaluated to ensure expeditious navigation of launching cases from the LIS or image management system, loading of other digital slides, and field of view navigation.
Validation is a requirement for any digital pathology system used for clinical purposes. Systems should be validated based on how they will be used clinically. Validations can include regulatory cleared and non-cleared devices for clinical use, with documented disclaimers on surgical pathology reports, if appropriate. The CAP has published updated guidelines for validation of whole slide imaging systems60,61. Numerous validation studies have been published following these guidelines2,3,25,26,53,54,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86. Clinical utilization of digital pathology systems should also be revalidated whenever a significant change is made to any of the prior validated components or the intended use.
Future
Clinical implementation of digital pathology systems requires planning and coordination with various stakeholders. Current LIS are lacking in extensive digital pathology functionality and digital pathology software suites are becoming increasingly available to support such workflows. These include integrations of clinical decision support tools through machine learning models or image analysis software that is embodied within a single workflow, without the need to launch several applications. Future success of digital pathology software will depend on interoperability and available support of routine pathology workflows. Evidence exists to show the non-inferiority of whole slide images to glass slides, however the real value in using digital pathology is not merely to replicate the microscope, but to offer ready access to digital slides from any location, innovative workflows, advancing pathology through clinical transformative solutions using machine learning and decision support tools.
Change history
13 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41379-021-00948-x
09 November 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41379-021-00968-7
References
Rogers E. M. Diffusion of Innovations, 5th edn, (Free Press, 2003).
Hanna, M. G. et al. Whole slide imaging equivalency and efficiency study: experience at a large academic center. Mod. Pathol. 32, 916–928 (2019).
Hanna, M. G. et al. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod. Pathol. 33, 2115–2127 (2020).
Weinstein, R. S., Holcomb, M. J. & Krupinski, E. A. Invention and Early History of Telepathology (1985-2000). J. Pathol. Inform. 10, 1 (2019).
Park, S. et al. The history of pathology informatics: a global perspective. J. Pathol. Inf. 4, 7 (2013).
Pantanowitz, L. et al. Twenty years of digital pathology: an overview of the road travelled, what is on the horizon, and the emergence of vendor-neutral archives. J. Pathol. Inform. 9, 40 (2018).
Farahani, N., Parwani, A. V. & Pantanowitz, L. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives. Pathol. Lab. Med. Int. 7, 23–33 (2015).
Hanna, M. G. et al. Implementation of digital pathology offers clinical and operational increase in efficiency and cost savings. Arch. Pathol. Lab. Med. 143, 1545–1555 (2019).
Hanna, M. G. & Pantanowitz, L. Bar coding and tracking in pathology. Surg. Pathol. Clin. 8, 123–135 (2015).
Slaw, R. J. et al. Doctor, We Shrunk the Cost (and the Tissue)! How Digitizing Control Slides Led to Cost Savings and Tissue Size Reduction. J Pathol Inform 8, 20 (2017).
Zarella, M. D. et al. A practical guide to whole slide imaging: a white paper from the digital pathology association. Arch. Pathol. Lab. Med. 143, 222–234 (2019).
Randell, R., Ruddle, R. A. & Treanor, D. Barriers and facilitators to the introduction of digital pathology for diagnostic work. Stud. Health Technol. Inform. 216, 443–447 (2015).
Browning, L. et al. Implementation of digital pathology into diagnostic practice: perceptions and opinions of histopathology trainees and implications for training. J. Clin. Pathol. 73, 223–227 (2020).
Retamero, J. A., Aneiros-Fernandez, J., Del & Moral, R. G. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch. Pathol. Lab. Med. 144, 221–228 (2020).
Fraggetta, F., Garozzo, S., Zannoni, G. F., Pantanowitz, L. & Rossi, E. D. Routine digital pathology workflow: the Catania experience. J. Pathol. Inform. 8, 51 (2017).
College of American Pathologists (2020) Policy PP. Minimum period of retention of laboratory records and materials, (CAP) https://elss.cap.org/elss/ShowProperty?nodePath=/UCMCON/Contribution%20Folders/WebApplications/pdf/retention-laboratory-records-and-materials.pdf (accessed 13 April 2021).
Hanna, M. G., Parwani, A. & Sirintrapun, S. J. Whole slide imaging: technology and applications. Adv. Anat. Pathol. 27, 251–259 (2020).
Baskota, S. U., Wiley, C. & Pantanowitz, L. The next generation robotic microscopy for intraoperative teleneuropathology consultation. J. Pathol. Inform. 11, 13 (2020).
Sirintrapun, S. J. et al. Robotic telecytology for remote cytologic evaluation without an on-site cytotechnologist or cytopathologist: a tale of implementation and review of constraints. J. Pathol. Inform. 8, 32 (2017).
Sirintrapun, S. J. et al. Robotic telecytology for remote cytologic evaluation without an on-site cytotechnologist or cytopathologist: an active quality assessment and experience of over 400 cases. J. Pathol. Inform. 8, 35 (2017).
Dunn, B. E. et al. Dynamic-robotic telepathology: department of veterans affairs feasibility study. Hum. Pathol. 28, 8–12 (1997).
Dunn, B. E., Choi, H., Recla, D. L., Kerr, S. E. & Wagenman, B. L. Robotic surgical telepathology between the Iron Mountain and Milwaukee Department of Veterans Affairs Medical Centers: a 12-year experience. Hum. Pathol. 40, 1092–1099 (2009).
Evans, A. J. et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: the University Health Network experience. Semin. Diagn. Pathol. 26, 165–176 (2009).
Leong, F. J. W.-M., Nicholson, A. G. & McGee, J. O. Robotic telepathology: efficacy and usability in pulmonary pathology. J. Pathol. 197, 211–217 (2002).
Menter, T., Nicolet, S., Baumhoer, D., Tolnay, M. & Tzankov, A. Intraoperative frozen section consultation by remote whole-slide imaging analysis -validation and comparison to robotic remote microscopy. J. Clin. Pathol. 73, 350–352 (2020).
Thrall, M. J., Rivera, A. L., Takei, H. & Powell, S. Z. Validation of a novel robotic telepathology platform for neuropathology intraoperative touch preparations. J. Pathol. Inform. 5, 1 (2014).
Pradhan, D. et al. Evaluation of panoramic digital images using Panoptiq for frozen section diagnosis. J. Pathol. Inform. 7, 26 (2016).
Roy, S. et al. Smartphone adapters for digital photomicrography. J. Pathol. Inform. 5, 24 (2014).
Groen, R. et al. Application of microscope-based scanning software (Panoptiq) for the interpretation of cervicovaginal cytology specimens. Cancer Cytopathol. 125, 918–925 (2017).
Hanna, M. G. et al. Comparison of glass slides and various digital-slide modalities for cytopathology screening and interpretation. Cancer Cytopathol. 125, 701–709 (2017).
Lin, O., Rudomina, D., Feratovic, R. & Sirintrapun, S. J. Rapid on-site evaluation using telecytology: a major cancer center experience. Diagn. Cytopathol. 47, 15–19 (2019).
Clunie, D. et al. Digital imaging and communications in medicine whole slide imaging connectathon at digital pathology association pathology visions 2017. J. Pathol. Inform. 9, 6 (2018).
Janowczyk, A., Zuo, R., Gilmore, H., Feldman, M. & Madabhushi, A. HistoQC: an open-source quality control tool for digital pathology slides. JCO Clin. Cancer Inform. 3, 1–7 (2019).
Chen, Y. et al. Assessment of a computerized quantitative quality control tool for whole slide images of kidney biopsies. J. Pathol. 253, 268–278 (2021).
Molin, J., Lundström, C. & Fjeld, M. A comparative study of input devices for digital slide navigation. J. Pathol. Inform. 6, 7 (2015).
Mateos E. A. et al. Research on Devices for Handling Whole Slide Images on Pathology Workstations. An Ergonomic Outlook. Diagn Pathol 2: (2016)
Kim, D. et al. (Re) Defining the high-power field for digital pathology. J. Pathol. Inform. 11, 33 (2020).
Abel, J. T. et al. Display characteristics and their impact on digital pathology: a current review of pathologists’ future ‘microscope’. J. Pathol. Inform. 11, 23 (2020).
Clarke, E. L., Munnings, C., Williams, B., Brettle, D. & Treanor, D. Display evaluation for primary diagnosis using digital pathology. J. Med. Imaging Bellingham Wash 7, 027501 (2020).
Williams, B. J., Bottoms, D. & Treanor, D. Future-proofing pathology: the case for clinical adoption of digital pathology. J. Clin. Pathol. 70, 1010–1018 (2017).
Luo, W. & Hassell, L. A. Use cases for digital pathology. In Digital Pathology: Historical Perspectives, Current Concepts & Future Applications, (eds Kaplan, K. J. & Rao, L. K. F.) pp. 5–15 (Springer International Publishing, 2016).
Jahn, S. W., Plass, M. & Moinfar, F. Digital pathology: advantages, limitations and emerging perspectives. J. Clin. Med. 9, 3697 (2020).
Niazi, M. K. K., Parwani, A. V. & Gurcan, M. N. Digital pathology and artificial intelligence. Lancet Oncol. 20, e253–e261 (2019).
Tizhoosh, H. R. & Pantanowitz, L. Artificial intelligence and digital pathology: challenges and opportunities. J. Pathol. Inform. 9, 38 (2018).
Higgins, C. Applications and challenges of digital pathology and whole slide imaging. Biotech. Histochem. 90, 341–347 (2015).
Pallua, J. D., Brunner, A., Zelger, B., Schirmer, M. & Haybaeck, J. The future of pathology is digital. Pathol. Res. Pract. 216, 153040 (2020).
Volynskaya, Z. et al. Integrated pathology informatics enables high-quality personalized and precision medicine: digital pathology and beyond. Arch. Pathol. Lab. Med. 142, 369–382 (2018).
Lujan, G. et al. Dissecting the business case for adoption and implementation of digital pathology: a white paper from the digital pathology association. J. Pathol. Inform. 12, 17 (2021).
Williams, B. J., Bottoms, D., Clark, D. & Treanor, D. Future-proofing pathology part 2: building a business case for digital pathology. J. Clin. Pathol. 72, 198–205 (2019).
Ho, J. et al. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. J. Pathol. Inform. 5, 33 (2014).
Evans, A. J., Vajpeyi, R., Henry, M. & Chetty, R. Establishment of a remote diagnostic histopathology service using whole slide imaging (digital pathology). J. Clin. Pathol. 74, 421–424 (2020).
Baidoshvili, A. et al. Evaluating the benefits of digital pathology implementation: time savings in laboratory logistics. Histopathology 73, 784–794 (2018).
Chong, T. et al. The California Telepathology Service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. J. Pathol. Inform. 10, 31 (2019).
Thrall, M. J., Wimmer, J. L. & Schwartz, M. R. Validation of multiple whole slide imaging scanners based on the guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch. Pathol. Lab. Med. 139, 656–664 (2015).
Mills, A. M. et al. Diagnostic efficiency in digital pathology: a comparison of optical versus digital assessment in 510 surgical pathology cases. Am. J. Surg. Pathol. 42, 53–59 (2018).
Raciti, P. et al. Novel artificial intelligence system increases the detection of prostate cancer in whole slide images of core needle biopsies. Mod. Pathol. 33, 2058–2066 (2020).
da Silva, L. M. et al. Independent real-world application of a clinical-grade automated prostate cancer detection system. J. Pathol. 254, 147–158 (2021).
Pantanowitz, L. et al. Accuracy and efficiency of an artificial intelligence tool when counting breast mitoses. Diagn. Pathol. 15, 80 (2020).
Pantanowitz, L. et al. Artificial intelligence-based screening for mycobacteria in whole-slide images of tissue samples. Am. J. Clin. Pathol. 156, 117–128 (2021).
Pantanowitz, L. et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch. Pathol. Lab. Med. 137, 1710–1722 (2013).
Evans, A. J. et al. Validating whole slide imaging systems for diagnostic purposes in pathology: guideline update from the College of American Pathologists in Collaboration With the American Society for Clinical Pathology and the Association for Pathology Informatics. Arch. Pathol. Lab. Med. (2021). Epub ahead of print.
Araújo, A. L. D. et al. Fully digital pathology laboratory routine and remote reporting of oral and maxillofacial diagnosis during the COVID-19 pandemic: a validation study. Virchows Arch. 479, 585–595 (2021).
Zelic, R. et al. Interchangeability of light and virtual microscopy for histopathological evaluation of prostate cancer. Sci Rep. 11, 3257 (2021).
Diniz, P.-B. et al. Comparison of the whole slide imaging and conventional light microscopy in the grading of oral epithelial dysplasia: a multi-institutional study. Med. Oral Patol. Oral Cir. Bucal 26, e8–e13 (2021).
Alassiri, A. et al. Whole slide imaging compared with light microscopy for primary diagnosis in surgical neuropathology: a validation study. Ann. Saudi Med. 40, 36–41 (2020).
Al Habeeb, A., Evans, A., Ghazarian, D. Virtual microscopy using whole-slide imaging as an enabler for teledermatopathology: a paired consultant validation study. J. Pathol. Inform. 3, 2 (2012).
Araújo, A. L. D. et al. Validation of digital microscopy in the histopathological diagnoses of oral diseases. Virchows Arch. 473, 321–327 (2018).
Bauer, T. W. et al. Validation of whole slide imaging for primary diagnosis in surgical pathology. Arch Pathol. Lab. Med. 137, 518–524 (2013).
Brunelli, M. et al. iPathology cockpit diagnostic station: validation according to College of American Pathologists Pathology and Laboratory Quality Center recommendation at the Hospital Trust and University of Verona. Diagn. Pathol. 9(Suppl 1), S12 (2014).
Buck, T. P., Dilorio, R., Havrilla, L. & O’Neill, D. G. Validation of a whole slide imaging system for primary diagnosis in surgical pathology: a community hospital experience. J. Pathol. Inform. 5, 43 (2014).
Fónyad, L. et al. Validation of diagnostic accuracy using digital slides in routine histopathology. Diagn. Pathol. 7, 35 (2012).
Jukić, D. M., Drogowski, L. M., Martina, J. & Parwani, A. V. Clinical examination and validation of primary diagnosis in anatomic pathology using whole slide digital images. Arch. Pathol. Lab. Med. 135, 372–378 (2011).
Mpunga, T. et al. Implementation and Validation of Telepathology Triage at Cancer Referral Center in Rural Rwanda. J. Glob. Oncol. 2, 76–82 (2016).
Campbell, W. S. et al. Concordance between whole-slide imaging and light microscopy for routine surgical pathology. Hum. Pathol. 43, 1739–1744 (2012).
Cheng, C. L. et al. Enabling digital pathology in the diagnostic setting: navigating through the implementation journey in an academic medical centre. J. Clin. Pathol. 69, 784–792 (2016).
Goacher, E., Randell, R., Williams, B. & Treanor, D. The diagnostic concordance of whole slide imaging and light microscopy: a systematic review. Arch. Pathol. Lab. Med. 141, 151–161 (2017).
Houghton, J. P. et al. Concordance between digital pathology and light microscopy in general surgical pathology: a pilot study of 100 cases. J. Clin. Pathol. 67, 1052–1055 (2014).
Mukhopadhyay, S. et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology. Am. J. Surg. Pathol. 42, 39–52 (2018).
Snead, D. R. J. et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology 68, 1063–1072 (2016).
Tabata, K. et al. Whole-slide imaging at primary pathological diagnosis: validation of whole-slide imaging-based primary pathological diagnosis at twelve Japanese academic institutes. Pathol. Int. 67, 547–554 (2017).
Al-Janabi, S. et al. Whole slide images for primary diagnostics of urinary system pathology: a feasibility study. J. Ren. Inj. Prev. 3, 91–96 (2014).
Arnold, M. A. et al. The College of American Pathologists guidelines for whole slide imaging validation are feasible for pediatric pathology: a pediatric pathology practice experience. Pediatr. Dev. Pathol. 18, 109–116 (2015).
Campbell, W. S. et al. Whole slide imaging diagnostic concordance with light microscopy for breast needle biopsies. Hum. Pathol. 45, 1713–1721 (2014).
Krishnamurthy, S. et al. Multi-institutional comparison of whole slide digital imaging and optical microscopy for interpretation of hematoxylin-eosin-stained breast tissue sections. Arch. Pathol. Lab. Med. 137, 1733–1739 (2013).
Ordi, J. et al. Validation of whole slide imaging in the primary diagnosis of gynaecological pathology in a University Hospital. J. Clin. Pathol. 68, 33–39 (2015).
Reyes, C., Ikpatt, O. F., Nadji, M. & Cote, R. J. Intra-observer reproducibility of whole slide imaging for the primary diagnosis of breast needle biopsies. J. Pathol. Inform. 5, 5 (2014).
Acknowledgements
The authors would like to thank the Warren Alpert Center for Digital and Computational Pathology for supporting clinical and investigational use of digital pathology at our Center. We also acknowledge the Operational Excellence team, digital scanning team, laboratory and pathology IT staff for continuous support throughout the Department’s digital pathology journey.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial interest in the products or companies described in this article. Unrelated competing interests include MGH and DK are consultants for PaigeAI.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to an error in the title.
The original online version of this article was revised: Due to one author being omitted.
Rights and permissions
About this article
Cite this article
Hanna, M.G., Ardon, O., Reuter, V.E. et al. Integrating digital pathology into clinical practice. Mod Pathol 35, 152–164 (2022). https://doi.org/10.1038/s41379-021-00929-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00929-0
This article is cited by
-
Artificial intelligence applications in histopathology
Nature Reviews Electrical Engineering (2024)
-
Full resolution reconstruction of whole-mount sections from digitized individual tissue fragments
Scientific Reports (2024)
-
Mitigating Bias in Clinical Machine Learning Models
Current Treatment Options in Cardiovascular Medicine (2024)
-
Quantitative multiplexed imaging technologies for single-cell analysis to assess predictive markers for immunotherapy in thoracic immuno-oncology: promises and challenges
British Journal of Cancer (2023)
-
Interoperable slide microscopy viewer and annotation tool for imaging data science and computational pathology
Nature Communications (2023)